Abstract
Chlamydia trachomatis infections represent the leading cause of bacterial sexually-transmitted disease in the United States and can cause serious tissue damage leading to infertility and ectopic pregnancies in women. Inflammation and hence the innate immune response to chlamydial infection contributes significantly to tissue damage, particularly by secreting proinflammatory cytokines such as interleukin (IL)-1β from monocytes, macrophages and dendritic cells. Here we demonstrate that C. trachomatis or Chlamydia muridarum infection of a monocytic cell line leads to caspase-1 activation and IL-1β secretion through a process requiring the NLRP3 inflammasome. Thus, secretion of IL-1β decreased significantly when cells were depleted of NLRP3 or treated with the anti-inflammatory inhibitors parthenolide or Bay 11-7082, which inhibit inflammasomes and the transcription factor NF-κB. As for other infections causing NRLP3 inflammasome assembly, caspase-1 activation in monocytes is triggered by potassium efflux and reactive oxygen species production. However, anti-oxidants inhibited IL-1β secretion only partially. Atypically for a bacterial infection, caspase-1 activation during chlamydial infection also involves partially the spleen tyrosine kinase (Syk), which is usually associated with a pathogen recognition receptor for fungal pathogens. Secretion of IL-1β during infection by many bacteria requires both microbial products from the pathogen and an exogenous danger signal, but chlamydial infection provides both the pathogen-associated molecular patterns and danger signals necessary for IL-1β synthesis and its secretion from human monocytes. Use of inhibitors that target the inflammasome in animals should therefore dampen inflammation during chlamydial infection.
Keywords: Inflammasome, Nod-like receptor, Innate immunity, Monocytes, Chlamydia
1. Introduction
Chlamydia trachomatis is responsible for the most common bacterial sexually-transmitted diseases and is the main cause of preventable blindness worldwide [1-4]. The Centers for Disease Control reported an increase in diagnosed cases of sexually-transmitted chlamydial infections in the U.S. from 102.5 to 401.3 cases per 100,000 populations from 1998 to 2008 respectively, with the highest prevalence being among young adults (18–26 years of age). A C. trachomatis strain, lymphogranuloma venereum (LGV2), causes inflammation and swelling of lymph nodes, and is becoming more common in North America and Europe [5-7]. This strain is commonly used in cellular studies of Chlamydia infection. Chlamydia muridarum, the chlamydial biovar that infects mouse species, gives a pathology similar to that seen in humans, and is used in in vivo and in vitro models of chlamydial pathogenesis [8].
Both innate and adaptive immune systems coordinate their responses in an attempt to eliminate chlamydial infection. At the same time, much of the pathogenesis due to C. trachomatis infection is due to chronic inflammation [9,10]. Epithelial cells, in response to chlamydial infection, secrete interleukin (IL)-6, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [11]. These chemokines recruit and activate immune cells including neutrophils, macrophages, dendritic cells (DCs) and natural killer (NK) cells, which in turn secrete more proinflammatory cytokines such as TNF-a. Moreover, previous studies demonstrated that dendritic cells, macrophages, and monocytes, secrete the proinflammatory cytokine IL-1β in response to chlamydial infection [12-15]. Excessive IL-1β secretion plays a role in tubal pathology associated with chlamydial infection [16]. More recently, the bulk of IL-1β secreted during chlamydial infection in genitally-infected mice was shown to be produced by macrophages and neutrophils, with very little produced by epithelial cells [17].
Infection by chlamydiae and other bacteria stimulate an innate immune response when pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS), upregulate the expression of proinflammatory mediators following ligation to pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs) and Nod-like receptors (NLRs)[18-21]. Most cytokines are secreted following ligation of PRRs; however, secretion of the key inflammatory cytokine interleukin IL-1β is under a stringent regulatory process. A first signal, following recognition of PAMP by its PRR, causes the production of the immature pro-IL-1β, and a second “danger signal”, derived from host-cell molecules that are released from stressed or infected cells, or detected as a PAMP such as flagellin that is present in the cytosol, can stimulate the assembly of an inflammasome that activates the protease caspase-1. Caspase-1, in turn, is responsible for processing and secretion of the mature IL-1β [21,22].
Previous studies have shown that caspase-1 could be activated during chlamydial infections [13,14,17,23,24]. Moreover, we recently demonstrated that C. trachomatis dependent caspase-1 activation in cervical epithelial cells is dependent on the NLRP3 inflammasome and requires K+ efflux and reactive oxygen species (ROS) production. Epithelial cells secrete little IL-1β [17], and we showed that caspase-1 activation in epithelial cells during C. trachomatis infection is required instead for chlamydial growth [25]. On the other hand, caspase-1 activation in immune cells during chlamydial infection is required for IL-1β secretion, which plays an important role in clearance and pathology associated with infection. However, the mechanism by which caspase-1 is activated, and whether an inflammasome is required, for IL-1β secretion in immune cells had yet to be investigated. In this study, we therefore demonstrated that unprimed human monocytes (THP-1 cells) secrete IL-1β in response to C. trachomatis and C. muridarum infections in a process that requires NLRP3-mediated caspase-1 activation. We also find that ROS production and K+ efflux are required for inflammasome activation in human monocytes.
Spleen tyrosine kinase (Syk) is a cytosolic tyrosine kinase that is expressed in immune and non-immune cells. Syk plays a key role in transmitting signals from a variety of cell surface receptors, such as FcγR, CR3, Dectin-1 and apoptotic cell-recognizing receptor [26]. Several recent studies have shown that Syk can be coupled to NLRP3 inflammasome to activate caspase-1 and induce NF-κB activation in response to fungal infection [27-30], which involves Syk recruitment to lipid raft domains [31]. As chlamydiae have been reported to enter host cells by lipid raft domains [32,33] and a protein secreted by Chlamydia is regulated by Syk phosphorylation [34], we also investigated whether Syk signaling is required for NLRP3-dependent caspase-1 activation and IL-1β secretion following chlamydial infection. Indeed we find that the ability of chlamydiae to activate caspase-1 relies at least partially on Syk kinase signaling.
2. Materials and methods
2.1. Cells, bacteria, and chemical reagents
The human acute monocytic leukemia cell line, THP-1, was from American Type Culture Collection (ATCC). The LGV/L2 strain of C. trachomatis and C. muridarum Nigg strain (also known as the mouse pneumonitis biovar, MoPn) were obtained from Roger Rank, University of Arkansas. THP-1 cells were cultured in a humidified incubator at 37 °C with 5% CO2 in RPMI medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen). C. trachomatis and C. muridarum were grown in infected HeLa cell monolayer cultures to determine the number of bacterial inclusion forming units as described previously[35,36]. After 2 days of infection, chlamydiae were harvested from infected cells by combining cells and supernatant and performing a freeze thaw cycle at −80 °C, and then centrifuging at 2000 rpm for 5 min at 4 °C to remove cell debris, followed by a high speed centrifugation at 15,000 rpm for 45 min at 4 °C. The resulting pellet was resuspended in an appropriate volume of DMEM with 10% FBS medium, and aliquoted and stored at −80 °C until ready for use. Diphenyliodonium chloride (DPI), parthenolide and bay 11-7082 were purchased from Enzo Life Sciences (Plymouth Meeting, PA), N-acetyl cysteine (NAC) was from Sigma (St. Louis, MO), KCl was from Fisher.
2.2. Cell culture, infection, and treatments
THP-1 cells were plated at 106 cells per well and infected with LGV/L2 C. trachomatis at a multiplicity of infection (m. o.i.) of 5.0 or infected with C. muridarum Nigg strain at an m. o.i. of 1.0, and incubated for 24 h in an incubator at 37 °C with 5% CO2. Treatment with inhibitors or other reagents was performed at the indicated times and concentrations.
2.3. Enzyme-linked immunosorbent assay (ELISA) for IL-1β secretion
Following 24 h of infection with Chlamydia, supernatant from cultured cells was collected and stored at −80 °C until ready for use in the assay. Measurement of IL-1β from THP-1 cells was carried out using Human IL1-b ELISA kit (eBioscience, San Diego, CA), following manufacturer’s instructions.
2.4. Generation of cells stably expressing shRNA
THP-1 stably expressing shRNA against NLRP3, ASC, Syk, MyD88 and caspase-1 were obtained by transducing THP-1 cells with lentiviral particles. The sequences 5′-CCGGGCGTTAGAAACACTTCAAGAACTCGAGTTCTTGAAGTGTTTC TAACGCTTTTTG-3′ for human NLRP3 (Sigma; catalog number NM_004895), 5′-CCGGCGGAAGCTCTTCAGTTT CACACTCGAGTGTGAAACTGAAGATTCCG TTTTTG-3′ for human ASC (Sigma; catalog number NM_013258), 5′-CCGGCCTGTCTCTGTTCTTGAACGTCTCGAGACGTTCAAG AACAGAGACAGGTTTTT-3′ for human MyD88 (Sigma; catalog number NM_002468), 5′-CCGGGCAGGCCATCAT CAGTCAGAACTCGAGTTCTGACTGATGATGGCCTGCT TTTT-3′ for human spleen tyrosine kinase (Syk) (Sigma; catalog number NM_003177), and five sequences for caspase-1 (Sigma; catalog number NM_001223): 5′-CCGGGAAGAGTTTGAGGATGATGCTCTCGAGAGCATCATCCTCAAACT CTTCTTTTT-3′, 5′-CCGGTGTATGAATGTCTGCTGGGCACTCGAGTGCCCAGCAGACATTCATACATTTTT-3′, 5′-CC GGCACACGTCTTGCTCTCATTATCTCGAGATAATGAGA GCAAGACGTGTGTTTTT-3′, 5′-CCGGCTACAACTCAATGCAATCTTTCTCGAGAAAGATTGCATTGAGTTGTAGTT TTT-3′, 5′-CCGGCCAGATATACTACAACTCAATCTCGAGATTGAGTTGTAGTATATCTGGTTTTT-3′ were used separately to silence gene expression following the manufacturer’s instructions. Nontarget shRNA control cells were also generated using an irrelevant sequence (Sigma; catalog number SHC002 V). Briefly, cells were plated at 35% confluency 24 h prior to transduction and then the corresponding lentiviral transduction particles were added at m.o.i. of 3 overnight. Fresh media were added the next day, and transduced cells were selected by addition of media containing 2 μg/ml puromycin (Sigma).
2.5. RNA isolation, PCR and real-time PCR
mRNA was isolated from cells after the indicated treatments or infections using the Qiagen RNeasy kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Quantitative PCR was performed with 1:50 of the cDNA preparation in the M×3000P (Stratagene, La Jolla, CA) in a 25 ml final volume with Brilliant QPCR master mix (Stratagene). The primers for human GAPDH were 5′-CTTCTCTGATGAGGCCCAAG-3′ forward and 5′-GCAGCAAACTGGAAAGGAAG-3′ reverse. Primers for human NLRP3 were 5′-CTTCCTTTCCAGTTTGCTGC-3′ forward and 5′-TCTCGCAGTCCACTTCCTTT-3′ reverse. Primers for human ASC were 5′-AGTTTCACACCAGCCTGGAA-3′ forward and 5′-TTTTCAAGCTGGCTTTTCGT-3′ reverse. Primers for Syk were 5′-AGAGCGAGGAGGAGCG GGTG-3′ forward, 5′-CCGCTGACCAAGTCGCAGGA-3′ reverse. Primers for MyD88 were 5′AGCGCTGGCAGACAA TGCGA-3′ forward, 5′-TCCGGCGGCACCTCTTTTCG-3′ reverse. Primers for caspase-1 were 5′-GCCCAAGTTTGAA GGACAAA-3′ forward, 5′-GGTGTGGAAGAGCAGAAA GC-3′ reverse. Real-time PCR included initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min, and one cycle of 95 °C for 1 min, 55 °C for 30 s, 95 °C for 30 s.
2.6. Western blotting
Samples were lysed using 1× RIPA Lysis Buffer (Millipore) with 1× protease inhibitor cocktail (Biovision) and loaded onto a 15% SDS-polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane (Millipore). For detection of the active caspase-1 subunit (p20), the blot was probed with 1 mg/ml rabbit anti-human caspase-1 antibody (Millipore), and then incubated again with conjugated 1:2000 dilution of anti-rabbit IgG horseradish peroxidase (Millipore). For confirmation of NLRP3 depletion by RNA interference, a 9% gel was used, and the blot was incubated with rabbit anti-human NLRP3 antibody (Sigma; cat. # HPA012878) at a dilution of 1:200 for 1 h. Immunoreactive proteins were detected by adding ECL Plus Western blotting detection reagents (Amersham Biosciences) following manufacturer’s instructions and chemiluminescence was detected using a gel doc system (Bio-Rad).
2.7. Statistical and flow cytometric analyses
The statistical analysis was performed using GraphPad Instat software (GraphPad Software Inc, La Jolla, CA) by Student’s t test and was considered significant at p < 0.05. Flow cytometry data were analyzed using FlowJo software (Tree Star Inc, Ashland, OR).
3. Results
3.1. Chlamydia induces caspase-1 activation in monocytes
Previous studies have shown that monocytes, macrophages and dendritic cells secrete IL-1β during chlamydial infection, although the mechanisms were not elucidated. Therefore we used a human monocytic cell line to determinewhether IL-1β secretion in Chlamydia-infected cells requires inflammasome-dependent caspase-1 activation. In fact, cells that were infected with either C. trachomatis (L2) at m.o.i of 5 or C. muridarum (MoPn) at m.o.i of 1 for 24 h exhibited an increase in the amount of active caspase-1 subunit (p20), compared to non-infected cells as shown by Western Blot analysis (Fig. 1 A). This result was quantitatively assessed by measuring the amount of active caspase-1 in the supernatant of infected THP-1 cells using a caspase-1 ELISA kit (Fig. 1 B). Furthermore, highlevelsof mature IL-1β were detected in the supernatant of these cells 24 h post infection (Fig. 1 C).
Fig. 1.
Chlamydia induces caspase-1 activation in monocytes. THP-1 cells treated with non-target control (sh Control) were infected with C. trachomatis (L2) at an m.o.i. of 5 or C. muridarum (MoPn) at an m.o.i. of 1 for 24 h. (A) Western blot analysis of the lysates was performed to monitor caspase-1 (Casp1) activation using an antibody that detects active caspase-1 (p20). (B) Caspase-1 ELISA was used to quantitatively measure caspase-1 activity in the supernatant of cultured cells. (C) IL-1β ELISA was used to measure IL-1β secretion in supernatants of cultured cells. Error bars represent standard deviation of at least three separate experiments. *** indicates p < 0.001.
To confirm that the IL-1β secretion was dependent on caspase-1 activation, we silenced caspase-1 gene expression by RNA interference. When compared with wild-type (WT) THP-1 cells or to cells treated with non-target shRNA (Sh Ctrl), cells treated with shRNA for caspase-1 showed a 40% reduction in mRNA expression as well as significantly lower protein expression (Fig. 2 A). When these cells were infected with C. trachomatis or C. muridarum for 24 h, they secreted significantly less IL-1β and activated caspase-1 into the supernatant, confirming that Chlamydia-induced IL-1β secretion relies on caspase-1 activation (Fig. 2 C,E).
Fig. 2.
Caspase-1 activation is required for Chlamydia-induced IL-1β secretion. (A) THP-1 cells were stably transfected with shRNAs that target caspase-1, and mRNA expression of caspase-1 was quantified by real-time PCR and compared with wild-type (WT) and non-target control (sh Ctrl) (one representative experiment is shown). Inset: Western blot analysis of wild-type THP-1 cells, cells treated with non-target control, and cells treated with shCasp1, confirming the decreased expression of the caspase-1 protein. The Western blot was performed with an anti-caspase-1 antibody that detects the p20 fragment of the enzyme. (B and D) THP-1 cells were infected with C. trachomatis (L2) at an m.o.i. of 5 or C. muridarum (MoPn) at an m.o.i. of 1 for 24 h and treated with control buffer or 10 mM parthenolide (Parth) or 12 mM Bay 11-7082 (Bay 11) for 3 h p.i. (B) Caspase-1 activation or (D) IL-1β secretion in the supernatants were measured by ELISA and plotted as a bar graph. (C and E) Caspase-1 (Casp1) or non-target control THP-1 cells were infected with L2 (m.o.i. 5) or MoPn (m.o.i. 1) for 24 h, and Chlamydia-induced (C) caspase-1 activation or (E) IL-1β secretion were measured by ELISA. The fold increase in IL-1β secretion in infected non-target controls (shCasp1-treated cells) with respect to uninfected cells was compared with the increase in 24 h-infected (C) non-target control treated cells or (E) wild-type cells. Error bars represent standard deviations from at least three separate experiments. * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001 compared with untreated infected cells.
A recent study shed light on the mechanisms of action of two known NF-κB pathway inhibitors: parthenolide and Bay 11-7082 [37]. Independently of its inhibitory effect on NF-κB activity, parthenolide also acts as a directinhibitor of caspase-1 and a potent inhibitor of multiple inflammasomes, whereas Bay 11-7082 selectively inhibits NLRP3 inflammasome activity. Therefore, to further confirm a caspase-1 requirement for IL-1β secretion, we incubated THP-1 cells with parthenolide for 3 h during chlamydial infection and measured the concentration of IL-1β and activated caspase-1 in the supernatant by ELISA. In fact, parthenolide induced a drastic reduction in both caspase-1 activation and IL-1β secretion in the supernatant of infected cells (Fig. 2 B, D). Since parthenolide inhibits both NF-κB and caspase-1 activation, we determined whether this result is due predominantly to inflammasome inhibition by treating THP-1 cells with Bay 11-7082, which selectively inhibits the NLRP3 inflammasome independently of its effect on NF-κB activity. Although the effect of Bay 11-7082 was less potent than parthenolide, IL-1β secretion was still remarkably diminished in the presence of Bay 11-7082 (Fig. 2 D). We therefore conclude that both C. trachomatis and C. muridarum induce caspase-1 dependent IL-1β secretion, and more importantly these results imply that the NLRP3 inflammasome is required for caspase-1 activation.
3.2. NLRP3 inflammasome is activated in response to chlamydial infection
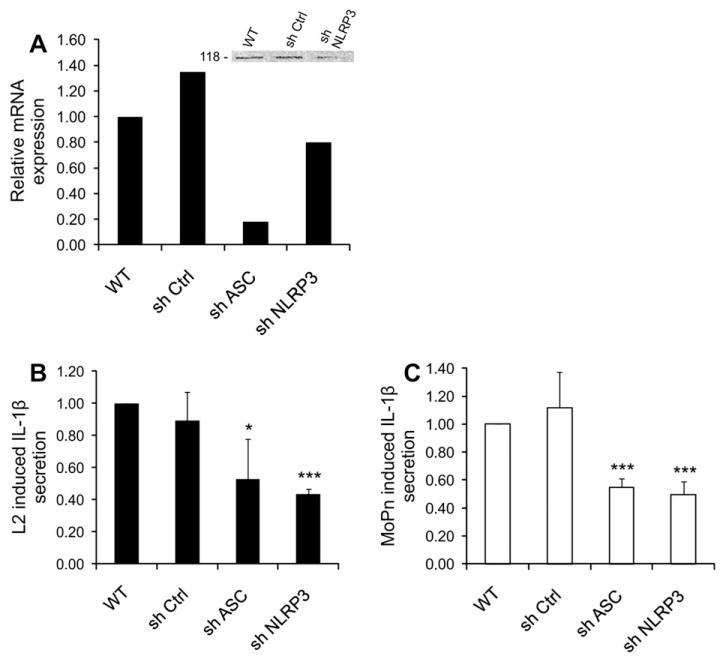
To further investigate the role of NLRP3 in activating caspase-1, we used shRNA to knockdown separately NLRP3 and its adaptor protein, ASC, in THP-1 cells. mRNA expression of either inflammasome component was significantly reduced in comparison with non-target shRNA, as measured by real-time PCR (Fig. 3 A), and NLRP3 protein depletion was confirmed by Western blotting (Fig. 3 A inset). We previously showed that C. trachomatis induces NLRP3/ASC inflammasome-dependent caspase-1 activation in epithelial cells [25], and we find here that Chlamydia uses the same effectors in monocytes, since both NLRP3 and ASC knockdown cells exhibited almost 45% reduction in the amount of secreted IL-1β when compared to wild-type and non-target (Sh Ctrl) cells during infection with C. trachomatis or C. muridarum (Fig. 3 B,C). The reduction of IL-1β secretion was partial, probably due to the partial mRNA depletion that was achieved by shRNA.
Fig. 3.
Chlamydia-induced IL-1β secretion requires the NLRP3 inflammasome. (A) THP-1 cells were stably transfected with shRNAs that target NLRP3 or ASC, and mRNA expression of NLRP3 and ASC was quantified by real-time PCR and compared with wild-type (WT) and non-target control (sh Ctrl). Inset: Western blot analysis of wild-type THP-1 cells, cells treated with non-target control, and cells treated with shNLRP3, confirming decreased expression of the NLRP3 protein after mRNA knockdown (one representative experiment is shown). The Western blot was performed with an anti-NLRP3 antibody, which detects the 118-kDa protein. (B) NLRP3, ASC, or non-target control knockdown cells were infected with L2 at an m.o.i. of 5 for 24 h, and C. trachomatis-induced IL-1β secretion in the supernatants was measured by ELISA. The fold increase in IL-1β secretion in infected non-target controls, shNLRP3-treated cells, and shASC-treated cells with respect to uninfected cells was compared with the increase in 24 h-infected wild-type cells. (C) Same as in panel B, but cells were infected with C. muridarum at an m.o.i. of 1 for 24 h. Error bars represent standard deviations of an experiment performed on three separate occasions. * indicates p < 0.05; *** indicates p < 0.001.
3.3. K+ efflux and ROS production are key elements in Chlamydia-induced NLRP3 activation
Despite the large variety of ligands that can induce NLRP3 inflammasome activation, the cell-signaling pathways often converge on K+ efflux and production of reactive oxygen species (ROS) [21,38-40]. To test the role of these elements, we neutralized ROS by adding the anti-oxidant reagent, N-acetyl cysteine (NAC), or by treating with an NADPH oxidase inhibitor, diphenyliodonium chloride (DPI). Treatment of C. trachomatis or C. muridarum infected cells with NAC diminished caspase-1 activation (Fig. 4 A). Consistent with this result, addition of DPI 9 h post infection (p.i.) caused a reduction in Chlamydia-induced IL-1β secretion (Fig.4 B,C). However, the effects of anti-oxidants on secretion of IL-1β from monocytes were not as dramatic as we previously observed for caspase-1 activation during C. trachomatis infection of epithelial cells [25].
Fig. 4.
IL-1β secretion during Chlamydia infection is caused by K+ efflux and, partially, by ROS production. (A) THP-1 cells were infected with C. trachomatis (L2) at an m.o.i. of 5 or C. Muridarum (MoPn) at an m.o.i. of 1 for 24 h, and treated with control buffer or 10 mM NAC. Caspase-1 activation was measured by ELISA (one representative experiment). (B and C) THP-1 cells were infected with C. trachomatis (L2) at an m.o.i. of 5 (A) or C. muridarum (MoPn) at an m.o.i. of 1 (B) for 24 h, and treated with control buffer or 10 mM NAC, 250 nM diphenyliodonium chloride (DPI), or 70 mM KCl during the last 15 h of infection. IL-1β levels in the supernatant were measured by ELISA and plotted as a bar graph. Error bars represent standard deviations from at least three separate experiments. * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001, compared with uninfected cells.
Next, we blocked K+ efflux by increasing the extracellular KCl concentration, and checked the secretion of IL-1β into the supernatant of infected cells 24 h post infection. Limiting K+ efflux was in fact able to significantly reduce IL-1β secretion from cells infected with C. trachomatis or C. muridarum (Fig. 4 B,C). Taken together, these results demonstrate that NLRP3 activation during chlamydial infection relies on a cell-signaling pathway involving, at least partially, K+ efflux and ROS production.
3.4. Syk kinase signaling couples with the NLRP3 inflammasome for inducing IL-1β release during chlamydial infection
Syk, through its interaction with immunoreceptor tyrosine-based activation motif (ITAMs)-based receptors, plays a major role in MyD88-independent signaling pathways of several receptors such as FcγR, CR3, Dectin-1 and apoptotic cell-recognizing receptor [26]. Recently, Syk signaling had been shown to be involved in NLRP3 inflammasome activation by fungi such as Candida albicans and Aspergillus fumigatus [27,30]. Interestingly, chlamydial translocated actin recruiting phosphoprotein (Tarp), a type three secretion (T3S) effector protein, is regulated by Syk phosphorylation [34]. At the same time, the TLR-adaptor protein MyD88 has been shown to participate in cytokine production during chlamydial infection[41-45]. To examine whether Syk and MyD88 are involved in NLRP3 inflammasome activation during Chlamydia infection, we depleted Syk and MyD88 in THP-1 cells using RNA interference. mRNA expression of Syk and MyD88 was significantly reduced in comparison with non-target shRNA, as measured by real-time PCR (Fig. 5 A). Individual knockdowns of Syk or MyD88 resulted in a reduction of ~40% in the amount of IL-1β secretion after 24 h of C. trachomatis (L2) infection (Fig. 5 D) or C. muridarum (MoPn) infection (Fig. 5 E), when compared to wild-type or non-target (Sh ctrl) THP-1 cells. Secretion of activated caspase-1 into the supernatants of infected Syk knockdown cells exhibited a ~60% reduction, when compared to control cells (Fig. 5 B,C). Our results thus demonstrate for the first time that NLRP3 activation can be coupled, at least partially, to tyrosine kinase Syk signaling in response to a bacterial infection in human monocytes.
Fig. 5.
Caspase-1 dependent IL-1β secretion during Chlamydia infection involves MyD88 and Syk signaling. (A) THP-1 cells were stably transfected with shRNAs that target Syk or MyD88, and mRNA expression of Syk and MyD88 was quantified by real-time PCR and compared with wild-type (WT) and non-target control (sh Ctrl) (one representative experiment is shown). (B and D) Syk, MyD88, or non-target control knockdown THP-1 cells were infected with L2 at an m.o.i. of 5 for 24 h, and C. trachomatis-induced (B) caspase-1 activation or (D) IL-1β secretion in the supernatants was measured by ELISA. The fold increase in IL-1β secretion in infected non-target controls, shSyk-treated cells, and shMyD88-treated cells with respect to uninfected cells was compared with the increase in 24 h-infected (B) sh Ctrl treated cells or (D) wild-type cells. (C and E) Same as in panels B and D, except that THP-1 cells were infected with MoPn at an m.o.i. of 1 for 24 h. (E) Error bars represent standard deviations of an experiment performed on three separate occasions. * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001.
4. Discussion
Previous studies have demonstrated that chlamydial infection of monocytes and macrophages leads to IL-1β secretion, and a requirement for caspase-1 was shown [13,14,17,23,24]. Despite the important role that IL-1β plays in pathogenesis of chlamydial infection, the mechanism of caspase-1 activation and the subsequent secretion of IL-1β had not been revealed yet. In this study, we demonstrate that the NLRP3 inflammasome is required for C. trachomatis or C. muridarum induced caspase-1 dependent IL-1β secretion by monocytes. The adaptor protein ASC was also needed for NLRP3-dependent caspase-1 activation.
Although the NLRP3 inflammasome could be activated by a wide range of triggers, most of these activators converge on a small number of shared mechanisms, including K+ efflux and ROS production [21,38,39]. We previously reported that K+ efflux precedes ROS production in C. trachomatis-induced NLRP3-dependent caspase-1 activation in epithelial cells [25]. Here we show that K+ efflux and ROS production are also required for NLRP3-dependent caspase-1 activation and IL-1β secretion in monocytes infected with C. trachomatis or C. muridarum. Blocking K+ efflux by increasing extracellular KCl caused a reduction in the amount of IL-1β secretion and caspase-1 activation in response to infection with both chlamydial strains, and scavenging ROS with the anti-oxidant NAC also led to lower levels of IL-1β production and caspase-1 activation, although the ability of anti-oxidants to block IL-1β secretion from monocytes was not as striking as for the effect of anti-oxidants on caspase-1 activation in epithelial cells, suggesting the existence of ROS-independent mechanisms for IL-1β secretion in human monocytes. We further demonstrated that ROS production induced by chlamydial infection of monocytes is produced, at least partially, by NADPH oxidase, because inhibiting NADPH oxidase with DPI caused a reduction in both IL-1β levels and caspase-1 activation following infection with either C. trachomatis or C. muridarum. In monocytes, our data further demonstrate that Syk signaling contributes to caspase-1 activation and IL-1β secretion during chlamydial infection.
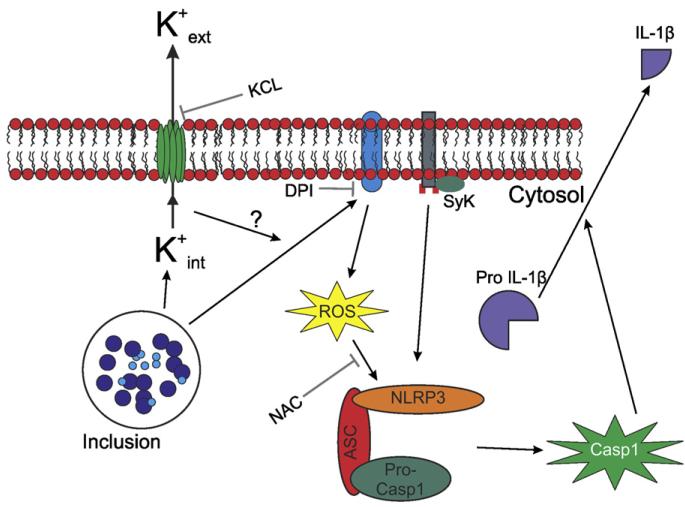
The mechanism of chlamydial entry into the host cell is not well understood, although previous studies have suggested that entry might taken place through lipid raft domains [32,33]. Independently of the mechanism of chlamydial entry, the Syk kinase is recruited to lipid raft domains [31], where the NADPH oxidase is also assembled [46-50]. Given the role that the NADPH oxidase plays in inflammasome activation in monocytes infected with chlamydiae, and recent reports that Syk signaling is coupled with NLRP3 inflammasome to activate caspase-1 and induce NF-κB activation [27,30], we investigated whether the Syk kinase might be involved in Chlamydia-induced NLRP3 inflammasome activation in monocytes. Indeed, we showed that both caspase-1 activation and IL-1β secretion following chlamydial infection of monocytes requires Syk signaling. Although the exact mechanism for Syk kinase involvement in Chlamydia-induced inflammasome activation will require further studies, we propose that K+ efflux during chlamydial infection leads to NADPH oxidase activation and ROS production, consistent with previous reports that plasma membrane depolarization can lead to ROS production [51-53]. ROS production results in NLRP3 inflammasome activation [40], which activates caspase-1. In parallel, chlamydial infection, through a process at least partially dependent on MyD88 [41,44,45], induces synthesis of pro-IL-1β. Activated caspase-1 cleaves pro-IL-1β, allowing its secretion (Fig. 6).
Fig. 6.
In monocytes, C. trachomatis and C. muridarum trigger inflammasome-mediated caspase-1 activation and IL-1β secretion through K+ efflux and ROS production, through a mechanism involving the Syk kinase. Chlamydial infection induces K+ efflux and ROS production, which promote assembly of the NLRP3 inflammasome through a pathway involving the Syk kinase. The inflammasome in turn activates caspase-1, which processes pro-IL-1β into the mature IL-1β form.
References
- [1].Gerbase AC, Rowley JT, Mertens TE. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351:2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- [2].Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- [3].Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. ASM Press; Washington, D.C: 1999. pp. 139–169. [Google Scholar]
- [4].Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull. World Health Organ. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- [5].Klint M, Lofdahl M, Ek C, Airell A, Berglund T, Herrmann B. Lymphogranuloma venereum prevalence in Sweden among men who have sex with men and characterization of Chlamydia trachomatis ompA genotypes. J. Clin. Microbiol. 2006;44:4066–4071. doi: 10.1128/JCM.00574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bebear C, de Barbeyrac B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009;15:4–10. doi: 10.1111/j.1469-0691.2008.02647.x. [DOI] [PubMed] [Google Scholar]
- [7].Stary G, Meyer T, Bangert C, Kohrgruber N, Gmeinhart B, Kirnbauer R, Jantschitsch C, Rieger A, Stary A, Geusau A. New Chlamydia trachomatis L2 strains identified in a recent outbreak of lymphogranuloma venereum in Vienna, Austria. Sex. Transm. Dis. 2008;35:377–382. doi: 10.1097/OLQ.0b013e31815d6df8. [DOI] [PubMed] [Google Scholar]
- [8].Cotter TW, Byrne GI. Immunity to Chlamydia: comparison of human infections and murine models. Res. Immunol. 1996;147:587–595. doi: 10.1016/s0923-2494(97)85226-1. [DOI] [PubMed] [Google Scholar]
- [9].Darville T, Andrews CW, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 2001;69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ward ME. Mechanisms of Chlamydia-induced disease. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. ASM Press; Washington, D.C: 1999. pp. 171–210. [Google Scholar]
- [11].Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Entrican G, Wilkie R, McWaters P, Scheerlinck J, Wood PR, Brown J. Cytokine release by ovine macrophages following infection with Chlamydia psittaci. Clin. Exp. Immunol. 1999;117:309–315. doi: 10.1046/j.1365-2249.1999.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gervassi A, Alderson MR, Suchland R, Maisonneuve JF, Grabstein KH, Probst P. Differential regulation of inflammation cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 2004;72:7231–7239. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ojcius DM, Souque P, Perfettini JL, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- [15].Rothermel CD, Schachter J, Lavrich P, Lipsitz EC, Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect. Immun. 1989;57:2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hvid M, Baczynska A, Deleuran B, Fedder J, Knudsen HJ, Christiansen G, Birkelund S. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell. Microbiol. 2007;9:2795–2803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- [17].Prantner D, Darville T, Sikes JD, Andrews CW, Jr., Brade H, Rank RG, Nagarajan UM. Critical role for interleukin-1beta (IL-1βeta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1βeta in mouse macrophages. Infect. Immun. 2009;77:5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Ann. Rev. Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- [19].Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends. Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [20].Bourhis LL, Werts C. Role of nods in bacterial infection. Microbes Infect. 2007;9:629–636. doi: 10.1016/j.micinf.2007.01.014. [DOI] [PubMed] [Google Scholar]
- [21].Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- [22].Abdul-Sater AA, Said-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today (Barc)(45 Suppl. B) 2009:105–112. [PMC free article] [PubMed] [Google Scholar]
- [23].Lu H, Shen C, Brunham RC. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J. Immunol. 2000;165:1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- [24].Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect. Immun. 2008;76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J. Biol. Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tohyama Y, Yamamura H. Protein tyrosine kinase, syk: a key player in phagocytic cells. J. Biochem. 2009;145:267–273. doi: 10.1093/jb/mvp001. [DOI] [PubMed] [Google Scholar]
- [27].Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- [28].Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu S, Huo J, Gunawan M, Su IH, Lam KP. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J. Biol. Chem. 2009;284:22005–22011. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jutras I, Abrami L, Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect. Immun. 2003;71:260–266. doi: 10.1128/IAI.71.1.260-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Norkin LC, Wolfrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell. Res. 2001;266:229–238. doi: 10.1006/excr.2001.5202. [DOI] [PubMed] [Google Scholar]
- [34].Mehlitz A, Banhart S, Hess S, Selbach M, Meyer TF. Complex kinase requirements for Chlamydia trachomatis Tarp phosphorylation. FEMS Microbiol. Lett. 2008;289:233–240. doi: 10.1111/j.1574-6968.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- [35].Perfettini JL, Ojcius DM, Andrews CW, Korsmeyer SJ, Rank RG, Darville T. Role of proapoptotic BAX in propagation of Chlamydia muridarum (the mouse pneumonitis strain of Chlamydia trachomatis) and the host inflammatory response. J. Biol. Chem. 2003;278:9496–9502. doi: 10.1074/jbc.M211275200. [DOI] [PubMed] [Google Scholar]
- [36].Ojcius DM, Degani H, Mispelter J, Dautry-Varsat A. Enhancement of ATP levels and glucose metabolism during an infection by Chlamydia. J. Biol. Chem. 1998;273:7052–7058. doi: 10.1074/jbc.273.12.7052. [DOI] [PubMed] [Google Scholar]
- [37].Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K + for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007;282:18810e18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- [40].Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- [41].Derbigny WA, Kerr MS, Johnson RM. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J. Immunol. 2005;175:6065–6075. doi: 10.4049/jimmunol.175.9.6065. [DOI] [PubMed] [Google Scholar]
- [42].Nagarajan UM, Ojcius DM, Stahl L, Rank RG, Darville T. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J. Immunol. 2005;175:450–460. doi: 10.4049/jimmunol.175.1.450. [DOI] [PubMed] [Google Scholar]
- [43].Buchholz KR, Stephens RS. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect. Immun. 2008;76:3150–3155. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J. Immunol. 2010;184:2602e2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- [45].Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 2009;183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- [47].Jin S, Zhou F. Lipid raft redox signaling platforms in vascular dysfunction: features and mechanisms. Curr. Atheroscler. Rep. 2009;11:220–226. doi: 10.1007/s11883-009-0034-6. [DOI] [PubMed] [Google Scholar]
- [48].Li H, Han W, Villar VA, Keever LB, Lu Q, Hopfer U, Quinn MT, Felder RA, Jose PA, Yu P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension. 2009;53:1054–1061. doi: 10.1161/HYPERTENSIONAHA.108.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Park SJ, Kim HY, Kim H, Park SM, Joe EH, Jou I, Choi YH. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free. Radic. Biol. Med. 2009;46:1694–1702. doi: 10.1016/j.freeradbiomed.2009.03.026. [DOI] [PubMed] [Google Scholar]
- [50].Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox. Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA. Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am. J. Physiol. Renal. Physiol. 2007;292:F1867eF1872. doi: 10.1152/ajprenal.00515.2006. [DOI] [PubMed] [Google Scholar]
- [52].Matsuzaki I, Chatterjee S, Debolt K, Manevich Y, Zhang Q, Fisher AB. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am. J. Physiol. Heart. Circ. Physiol. 2005;288:H336eH343. doi: 10.1152/ajpheart.00025.2004. [DOI] [PubMed] [Google Scholar]
- [53].DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]