Abstract
Integrins are functionally regulated by “inside-out” signaling, in that stimulus-induced signaling pathways act on the intracellular integrin tail to regulate the activity of the receptor on the outside. Both a change in conformation (affinity) and clustering (avidity/valency) of the receptors occurs, but the mechanisms that regulate inside out signaling are not completely understood. Previously, we identified gelsolin in a proteomics screen to identify proteins involved in inside-out control of integrins using the lymphocytic leukemia cell line L1210. Furthermore, we showed that gelsolin was involved in affinity regulation of β1-integrins in the leukemic cell line U937. Here, we examined how gelsolin regulates β1-integrin affinity in the leukemia cell line L1210. We show that gelsolin is mainly expressed at the cell membrane and is present near β1-integrins. The role for actin polymerization in integrin affinity regulation was examined using the actin modulating agent jasplakinolide, which decreased β1-integrin affinity. Similarly, knock-down of gelsolin in L1210 cells also decreased β1-integrin affinity and cell adhesion to collagen. These data suggest that increased actin polymerization through gelsolin regulates β1-integrin affinity and cell adhesion.
Introduction
Adhesion of leukocytes to the endothelium is essential for their resistance to shear forces present in the blood stream and subsequent migration into tissues (Ley et al. 2007). To resist shear forces, leukocytes express adhesion molecules such as selectins and integrins. The latter belong to a family of at least 26 transmembrane adhesion molecules which are composed of non-covalently linked α and β subunits (Hynes 1992). In resting conditions, circulating peripheral blood leukocytes express integrins in an inactive state that are unable to bind ligand. Upon stimulation by inflammatory mediators such as cytokines and chemokines signals are induced in the leukocytes that converge at the intracellular integrin tail and lead to activation of the integrin, a process generally referred to as inside-out control (Constantin et al. 2000; Laudanna et al. 2002). Activation of integrins is determined by two processes, a change in conformation (affinity) and/or clustering of integrins on the cell membrane (avidity/valency) (Carman and Springer 2003). Although the list of signaling and adaptor proteins involved in inside-out signaling is increasing (Kinashi 2005) the precise mechanism by which all these proteins function at the intracellular tail is largely unknown.
The complex architecture of proteins that regulate integrin function is part of the so-called adhesome (Kinashi 2007; Zaidel-Bar et al. 2007). Talin and kindlins are essential components of this complex and are seen as the common last step of integrin activation in leukocytes (Harburger et al. 2009; Moser et al. 2008; Tadokoro et al. 2003). The linkage of integrins to the cytoskeleton through talin and possibly through kindlins, is thought to be very important in the induction of integrin affinity configuration. However, recent data shows that talin-1 and kindlin-3 are not involved in α4β1-integrin affinity regulation (Hyduk et al. 2011). Furthermore, many cytoskeletal proteins are described to play a role in the linkage of integrin chains to the actin cytoskeleton such as vinculin and paxillin that directly bind integrin chains to actin (Critchley 2004; Liu et al. 1999; Ziegler et al. 2008). Also, actin bundling proteins such as L-plastin have been shown to play a role in integrin activation (Jones et al. 1998). Next to these adaptor proteins, actin cytoskeleton modulating proteins such as ARP2/3 and cofilin but also gelsolin are involved in the dynamics of F actin formation and degradation (Burtnick et al. 1997; Tellam and Frieden 1982).
Previously, two variants of a mouse acute lymphocytic leukemia cell line (L1210), a suspension growing (L1210-S) and an adherent growing variant (L1210-A) (Brandsma et al. 2006) that differed in integrin activity were compared using a proteomics approach. We found that gelsolin was differentially expressed in the cell lines and confirmed a role for gelsolin in the induction of integrin affinity and function by ectopic expression of gelsolin in a monocytic cell line U937 (Langereis et al. 2009). Gelsolin is widely expressed in mammalian tissue and is particularly abundant in cells that migrate or can change shape rapidly (Cunningham et al. 1991; Hartwig 1992; Howard et al. 1990; Yin and Stossel 1979). Gelsolin functions as an F-actin severing and capping protein and is dependent on Ca2+ and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) (Kwiatkowski et al. 1988a; Kwiatkowski et al. 1988b).
Here, we report that gelsolin is abundantly present at the cell membrane of L1210 cells. Treatment of L1210-S cells with latrunculin B (inducing depolarization of F-actin) induced β1-integrin activtion, whereas jasplakinolide (inducing actin polymerization) reduced β1-integrin activation on L1210-A cells. Knock down of gelsolin in L1210-A cells correlated with a loss of active β1-integrins as well as reduced cell adhesion, whereas over expression of gelsolin in L1210-S cells increased active β1-integrins. Therefore, we hypothesized that gelsolin increases β 1-integrin affinity by modulating actin polymerization.
Materials and Methods
Reagents
Anti-mouse gelsolin was from R&D systems (Abingdon, United Kingdom), and anti-mouse α-tubulin was from Santa Cruz (Santa Cruz, CA, USA). Mouse CD29-PE-Cy5 was from Biolegend (San Diego, CA). Rabbit Anti-Goat IgG, F(ab′)2 fragment FITC conjugated was from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-β1 (9EG7) was from BD Biosiences (San Diego, CA). Goat anti-rat IgG Alexa Fluor 647 conjugated was from Invitrogen (Carlsbad, CA). Collagen and DAPI were from Sigma-Aldrich (St. Louis, MO). Jasplakinolide was from Calbiochem (San Diego, CA). All other materials were reagent grade.
Cell culture
L1210 cells were cultured in RPMI 1640 supplemented with 10% FCS, 1% penicillin–streptomycin-l-glutamine, and 60 μM β-mercaptoethanol. Cells were incubated in 5% CO2-95% air at 37°C. The L1210-A cells were harvested by treatment with 10 mM EDTA followed by two washing steps with phosphate buffered saline (PBS).
Gelsolin knockdown
Knockdown of gelsolin was accomplished by using stable lentiviral delivery of shRNA to gelsolin purchased from Open Biosystems (Huntsville, AL). Phoenix Ampho cells were transfected with 6 μg pLKO.1-puro shRNA construct by lipofectamin-2000 (Invitrogen, Carlsbad, CA). Virus production was performed at 32°C. L1210-A cells were infected with virus for 6h in the presence of 8 μg/mL polybrene. Positive cells were selected with 4 μg/mL puromycin.
Over expression of gelsolin in L1210-S cells
Mouse gelsolin was PCR amplified from pSPORT6-mGelsolin plasmid (Open biosystems, Huntsville, AL) with forward primer CTCGAGCCGCTCCGTACCGCTCTTCACT and reverse primer GTCGACTCAGGCAGCCAGCTCAGC containing XhoI and SalI restriction sites. The PCR product was ligated into pGEMteasy (Promega, Madison, WI), digested with XhoI and SalI and subsequently ligated into pEGFP-C1, resulting in pEGFP-C1-mGelsolin plasmid. Gelsolin sequence was verified by plasmid sequencing.
L1210-S cells (2 × 106 cells/100 μL) were transfected with 1 μg of either pEGFP-C1 empty vector or pEGFP-C1-mGelsolin using Amaxa. Fluorescent microscopy was conducted 24 hours after transfection.
Immunofluorescence confocal microscopy
Glass coverslips were acid-washed and coated with 10 μg/ml collagen. L1210 cells were loaded with 1 μM Calcein-AM and stained with CD29 of 9EG7 for 30 min in PBS with 5% normal mouse serum at 4°C, added to coverslips and allowed to adhere for 30 min at 4°C. Cells were fixed in 3.7% formaldehyde for 10 min, quenched with 0.15 M glycine for 10 min, permeabilized with 0.2% Triton X-100 for 10 min and blocked with 5% goat serum. The cells were incubated with primary antibodies for 30 min, washed and then with fluorophore conjugated secondary antibodies for 30 min. DAPI was incubated for 15 min. Confocal images were collected using a laser-scanning confocal microscope (Fluoview FV-1000; Olympus) using a 60× Plan Apo/1.45 oil immersion objective with a 2.5 zoom factor and were captured into Fluoview software (FV10-ASW version 01.07; Olympus). Images were processed using Olympus Fluoview FV1000 or ImageJ software.
Treatment L1210-S and L1210-A cells with latrunculin B or jasplakinolide
L1210 cells were centrifuged, washed twice in PBS at room temperature and resuspended to 5×106 cells/mL. L1210 cells were treated with latrunculin B or jasplakinolide for 20 or 15 minutes at room temperature, respectively, and fixed with 2% formaldehyde. Expression of total β1-integrin and active β1-integrin was assessed by flow cytometry on a FACScalibur (Becton Dickinson, San Jose, CA). Data are reported as median channel fluorescence (MFI) in arbitrary units (AU).
Determination of total and active integrin expression by flow cytometry
Flow cytometry was used to measure expression levels of total and active integrins. L1210 cells were centrifuged and washed twice in phosphate buffered saline (PBS) at room temperature. Cells were resuspended in Hepes incubation buffer (20 mM HEPES, 132 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1.2 mM KH2 PO4, 5 mM glucose, 1.0 mM CaCl2, and 0.5% (w/v) Human Serum Albumin) and put in a concentration of 2×105 cells/sample. Directly labeled antibodies were incubated for 1 h at 4°C. Subsequently, cells were washed twice in Hepes incubation buffer and, fixed with 2% formaldehyde and analyzed by flow cytometry on a FACScalibur (Becton Dickinson, San Jose, CA). Data are reported as median channel fluorescence (MFI) in arbitrary units (AU).
Static adhesion assays on collagen
Adhesion assays were performed as described before (Brandsma et al. 2006) with some minor modifications. In short, flat bottom plates were coated with 10 μg/ml collagen for 2h at 37°C. Subsequently, wells were blocked with 2.5% BSA in PBS for 1 h at room temperature. L1210 cells were labeled with 1 μM calcein (Molecular Probes, Invitrogen, Carlsbad, CA) for 30 min at 37°C and washed twice with Hepes incubation buffer. Adhesion assays were performed in Hepes incubation buffer by administering 5 x105 L1210 cells per well in a 96 wells plate coated with collagen during 30 min at 37°C. EDTA (10 mM) served as a control for divalent cation dependent binding. Fluorescence per well was measured before and after three washes using FLUOstar OPTIMA (BMG Labtech GmbH, Offenburg Germany). The percentage adherence was determined by calculating the fluorescence after three washes as a percentage of the total fluorescence before washing
Western blotting
The L1210 cells were washed once with PBS, lysed in sample buffer (2% β-mercaptoethanol, 2% SDS, 10% glycerol, 30mM Tris-HCL pH 6.8) and boiled for 5 min. Protein samples were analyzed on 10–12% SDS-polyacrylamide gels. Proteins were transferred to Immobilon-P (Millipore). The blots were blocked in hybridization buffer (10 mM Tris, 150 mM NaCl, and 0.1% Tween 20) containing 5% (w/v) milk powder (ELK, Campina, the Netherlands) for 1 h followed by incubation with first specific antibody (1:1000) in hybridization buffer with 5% (w/v) BSA or 0.5% (w/v) milk powder overnight at 4°C. After incubation with the first antibody, the blots were washed three times for 5 min in hybridization buffer. The second antibody (HRP-coupled; 1:3000) was incubated in hybridization buffer containing 5% (w/v) milk for 2h at room temperature followed by three washings for 5 min in hybridization buffer and a last wash step in PBS. Detection of all Western blots was performed by ECL plus (GE Healthcare, Uppsala, Sweden) and detected using a Typhoon 9410 (GE Healthcare, Uppsala, Sweden). The western blot results are expressed as mean ± standard error of the mean.
RNA isolation and cDNA synthesis
L1210 cells were lysed in Trizol (Invitrogen, Carlsbad, CA), and mRNA was isolated according to the manufacturer’s protocol. cDNA was synthesized with iScript (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s protocol.
Real-time PCR
Gene expression was analyzed by real-time PCR. All primers used in this study were described previously (Oudijk et al. 2005). Real-time PCR was performed using 5.0–12.5 ng cDNA, 12.5 μL iQ SYBR Green Supermix (Bio-Rad), and 400 nM of each primer (total volume 25 μL). Amplification and detection were performed with a MyiQ real-time PCR detection system (Bio-Rad) under the following conditions: 3 min at 95°C and 40 cycles of 30 s at 95°C, 30 s at 61°C, and 30 s at 72°C. The results represent relative quantity of mRNA levels normalized for the housekeeping gene GAPDH.
Statistical analysis
All statistical analyses were performed in Graphpad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA), where p<0.05 was considered significant.
Results and discussion
Increased active β1-integrin expression on L1210-A cells compared to L1210-S cells
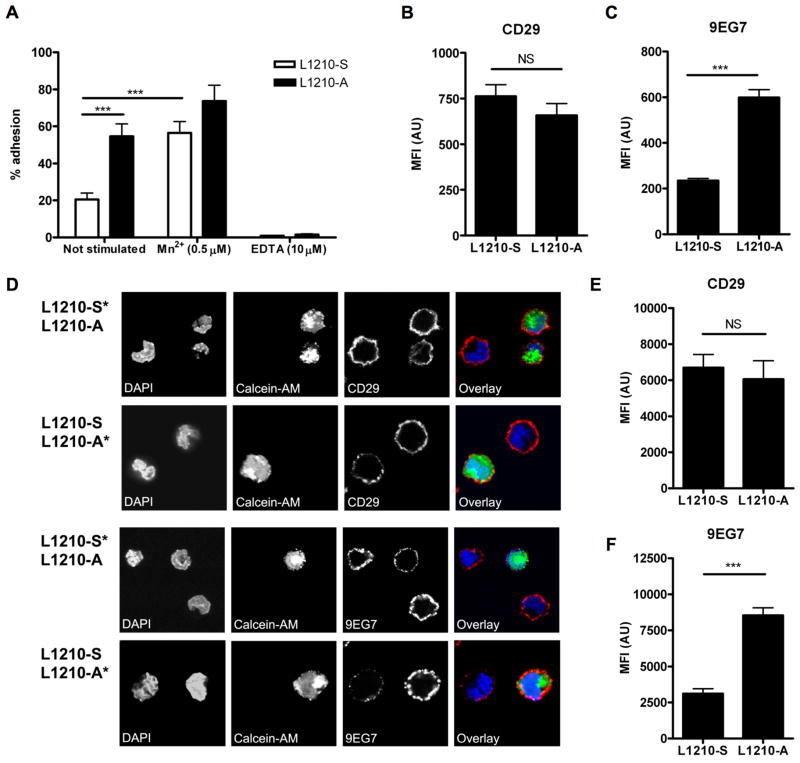
Previous research identified two variants of the mouse acute lymphocytic leukemia cell line L1210, a suspension growing (L1210-S) and an adherent growing variant (L1210-A) (Brandsma et al. 2006). The L1210-A showed increased adhesion to collagen compared to the L1210-S (Figure 1A). Adhesion of the L1210-S cells could be induced with Mn2+, which showed that the integrins on the L1210-S cells were functional when activated, whereas addition of EDTA blocked adhesion for both the L1210-A and L1210-S cells (Figure 1A). Previously, we showed that L1210-A cells expressed increased levels of gelsolin and that over expression of gelsolin in U937 cells increased cell adhesion and expression of the high affinity epitope of integrin β1, as detected by N29 antibody (Langereis et al. 2009). To confirm a relationship between increased gelsolin expression in L1210-A cells and the expression of the high affinity epitope of integrin β1 we used the 9EG7 antibody for flow cytometry and confocal immunofluorescence microscopy experiments. This 9EG7 antibody specifically recognizes the high affinity β1-integrin epitope, further referred as active β1-integrins (Lenter et al. 1993). However, 9EG7 is also able to activate “resting” β1-integrins (Driessens et al. 1995), therefore, incubation with 9EG7 was conducted at 4°C. Expression of CD29 (total β1-integrins) was not significantly different between L1210-S and L1210-A cells (Figure 1B), whereas binding of the 9EG7 antibody was clearly increased on L1210-A cells (Figure 1C). Therefore, these results support our earlier observation that increased gelsolin expression corresponds with increased active β1-integrin expression on U937 cells.
Figure 1. Increased active β1-integrin expression on L1210-A cells.
(A) L1210-S and L1210-A cells adhesion to collagen. Expression of (B) CD29 and (C) binding of 9EG7 to L1210-S and L1210-A cell by flow cytometry. (D) L1210-S or L1210-A cells were stained with Calcein-AM and mixed with unstained L1210-A or L1210-S, respectively. The mixed cell populations were stained with CD29 or 9EG7 and DAPI as nuclear stain and imaged by confocal microscopy. Fluorescence intensity quantification of (E) CD29 expression (n=15) and (F) binding of 9EG7 (n=15) to L1210-S and L1210-A cells.
This increased expression of active β1-integrins was confirmed by confocal microscopy. To exclude staining differences between slides and biased cell selection methods, we stained either L1210-A or L1210-S cells with calcein-AM (indicated with *) and mixed them with the other non-stained cell line. These mixed cell population were stained for 9EG7 or CD29 and DAPI as nuclear stain. Expression of CD29 on L1210-A and L1210-S cells was not different, whereas L1210-A cells showed a marked increase in 9EG7 binding compared to L1210-S (Figure 1D). Images at low magnification bearing 20–50 cells were made and fluorescence intensity of CD29 or 9EG7 binding was quantified. Expression of CD29 was not statistically different between L1210-A and L1210-S cells (Figure 1E), whereas L1210-A cells showed a 3-fold increase in 9EG7 binding (Figure 1F), which corresponded to the flow cytometry data (Figure 1C).
Gelsolin, β1-integrins and the active β1-integrin are expressed at the cell membrane of L1210 cells
Subsequently, we examined the localization of gelsolin in L1210-A and L1210-S cells. To date, few studies have examined gelsolin localization. Gelsolin is particularly abundant in the cytoplasm of cells that migrate or change shape rapidly (Howard et al. 1990; Yin and Stossel 1979) (Cunningham et al. 1991; Hartwig 1992) and gelsolin is a known marker for podosomes in osteoclasts (Chellaiah et al. 2000). A z-stack of L1210 cells by confocal microscopy showed that gelsolin was abundantly present at the cell membrane, detectible in the cytoplasm and absent from the nucleus (Figure 2A). The localization of gelsolin was similar in both cell lines although an increased gelsolin expression was present in L1210-A cells, as was also observed by Western blot analysis (Langereis et al. 2009). Gelsolin was abundantly present at the cell membrane, located at sites where β1-integrins (CD29) as well as active β1-integrins were present (Figure 2B). Previously, expression of gelsolin has been shown to be localized in pseudopodia that are in contact with phagocytic particles (Yin et al. 1981), implicating a role in adhesion. This observation corresponds to more recent data that gelsolin knock-out fibroblasts showed decreased binding capacity to beads coated with β1-integrin ligand collagen (Arora et al. 2005; Arora et al. 2004).
Figure 2. Gelsolin, total β1- and the high affinity β1-integrins are expressed at the cell membrane of L1210 cells.
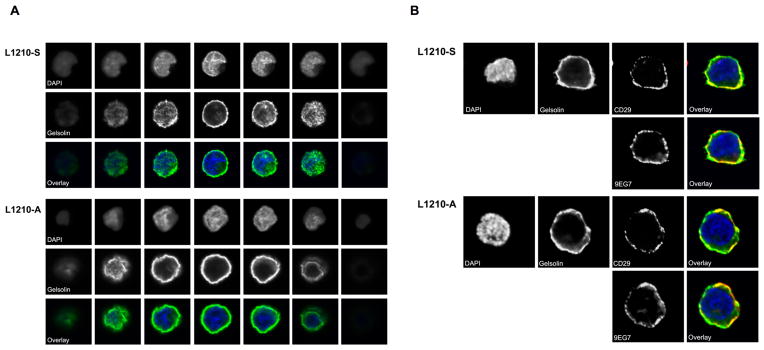
(A) L1210-S and L1210-A cells were stained with anti-gelsolin (green) and DAPI (blue) as nuclear stain and imaged by confocal microscopy. (B) L1210-S and L1210-A cells were stained with CD29 (red), 9EG7 (red), anti-gelsolin (green) and DAPI (blue) as nuclear stain and imaged by confocal microscopy. Overlays of CD29 or 9EG7 with gelsolin are depicted.
Effects of actin polymerization on β1-integrin affinity regulation
Because gelsolin is an actin-severing and capping protein (Burtnick et al. 1997; Tellam and Frieden 1982), we tested whether actin polymerization (treatment with jasplakinolide) and depolymerization (treatment with latrunculin B) affected β1-integrin activity. Of note, as shown in Figure 1C, binding of 9EG7 to L1210-A was increased compared to L1210-S cells, therefore, expression of each cell line was normalized to 1 for easy comparison between the two cell lines. Inhibition of actin polymerization with latrunculin B increased integrin activity on L1210-S cells as measured by increased reactivity with the 9EG7 antibody, but did not further induce integrin activity on L1210-A cells. Total β1 integrin expression was not affected by latrunculin treatment in either cell lines (Figure 3A). The reciprocal experiment was performed by treatment of the cells with jasplakinolide that induces actin polymerization. Jasplakinolide treatment reduced active β1-integrin expression on L1210-A cells whereas the already low active β1-integrin expression on L1210-S cells was not further decreased (Figure 3B). These data corroborate that inhibition of platelet actin polymerization increased αIIbβ3-integrin affinity and binding of soluble fibrinogen, which was inhibited by treatment with jasplakinolide (Bennett et al. 1999). The authors propose that the actin cytoskeleton in unstimulated platelets constrains the αIIbβ3-integrins in a low affinity state and increased actin filament turnover releases this cytoskeletal constrain enabling high affinity αIIbβ3-integrin expression and soluble fibrinogen binding. Increased gelsolin expression in L1210-A cells, apparently, increases basal actin filament turnover and integrin activity.
Figure 3. Actin dynamics affect L1210 β1-integrin affinity regulation.
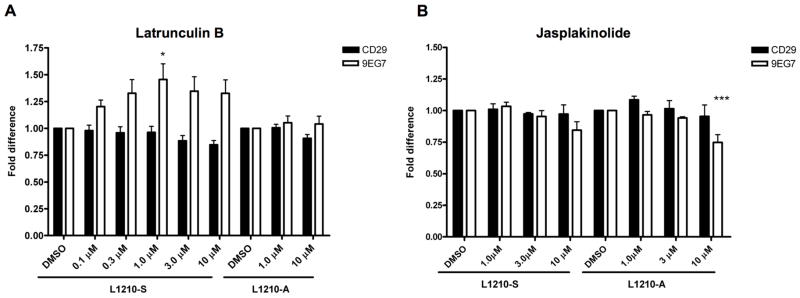
L1210-S and L1210-were treated with (A) latrunculin B or (B) jasplakinolide and stained for total β1-integrin (CD29) and active β1-integrins (9EG7) by flow cytometry. One-way ANOVA with Dunnett’s multiple comparison test was used to perform statistical analysis (* = P<0.05, *** = P<0.001).
Inhibition of gelsolin decreased active β1-integrin expression and cell adhesion
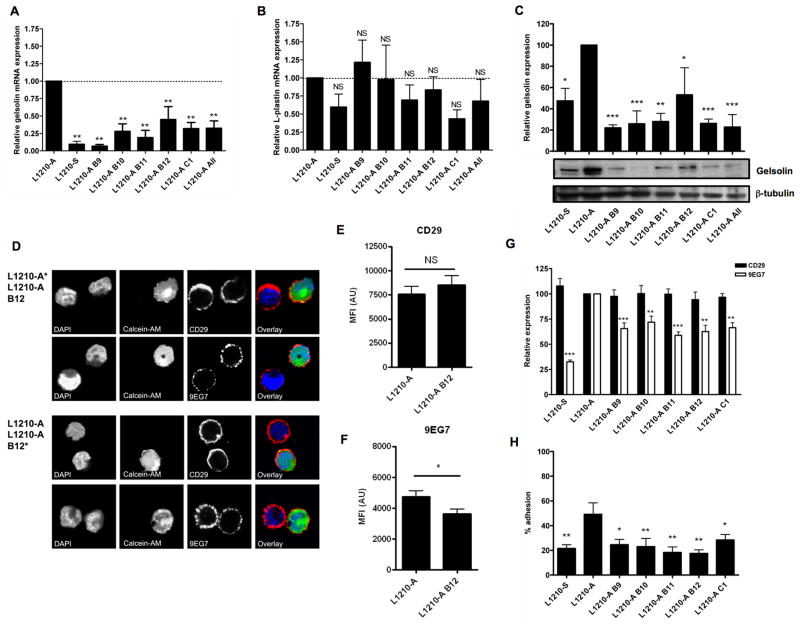
Modulation of actin polymerization with latrunculin B and jasplakinolide clearly affected active β1-integrin expression on L1210-A and L1210-S cells. To link a role for gelsolin in this regulation of β1-integrins we decreased gelsolin expression in L1210-A cells using lentiviral introduction of shRNA. We used 5 lentiviral constructs (B9, B10, B11, B12, and C1) or a pool of all constructs (All), which all resulted in a decreased gelsolin mRNA expression to 10–50% of the gelsolin expression level of control L1210-A cells (Figure 4A), whereas mRNA expression of the actin bundling protein L-plastin was not significantly affected (Figure 4B). More importantly, the decreased mRNA expression resulted in decreased gelsolin protein expression (Figure 4C).
Figure 4. Gelsolin knock down decreases cell adhesion.
Relative mRNA expression of (A) gelsolin and (B) L-plastin in L1210-S, L1210-A and L1210-A geslolin shRNA knock-down cells. (C) Gelsolin protein expression in L1210-S, L1210-A and L1210-A geslolin shRNA knockdown cells were analyzed by westernblot analysis with anti-gelsolin and anti-β-tubulin as loading control. The western blots shown are representative for five experiments. Mean values are represented ± SEM (n=5). One-way ANOVA with Dunnett’s multiple comparison test was used to perform statistical analysis (* = P<0.05, ** = P<0.01, *** = P<0.001). (D) L1210-A or L1210-A B12 gelsolin shRNA knock-down cells were stained with Calcein-AM and mixed with unstained L1210-A or L1210-A B12 gelsolin shRNA knock-down cells, respectively. The mixed cell populations were stained with CD29 or 9EG7 and DAPI as nuclear stain and imaged by confocal microscopy. Fluorescence intensity quantification of (E) CD29 expression (n=25) and (F) binding of 9EG7 (n=19) on L1210-A or L1210-A B12 gelsolin shRNA knock-down cells. (G) Expression of CD29 and binding of 9EG7 on L1210-S, L1210-A and L1210-A gelsolin shRNA knock-down cells were analyzed by flow cytometry.
We analyzed expression of CD29 and binding of 9EG7 to L1210-A and L1210-A B12 gelsolin shRNA knock-down cells by confocal microscopy. Expression of CD29 on L1210-A and L1210-A B12 gelsolin shRNA knock-down cells was not different, whereas L1210-A B12 gelsolin shRNA knock-down cells showed decreased reactivity of 9EG7 compared to L1210-A cells (Figure 4D). Fluorescence intensity of CD29 or 9EG7 was quantified and expression of CD29 was not statistically different between L1210-A and L1210-A B12 gelsolin shRNA knock-down cells (Figure 4E), whereas L1210-A B12 gelsolin shRNA knock-down cells showed a significant decrease in 9EG7 binding (Figure 4F). Additionally, flow cytometry analysis also showed decreased binding of 9EG7 to L1210-A gelsolin shRNA knock-down cells whereas expression of CD29 expression was not changed (Figure 4G). Potential shortcoming in these knock-down experiments is that we cannot exclude an off-target effect of the gelsolin shRNA, although we observe a similar decrease in binding of 9EG7 for all 5 shRNA construct used in our experiments. Besides gelsolin protein expression, the severing and capping activity of this protein is dependent on Ca2+ and PI(4,5)P2 (Kwiatkowski et al. 1988a; Kwiatkowski et al. 1988b). Our data indicate that expression of gelsolin in U937 and L1210 cells modulates β1-integrin activity, which would mean that the basal severing and capping activity is present in resting L1210 cells. This is supported by the fact that gelsolin activity and active β1-integrin expression on L1210-A cells could be inhibited with jasplakinolide (Figure 3B).
Next, we tested the ability of the L1210-A knock-down cells to adhere to the β1-integrin substrate collagen because down-regulation of active β1-integrins would suggest loss of adhesion. Indeed, the L1210-A knock-down cells showed significantly impaired adhesion (17 – 25%) compared to wild type L1210-A cells (50%) but was comparable with the adhesion capacity of L1210-S cells (Figure 4H). Addition of EDTA blocked adhesion completely which showed that this process is integrin mediated (data not shown).
Although expression of most L1210-S gelsolin knock-down cell lines show equal gelsolin levels compared to the L1210-S cells (Figure 4C), binding of 9EG7 is still significantly higher (Figure 4G). It is very well possible that a small decrease in 9EG7 reactivity to the L1210-A gelsolin knock-down cells has already major effects on cell adhesion. Alternatively, expression of gelsolin might not only affect β1-integrin activity, but also β1-integrin clustering, and thereby cell adhesion. This hypothesis is supported by the fact that activation of actin polymerization by jasplakinolide decreased β2αL-integrin clustering and subsequently decreased binding to ICAM-1 (Stewart et al. 1998).
Increased gelsolin expression in L1210-S cells increases active β1-integrin expression
Knock-down of gelsolin in L1210-A cells decreased β1-integrin activity and cell adhesion, therefore, we wondered whether over expression of gelsolin in L1210-S cells would increase β1-integrin activity. Over expression of gelsolin in L1210-S cells increased binding of 9EG7, whereas the empty vector had no effect (Figure 5A). Fluorescence intensity 9EG7 was quantified and overexpression of gelsolin showed a significant 3-fold increase in 9EG7 binding (Figure 5B), whereas this was not observed for the empty vector control (Figure 5C). Of interest, EGFP-tagged gelsolin showed a similar cell membrane-associated localization as gelsolin in L1210-A cells (Figure 2), whereas EGFP of the vector control was localized in the cytoplasm.
Figure 5. Over expression of gelsolin increases β1-integrin activity on L1210-S cells.
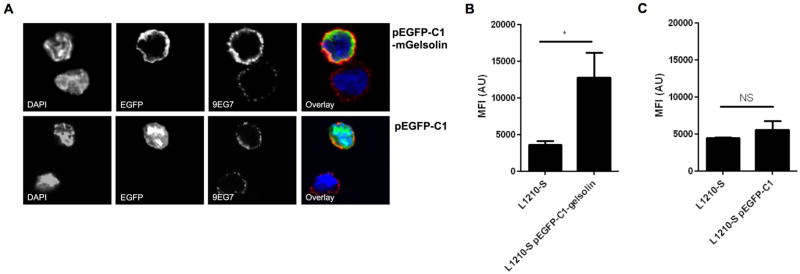
(A) L1210-S cells transfected with pEGFP-C1 or pEGFP-C1-mGelsolin were stained with 9EG7 and DAPI as nuclear stain and imaged by confocal microscopy. Fluorescence intensity quantification of (B) L1210-S transfected with pEGFP-C1-mGelsolin (n=4) or (C) L1210-S transfected with pEGFP-C1 (n=4).
Mechanism for gelsolin-mediated β1-integrin activity regulation
Previous study has linked actin polymerization to regulation of αIIbβ3-integrin affinity, although the exact mechanism has also not been determined (Bennett et al. 1999). It is tempting to speculate that the level of gelsolin, and thereby actin polymerization, determines the availability of integrin-interacting proteins such as talin or kindlin at the intracellular tail. This hypothesis could also explain the residual binding of L1210-S and L1210-A shRNA knock-down cells since low levels of gelsolin was present (Figure 4C). Whether gelsolin expression affects the availability of integrin-interacting proteins remains to be determined.
Taken together, these data demonstrate that gelsolin might play an important role in the regulation of β1-integrin affinity in L1210 cells, which corroborates our previous observation that increased gelsolin expression increased β1-integrin affinity and adhesion of U937 cells.
Acknowledgments
We want to thank dr. Sa Kan Yoo for his technical assistance with the confocal microscopy. This research was supported by a research grant of the Netherlands Asthma Foundation (NAF) project number 3.2.03.63 and an “internationaliseringsbeurs” of the University Medical Center Utrecht WP/U-06-05939.
Footnotes
The authors declare no conflict of interest.
References
- Arora PD, Chan MW, Anderson RA, Janmey PA, McCulloch CA. Separate functions of gelsolin mediate sequential steps of collagen phagocytosis. Mol Biol Cell. 2005;16(11):5175–90. doi: 10.1091/mbc.E05-07-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora PD, Glogauer M, Kapus A, Kwiatkowski DJ, McCulloch CA. Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol Biol Cell. 2004;15(2):588–99. doi: 10.1091/mbc.E03-07-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin alpha(IIb)beta(3) for fibrinogen. J Biol Chem. 1999;274(36):25301–7. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- Brandsma D, Ulfman L, Reijneveld JC, Bracke M, Taphoorn MJ, Zwaginga JJ, Gebbink MF, de Boer H, Koenderman L, Voest EE. Constitutive integrin activation on tumor cells contributes to progression of leptomeningeal metastases. Neuro Oncol. 2006;8(2):127–36. doi: 10.1215/15228517-2005-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell. 1997;90(4):661–70. doi: 10.1016/s0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15(5):547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska KA. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol. 2000;148(4):665–78. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13(6):759–69. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- Critchley DR. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem Soc Trans. 2004;32(Pt 5):831–6. doi: 10.1042/BST0320831. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251(4998):1233–6. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Driessens MH, Van Rijthoven EA, Kemperman H, Roos E. Adhesion of lymphoma cells to fibronectin: differential use of alpha 4 beta 1 and alpha 5 beta 1 integrins and stimulation by the 9EG7 mAb against the murine beta 1 integrin subunit. Cell Adhes Commun. 1995;3(4):327–36. doi: 10.3109/15419069509081017. [DOI] [PubMed] [Google Scholar]
- Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284(17):11485–97. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118(6):1421–42. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T, Chaponnier C, Yin H, Stossel T. Gelsolin-actin interaction and actin polymerization in human neutrophils. J Cell Biol. 1990;110(6):1983–91. doi: 10.1083/jcb.110.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyduk SJ, Rullo J, Cano AP, Xiao H, Chen M, Moser M, Cybulsky MI. Talin-1 and kindlin-3 regulate alpha4beta1 integrin-mediated adhesion stabilization, but not G protein-coupled receptor-induced affinity upregulation. J Immunol. 2011;187(8):4360–8. doi: 10.4049/jimmunol.1003725. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones SL, Wang J, Turck CW, Brown EJ. A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function. Proc Natl Acad Sci U S A. 1998;95(16):9331–6. doi: 10.1073/pnas.95.16.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5(7):546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Integrin regulation of lymphocyte trafficking: lessons from structural and signaling studies. Adv Immunol. 2007;93:185–227. doi: 10.1016/S0065-2776(06)93005-3. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Mehl R, Izumo S, Nadal-Ginard B, Yin HL. Muscle is the major source of plasma gelsolin. J Biol Chem. 1988a;263(17):8239–43. [PubMed] [Google Scholar]
- Kwiatkowski DJ, Mehl R, Yin HL. Genomic organization and biosynthesis of secreted and cytoplasmic forms of gelsolin. J Cell Biol. 1988b;106(2):375–84. doi: 10.1083/jcb.106.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, Prinsen BH, de Sain-van der Velden MG, Coppens CJ, Koenderman L, Ulfman LH. A 2D-DIGE approach to identify proteins involved in inside-out control of integrins. J Proteome Res. 2009;8(8):3824–33. doi: 10.1021/pr8010815. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A. 1993;90(19):9051–5. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402(6762):676–81. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–30. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Oudijk EJ, Nijhuis EH, Zwank MD, van de Graaf EA, Mager HJ, Coffer PJ, Lammers JW, Koenderman L. Systemic inflammation in COPD visualised by gene profiling in peripheral blood neutrophils. Thorax. 2005;60(7):538–44. doi: 10.1136/thx.2004.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MP, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140(3):699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302(5642):103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tellam R, Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982;21(13):3207–14. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Yin HL, Albrecht JH, Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981;91(3 Pt 1):901–6. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HL, Stossel TP. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281(5732):583–6. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler WH, Gingras AR, Critchley DR, Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans. 2008;36(Pt 2):235–9. doi: 10.1042/BST0360235. [DOI] [PubMed] [Google Scholar]