Abstract
Bombyx mori (B. mori), silkworm, is one of the most important economic insects in the world, while phoxim, an organophosphorus (OP) pesticide, impact its economic benefits seriously. Phoxim exposure can damage the brain, fatbody, midgut and haemolymph of B. mori. However the metabolism of proteins and carbohydrates in phoxim-exposed B. mori can be improved by Titanium dioxide nanoparticles (TiO2 NPs). In this study, we explored whether TiO2 NPs treatment can reduce the phoxim-induced brain damage of the 5th larval instar of B. mori. We observed that TiO2 NPs pretreatments significantly reduced the mortality of phoxim-exposed larva and relieved severe brain damage and oxidative stress under phoxim exposure in the brain. The treatments also relieved the phoxim-induced increases in the contents of acetylcholine (Ach), glutamate (Glu) and nitric oxide (NO) and the phoxim-induced decreases in the contents of norepinephrine (NE), Dopamine (DA), and 5-hydroxytryptamine (5-HT), and reduced the inhibition of acetylcholinesterase (AChE), Na+/K+-ATPase, Ca2+-ATPase, and Ca2+/Mg2+-ATPase activities and the activation of total nitric oxide synthase (TNOS) in the brain. Furthermore, digital gene expression profile (DGE) analysis and real time quantitative PCR (qRT-PCR) assay revealed that TiO2 NPs pretreatment inhibited the up-regulated expression of ace1, cytochrome c, caspase-9, caspase-3, Bm109 and down-regulated expression of BmIap caused by phoxim; these genes are involved in nerve conduction, oxidative stress and apoptosis. TiO2 NPs pretreatment also inhibited the down-regulated expression of H+ transporting ATP synthase and vacuolar ATP synthase under phoxim exposure, which are involved in ion transport and energy metabolism. These results indicate that TiO2 NPs pretreatment reduced the phoxim-induced nerve toxicity in the brain of B. mori.
Introduction
Silkworm, Bombyx mori (B. mori, Bombycidae: Lepidoptera), is one of the most important economic insects in Asia, Africa, Europe and Latin America. B. mori has been domesticated for about 5,700 years in China, and it produces more than 80% of raw silk around the world [1]. However, B. mori is highly sensitive to adverse environmental conditions, especially pesticides. Every year, pesticide contamination causes as much as 30% of the reduction in raw silk production in China [2]. Due to its short growth cycle and pesticide sensitivity, B. mori has been a widely used model insect for pesticide toxicology studies. Phoxim is an efficient broad-spectrum organophosphorus (OP) pesticide, but its indiscriminate use has generated serious environmental problems.
Phoxim may trigger oxidative stress, which is mainly reflected in altered Malondialdehyde (MDA) content and Glutathione S-transferase (GST) activity in the fat body and midgut of B. mori [3]. Our previous study demonstrated that phoxim destroyed the carbohydrate and lipid metabolism in the haemolymph of B. mori [4]. Exposure of B. mori to phoxim also affected the activities of acetylcholinesterase (AChE) and detoxification enzymes, which play important roles in organophosphorus pesticide resistance and metabolism [5]. The catalytic substrate of AChE, Acetylcholine (ACh), is a chemical transmitter of cholinergic neurons that are exclusively in the central nervous system (CNS) of insects [6]. However, clear understanding of phoxim’s effects on the the brain of B. mori is still lacking. We hypothesized that nerve toxicity of phoxim in B. mori is associated with brain damages and gene expression profile alterations.
Titanium dioxide nanoparticles (TiO2 NPs) are widely used as whitening agents in paper, cosmetics, and food industries because of their whitening effects. TiO2 NPs may also be used for photocatalytic degradation of pesticide in water, soil, and air [7]–[9]. The growth of plants can be promoted by TiO2 NPs that improve their antioxidative capacity [10], [11]. Recently, it was reported that TiO2 NPs increased the cold-tolerance of Chickpea [12]. Our previous studies have shown that TiO2 NPs improve protein and carbohydrate metabolism to meet required energy demands and increase antioxidant capacity of midgut in B. mori exposed to phoxim [4], [13]. It was also found TiO2 NPs pretreatment decreased phoxim-induced toxicity to silkworms by greatly reducing the phoxim residue [14]. Therefore, we speculated that TiO2 NPs treatments may relieve phoxim-induced damage by modulating gene expression and enzymatic activities in the brain of B. mori.
Digital Gene Expression Profile (DGE) with massive parallel sequencing has been shown to have high sensitivity and reproducibility for transcriptome profiling [15]. DGE is based on new generation high-throughput sequencing technologies and high-performance computing analyses. Nowadays DGE has been widely used in biological, medical and pharmaceutical research [16]–[18].
In this study, we investigated the nerve toxicity of phoxim and the effects of TiO2 NPs in the brain of B. mori. To further explore the mechanisms of toxicity, we adopted DGE assay and real time quantitative PCR (qRT-PCR) to detect the alterations of genes participated in regulating neurotransmitter contents, oxidative stress and apoptosis. These findings may promote future mechanistic studies on the effects of TiO2 NPs on the toxicity of insecticides in B. mori.
Results
Body weight and Survival rate
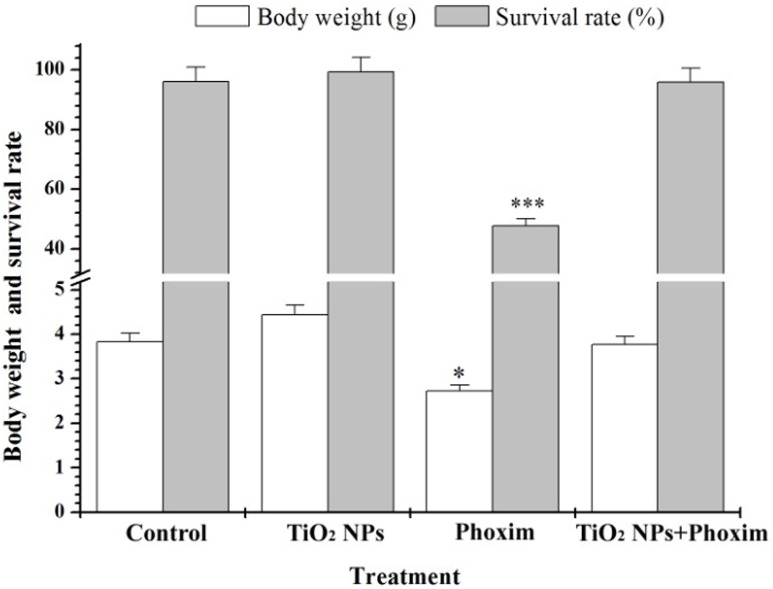
We observed that the fifth-instar larvae appeared as gastric juice spit, head nystagmus, body distortion, body shrink, paralysis and other symptoms after 48 h of phoxim exposure. However, the larvae in the control group, the TiO2 NPs group, and the TiO2 NPs + phoxim group did not show such symptoms. As shown in Figure 1, phoxim exposure significantly decreased the body weight (P<0.05) and survival rate (P<0.001) of the larvae, while TiO2 NPs promoted their body weights and survival rate.
Figure 1. Effects of TiO2 NPs on body weight, survival of phoxim-exposed fifth-instar larvae.
*P<0.05, and ***P<0.001. Values represent means ± SEM (n = 5).
Histopathological evaluation
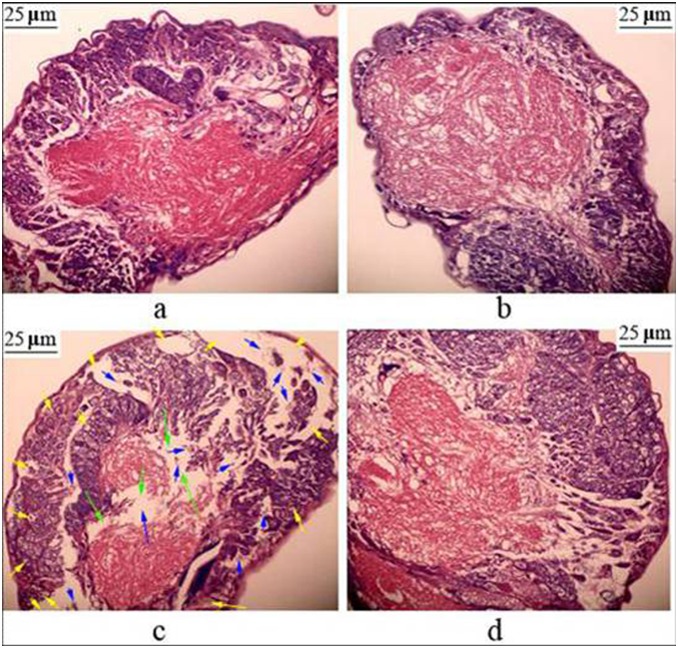
The brains of the larvae of both the control group (Fig. 2a) and the TiO2 NPs-treated group (Fig. 2b) had normal morphology. In the phoxim-exposed group, we observed widespread gaps among plasma membrane, breakage of nerve fibers, protein aggregation, adipose degeneration, and cell debris (Fig. 2c). However, the TiO2 NPs + phoxim-treated group did not show such pathological changes (Fig. 2d). It demonstrated that phoxim exposure caused brain damages, while TiO2 NPs treatments were able to reduce such damages.
Figure 2. Histopathology of the brain tissue in fifth-instar larvae after phoxim exposure 48 h.
(a) Control; (b) TiO2 NPs; (c) Phoxim; (d) TiO2 NPs + Phoxim. Green arrows indicate breakage of nerve fibers, yellow arrows show adipose degeneration, blue arrows indicate cell debris.
Brain ultrastructure evaluation
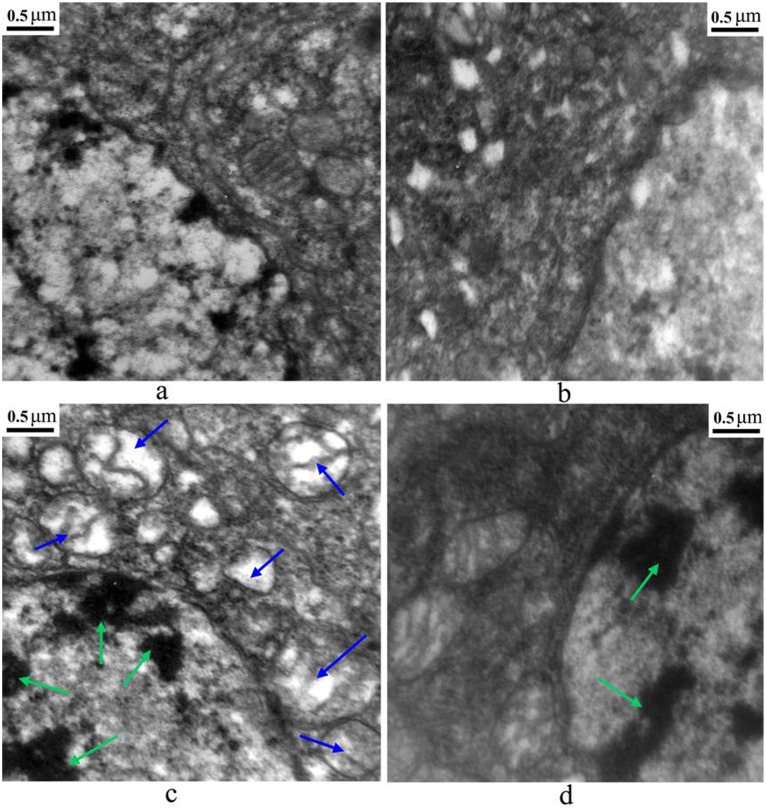
As shown in Figure 3, the ultrastructure of cells in the control group and the TiO2 NPs group was normal with well distributed chromatin and integral mitochondria crista (Fig. 3a, 3b), compared with karyopyknosis, chromatin marginalization, and mitochondria swelling in the phoxim exposure group (Fig. 3c) at 48 h after phoxim exposure. However, only chromatin marginalization was observed in the TiO2 NPs + phoxim group (Fig. 3d), indicating that TiO2 NPs reduced the damage in B. mori brain cells caused by phoxim exposure.
Figure 3. Ultrastructure of the brain tissue in fifth-instar larvae after phoxim exposure 48 h.
(a) Control; (b) TiO2 NPs; (c) Phoxim; (d) TiO2 NPs + Phoxim. Green arrows indicate karyopyknosis and chromatin marginalization, blue arrows show mitochondria swelling and became deformed, crest broken.
Neurotransmitter contents and enzyme activities in the brain
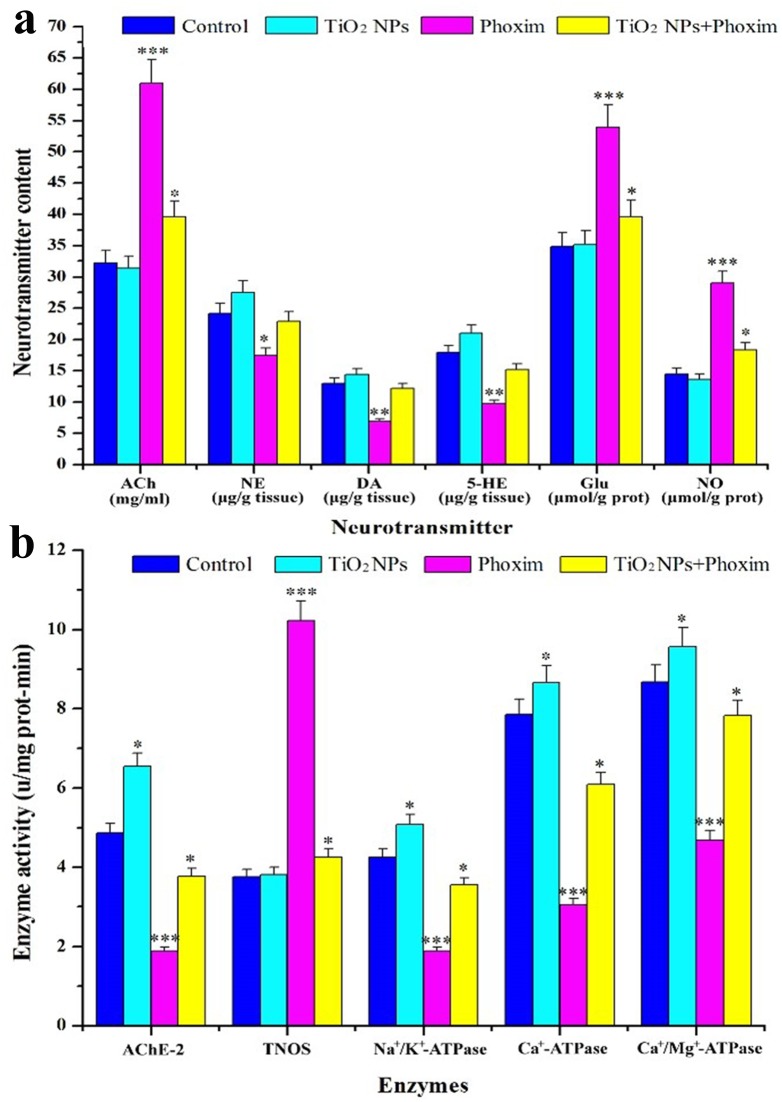
The contents of neurotransmitters, including ACh, Glutamate (Glu), and nitric oxide (NO), in the brains of fifth-instar larvae in the phoxim-exposed group were higher than those of the control, while the contents of norepinephrine (NE), dopamine (DA), and 5-hydroxytryptamine (5-HT) were otherwise decreased significantly by phoxim exposure (Fig. 4a). Pretreatments with TiO2 NPs reversed the changes in the contents of NE, DA, 5-HT, ACh, Glu, and NO (Fig. 4a). We also observed that phoxim exposure significantly inhibited the activities of AChE, Na+/K+-ATPase, Ca2+-ATPase, and Ca2+/Mg2+-ATPase and promoted the activity of total nitric oxide synthase (TNOS) in the brain, while TiO2 NPs significantly promoted the activities of AChE, Na+/K+-ATPase, Ca2+-ATPase, Ca2+/Mg2+-ATPase, and AChE and inhibited the activity of TNOS (Fig. 4b). These results demonstrated that phoxim exposure altered the releases of neurotransmitters and the activities of several important enzymes in the nerve conduction in B. mori larvae brain, while TiO2 NPs were able to reverse such changes.
Figure 4. Effects of TiO2 NPs on nerve conduction in the brain of phoxim-exposed fifth-instar larvae.
*p<0.05, **p<0.01, and ***p<0.001. Values represent means ± SEM (N = 5). (a) Neurotransmitter contents, (b) Enzyme activity.
Oxidative stress
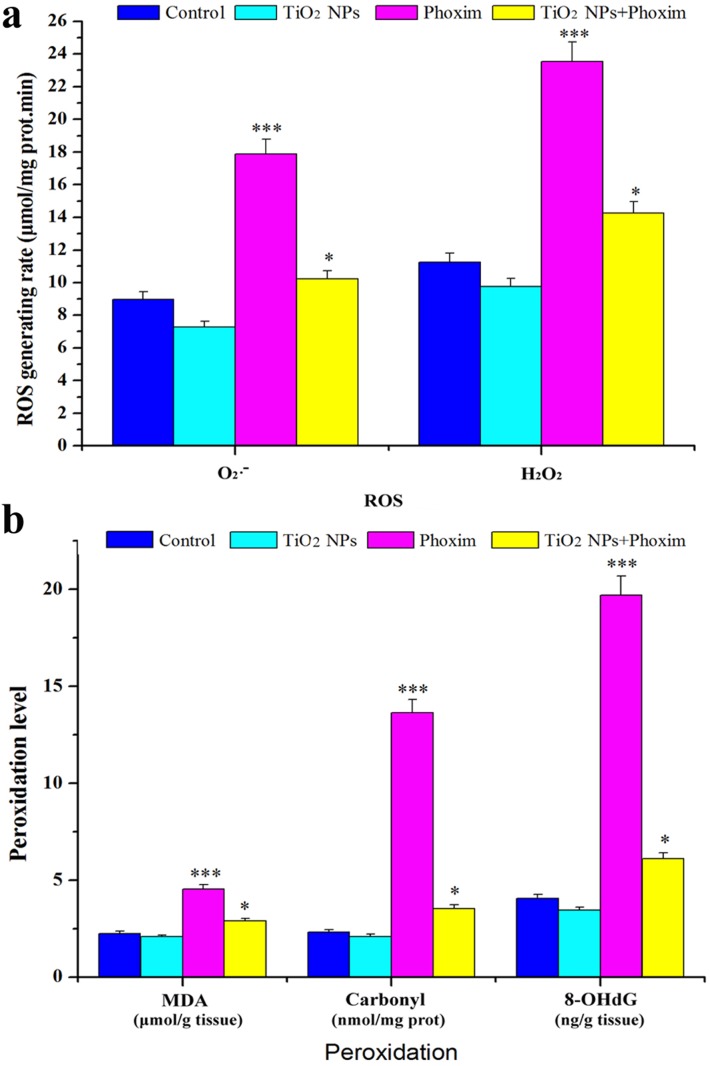
As shown in Figure 5a, phoxim exposure significantly promoted the production of ROS species, such as O2 − and H2O2, in larval brains (P<0.001) at 48 h, while TiO2 NPs attenuated such enhancement in ROS production (P<0.05). The ROS production was further demonstrated by the measurements of the levels of lipid peroxidation (MDA), protein peroxidation (protein carbonyl, PC), and DNA damage (8-hydroxy deoxyguanosine, 8-OHdg) in the larval brain (Fig. 5b). Significantly increased MDA, protein carbonyl, and 8-OHdG were observed in the phoxim-exposed midguts, but the increases became much lower with the combined treatments (Fig. 5b). It suggested that TiO2 NPs treatments decreased ROS accumulation, which may lead to attenuated peroxidation of lipids, proteins, and DNAs in the larval brains under phoxim-induced toxicity.
Figure 5. Effects of TiO2 NPs on oxidative stress in brain of phoxim-exposed fifth-instar larvae.
*p<0.05, and ***p<0.001. Values represent means ± SEM (N = 5). (a) ROS production, (b) Levels of lipid, protein, and DNA peroxidation.
Digital Gene Expression Profile
To investigate the molecular mechanisms of reduced nerve toxicity by TiO2 NPs under phoxim stress in the brain of B. mori, we adopted the DGE method to detect the differences in gene expression in the brain among the control-, TiO2 NPs-, phoxim-, and TiO2 NPs + phoxim-treated larvae at 48 h. Compared with those of the control group, 288, 295, and 472 genes were expressed significantly differently in the TiO2 NPs group (Fig. S1), the phoxim group (Fig. S2), and the TiO2 NPs + phoxim group (Fig. S3), respectively, with 117, 64, and 48 genes being up-regulated, respectively, and 171, 231, and 424 genes being down-regulated, respectively. The genes with differential expression were classified by Gene Ontology (GO) classification analysis into 12 groups, which were oxidative stress, stress response, metabolic process, cell component, transport, transcription-related, translation-related, growth and development, nerve conduction, immune response, cell cycle, and apoptosis (Tab. S1, S2, S3).
Gene Expression Detection by qRT-PCR
Combine with DGE assay, histopathological and ultrastructure evaluation, we hypothesized TiO2 NPs pretreatment might decrease the expression changes of genes essential in maintain normal physiological activity. To validate the hypothesis, we performed qRT-PCR for several genes that are involved in neurotoxicity, ion transport, oxidative stress and apoptosis. In the present study, actin3 was used as the internal reference gene. As shown in Table 1, the expression level of ace1 was significantly increased by 19.45-fold after 48 h of phoxim exposure, while the expression levels of H+ transporting ATP synthase, vacuolar ATP synthase, SOD and TPx were significantly reduced by 63%, 55%, 74% and 35% respectively. However, the expression changes of ace1, H+ transporting ATP synthase and vacuolar ATP synthase were 6.69-fold, 0.78-fold, 0.93-fold, 0.81-fold and 0.93-fold respectively for TiO2 NPs + phoxim-treated brains. Moreover, the expression of cytochrome-c was up-regulated by 1.21-fold in the phoxim-exposed brain, but by 1.02-fold in the TiO2 NPs + phoxim treated group. All the qRT-PCR data were consistent with those of DGE assay (Tab. 1). In order to explore whether phoxim stress induced apoptosis through the mitochondria/cytochrome-c pathway, the expression of four additional genes regulating mitochondria apoptosis pathway were determined by qRT-PCR. As shown in Table 1, compared with the control group, the expression levels of three pro-apoptotic genes, Bm109, caspase-9, and caspase-3 were changed by 3.0-fold, 2.51-fold, and 3.07-fold, respectively in the phoxim-exposed group, and by 2.42-fold, 2.2-fold, and 2.45-fold, respectively in the TiO2 NPs + phoxim-treated group. On the other hand, the mRNA levels of BmIAP, an apoptosis inhibitor gene, were down-regulated by 0.785-fold under phoxim stress, but by 0.91-fold in the TiO2 NPs + phoxim-treated group, respectively. These results indicated that TiO2 NPs treatment decreased expression alterations of these genes involved in neurotoxicity, ion transport, oxidative stress and apoptosis in the brain under phoxim stress.
Table 1. Comparison between fold-difference with qRT-PCR results and DGE assay in each group.
| Gene | TiO2 NPs/Control | Phoxim/Control | TiO2 NPs + Phoxim/Control | |||
| qRT-PCR(Fold) | DGE(log2 value) | qRT-PCR(Fold) | DGE(log2 value) | qRT-PCR(Fold) | DGE(log2 value) | |
| ace1 | 0.823 | 0.068 | 19.453*** | 0.956 | 6.689*** | 0.583 |
| H+ tATPase | 2.143 | 1.324 | 0.373*** | −0.303 | 0.779** | −0.148 |
| vATPase | 1.065 | 0.547 | 0.453** | −1.236 | 0.933 | −1.061 |
| SOD | 1.317 | 0.218 | 0.263** | −0.627 | 0.808 | −0.472 |
| TPx | 1.204 | 0.484 | 0.649* | −0.680 | 0.930 | −0.406 |
| Bm109 | 0.849 | No difference | 2.999** | No difference | 2.416** | No difference |
| BmIap | 1.026 | No difference | 0.785* | No difference | 0.905 | No difference |
| caspase-9 | 0.979 | No difference | 2.513** | No difference | 2.204** | No difference |
| caspase-3 | 0.970 | No difference | 3.065*** | No difference | 2.451** | No difference |
| cytochrome c | 0.953 | −0.085 | 1.214* | 0.294 | 1.021 | 0.099 |
*p<0.05, **p<0.01, and ***p<0.001.
Values represent means ± SEM (n = 5).
Discussion
The insect brain, a part of CNS, is essential in regulating nerve conduction, growth and development. It has been reported that the brain of B. mori is the target organ of nerve agent phoxim. In the present study, the body weight and survival rate of B. mori were significantly reduced by phoxim (Fig. 1), and severe brain damage was observed (Fig. 2), while pretreatment with TiO2 NPs protected the brain (Fig. 2). In addition, TiO2 NPs decreased the severe apoptosis of brain cells after phoxim exposure (Fig. 3) and protected larvae from anomalous nerve conduction (Fig. 4) and excessive ROS production (Fig. 5). Furthermore, we adopted DGE assay and qRT-PCR method to explore the molecular mechanisms of reduced nerve toxicity by TiO2 NPs in the phoxim-exposed brain of B. mori, the main results were divided into three parts and discussed below.
Nerve conduction
It has been reported that vacuolar-type ATPases (V-ATPases) produce proton-motives that are indispensable for ion transports and the energization of membrane transport in insect systems [19]. In the present study, the expression of H+ transporting ATP synthase and vacuolar ATP synthase was down-regulated under phoxim stress in the brain of B. mori larvae, which was reversed by TiO2 NPs. Furthermore, the activity of Na+/K+-ATPase that maintains the balance of K+ and Na+ concentrations in the organisms was inhibited in the brain under phoxim stress, which resulted in physiological damages and cellular homeostasis disturbance [20]; these changes could also be mitigated by TiO2 NPs pretreatments. Besides, Ca2+ concentration that is essential for ion transport and nerve conduction is regulated by Ca2+-ATPase and Ca2+/Mg2+-ATPase in eukaryotic cells, and defects in these enzymes seriously compromise the normal functions of cells [21]. Similar to the finding of inhibited activity of Ca2+/Mg2+-ATPase by pyrethroids [22], we observed inhibited activities of Ca2+-ATPase and Ca2+/Mg2+-ATPase by phoxim in the brain of B. mori in our study. However, the activities of the two enzymes were only slightly inhibited in the TiO2 NPs + phoxim group. These changes in gene expression and enzymatic activity in the brain are expected to lead to abnormal concentrations of neurotransmitters that are important in nerve conduction. Monoamine neurotransmitters, such as 5-HT, DA, and NE, are closely related to learning, memory, and normal behaviors [14], [23]. In this study, the contents of 5-HT, DA, and NE were decreased significantly by phoxim exposure, while those in the TiO2 NPs + phoxim-treated larvae were similar to the control. The contents of several amino acid neurotransmitters, such as ACh, Glu, and NO, were increased significantly by phoxim exposure.
It was reported that inhibition of the amino acid neurotransmitter AChE is the main mechanism of OP pesticides [2]. AChE catalyzes the hydrolysis of the excitatory neurotransmitter ACh into choline and acetic acid, which terminates nerve impulses on postsynaptic membrane [24]. Therefore, inhibited AChE activity results in increased ACh contents and continuing nerve impulses. In the current study, a significant inhibition of AChE activity was observed in the brain of phoxim-exposed larvae (Fig. 4b). However, the expression of ace1 was actually up-regulated, likely a compensation for the inhibited AChE activity. This is consistent with the finding in a previous study [1]. TiO2 NPs treatments mitigated the inhibition of AChE activity (Fig. 4b) and down-regulated the ace1 expression.
Glu, another excitatory amino acid neurotransmitter, binds to NMDA receptors to promote the influx of extracellular Ca2+ [25], [26] and enhance the activity of calcium-dependent proteases, such as TNOS that is involved in the release of NO. We observed that phoxim exposure significantly increased Glu contents, TNOS activity, and NO contents, while TiO2 NPs pretreatments reversed such increases. The free radical NO modulates neuronal functions by increasing the release of neurotransmitters [27], and NO can be oxidized to peroxinitrite (ONOO-) that may cause neuronal damages and induce apoptosis [26]. Therefore, the reversed changes in Glu contents, TNOS activity, and NO contents may further explain the protective effects of TiO2 NPs against phoxim-induced damages in the brain of B. mori.
Oxidative stress
Previous study had shown mitochondria may be the primary target of OP-initiated cytotoxicity [28]. They play crucial roles in oxidative stress [29], as the levels of ROS species, such as O2 − and H2O2, are related to the respiratory chain, substrate dehydrogenases in the matrix, monoamine oxidase, and cytochrome P450 [30]. When mitochondria are damaged, the ROS levels are usually increased significantly, causing oxidative damages to lipids, proteins, and DNA. These oxidative damages generate peroxidation products, such as MDA, PC, and 8-OHdG [31]–[33], which may induce apoptosis and necrosis. However, many stress response proteins are associated with the removal of ROS in insects, such as SOD that is the primary antioxidant enzyme catalyzing the dismutation of superoxide radicals to hydrogen peroxide and oxygen [34] and TPx that removes hydrogen peroxide and alkyl hydroperoxides [35]. In the present study, the expression of both SOD and TPx was significantly inhibited after 48 h of phoxim exposure, along with significantly increased contents of O2 •− and H2O2 (Fig. 5a), significantly improved levels of MDA, PC, and 8-OHdG (Fig. 5b), and swelling mitochondria and broken mitochondria crista (Fig. 3c). However, in the TiO2 NPs group and the TiO2 NPs + phoxim group, the morphology of cells and mitochondria were normal, indicating that TiO2 NPs protected the brain of B. mori from phoxim stress by regulating the expression of genes important in oxidative stress and mitochondria respiratory chain.
Apoptosis
Many pesticides have been shown to cause apoptosis and necrosis [36]. The accumulation of ROS and peroxidation may also promote the release of mitochondrial cytochrome c [37]. Once released to the cytoplasm, cytochrome c binds to the apoptotic protease activating factor-1 (Apaf-1) and pro-caspase-9 to form a tripolymer protein complex. The tripolymers form apoptosomes that activate caspase-9, an initiator caspase in the mitochondria/cytochrome-c pathway, and caspase-3 [38]. In the present study, over-expression of cytochrome-c, caspase-9 and caspase-3 was observed in phoxim-exposed brains, while TiO2 NPs treatments mitigated the over-expression. Moreover, apoptosis is regulated by many apoptotic associated proteins, such as Bcl-2 and the inhibitor of apoptosis proteins (IAP) [39], [40]. In B. mori, Bm109 is homologous to the anti-apoptotic Bcl-2 family proteins within the four conserved BH regions. Bm109 has been reported to up-regulate apoptosis by participating in the translocation of Bax to mitochondria and the release of cytochrome c [41], [42]. In this study, TiO2 NPs mitigated the over-expression of Bm109 and the down-expression of BmIAP, a specific inhibitor of caspase-9 [43], under phoxim stress in the brain of B. mori. We also observed cell debris, swelled mitochondria, and broken mitochondria crista by histological and ultrastructure photomicrographs (Figs. 2c, 3c), indicating that phoxim induces apoptosis through the mitochondria/cytochrome-c pathway, and that TiO2 NPs treatments can mitigate mitochondrial damages and block apoptosis in the brain of B. mori under phoxim stress.
Conclusion
The results from this study indicate that TiO2 NPs can reduce the phoxim-induced changes in the expression of genes and the activity of enzymes that regulate nerve conduction, oxidative stress and apoptosis, and relieve phoxim-induced physiological disorders and brain damages in B. mori. Our study may promote the application of TiO2 NPs in reducing pesticide toxicity in B. mori in the future, although further investigations are needed to reveal the specific mechanisms of the effects of TiO2 NPs on phoxim exposure.
Materials and Methods
Insects and Chemicals
The larvae of B. mori (Bombyx mori L Qiufeng × baiyu), which were maintained in our laboratory, were reared on mulberry leaves under 12-h light/12-h dark cycles for this study.
Nanoparticulate anatase TiO2 was prepared through controlled hydrolysis of titanium tetrabutoxide. Detailed synthesis and characterization of TiO2 NPs have been described previously [44].
Phoxim was purchased from Sigma Co. at 98.1% purity.
Preparation of phoxim and TiO2 NPs solutions
TiO2 NPs powder was dispersed onto the surface of 0.5% Hydroxypropylmethylcellulose (HPMC) (w/v), suspended, sonicated for 30 min, and mechanically vibrated for 5 min. Phoxim was dissolved in acetone to prepare the stock solution, which was diluted with water into different concentrations for analysis. 0.5% HPMC was used as the suspending agent.
Resistance measurement
In the pre-experiment, we tried 1, 2, 5, 10, and 15 mg/L TiO2 NPs suspensions in fifth-instar larvae and determined that the optimum concentration for larvae growth was 5 mg/L for further experiments. The Lethal Concentration 50 (LC 50) of phomix in B. mori was 7.86 µg/mL, and 4 µg/mL was used as the concentration in further experiments. 100 g of fresh mulberry, Morus albus (L.), leaves were dipped in 5 mg/L TiO2 NPs suspension for 1 min, followed by dipping in the solution of 4 µg/mL phoxim for 1 min.
After being air-dried, TiO2 NPs-treated leaves were used to feed B. mori instar larvae three times a day until the second day of fifth-instar larvae. Fresh leaves treated with 0.5% HPMC served as controls. Later, phoxim-treated leaves were used to feed B. mori larvae three times a day from the third day, before the silkworms (1,000 in each group) were fed with either TiO2 NPs-treated leaves or the control fresh leaves under long-day photoperiods (16 h light: 8 h dark) at 25°C and about 75% relative humidity. Each experiment was repeated three times with 200 larvae. The mortality of larvae was counted 48 h later.
To measure the resistance of larvae, the body weight and survival rate of larvae was counted 48 h later by the method of our previous study [4] with minor modifications.
Brain tissue collection
Forty eight hours after phoxim treatments, 100 fifth-instar larvae were selected randomly from each group. Larval brains were collected and frozen at −80°C for subsequent antioxidant assay.
Histopathological evaluation of brain
All histopathological examinations were performed using standard laboratory procedures. Brains of five larvae of each group were embedded in paraffin, sliced (5 µm thickness), placed onto glass slides, and stained with hematoxylin–eosin (HE). Stained sections were evaluated by a histopathologist who was unaware of the treatments using an optical microscope (Nikon U-III Multi-point Sensor System, Japan).
Observation of brain ultrastructure
Brains of five larvae of each group were fixed in freshly prepared 0.1 M sodium cacodylate buffer with 2.5% glutaraldehyde and 2% formaldehyde, before being treated at 4°C with 1% osmium tetroxide in 50 mM sodium cacodylate (pH 7.2–7.4) for 2 h. Staining was performed overnight with 0.5% aqueous uranyl acetate. After serial dehydration with ethanol (75, 85, 95, and 100%), the specimens were embedded in Epon 812 and sliced. Ultrathin sections were treated with uranyl acetate and lead citrate and observed with a HITACHI H600 TEM (HITACHI Co., Japan). The apoptosis in brain was determined by observing the changes in nuclear morphology (e.g., chromatin condensation and fragmentation).
Oxidative stress assay of brain
ROS (O2 − and H2O2) production, MDA levels, protein carbonyl (PC), and 8-OHdG in brain tissues were assayed using commercial enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) following the manufacturer’s instructions.
Assay of enzymatic activities
For enzymatic activity determinations, brain tissues were homogenized in 10 volumes of 0.15 M NaCl. A quantity of homogenate was used to the activies of different enzymes. The activities of AChE, Ca2+-ATPase, Ca2+/Mg2+-ATPase, Na+/K+-ATPase, and TNOS in the brain were spectrophotometrically measured with commercial kits (Nanjing Jiancheng Bioengineering Institute, China) targeting the oxidation of oxyhaemoglobin to methaemoglobin by nitric oxide.
Determination of neurochemicals
The homogenate of brains was centrifuged at 12,000 g for 20 min at 4°C. The concentrations of DA, 5-HT, NE, and ACh were spectrophotometrically measured with commercially kits (Nanjing Jiancheng Bioengineering Institute, China).
Glu concentrations were measured using commercial kits (Nanjing Jianchen Biological Institute, China), and the standard curves were produced by using standard Glu stock solutions. Glu levels in the samples were detected using a spectrophotometer at 340 nm and expressed as µmol/g prot. The concentration of NO in the brain was measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China). The OD value was determined by a spectrophotometer (U-3010, Hitachi, Japan). Results of NO were read with OD value at 550 nm. The results were calculated using the following formula: NO (µmol/L) = (Asample−Ablank)/(Astandard- Ablank)×20(µmol/L).
Total RNA isolation
Total RNA was extracted from brain samples using the Trizol reagent (Takara, Japan) and treated with DNase to remove potential genomic DNA contamination. The quality of RNA was assessed by formaldehyde agarose gel electrophoresis and was quantitated spectrophotometrically.
DGE library preparation, sequencing, tag mapping and evaluation of DGE libraries
For RNA library construction and deep sequencing, equal quantities of brain RNA samples (n = 3) were pooled for the control group and the treated group, respectively. Approximately 6 µg of RNA representing each group were submitted to Solexa (now Illumina Inc.) for sequencing. The detailed methodology were performed in our previous study [45].
qRT-PCR analysis
The specific primers for the 11 genes are listed in Table S4. The internal reference gene was actin3. qRT-PCR was performed using the 7500 Real-time PCR System (ABI) with SYBR Premix Ex Taq™ (Takara, Japan) according to the manufacturer’s instructions. The qRT-PCR analysis was carried out following the method described in previous studies [1], [5].
Statistical Analysis
Statistical analyses were performed using the SPSS 19 software. Data are expressed as means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was carried out to compare the differences of means of the multigroup data. Dunnett’s test was performed when each dataset was compared with the solvent-control data. Statistical significance for all tests was judged at a probability level of 0.05 (P<0.05).
Supporting Information
Functional categorization of 295 genes which significantly altered by phoxim exposure. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Functional categorization of 288 genes which altered by TiO2 NPs pretreatment. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Functional categorization of 472 genes which altered by TiO2 NPs + phoxim exposure. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by phoxim exposure.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by TiO2 NPs exposure.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by TiO2 NPs + phoxim exposure.
(DOC)
Primer pairs for qRT-PCR in the gene expression analysis.
(DOC)
Funding Statement
This work was supported by the National High Technology Research and Development Program of China (863 Program) (Grant No. 2013AA102507), the transformation project of agriculture scientific and technological achievements (2013GB2C100180), the projects sponsored by the national cocoons silk development funds in 2014, the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Doctoral Fund of Ministry of Education of China (20113201110008), the China Agriculture Research System (CARS-22-ZJ0305), and the Science & Technology support Program of Suzhou (ZXS2012005, SNG201352). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Peng GD, Wang JM, Ma L, Wang YH, Cao YQ, et al. (2011) Transcriptional characteristics of acetylcholinesterase genes in domestic silkworms (Bombyx mori) exposed to phoxim. Pesticide Biochemistry and Physiology 101: 154–158. [DOI] [PubMed] [Google Scholar]
- 2. Li B, Wang D, Zhao HQ, Shen WD (2010) Comparative analysis of two acetylcholinesterase genes of Bombyx mandarina and Bombyx mori . Afr J Biotechnol 9: 8477–8485. [Google Scholar]
- 3. Yu QY, Fang SM, Zuo WD, Dai FY, Zhang Z, et al. (2011) Effect of organophosphate phoxim exposure on certain oxidative stress biomarkers in the silkworm. J Econ Entomol 104: 101–106. [DOI] [PubMed] [Google Scholar]
- 4. Li B, Hu RP, Cheng Z, Cheng J, Xie Y, et al. (2012) Titanium dioxide nanoparticles relieve biochemical dysfunctions of fifth-instar larvae of silkworms following exposure to phoxim insecticide. Chemosphere 89: 609–614. [DOI] [PubMed] [Google Scholar]
- 5. Wang YH, Gu ZY, Wang JM, Sun SS, Wang BB, et al. (2013) Changes in the activity and the expression of detoxification enzymes in silkworms (Bombyx mori) after phoxim feeding. Pesticide Biochemistry and Physiology 105: 13–17. [DOI] [PubMed] [Google Scholar]
- 6. Surendra Nath B, Surendra Kumar RP (1999) Toxic impact of organophosphorus insecticides on acetylcholinesterase activity in the silkworm, Bombyx mori L. Ecotoxicology and environmental safety. 42: 157–162. [DOI] [PubMed] [Google Scholar]
- 7. Higarashi MM, Jardim WE (2002) Remediation of pesticide contaminated soil using TiO2 mediated by solar light. Catal Today 76: 201–207. [Google Scholar]
- 8. Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations-A review. Appl Catal B-Environ 49: 1–14. [Google Scholar]
- 9. Esterkin CR, Negro AC, Alfano OM, Cassano AE (2005) Air pollution remediation in a fixed bed photocatalytic reactor coated with TiO2 . Aiche J 51: 2298–2310. [Google Scholar]
- 10. Hong FH, Yang F, Liu C, Gao Q, Wan ZG, et al. (2005) Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biological trace element research 104: 249–260. [DOI] [PubMed] [Google Scholar]
- 11. Zheng L, Hong FS, Lu SP, Liu C (2005) Effect of nano-TiO2 on strength of naturally and growth aged seeds of spinach. Biological trace element research 104: 83–91. [DOI] [PubMed] [Google Scholar]
- 12. Mohammadi R, Maali-Amiri R, Abbasi A (2013) Effect of TiO2 Nanoparticles on Chickpea Response to Cold Stress. Biological trace element research 152: 403–410. [DOI] [PubMed] [Google Scholar]
- 13.Su JJ, Li B, Cheng S, Zhu Z, Sang XZ, et al. (2013) Phoxim-induced damages of Bombyx mori larval midgut and titanium dioxide nanoparticles protective role under phoxim-induced toxicity. Environmental toxicology. DOI:10.1002/tox.21866. [DOI] [PubMed]
- 14. Li B, Yu XH, Gui SX, Xie Y, Hong J, et al. (2013) Titanium Dioxide Nanoparticles Relieve Silk Gland Damage and Increase Cocooning of Bombyx mori under Phoxim-Induced Toxicity. J Agric Food Chem 61: 12238–12243. [DOI] [PubMed] [Google Scholar]
- 15. Asmann YW, Klee EW, Thompson EA, Perez EA, Middha S, et al. (2009) 3′ tag digital gene expression profiling of human brain and universal reference RNA using Illumina Genome Analyzer. Bmc Genomics 10: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishiyama T, Miyawaki K, Ohshima M, Thompson K, Nagashima A, et al. (2012) Digital gene expression profiling by 5′-end sequencing of cDNAs during reprogramming in the moss Physcomitrella patens. Plos One 7(5): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordlund J, Kiialainen A, Karlberg O, Berglund EC, Goransson-Kultima H, et al. (2012) Digital gene expression profiling of primary acute lymphoblastic leukemia cells. Leukemia 26: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Luoh SM, Hon LS, Baertsch R, Wood WI, et al. (2007) GeneHub-GEPIS: digital expression profiling for normal and cancer tissues based on an integrated gene database. Nucleic Acids Res 35: W152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azuma M, Ohta Y (1998) Changes in H+-translocating vacuolar-type ATPase in the anterior silk gland cell of Bombyx mori during metamorphosis. The Journal of experimental biology 201: 479–486. [DOI] [PubMed] [Google Scholar]
- 20. Kopecka-Pilarczyk J (2010) Effect of exposure to polycyclic aromatic hydrocarbons on Na+-K+-ATPase in juvenile gilthead seabream Sparus aurata. Journal of fish biology 76: 716–722. [DOI] [PubMed] [Google Scholar]
- 21. Brini M, Carafoli E (2009) Calcium pumps in health and disease. Physiological reviews 89: 1341–1378. [DOI] [PubMed] [Google Scholar]
- 22. Alrajhi DH (1990) Properties of Ca2++Mg2+-Atpase from Rat-Brain and Its Inhibition by Pyrethroids. Pesticide Biochemistry and Physiology 37: 116–120. [Google Scholar]
- 23. Meneses A, Liy-Salmeron G (2012) Serotonin and emotion, learning and memory. Reviews in the neurosciences 23: 543–553. [DOI] [PubMed] [Google Scholar]
- 24. Fournier D, Mutero A (1994) Modification of acetylcholinesterase as a mechanism of resistance to insecticides. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 108: 19–31. [Google Scholar]
- 25. Fu W, Ruangkittisakul A, Mactavish D, Baker GB, Ballanyi K, et al. (2013) Activity and metabolism related Ca2+ and mitochondrial dynamics in co-cultured human fetal cortical neurons and astrocytes. Neuroscience 250: 520–535. [DOI] [PubMed] [Google Scholar]
- 26. Hu RP, Gong XL, Duan YM, Li N, Che Y, et al. (2010) Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 31: 8043–8050. [DOI] [PubMed] [Google Scholar]
- 27. Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Progress in neurobiology 64: 51–68. [DOI] [PubMed] [Google Scholar]
- 28. Carlson K, Ehrich M (1999) Organophosphorus compound-induced modification of SH-SY5Y human neuroblastoma mitochondrial transmembrane potential. Toxicol Appl Pharmacol 160: 33–42. [DOI] [PubMed] [Google Scholar]
- 29. Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87: 1157–1180. [DOI] [PubMed] [Google Scholar]
- 30. Chernyak BV, Izyumov DS, Lyamzaev KG, Pashkovskaya AA, Pletjushkina OY, et al. (2006) Production of reactive oxygen species in mitochondria of HeLa cells under oxidative stress. Biochimica et biophysica acta 1757: 525–534. [DOI] [PubMed] [Google Scholar]
- 31. Yang ZP, Dettbarn WD (1998) Lipid peroxidation and changes in cytochrome c oxidase and xanthine oxidase activity in organophosphorus anticholinesterase induced myopathy. Journal of physiology, Paris 92: 157–161. [DOI] [PubMed] [Google Scholar]
- 32. Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino acids 25: 207–218. [DOI] [PubMed] [Google Scholar]
- 33. Tokiwa H, Sera N, Nakanishi Y, Sagai M (1999) 8-Hydroxyguanosine formed in human lung tissues and the association with diesel exhaust particles. Free radical biology & medicine 27: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 34. Park SY, Nair PM, Choi J (2012) Characterization and expression of superoxide dismutase genes in Chironomus riparius (Diptera, Chironomidae) larvae as a potential biomarker of ecotoxicity. Comparative biochemistry and physiology Toxicology & pharmacology: CBP 156: 187–194. [DOI] [PubMed] [Google Scholar]
- 35. Lee KS, Kim SR, Park NS, Kim I, Kang PD, et al. (2005) Characterization of a silkworm thioredoxin peroxidase that is induced by external temperature stimulus and viral infection. Insect biochemistry and molecular biology 35: 73–84. [DOI] [PubMed] [Google Scholar]
- 36. Astiz M, de Alaniz MJ, Marra CA (2009) Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicology and environmental safety 72: 2025–2032. [DOI] [PubMed] [Google Scholar]
- 37. Zamzami N, Larochette N, Kroemer G (2005) Mitochondrial permeability transition in apoptosis and necrosis. Cell death and differentiation 12 Suppl 21478–1480. [DOI] [PubMed] [Google Scholar]
- 38. Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM, et al. (1999) Caspase-9 can be activated without proteolytic processing. The Journal of biological chemistry 274: 8359–8362. [DOI] [PubMed] [Google Scholar]
- 39. Yano M, Terada K, Gotoh T, Mori M (2007) In vitro analysis of Bcl-2 proteins in mitochondria and endoplasmic reticulum: similarities in anti-apoptotic functions and differences in regulation. Experimental cell research 313: 3767–3778. [DOI] [PubMed] [Google Scholar]
- 40. Deveraux QL, Reed JC (1999) IAP family proteins–suppressors of apoptosis. Genes & development 13: 239–252. [DOI] [PubMed] [Google Scholar]
- 41. Tambunan J, Kan Chang P, Li H, Natori M (1998) Molecular cloning of a cDNA encoding a silkworm protein that contains the conserved BH regions of Bcl-2 family proteins. Gene 212: 287–293. [DOI] [PubMed] [Google Scholar]
- 42. Wu WX, Wei W, Ablimit M, Ma Y, Fu T, et al. (2011) Responses of two insect cell lines to starvation: autophagy prevents them from undergoing apoptosis and necrosis, respectively. J Insect Physiol 57: 723–734. [DOI] [PubMed] [Google Scholar]
- 43. Huang QH, Deveraux QL, Maeda S, Stennicke HR, Hammock BD, et al. (2001) Cloning and characterization of an inhibitor of apoptosis protein (IAP) from Bombyx mori . Biochimica et biophysica acta 1499: 191–198. [DOI] [PubMed] [Google Scholar]
- 44. Hu RP, Zheng L, Zhang T, Gao GD, Cui YL, et al. (2011) Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. Journal of hazardous materials 191: 32–40. [DOI] [PubMed] [Google Scholar]
- 45. Gu ZY, Zhou YJ, Xie Y, Li FC, Ma L, et al. (2013) The adverse effects of phoxim exposure in the midgut of silkworm, Bombyx mori. Chemosphere 96: 33–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Functional categorization of 295 genes which significantly altered by phoxim exposure. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Functional categorization of 288 genes which altered by TiO2 NPs pretreatment. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Functional categorization of 472 genes which altered by TiO2 NPs + phoxim exposure. Genes were functionally classified based on the ontology-driven clustering approach of PANTHER.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by phoxim exposure.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by TiO2 NPs exposure.
(DOC)
Genes related to oxidative stress, stress response, metabolic process, cell component, transport, transcription, translation, growth and development, signal transduction, immune response, cell cycle and apoptosis altered significantly by TiO2 NPs + phoxim exposure.
(DOC)
Primer pairs for qRT-PCR in the gene expression analysis.
(DOC)