Abstract
Background
Light touch with a stable object reduces postural sway by increasing axial postural tone in healthy subjects. However, it is unknown whether subjects with Parkinson’s disease (PD), who have more postural sway and higher axial postural tone than healthy subjects, can benefit from haptic touch.
Objective
To investigate the effect of light and heavy touch on postural stability and hip tone in subjects with PD.
Methods
Fourteen subjects with mid-stage PD, and 14 healthy control subjects were evaluated during quiet standing with eyes closed with their arms: 1) crossed, 2) lightly touching a fixed rigid bar in front of them and 3) firmly gripping the bar. Postural sway was measured with a forceplate and axial hip tone was quantified using a unique device that measures the resistance of the hips to yaw rotation while maintaining active stance.
Results
Subjects with PD significantly decreased their postural sway with light or heavy touch (p<0.001 vs. arms crossed), similarly as control subjects. Without touch, hip tone was larger in PD subjects. With touch, however, tone values were similar in both groups. This change in hip tone with touch was highly correlated with the initial amount of tone (PD: r=− 0.72 to −0.95 and controls: r=−0.74 to−0.85).
Conclusions
We showed, for the first time, that subjects with PD benefit from touch similarly to control subjects and that despite higher axial postural tone, PD subjects are able to modulate their tone with touch. Future studies should investigate the complex relationship between touch and postural tone.
Keywords: Balance, postural stability, haptic touch, postural tone, center of pressure
Introduction
It is well-known that haptic touch or light touch with a stable object reduces postural sway in young (1–3) and elderly subjects (4) as well as in patients with peripheral neuropathy (5), vestibular loss (6) and stroke (7). Yet, it is unknown whether subjects with Parkinson’s disease (PD) can benefit from haptic touch to improve postural instability, one of their cardinal symptoms. Understanding the benefits of light touch during standing or walking may have significant implications in rehabilitation for the use of walking aids (e.g., cane, walking sticks, walker, see Constantinescu et al (8)) and development of strategies to enhance stability and prevent falls.
Haptic sense combines cutaneous and kinaesthetic (proprioceptive) inputs and numerous studies have observed haptic and kinesthetic deficits in both the early- and mid-stages of PD (9–16) along with difficulties in integrating somatosensory information (15, 17, 18). These difficulties in processing somatosensory information might reduce the stabilizing effect of light or heavy touch with a stable object.
During light touch contact, when forces applied are physically inadequate to stabilize the body, cutaneous cues from the fingertip and proprioceptive arm inputs are important in providing sensory feedback to reduce postural sway (2, 3, 16). Nevertheless, the neural mechanism that mediates this effect is controversial. Correlations among body sway, fingertip shear forces and postural muscle activity, suggest that a feedforward activation of postural muscles based on fingertip shear force is involved in stabilizing postural sway during light touch (16). We recently observed a relationship between postural stability and axial postural tone during haptic touch suggesting that touch decreases postural sway because of increased axial postural tone due to a change in earth-stable reference frame (1). With our unique ‘Twister’ device, axial hip tone can be measured while subjects actively maintain equilibrium during stance (1, 11, 19, 20). We have shown a significantly increased hip tone during both light and heavy touch of a stable object in young healthy controls. In addition, we found that the subjects’ perception changed with the touch condition. Without touch, subjects wrongly perceived that their trunk rotated in space when in fact, it was their feet that were rotated. During the light or heavy touch condition, these same subjects correctly perceived their feet rotation (1). Thus, reduced postural sway was associated with significant increases in axial tone and perception of motion consistent with the central nervous system changing from using a global reference to a local, trunk reference frame for the control of posture during touch.
Since subjects with PD have shown increased axial postural tone (11, 19), increased postural sway (21, 22), and impaired somatosensory integration (15, 17, 18) they may also have difficulty modifying their postural tone and sway with touch of a stable surface. In this study, we hypothesized that subjects with PD would not be able to significantly decrease postural sway with light touch because they could not increase their already high axial postural tone (11, 19).
Methods
Subjects
Fourteen healthy male control subjects (64 ± 9 years old) and 14 male subjects (64 ± 8 years) with a clinical diagnosis of “idiopathic” PD, treated with levodopa participated in the study. The subjects were matched for age, weight and height. PD subjects’ characteristics are presented in Table I and more details are available in Franzén et al (19). None of the subjects with PD had a history suggesting “atypical” PD symptoms, as defined by Hughes et al (23), or other existing neuromuscular disorders, including severely stooped posture. Subjects with PD with a Hoehn &Yahr score of 2 or 3 were included. The healthy control subjects did not present with recent or unresolved history of musculoskeletal, neuromuscular or motor disorders. All subjects participating in our study could stand independently for at least 10 minutes. All subjects provided informed consent in accordance to the Oregon Health & Science University Internal Review Board regulations for human subjects’ studies and the Helsinki Declaration.
Table I.
Subject characteristics in the PD and control group, mean (SD)
| PD | Controls | |
|---|---|---|
| Age (yrs) | 64 (8) | 64 (9) |
| Weight (kg) | 81 (10) | 78 (9) |
| Height (cm) | 176 (6) | 174 (6) |
| Duration of PD (yrs) | 7 (4) | - |
| Side (R/L) | 9R/5L | - |
| UPDRS Motor part (ON/OFF) | 24 (8) / 33 (10) | - |
| Hoehn & Yahr | 2.5 (0.5) | - |
| UPDRS ADL part | 12 (6) | - |
Duration of PD-years since the PD diagnosis, Side – affected side, L - left, R - right, ADL-Activities of Daily Living.
Protocol
The subjects with PD were assessed OFF medication (OFF), the morning after abstaining from levodopa overnight (washout period ≥12 h). The measurement of postural sway and axial tone at the hips was first assessed in three different touch conditions: no touch (NT), light touch (LT) and heavy touch (HT). Then, the motor part of the UPDRS (24) was administered after which PD subjects took their usual morning dose of levodopa medication. During the following rest period (1 hour), the subjects rated themselves on activities of daily living (ADL) as part of the UPDRS (24) and on the Activities of Balance Confidence scale (ABC) (25), to quantify activity limitations and balance confidence. Once the subjects with PD reported feeling “ON” (PD ON, ~60 min. after levodopa intake), the same postural sway, tone and UPDRS measurements were repeated, starting with the motor part of the UPDRS. The large decrease in UPDRS motor scores (27%, p<0.000002) from the OFF state to the ON state clearly indicates that PD subjects were ON levodopa at the time of testing. Control subjects performed all tests only once.
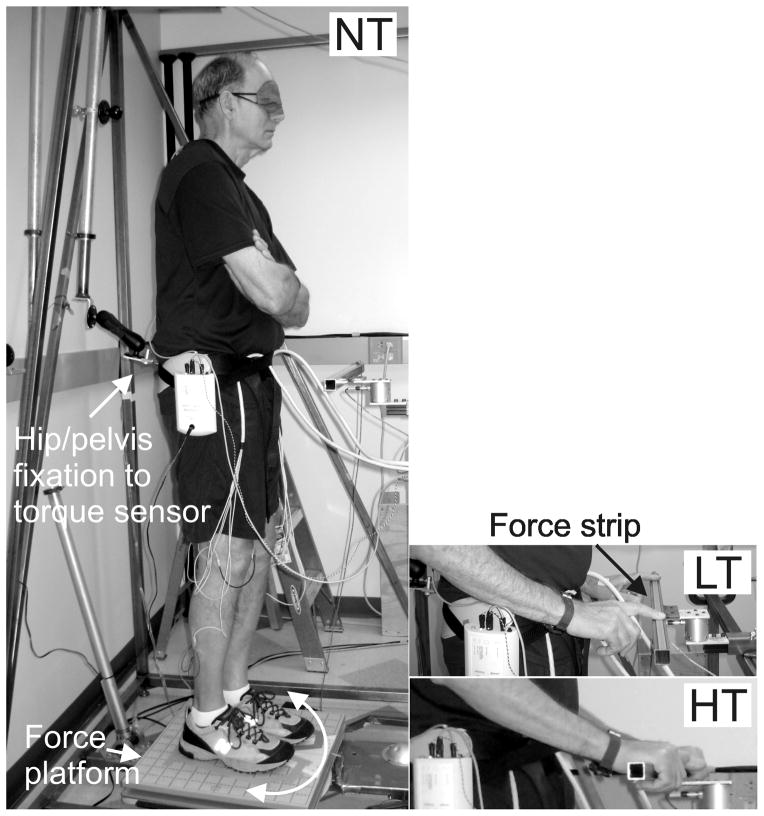
In all three conditions (NT, LT and HT), the subjects stood with eyes closed on a forceplate fixed to the support surface. Axial hip tone was measured by slowly rotating the surface while the hips were fixed to a suspension, which prevents rotation, without restricting horizontal and vertical motion (Figure 1A). In each condition, the surface moved clockwise and counterclockwise in relation to the hips, for 4 cycles (one trial of 4 cycles per condition). In the NT conditions, the subjects were instructed to stand with their arms crossed over their chest. Subjects were instructed to adapt to a natural upright standing position, to relax and to not resist the platform motion. During the LT condition, the subjects were instructed to lightly touch a stable bar with both index fingers without using the bar to provide support or resisting rotation (Figure 1B). During the HT condition, the subjects were instructed to grasp the stabilizing bar with both hands using a palmar grip and comfortably hold on without resisting rotation (Figure 1C). Adhesive tape was used to mark the feet location on the platform so that the same position was repeated for each trial. After every complete set of cycles, the subjects were encouraged either to sit or to walk about to prevent fatigue or stiffness. The order of the three conditions was randomized and the very first condition, considered a training trial, was rejected from analysis. This condition was later repeated and retained for analysis.
Figure 1.
Left: The force platform rotates the feet relative to the fixed hip/pelvis while the torque sensor measures hip resistance/torque during the no touch (NT) condition. Right top: During the light touch (LT) condition, subjects were touching with both index fingers on an adjustable rigid metal bar that was integrated into the external frame of the Twister device. A force sensing strip on the bar beeped if the subjects exceeded the threshold of 2 N. Right bottom: During the heavy touch (HT) condition, subjects were gripping the stabilizing bar with both hands.
Measurement of postural stability
An AMTI AccuSwayplus force platform (50 × 50 cm) (Advanced Mechanical Technology, Inc., Watertown, MA) was integrated into the device measuring hip torque. The force platform measured 3-dimensional forces (Fx, Fy, Fz) and 3-dimentional moments (Mx, My, Mz) to provide the location of the center of pressure (CoP).
Measurement of axial tone
Using the ‘Twister’ device, we quantified axial tone at the hips in all subjects during standing with and without touch. This device is uniquely designed to measure axial torque during active control of postural stability in standing while restricting movement only in axial torsion during yaw twisting. When standing in this device, subjects must use their axial and leg musculature to counteract gravity and to maintain equilibrium, thereby activating their postural tone. As illustrated in Figure 1, subjects stood blindfolded on a horizontal platform that slowly (1 deg/s) rotated left and right (±10 deg). The subjects were fixated at the hips with a double-hinged suspension apparatus, preventing only yaw motions in the axial plane and not allowing any support or earth reference in the horizontal or vertical directions (20). The twister device provides a repeatable and reliable measure of axial postural tone in healthy individuals (1, 20) and PD subjects (11, 19). In this protocol, torsional strain/resistance was measured about the hips’ yaw axis with a torque sensor (Futek TFF400, Irvine, CA) located between the frame and suspension apparatus, which was attached to a pelvic belt preventing any yaw rotation. A potentiometer signaled the angular position of the platform. The rate of rotation was constant to minimize effects of inertia, except during directional changes where the rotation followed a parabolic trajectory, reducing angular acceleration to <12 deg/s2 to ensure smooth changes in rotational direction.
For the trials with a touch condition, a 40 cm horizontal rigid metal bar was integrated into the external frame of the Twister (Figure 1). This adjustable stabilizing bar was positioned at waist height in front of the subject at a comfortable distance from the body midline for touching with elbows flexed at approximately 90 degrees. During the LT condition, subjects touched the bar with the tip of their two index fingers and during the HT condition, they grabbed the bar with both hands. A force sensing strip (Interlink electronics, Camarillo, CA, part no 408) mounted on the bar and coupled to a circuit with an adjustable threshold beeped if the subjects exceeded the threshold of 2 N (200g) as negative feedback for the LT condition. Only two PD subjects exceeded the threshold and the trials were rejected and repeated. One of those subjects needed to perform the LT condition with the left index finger only because of severe tremor in the right hand. This subject was unable to control the pressure with the right hand or maintain it on the bar.
The resistive torque of the hips and force platform data were recorded at 100 Hz using Spike2 software (Cambridge Electronic Devices, Cambridge, UK) and analyzed offline using Matlab software (Mathworks, Natick, MA).
Data analysis
Postural sway was measured from the displacement of the CoP during 2–4 cycles and cropped into the respective cycle and averaged. Motion of the CoP was quantified separately in the antero-posterior and medio-lateral directions by computing the root mean square (RMS) and the RMS mean velocity (26). The resistive torque in the hip during HT, LT and NT conditions was quantified as the mean peak-to-peak amplitude for 2–4 cycles from one direction of rotation to the next. The first cycle of rotation was excluded from the analysis of the torsional resistance since that cycle might be affected by the subject’s initial postural set or resting short-range stiffness (19). Torque asymmetry was analyzed as the difference in average torque during CW rotations and that during CCW rotation (11).
Statistical analysis
Statistical analysis for both postural stability and hip tone measures consisted of a two-way analysis of variance (ANOVA) on Group (PD OFF meds vs. controls) X Condition (NT, LT and HT) with repeated measures on the last factor to compare PD subjects to control subjects. To test for the effect of medication in the PD group (ON vs. OFF) X the conditions (NT, HT and LT), an ANOVA with repeated measurement on both factors was used. The significance level was set at p<0.01 to adjust for multiple ANOVAs. When significant main effects were found, Tukey HSD was used for post hoc analysis. The Pearson product moment correlation (r) and the coefficient of determination ( R2) was used to investigate the relationship between tone during touch (LT or HT) and tone without touch (NT) as well as the relation between tone and sway.
Results
Effects of haptic touch on postural stability
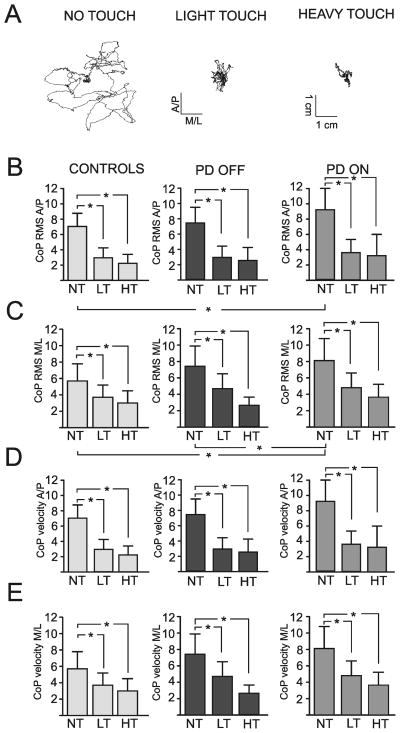
The groups were matched for age, weight and height (p>0.01). Stabilogram sway data from a representative subject with PD in the OFF state shows how much postural sway amplitude was reduced by both light touch and heavy touch during 30 seconds of stance (Figure 2A). As shown in Figure 2B and C, when lightly touching or gripping the bar, both PD OFF and control subjects significantly decreased their antero-posterior RMS sway amplitude by about 60% and the medio-lateral sway by ~45% compared to the no touch condition (F(2,52)=113.35, p=0.000 and F(2,52)=46.73, p=0.000, respectively). All subjects benefited equally from the touch or grip condition with no main effect of group (F(1,26)=0.26, p=0.616 and F(1,26)=2.52, p=0.124).
Figure 2.
A) xy-plot of the displacement of the CoP in both the anterio-posterior (A/P) and in the medio-lateral (M/L) direction that illustrates the changes in the center of pressure during light and heavy touch in comparison to the no touch condition in a representative subject with Parkinson’s disease without medication (OFF). B) The mean and SD of the root mean square (RMS) of the center of pressure (CoP) in A/P and C) M/L direction as well as the CoP velocity in D) A/P and E) M/L direction during no touch (NT), heavy touch (HT) and light touch (LT) in age-matched control subjects as well as in subjects with Parkinson’s disease (PD) OFF and ON their medication.
When gripping the bar, both PD OFF and control subjects also reduced their sway velocity significantly from the no touch condition in both antero-posterior and medio-lateral directions (F(2,52)=46.69, p=0.000 and F(2,52)=33.88, p=0.000, respectively, Figure 2C and D). Both groups also benefited from the LT condition by significantly reducing their sway velocity but significantly less than for the HT condition (p=0.015 and p=0.006). No main effects of group in either anterio-posterior or medio-lateral directions were evident (F(1,26)=1.03, p=0.319 and F(1,26)=0.73, p=0.404, respectively).
Effect of dopamine on postural stability during haptic touch
When subjects with PD were ON levodopa, their sway amplitude (RMS) increased significantly compared to OFF levodopa. The antero-posterior sway amplitude increased by 25% but did not reach significance (F(1,13)=6.36, p=0.026) whereas the medio-lateral sway significantly increased by15% (F(1,13)=9.22, p=0.010) ON levodopa. Consequently, with no touch, PD subjects in the ON state, but not the OFF state, showed larger postural sway (RMS in the AP and ML directions) than control subjects (p=0.027 and p=0.011, respectively). However, the improvement from HT or LT on sway variability (F(2,26)=50.36, p=0.000) was not affected by the use of levodopa. Sway velocity was not affected by the use of levodopa in either antero-posterior or medio-lateral directions (F(1,13)=2.10, p=0.171 and F(1,13)=0.17, p=0.688, respectively).
Effects of haptic touch on postural tone
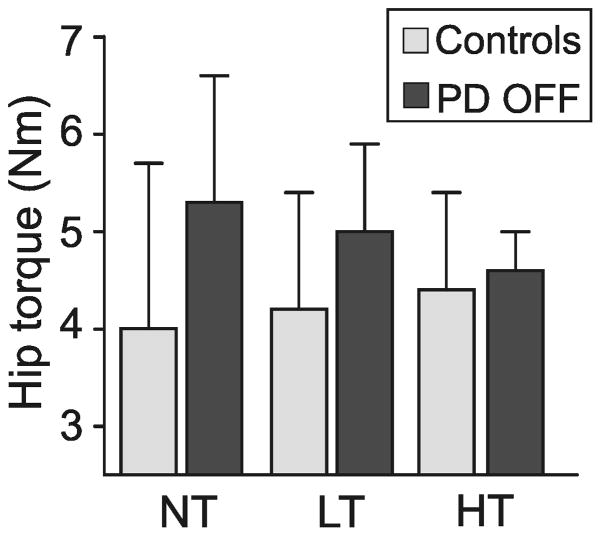
Overall, the hip postural tone was 16% higher in the PD OFF group than in the control group, but this difference did not reach significance (F(2,26)=4.77, p=0.038). When touching or gripping the bar, there were no significant group differences in hip tone (PD OFF vs. controls, p>0.01; see Figure 3).
Figure 3.
Illustrates the group main effect [F(2,26)=4.77, p=0.038] as well as the group mean and SD of the hip resistance to twisting the feet and legs ±10° at 1°/s during no touch (NT), light touch (LT) and heavy touch (HT) in subjects with Parkinson’s disease (PD) OFF their medication and in age-matched control subjects.
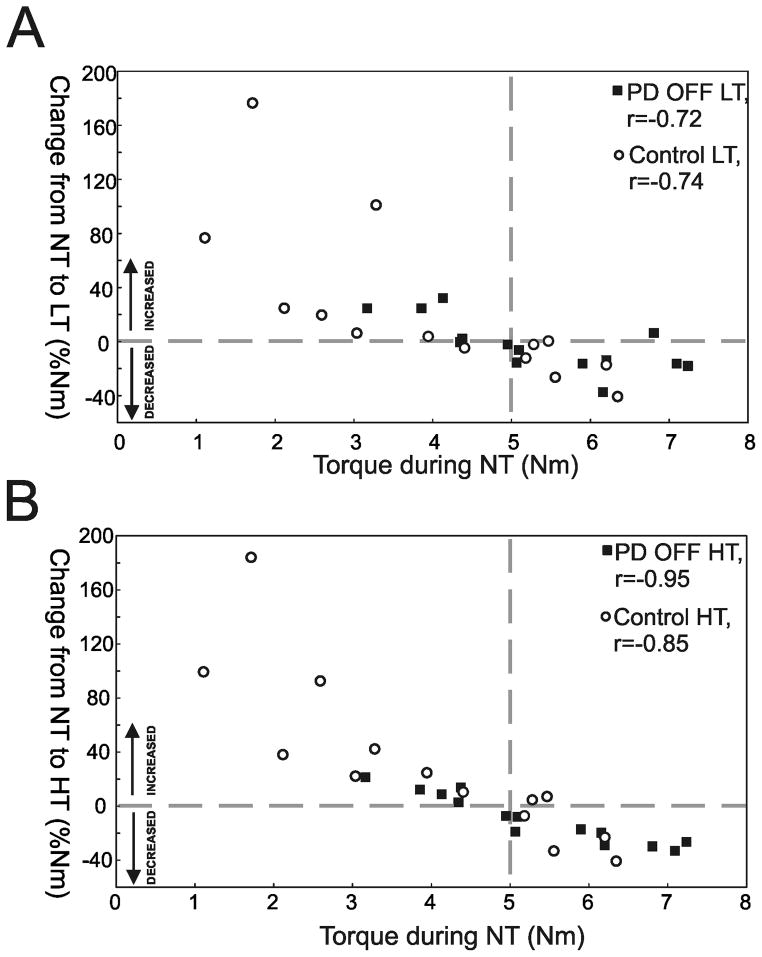
Figure 4 shows the correlation between the hip postural tone without touch and the percentage change in hip postural tone from NT to LT (4A) and from NT to HT (4B). This change in hip tone with touch was highly correlated with the amount of hip tone without touch (PD OFF: LT vs. NT r=−0.72 and R2=0.52, HT vs. NT r=−0.95 and R2=0.90 as well as controls: LT vs. NT r=−0.74 and R2=0.55, HT vs. NT r=−0.85 and R2=0.72). When touching or gripping the bar, control subjects, who have significantly smaller hip postural tone in the NT condition, increase their hip tone. Alternatively, subjects with PD who generally have higher tone tend to decrease it when touching or grabbing. As illustrated in Figure 4, only small changes in hip postural tone when touching or gripping the bar were observed from subjects (PDs and controls) that had hip tone values near 5 Nm (vertical dashed line). The use of levodopa in subjects with PD had no effect on hip postural tone (p>0.01) [PD ON: NT= 5.2 SD 1.7 Nm, LT= 4.7 SD 1.3 Nm and HT= 4.5 SD 1.0 Nm]. Further, no difference in torque asymmetry between groups (p=0.633) or conditions were found (p=0.095).
Figure 4.
Relation between the hip torque (Nm) during the no touch (NT) condition and the percentage change (%Nm) in hip torque from the NT condition to A) the light touch (LT) and B) the heavy touch (HT) condition in subjects with Parkinson’s disease OFF medication (PD OFF, black squares) and control subjects (open circles). Above the horizontal dashed line subjects are increasing in hip axial tone during LT or HT in comparison to NT and below subjects are decreasing. The vertical dashed line represents the intersection (approximate 5 Nm) between the zero line and the regression lines.
Relation between postural tone and stability
Our analysis showed a significant correlation between the level of postural hip tone and antero-posterior sway variability in PD subjects (r=0.47, R2=0.22). This correlation however, was not observed in control subjects (r=0.00, R2=0.00). While 22% of sway can be explained by hip torque in the PD group, it does not contribute to sway variability in the control group. Analyzing the NT, HT and LT conditions separately, revealed significant correlations between the postural tone and sway variability in the PD group during LT (r=0.59, R2=0.35) and HT (r=0.61, R2=0.37) but not during NT (r=0.40, R2=0.16). There were no correlation in the control group, regardless of the touch condition (NT: r=0.29 and R2=0.08, LT: r=−0.24 and R2=0.06, HT: r=0.24 and R2=0.06). Finally, no correlation between the change in postural tone and the change in sway variability from NT to HT or from NT to LT was observed (PD: r=0.02 and R2=0.00 and controls: r=0.20 and R2=0.04).
The PD group estimated their balance confidence in relation to everyday activities lower as compared to the control group (PD: mean 89, range 61–100 and controls: mean 97, range 91–100, p=0.005)
Discussion
This study showed, for the first time, that subjects with PD ON or OFF medication significantly improve postural stability with light or heavy touch, a benefit similar to sway reduction observed in control subjects. Reduction in postural sway was associated with reduced axial postural tone suggesting that tone changes may mediate the effects of light touch on postural stability.
PD subjects benefit from haptic input
Postural sway decreased by ~60% when both subjects with PD and controls lightly touched or gripped a rigid bar during stance on a very slowly rotating surface with eyes closed. The stabilizing effects from touch on postural sway were very similar between light and heavy touch suggesting that subjects with PD benefit primarily from somatosensory input associated with touch rather than the mechanical support provided by heavy touch.
The effects of light touch are relayed through the somatosensory inputs from the finger tip in combination with whole arm proprioception (2, 16, 27), providing an additional spatial reference (1, 27, 28) to stabilize posture. Contrary to our hypothesis, subjects with PD significantly reduced their postural sway with touch in a similar way as healthy control subjects. PD subjects benefited from touch to reduce their sway despite previous studies showing deficits in sensing tactile (29) and proprioceptive information (9, 11–13) as well as in integrating sensory cues (15, 17, 18). Light touch might act to reduce postural sway like external cues improve motor function in people with PD, via bypassing dysfunctional neural loops with compensatory pathways. External sensory cues, such as auditory, visual and somatosensory, have been shown to improve motor function in subjects with PD, including improved postural stepping responses and improved gait (30–33). Sensory cuing to improve gait, for example, has been associated with reduced activation of supplementary motor area and basal ganglia pathways and increased function in dorsolateral premotor cortex and cerebellar pathways (34, 35).
Another explanation to the reduced sway might be that being adjacent to a stable object and thereby having the option to grab on to it, should balance be perturbed, could increase balance confidence and indirectly reduce sway. However, this is less likely since the balance confidence scores in this study were very high in the control group even though they did reduce their sway in the same manner as the PD subjects.
We found that subjects with PD tended to increase sway, as reported in the literature, during NT when ON their medication compared to OFF medication (22). In fact, deficits in axial kinesthesia (13) as well as proximal proprioception (36) have been observed in subjects with PD using levodopa and could explain the change in sway between OFF and ON medication. On the other hand, when movement ability increases in the ON state, subjects with PD might unconsciously ‘allow’ themselves to sway more than in the OFF state. In the present study, light touch resulted in comparable postural sway when PD subjects were ON or OFF medication and similar to control subjects. These results indicate that touch can attenuate the negative effects of PD and of levodopa on postural sway.
Haptic input modifies axial postural tone
This is the first study showing that axial tone might be modified by additional sensory input in subjects with PD. Our previous study (1) in young, healthy adults, showed that postural sway decreased as tone increased when touching or gripping a stable bar. Since subjects with PD have high axial tone (11, 19) and show deficits in processing somatosensory input (15, 17, 18), we hypothesized that subjects with PD would be unable to modify their tone with haptic touch. In contrast, our results indicated that PD subjects are able to modulate axial tone and generally decrease it.
Most (~65%) of our subjects with PD had higher than normal axial tone during standing without touch (NT) and decreased their tone when touching or gripping the bar. On the other hand, most age-matched control subjects had lower tone during NT and increased hip tone with touch. Interestingly, with touch, both groups reached similar axial tone values of approximately 5 Nm. Our previous study showed that an increase in axial tone in young adults during light touch was associated with unmodulated axial muscle activity and reduced variability in the hip torque, reflecting reduced active postural shortening reactions that kept axial tone low (1). Results from the current study suggest that there is a preferred, or optimal, level of axial hip tone during haptic touch that stabilizes the trunk in space (37). This level of axial hip tone might be optimal to be able to make postural corrections based on a local trunk reference frame instead of a global reference frame (1). Consistent with a change in the postural reference frame during light touch, both control and PD subjects reported similar change in perception when touching the bar. As seen in the younger adults (1), the elderly and PD subjects perceived the relative motion between the legs and the trunk as if the trunk was rotating. With light or heavy touch this sensation changed from trunk to surface rotation.
Despite high levels of axial tone, PD subjects in our study were able to modulate their axial hip tone when touching or gripping a stable bar to a similar level as age-matched, control subjects. Unlike previous studies showing that PD subjects with rigidity are unable to flexibly inhibit phasic leg muscle responses to postural perturbations when the support conditions change (38, 39) or when gripping a stable object (40), our results imply that remote sensory input can modulate the background tonic postural activity during stance in subjects with PD. This disparity in our results could be due to different structures in the CNS controlling tonic versus phasic postural activity (41) or controlling feedback (postural perturbations) versus feedforward control (postural sway) (42), which might not be affected by the disease in a similar way.
In the present study, we show that the relationship between postural tone and stability is weaker than we observed in our earlier study in healthy young adults (1). In that study, 30% of the variance in sway could be explained by the change in tone, while we found no such relationship (R2=0.00) in our current, older control group. A few factors may explain the differences observed in these studies. There is an important age difference (24 years) between these control groups which may explain the larger variance and higher tone values in the current control group. Larger axial hip tone observed in the healthy elderly in the current study might be caused by age-related changes in the muscle properties or in the neuromuscular system, such as co-contraction during stance (43). Nevertheless, the relationship between postural tone and postural stability seems to be more complex than we initially hypothesized and future studies need to investigate this relationship.
If haptic touch can reduce high axial tone and excessive postural sway during quiet stance, this has important implications for rehabilitation. Our results suggest that training programs should encourage the use of assistive devices and touching of stable references in the environment by individuals with PD, in addition to other rehabilitation methods. This combination of strategies may be most important in individuals with high axial tone and severe PD, since postural sway seems to increase with disease severity and duration (21, 22).
In conclusion, subjects with mid-stage PD with excessive postural sway and excessive axial postural tone benefit from haptic touch. Rehabilitation strategies can draw upon this finding to improve functional standing and walking.
Acknowledgments
Funding
Supported by NIH R37-AG006457, Center for Health Care Sciences at Karolinska Institutet and Loo and Hans Osterman’s foundation.
We thank all the participants for their time and patience and Triana Nagel, BS, for her help with subject recruitment. We also thank Edward King, MS, and Charley Russell, PhD, for assistance with equipment and programming.
Footnotes
Declaration of conflicting Interests
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Franzén E, Gurfinkel VS, Wright WG, Cordo PJ, Horak FB. Haptic touch reduces sway by increasing axial tone. Neuroscience. 2011;174:216–23. doi: 10.1016/j.neuroscience.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Exp Brain Res. 1994;100:495–502. doi: 10.1007/BF02738408. [DOI] [PubMed] [Google Scholar]
- 3.Jeka JJ. Light touch contact as a balance aid. Phys Ther. 1997;77:476–87. doi: 10.1093/ptj/77.5.476. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay F, Mireault AC, Dessureault L, Manning H, Sveistrup H. Postural stabilization from fingertip contact: I. Variations in sway attenuation, perceived stability and contact forces with aging. Exp Brain Res. 2004;157:275–85. doi: 10.1007/s00221-004-1830-4. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein R, Shupert CL, Horak FB. Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture. 2001;14:238–47. doi: 10.1016/s0966-6362(01)00161-8. [DOI] [PubMed] [Google Scholar]
- 6.Lackner JR, DiZio P, Jeka J, Horak F, Krebs D, Rabin E. Precision contact of the fingertip reduces postural sway of individuals with bilateral vestibular loss. Exp Brain Res. 1999;126:459–66. doi: 10.1007/s002210050753. [DOI] [PubMed] [Google Scholar]
- 7.Boonsinsukh R, Panichareon L, Phansuwan-Pujito P. Light touch cue through a cane improves pelvic stability during walking in stroke. Arch Phys Med Rehab. 2009;90:919–26. doi: 10.1016/j.apmr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Constantinescu R, Leonard C, Deeley C, Kurlan R. Assistive devices for gait in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:133–8. doi: 10.1016/j.parkreldis.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Zia S, Cody FWJ, O’Boyle DJ. Joint position sense is impaired by Parkinson’s disease. Ann Neurol. 2000;47:218–28. [PubMed] [Google Scholar]
- 10.Zia S, Cody FW, O’Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson’s disease. Clin Anat. 2002;15:23–31. doi: 10.1002/ca.1087. [DOI] [PubMed] [Google Scholar]
- 11.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson’s disease: direct measurements of trunk and hip torque. Expl Neurol. 2007;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright WG, Gurfinkel V, King L, Horak F. Parkinson’s disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett. 2007;417:10–5. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson’s disease: Effects of levodopa. Exp Neurol. 2010;225:202–9. doi: 10.1016/j.expneurol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maschke M, Tuite PJ, Krawczewski K, Pickett K, Konczak J. Perception of heaviness in Parkinson’s disease. Mov Disord. 2006;21:1013–8. doi: 10.1002/mds.20876. [DOI] [PubMed] [Google Scholar]
- 15.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–22. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 16.Jeka JJ, Lackner JR. The role of haptic cues from rough and slippery surfaces in human postural control. Exp Brain Res. 1995;103:267–76. doi: 10.1007/BF00231713. [DOI] [PubMed] [Google Scholar]
- 17.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–40. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 18.Konczak J, Corcos DM, Horak F, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543–52. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 19.Franzén E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol. 2009;219:430–8. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96:2678–87. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- 21.Viitasalo MK, Kampman V, Sotaniemi KA, Leppavuori S, Myllyla VV, Korpelainen JT. Analysis of sway in Parkinson’s disease using a new inclinometry-based method. Mov Disord. 2002;17:663–9. doi: 10.1002/mds.10023. [DOI] [PubMed] [Google Scholar]
- 22.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahn S, Elton R. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden D, Calne D, editors. Recent Developments in Parkinson Diseases. London: Macmillan; 1987. pp. 153–63. [Google Scholar]
- 25.Powell LE, Myers AM. The Activities-specific balance confidence (ABC) scale. Journal of Gerontology. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 26.Rocchi L, Chiari L, Cappello A, Horak FB. Identification of distinct characteristics of postural sway in Parkinson’s disease: a feature selection procedure based on principal component analysis. Neurosci Lett. 2006;394:140–5. doi: 10.1016/j.neulet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Lackner JR, Rabin E, DiZio P. Stabilization of posture by precision touch of the index finger with rigid and flexible filaments. Exp Brain Res. 2001;139:454–64. doi: 10.1007/s002210100775. [DOI] [PubMed] [Google Scholar]
- 28.Gurfinkel VS, Levik YS. The suppression of cervico-ocular response by the haptokinetic information about the contact with a rigid, immobile object. Exp Brain Res. 1993;95:359–64. doi: 10.1007/BF00229794. [DOI] [PubMed] [Google Scholar]
- 29.Artieda J, Pastor MA, Lacruz F, Obeso JA. Temporal discrimination is abnormal in Parkinson’s disease. Brain. 1992;115(Pt 1):199–210. doi: 10.1093/brain/115.1.199. [DOI] [PubMed] [Google Scholar]
- 30.Lim I, van Wegen E, de Goede C, et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil. 2005;19:695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- 31.Burleigh A, Horak FB, Nutt JG, Obeso JA. Step Initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–15. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 32.Nieuwboer A, Baker K, Willems AM, et al. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil Neural Repair. 2009;23:831–6. doi: 10.1177/1545968309337136. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural resposes of subjects with parkinson’s disease. Neuroscience. 2006;141:999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Delolmo M, Arias P, Furio M, Pozo M, Cudeiro J. Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients—a combined motor and [18F]-FDG PET study. Parkinsonism Relat Disord. 2006;12:155–64. doi: 10.1016/j.parkreldis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Hanakawa T, Katsumi Y, Fukuyama H, et al. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999;122:1271–82. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- 36.O’Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. 2001;71:607–10. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creath R, Kiemel T, Horak F, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res. 2008;18:39–49. [PMC free article] [PubMed] [Google Scholar]
- 38.Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci. 2000;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 39.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 40.Schieppati M, Nardone A. Free and supported stance in Parkinson’s disease: the effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain. 1991;114 (3):1227–44. doi: 10.1093/brain/114.3.1227. [DOI] [PubMed] [Google Scholar]
- 41.Kuypers HG. The Descending Pathways to the Spinal Cord, Their Anatomy and Function. Prog Brain Res. 1964;11:178–202. doi: 10.1016/s0079-6123(08)64048-0. [DOI] [PubMed] [Google Scholar]
- 42.Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. McGraw-Hill; 2000. [Google Scholar]
- 43.Cacciatore TW, Gurfinkel VS, Horak FB, Cordo PJ, Ames KE. Increased dynamic regulation of postural tone through Alexander Technique training. Hum Mov Sci. 2011;30:74–89. doi: 10.1016/j.humov.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]