Abstract
Background
Mycoplasma pneumoniae is one of the causative organisms of community-acquired pneumonia which is found commonly in younger patients. Extrapulmonary complications similar to autoimmune disease are caused by M. pneumoniae following the initial infection. The mechanism and pathology of onset is not clear, but it is considered that excessive host immunoreactions play a part in the onset of mycoplasmal pneumonia and its extrapulmonary complications. In this study, we investigated the participation of the immune response, excluding the participation of Th1 and Th2 which has previously been investigated.
Results
In this study, the host immune response of an antigen induced inflammation model using SPF mice repeatedly sensitized with M. pneumoniae antigens was analyzed. The specificity of M. pneumoniae antigens in the Th17 response of murine lymphocytes in vitro was also examined. Frequent and concentrated sensitization induced exacerbation of lung inflammation immunologically and pathologically, and evoked intrapulmonary IL-17A and IL-10 production. M. pneumoniae antigen stimulation induced proliferation of mouse lymphocytes and caused production of IL-17A and IL-10. In addition, it was shown that IL-17A and IL-10 production was increased in the presence of IL-6 and TGF-β1.
Conclusions
It was shown that M. pneumoniae antigens induced potent immunoreaction and enhanced the Th17 cell response both in vivo and in vitro, and that both Treg and IL-10 are involved in the suppression of IL-17A production. This raises the possibility that breakdown of the immune balance may be part of the process leading to subsequent development of extrapulmonary mycoplasmal pneumonia.
Keywords: Mycoplasma pneumoniae, Th17, Animal models, Immune response, Cytokines
Background
Mycoplasmas are the smallest bacteria capable of autonomous replication, and these microorganisms are unique in that they lack a bacterial cell wall. M. pneumoniae is an etiologic agent responsible for community-acquired respiratory tract infections (primary atypical pneumonia, PAP) mainly in school-age children and young adults. M. pneumoniae can spread from person to person via droplets, attaching to human airway epithelial cells via the P1 protein, one of the tip components of an adherent organ on the bacterial cell surface [1,2].
Recently, it has been reported that the community-acquired respiratory distress syndrome toxin (CARDS Tx) which possesses adenosine diphosphate-ribosyltransferase activity similar to Bordetella pertussis toxin is produced by M. pneumoniae[3]. CARDS Tx was not secreted into the culture supernatant, but localized to the cytoplasmic and cell membranes, inducing vacuolating cytotoxicity. However, it is difficult to explain the pathogenic mechanisms of mycoplasmal pneumonia in relation to M. pneumoniae virulence factors. Clinical symptoms of mycoplasmal pneumonia in early childhood are not marked and manifestations of M. pneumoniae infection such as pneumonia appear only in school-age or older children [4]. Severe inflammatory responses in the lung are also not commonly observed in M. pneumoniae infected immunocompromised hosts [5]. According to the report by Tanaka et al. [6], it was inferred that accumulation of lymphocytes and monocytes activated by M. pneumoniae infection in the bronchi and lung tissue leads to both insufficiency of lymphocytes at the periphery and negative conversion in the tuberculin test. Furthermore, it was reported that the onset of various autoimmune type extrapulmonary complications such as Guillain-Barré syndrome, Stevens-Johnson syndrome, hepatitis, myocarditis and arthritis were observed subsequent to M. pneumoniae infections [7-10]. Consequently, the participation of the excessive host immune response is thought to be involved in the severity of mycoplasmal pneumonia and also the onset of complications [11,12].
In recent years, a third positive effector T cell subset known as Th17 cells were characterized by abundant production of IL-17 [13,14]. IL-17 is more important than IFN-γ in onset and exacerbation of autoimmune diseases such as collagen-induced arthritis (CIA) and experimental allergic encephalitis (EAE), which are thought to be pathogenetically induced by the Th1 immune response [15,16].
On the other hand, inducible regulatory T cells (iTreg) such as Tr1 and Th3 have been reported to contribute to the suppression of the hyperimmune response [17,18].
It was reported that the Th17 cells are induced by segmented filamentous bacteria (SFB) which colonize the intestinal tract [19]. However, the relationship of Th17 cells with the pathogenic mechanisms of mycoplasmal pneumonia and its extrapulmonary complications are not clear. Treg has not previously been identified as an inhibiting factor of the M. pneumoniae inflammatory response.
We have previously reported that experimental pneumonia can be caused by intranasal inoculation of M. pneumoniae soluble sonicated antigens to specific pathogen-free (SPF) mice [20,21]. In the present study, we prepared a M. pneumoniae antigen induced inflammation model by use of SPF mice recurrently inoculated with M. pneumoniae antigens and performed pathological and immunological analyses to examine the induction mechanisms of Th17 and Treg cells. Additionally, we investigated the specificity of Th17 and Treg cell inducibility with mouse lymphocytes in vitro by using various bacterial antigens and immunoactivatory components.
Methods
Bacterial strains and culture conditions
The reference strain M. pneumoniae M129, stocked at the Department of Infectious Diseases, Kyorin University School of Medicine was used in this study.
M. pneumoniae cells were cultured at 37°C under a 5% CO2 atmosphere for 7 days in PPLO broth (Oxoid, Hampshire, UK) containing mycoplasma supplement-G (Oxoid) for the preparation of soluble M. pneumoniae antigens. Klebsiella pneumoniae (ATCC 13883; American Type Culture Collection, Rockville, MD) and Streptococcus pneumoniae (ATCC 33400) were cultured at 37°C under aerobic conditions for 18 hours in brain heart infusion broth (BHI; Becton Dickinson, MD) (BD Difco Franklin Lakes, NJ).
Experimental animals and breeding conditions
Specific pathogen-free (SPF) female BALB/cAJcl mice (5 weeks of age for antigen induced inflammation model, and 6 weeks of age for harvest of splenocytes) were purchased from Clea Japan (Tokyo, Japan) and bred in isolators.
Preparation of sonicated M. pneumoniae crude antigens
M. pneumoniae soluble antigens were prepared as previously described [20,21]. The cultured bacteria were harvested and washed 5 times by centrifugation at 10000 × g for 20 min (M. pneumoniae) or 3000 × g for 15 min (K. pneumoniae and S. pneumoniae) in Hanks’ balanced salt solution (Gibco, New York, USA). The cells were suspended in saline and sonicated 10 times for 1 min per burst at output 7 (Sonifier 250, Branson Ultrasonic Corporation, Danbury, CT, USA). The supernatant was decanted after centrifugation at 10000 × g for 5 min, and served as crude soluble antigen. The protein concentration of the suspension was measured using the Bio-Rad Protein Assay (Hercules, CA, USA).
Inoculation and sensitization conditions
Animal experiments were approved by the Institutional Animal Care and Use Committee of Kyorin University School of Medicine (Approval No. 95, 95–1, 95–2).
Mice were anaesthetized intraperitoneally with 25 mg/kg body weight of sodium pentobarbital (Dainippon Sumitomo Pharma, Osaka, Japan). SPF mice in Group A were intranasally inoculated once a week for 5 weeks with sonicated crude antigens prepared from M. pneumoniae strain M129 (1 mg protein/kg/5 times). The inoculated protein doses were changed in Groups B and C. In Group B, lower doses (0.1 mg/kg) of the antigen were inoculated once a week at day 0, 7 and 14, and higher doses (1 mg/kg) of the antigen were used for the last inoculation at day 28. In Group C, crude antigen (1 mg/kg) was inoculated at day 0 and 28 only. Control mice in Group D were inoculated with saline once a week for 5 weeks (n = 5 or 6 in each group).
Pathological examination
Mice were sacrificed on the day after the last sensitization. The intermediate and lower lobes of the right lungs of the mice were fixed in 5% formalin. Sections of paraffin-embedded tissues were stained with hematoxylin and eosin and analyzed by light microscopy.
Intrapulmonary mRNA gene expression analysis
Total RNA was extracted from the upper lobe of the right lungs of the mice using the QIAzol, QIAshredder and RNeasy Mini spin column RNA isolation Kit (QIAGEN GmbH, Hilden, Germany). cDNA was synthesized from sample RNA using ReverTra Ace RT PCR Kit (TOYOBO CO., LTD, Osaka, Japan). All real-time PCRs were performed with SYBR Green Premix Ex Taq (TaKaRa Bio Inc., Shiga, Japan) by the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Inc. Carlsbad, California, US) as described previously [22-25] using specific primers for individual genes. Fold changes of targeted genes of each sample were relatively quantified using threshold cycle (Ct) values and calculated using the ddCT method normalizing B-actin or 18S RNA values.
In vitro analysis for specificity of differentiation inducing activity of Th17 cells by M. pneumoniae antigens
The spleens were removed from three mice for each experiment. Lymphocytes were separated from the spleens of BALB/c mice by Lympholyte M (Cedarlane Laboratories Limited, Hornby, Ontario, Canada). Lymphocytes (8 × 104 cells/0.2 ml) were then incubated with 20 ng/ml of mouse IL-6 (R&D Systems, Minneapolis, MN, USA) plus 2 ng/ml of human TGF-β1(R&D Systems) at 37°C under 5% CO2 for 4 days in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; Gibco), 10 μM 2-mercaptoethanol (MP Biomedicals, Fountain Parkway, Solon, OH), 50 μg/ml gentamicin (Schering Plough, Osaka, Japan) and 2.5 μg/ml amphotericin B (Bristol-Myers Squibb, Tokyo, Japan) [26].
In addition, lymphocytes were stimulated with the Dynabeads Mouse CD3/CD28 T Cell Expander (Invitrogen, Carlsbad, CA) during the incubation period. The sonicated crude antigens from M. pneumoniae strain M129, K. pneumoniae ATCC 13883, S. pneumoniae ATCC 33400, lipopolysaccharide from Escherichia coli O127:B7 (SIGMA-ALDRICH, St. Louis, MO, USA), and zymosan A from Saccharomyces cerevisiae (SIGMA-ALDRICH) were added to the culture. A culture without the addition of IL-6, TGF-β1 or antigens was included as control.
After 4-day culture, cell viability, based on mitochondrial succinic dehydrogenase activity was measured using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) consisting of a WST-8 assay (2-{2-methoxy-4-nitrophenyl}-3-{4-nitrophenyl}-5-{2, 4-disulfophenyl}-2H-tetrazolium, monosodium salt). Culture supernatants were also harvested and assayed for cytokine activities by ELISA.
Statistical analysis
Statistical evaluations were performed with Dunnett multiple comparison statistical test and Student’s t-test for comparisons between groups.
A value of p < 0.05 was considered to be statistically significant. Data are expressed as the mean ± the standard deviation.
Results
Histopathological analysis
High dose and frequent M. pneumoniae antigen sensitization caused severe inflammatory changes including neutrophil infiltration and bronchial wall thickening in the lung tissues of Group A mice (Figure 1a). Low dose and frequent sensitization also induced neutrophilic infiltration in the lungs of the mice in Group B, but this inflammation was milder than that in Group A (Figure 1b). In Group C mice with high dose and infrequent sensitization, the inflammatory levels differed according to lung site and localized inflammation with neutrophil infiltration was observed (Figure 1c). No inflammatory cell infiltration was observed in any of the tissues in the saline control Group D mice (Figure 1d). These results demonstrated that high dose and frequent M. pneumoniae antigen sensitization induce significant inflammation in the lung.
Figure 1.
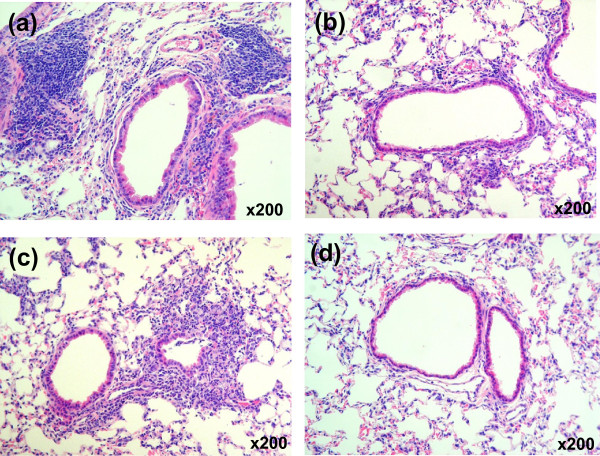
Histopathology of the lung of BALB/c mice after intranasal sensitization with M. pneumoniae-sonicated antigens. The figure shows hematoxylin and eosin staining of lung sections from mice repeatedly inoculated with M. pneumoniae antigens (day 29). Lung tissue sections from (a) Group A, (b) Group B, (c) Group C and (d) Group D (control) (magnification: × 200).
Immunological analysis for intrapulmonary cytokine protein quantification
In Group A mice, IL-17A levels in lung tissues were markedly increased (Figure 2a). Sensitization by lower doses of M. pneumoniae antigens also led to a rise in IL-17A levels in Group B mice. However, no significant changes were found in Group C mice. The levels of intrapulmonary IFN-γ and IL-4 in all mice were undetectable by ELISA (data not shown).
Figure 2.
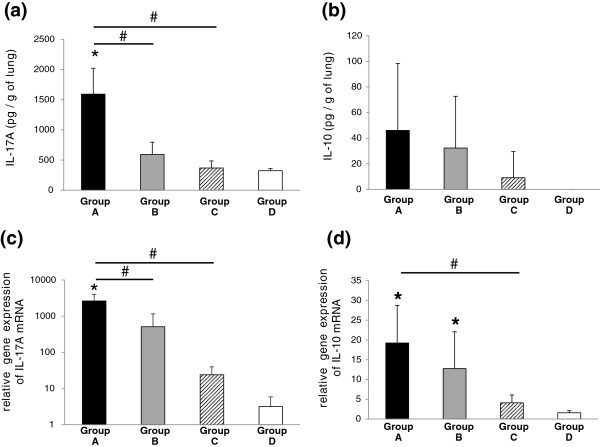
Cytokine levels and relative quantification of cytokine mRNA levels in lung tissues of BALB/c mice. (a) IL-17A levels per gram of lung tissue. (b) IL-10 levels per gram of lung tissue. (c) Relative quantification of IL-17A mRNA levels. (d) Relative quantification of IL-10 mRNA levels. Black bars, Group A mice; Grey bars, Group B mice; hatched bars, Group C mice; white bars, Group D mice. *p < 0.05, inoculate vs. Group D (control) by Dunnett multiple comparison statistical test, #p < 0.05 by Student’s t-test.
Intrapulmonary IL-10 production was not detected in control Group D mice, but sensitization with M. pneumoniae antigens induced the production of IL-10 in Groups A, B and C (Figure 2b).
Statistically significant increases in IL-17A and IL-10 mRNA expression were shown to depend on frequency of sensitization and concentration of M. pneumoniae antigens used (Figure 2c,d). Relative quantification of tumor necrosis factor (TNF)-α mRNA and Keratinocyte-derived chemokine (KC) mRNA expression as an index of lung inflammation is shown in Figure 3a and b. Up-regulation of TNF-α mRNA and KC mRNA was observed in Groups A, B and C mice as expected according to histopathological findings. Forkhead box p3 (Foxp3) is a master regulator of CD4+CD25+ naturally occurring regulatory T cells (nTreg). Foxp3 mRNA was highly expressed in only Group A mice (Figure 3c). In contrast, no significant effect of M. pneumoniae antigens on TGF-β1 mRNA expression was observed in the lung (Figure 3d).
Figure 3.
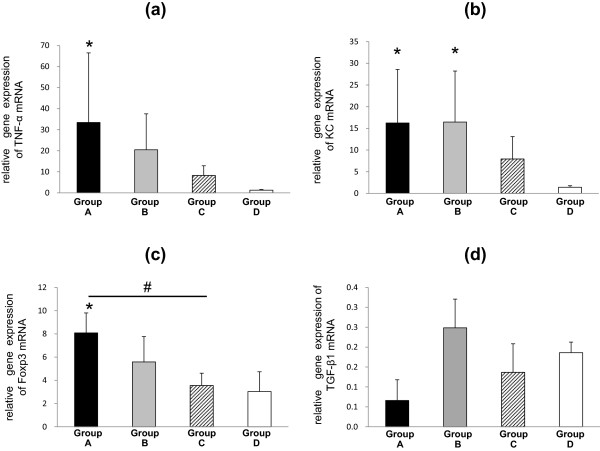
Relative quantification of cytokine mRNA levels in lung tissues of BALB/c mice. (a) Relative quantification of TNF-α mRNA levels. (b) Relative quantification of KC mRNA levels. (c) Relative quantification of Foxp3 mRNA levels. (d) Relative quantification of TGF-β1 mRNA levels. Black bars, Group A mice; Grey bars, Group B mice; hatched bars, Group C mice; white bars, Group D mice. *p < 0.05, inoculate vs. Group D (control) by Dunnett multiple comparison statistical test, #p < 0.05 by Student’s t-test.
In vitro analysis for specificity of differentiation inducing activity of Th17 cells by M. pneumoniae antigens
Chronological cytokine production by M. pneumoniae antigens was examined. Lymphocytes were cultured with 50 μg protein/ml of M. pneumoniae antigens in the presence of IL-6 and TGF-β1. IL-17A concentration in the culture media was elevated from day 1 to day 4 and maintained at 600–700 pg/ml (Figure 4a). IL-10 production induced by M. pneumoniae antigens was observed to be maintained at 400–500 pg/ml (Figure 4b). In the saline control, elevation of IL-17A and IL-10 concentrations was up to 100 pg/ml at day 4 (Figure 4a,b).
Figure 4.
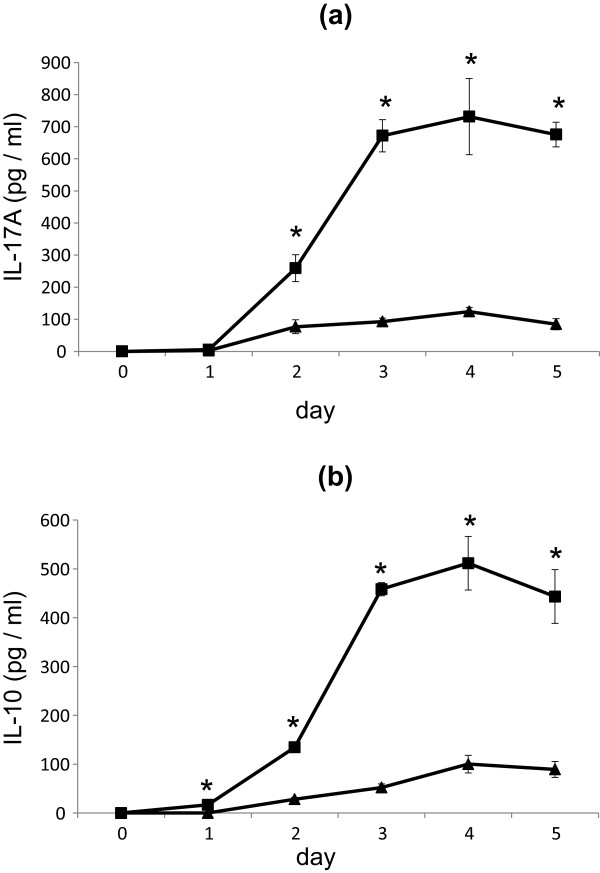
Effects of M. pneumoniae antigens on cytokine production by murine lymphocytes. Lymphocyte culture supernatant concentrations of (a) IL-17A (pg/ml), (b) IL-10 (pg/ml). Closed squares (■) show stimulation with 50 μg protein/ml of M. pneumoniae antigen. Closed triangles (▲) show saline control. *p < 0.05 vs. saline control by Student’s t-test.
Effects of M. pneumoniae and other bacterial antigens on lymphocyte growth
Without IL-6 and TGF-β1, only 50 μg protein/ml of M. pneumoniae antigens promoted the proliferation of lymphocytes (Table 1). In the presence of IL-6 and TGF-β1, proliferation of lymphocytes was increased by either 10 or 50 μg protein/ml of M. pneumoniae antigens, while 50 μg protein/ml of either S. pneumoniae or K. pneumoniae sonicated antigens markedly decreased viable lymphocyte count. Similarly, in the presence of IL-6 and TGF-β1, sonicated antigens of S. pneumoniae (10 and 50 μg protein/ml) and K. pneumoniae (5, 10 and 50 μg protein/ml) reduced the growth of lymphocytes (Table 1). In the absence of IL-6 and TGF-β1, growth of lymphocytes was not inhibited by LPS. However in the presence of IL-6 and TGF-β1, high concentrations (10 and 50 μg protein/ml) of LPS suppressed the multiplication of lymphocytes (Table 1). On the other hand, zymosan A promoted the proliferation of lymphocytes with or without IL-6 and TGF-β1 (Table 1).
Table 1.
Effects of microbial antigens on lymphocyte growth with or without IL-6 and TGFβ1
| Antigen | IL-6(-), TGF-β1(-) a | IL-6(+), TGF-β1(+) a | |||||
|---|---|---|---|---|---|---|---|
| 0 μg/ml | 50 μg/ml | 0 μg/ml | 1 μg/ml | 5 μg/ml | 10 μg/ml | 50 μg/ml | |
| M. pneumoniae M129 | 229.6±19.1b | 81.9±5.8 | 101.5±10.9 | 134.7±15.6c | 147.8±6.3c | ||
| S. pneumoniae ATCC 33400 | 18.4±1.2b | 110.1±6.3 | 100.9±12.9 | 66.8±5.2c | 22.3±2.4c | ||
| K. pneumonia ATCC 13883 | 111.7±13.0 | 6.8±4.2b | 100.0±8.1 | 109.2±4.1c | 44.3±1.2c | 27.3±1.6c | 6.1±0.7c |
| LPS from E. coli 0127: B8 | 128.8± 6.1b | 86.5±2.7c | 89.4±8.1 | 81.2±5.0c | 56.5±7.0c | ||
| Zymosan A from S. cerevisiae | 197.9±10.2b | 104.5±10.1 | 114.8±9.6c | 124.9±4.0c | 159.1±5.4 | ||
aRelative ratio (%) of viable lymphocyte count with or without IL-6 (20 ng/ml) and TGF-β1 (2 ng/ml) stimulated with M. pneumoniae and other antigens. Relative ratio is the mean ± standard deviation (four or five samples per group) of the number of viable lymphocytes at day 4.
bSignificantly different (p < 0.05) from value for cytokine (−), antigen 0 μg/ml by Student’s t-test.
cSignificantly different (p < 0.05) from value for 20 ng/ml of IL-6 and 2 ng/ml of TGF-β1 (+), antigen 0 μg/ml by Dunnett multiple comparison statistical test.
Effect of M. pneumoniae and other antigens on lymphocyte IL-17A production
M. pneumoniae antigens promoted the production of IL-17A. Furthermore, in the presence of IL-6 and TGF-β1, IL-17A production by lymphocytes markedly increased in an antigen concentration-dependent manner (Figure 5a). IL-17A production by lymphocytes induced by either S. pneumoniae, K. pneumoniae antigens or LPS was increased only twice as much as control in the presence of IL-6 and TGF-β1 (Figure 5b,c,d). The addition of 50 μg protein/ml of S. pneumoniae antigens and 50 μg/ml LPS could not induce the levels of IL-17A compared to M. pneumoniae antigens (Figure 5b,d). Moreover, very low levels of IL-17A production were observed in the presence of 50 μg protein/ml of K. pneumoniae sonicated antigens (Figure 5c) and IL-17A production was not increased by zymosan A stimulation at all (Figure 5e).
Figure 5.
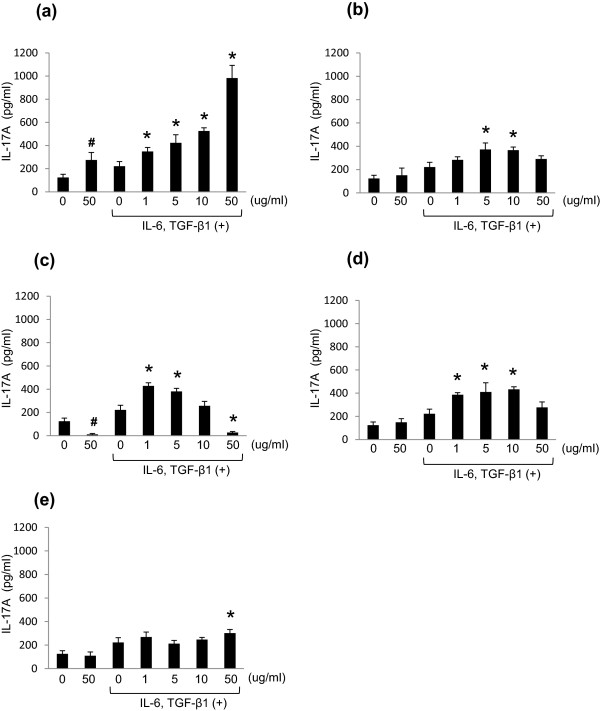
Effects of M. pneumoniae and other antigens on IL-17A production in murine lymphocytes. IL-17A concentration (pg/ml) in the culture supernatant of murine lymphocytes stimulated with antigens of: M. pneumoniae strain M129 (a), S. pneumoniae strain ATCC 33400 (b), K. pneumoniae strain ATCC 13883 (c), LPS from E. coli O127:B8 (d), Zymosan A from S. cerevisiae(e). *p < 0.05 vs. TGF-β1 and IL-6 (+), Ag (−) by Dunnett multiple comparison statistical test; #p < 0.05 vs. cytokine (−), Ag (−) by Student’s t-test.
Effect of M. pneumoniae and other antigens on lymphocyte IL-10 production
M. pneumoniae antigens promoted the production of IL-10 (Figure 6a). Furthermore, as for IL-17A, IL-6 and TGF-β1 increased IL-10 production by lymphocytes in an antigen concentration-dependent manner (Figure 6a). IL-10 production by lymphocytes induced by S. pneumoniae and K. pneumoniae antigens increased only twice as much as control in the presence of IL-6 and TGF-β1 (Figure 6b,c). However, LPS did not induce significant lymphocyte IL-10 production, even in the presence of IL-6 and TGF-β1 (Figure 6d). IL-10 production by zymosan A induction was increased in the presence of IL-6 and TGF-β1, though this was only approximately 50% of that observed in M. pneumoniae antigen experiments (Figure 6e).
Figure 6.
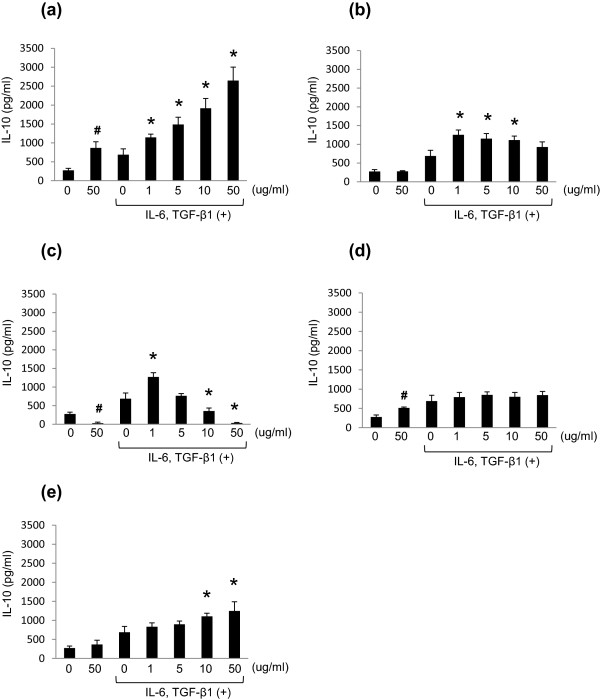
Effects of M. pneumoniae and other antigens on IL-10 production in murine lymphocytes. IL-10 concentration (pg/ml) in the culture supernatant of murine lymphocytes stimulated with antigens of M. pneumoniae strain M129 (a), S. pneumoniae strain ATCC 33400 (b), K. pneumoniae strain ATCC 13883 (c), LPS from E. coli O127:B8, (d), Zymosan A from S. cerevisiae(e). *p < 0.05 vs. TGF-β1 and IL-6 (+), Ag (−) by Dunnett multiple comparison statistical test; #p < 0.05 vs. cytokine (−), Ag (−) by Student’s t-test.
Discussion
The pathogenic mechanism by which the diverse extrapulmonary symptoms subsequent to mycoplasma infection occur is thought to be possibly due to indirect tissue injury caused by an overzealous host immune response [11,12].
In this study we investigated the Th17 and Treg based immune response to mycoplasmal diseases using IL-17A and IL-10 as index markers. It was therefore suggested that extrapulmonary complications subsequent to the development of mycoplasmal pneumonia were due to breakdown of the immune response.
Histological inflammation was induced in the murine lung by nasal inoculation of M. pneumoniae antigens, and the levels of inflammation correlated with sensitization conditions in this in vivo study. Severe inflammation was observed in the higher-dose and frequent sensitization group (Group A). Moreover, mRNA expression of TNF-α and KC proinflammatory cytokines supported the histopathological findings. This in vivo analysis revealed that M. pneumoniae antigens were also capable of inducing chemokines in our antigen induced inflammation model. Intrapulmonary concentrations of IL-17A in BALB/c mice were increased in Group A and B which were sensitized frequently or sensitized with higher amounts of M. pneumoniae antigens. We inferred that the positive effector T cell balance (Th1-Th2-Th17) of the antigen induced inflammation model was a persistent Th17 dominant condition, as intrapulmonary Th1 and Th2 cytokines IFN-γ and IL-4 were not detected but high concentrations of IL-17A and high expression levels of IL-17A mRNA were detected in the lung of BALB/c mice. The immunological response causes migration and generation of neutrophils, which plays a part not only in host defense from bacterial infection but also as a pathological mechanism for autoimmune diseases such as chronic rheumatoid arthritis [27,28]. Our experimental results demonstrated that even repetitive sensitization with a small amount of M. pneumoniae antigens induced a Th17 dominant immune response. This discovery raises the possibility that clinically mild symptoms observed in mycoplasmal pneumonia caused by a small bacterial colonization load may still result in enhancement of the Th17 response, eliciting host autoimmune diseases by persistent infection. Therefore, it is not only simple infection but the antigen inoculation conditions that are involved in the onset of extrapulmonary complications resembling autoimmune disease.
It was recently reported that polysaccharide derived from Bacteroides fragilis activated Treg cells and promoted a production of IL-10 in the intestinal tract [29]. Both factors elevate the intrapulmonary concentration of IL-10 and up regulate IL-10 mRNA expression in the lungs of BALB/c mice representing persistent IL-10 production in this M. pneumoniae antigen induced inflammation model.
It was previously reported that IL-10 deficient mice developed spontaneous enterocolitis similar to human inflammatory bowel disease [30], and it was proven that large quantities of IL-10 improved formalin or dextran sulfate sodium (DSS) induced colitis [31,32]. We therefore suspected that IL-10 was produced in our antigen induced inflammation model as demonstrated previously. Thus when IL-10 production is decreased by inhibition of Tr1 differentiation, lung inflammation induced by M. pneumoniae antigens cannot be mitigated, and extrapulmonary complications similar to autoimmune diseases may also occur in vivo. These results suggest that inflammation induced by IL-17A production in Th17 cells is mitigated by Tr1 production of IL-10.
We could not confirm the inhibitory effect of Th3 cells on immune responses at inflammatory sites, as TGF-β1 mRNA expression did not correlate with the frequency of sensitization or dose in this antigen induced inflammation model.
CD4+CD25+T cells express cytotoxic T-lymphocyte antigen 4 (CTLA-4) with membrane-associated TGF-β on the cell surface, which suppresses multiplication of positive effector T cells by direct cytoadherence [33,34]. Foxp3, a master regulatory gene is constitutively expressed in CD4+CD25+T cells [35], and both Tr1 and Th3 cells are negative for Foxp3 [36,37]. It was assumed that intrapulmonary Foxp3 mRNA expression is not increased as drastically in comparison with IL-10, as frequent and large quantity sensitization with M. pneumoniae antigens induced CD4+CD25+T cell translocation from thymus to the lung.
Additionally, we performed an in vitro analysis aimed to evaluate the specificity of immuno-inducibility and Th17-differentiation enhancability of M. pneumoniae antigens. It was reported that IL-6 and TGF-β1 are necessary for early differentiation of the Th17 cell from naïve T cells [38]. Therefore, mouse lymphocytes were primed with M. pneumoniae antigens in the presence of IL-6 and TGF-β1. Furthermore, in order to simulate the presentation of M. pneumoniae antigens by dendritic cells in vitro, we added anti-CD3 antibodies and anti-CD28 antibodies.
Compared to saline control, 50 μg protein/ml of M. pneumoniae antigen stimulation significantly induced IL-17A production by mouse lymphocytes from day 2 to 5, with greater than sixfold production observed on day 3 (Figure 4a). Additionally, IL-10 production showed a significant increase from day 1 to 5 (Figure 4b). This showed that IL-17A and IL-10 production in vitro induced by M. pneumoniae antigen sensitization mirrored the in vivo antigen induced inflammation model.
When we compared viable cell count at the peak of IL-17A and IL-10 production on day 4, 50 μg protein/ml of M. pneumoniae antigens induced multiplication of mouse lymphocytes approximately twofold compared to saline control. Though mildly increased growth rates were observed in the presence of IL-6 and TGF-β1, higher concentrations of M. pneumoniae antigens induced activation and proliferation of lymphocytes (Table 1). IL-17A and IL-10 production were enhanced in a concentration-dependent manner by M. pneumoniae antigens, and the presence of IL-6 and TGF-β1 led to further production of IL-17A and IL-10 (Figures 5a, 6a), showing induction of the two genes under a Th17 dominant immune balance both in vivo and in vitro.
With respect to the effects of antigens prepared from bacteria causing a classical pneumonia, 50 μg protein/ml of S. pneumoniae sonicated antigens imposed a lethal effect on lymphocytes, with decreased viability to 18% of saline control, possibly through the effect of pneumolysin (Table 1). S. pneumoniae is well known to produce various virulence factors, and pneumolysin is an intracellular cytotoxin causing lysis of the cytoplasmic membrane of host cells by perforation [39]. Due to lymphocyte death and reduction of activity by pneumolysin containing S. pneumoniae sonicated antigens, IL-17A and IL-10 production was not observed in a concentration-dependent manner (Figures 5b, 6b).
Regardless of the addition of IL-6 and TGF-β1, 50 μg protein/ml of K. pneumoniae antigens had a distinct lethal effect on mouse lymphocytes, with a viability of approximately 6% at 4 days. Cell death in this experiment was observed in a K. pneumoniae antigen concentration-dependent manner (Table 1). Recently, it was reported that K. pneumoniae produced various lethal active metabolites including cytotoxins, hydrolytic enzymes and haemolysins similar to S. pneumoniae[40,41] and both IL-17A and IL-10 production were decreased as expected by exposure to K. pneumoniae antigens (Figures 5c, 6c).
The difference in pathogenic mechanism between M. pneumoniae and other pulmonary pathogenic bacteria can be explained by the results of in vitro analyses. The antigens derived from bacteria causing pneumonia showed lethality to immunocytes, but M. pneumoniae antigens lead to activation of host immune responses.
LPS is recognized by TLR 4 and activates macrophages [42,43]. However, in this in vitro study, LPS did not induce proliferation of lymphocytes (Table 1). In addition, LPS stimulated IL-17A and IL-10 production did not occur in a concentration-dependent manner (Figures 5d, 6d). It was considered that in comparison with M. pneumoniae antigen, LPS has minimal effect on Th17 cell differentiation.
Zymosan A is recognized by a polymer of TLR2 and TLR1 or TLR6, causing macrophage activation [44]. Zymosan A induced proliferation of lymphocytes and IL-10 production in a concentration-dependent manner similar to M. pneumoniae antigens (Table 1, Figure 6e). However, there was no significant dose-dependant increase in IL-17A production (Figure 5e) and so we did not consider Zymosan A to be a major player in Th17 cell differentiation. Zymosan A induces not only innate immunity but a Th17 response via Jagged1 activation on the dendritic cell and was recently reported as a Th17 adjuvant [45]. From the above, we can conclude that Zymosan A alone without other immune cells can activate the proliferation of lymphocytes, but cannot induce a potent Th17 response even in the presence of IL-6 and TGF-β1.
Conclusions
In this study, it was shown that M. pneumoniae antigens induced potent immunoreaction and enhanced the Th17 cell response both in vivo and in vitro, and that both Treg and IL-10 are involved in the suppression of IL-17A production. This raises the possibility that breakdown of the immune balance may be part of the process leading to subsequent development of extrapulmonary mycoplasmal pneumonia.
Competing interests
The authors declare no competing interests concerning this work.
Authors’ contributions
SKu and SKa conceived and designed the experiments. SKu and TO performed animal experiments. SKu and HY performed real time PCR procedures. SKu, SKa and HT analyzed the data. TO, HY and KA contributed reagents/materials/analysis tools. All authors read and approved the final manuscript.
Contributor Information
Satoshi Kurata, Email: kura@ks.kyorin-u.ac.jp.
Takako Osaki, Email: osaki@ks.kyorin-u.ac.jp.
Hideo Yonezawa, Email: yonezawa@ks.kyorin-u.ac.jp.
Ken Arae, Email: araek@ks.kyorin-u.ac.jp.
Haruhiko Taguchi, Email: taguchi@ks.kyorin-u.ac.jp.
Shigeru Kamiya, Email: skamiya@ks.kyorin-u.ac.jp.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 24591175.
References
- Dallo SF, Su CJ, Horton JR, Baseman JB. Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 1988;167:718–723. doi: 10.1084/jem.167.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Plummer J, Lazzell A, Baseman JB. Shared epitopes between Mycoplasma pneumoniae major adhesin protein P1 and a 140-kilodalton protein of Mycoplasma genitalium. Infect Immun. 1987;55:49–56. doi: 10.1128/iai.55.1.49-56.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103:6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy HM, Kenny GE, McMahan R, Kaiser G, Grayston JT. Mycoplasma pneumoniae in the community. Am J Epidemiol. 1971;93:55–67. doi: 10.1093/oxfordjournals.aje.a121228. [DOI] [PubMed] [Google Scholar]
- Foy HM, Ochs H, Davis SD, Kenny GE, Luce RR. Mycoplasma pneumoniae infections in patients with immunodeficiency syndromes: report of four cases. J Infect Dis. 1973;127:388–393. doi: 10.1093/infdis/127.4.388. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Koba H, Honma S, Sugaya F, Abe S. Relationships between radiological pattern and cell-mediated immune response in Mycoplasma pneumoniae pneumonia. Eur Respir J. 1996;9:669–672. doi: 10.1183/09031936.96.09040669. [DOI] [PubMed] [Google Scholar]
- Ginestal RC, Plaza JF, Callejo JM, Rodríguez-Espinosa N, Fernández-Ruiz LC, Masjuán J. Bilateral optic neuritis and Guillain-Barré syndrome following an acute Mycoplasma pneumoniae infection. J Neurol. 2004;251:767–768. doi: 10.1007/s00415-004-0441-0. [DOI] [PubMed] [Google Scholar]
- Stutman HR. Stevens-Johnson syndrome and Mycoplasma pneumoniae: evidence for cutaneous infection. J Pediatr. 1987;111:845–847. doi: 10.1016/s0022-3476(87)80200-7. [DOI] [PubMed] [Google Scholar]
- Yamane Y, Kawai C. A case of myocarditis caused by Mycoplasma pneumoniae. Jpn Circ J. 1978;42:1279–1287. doi: 10.1253/jcj.42.1279. [DOI] [PubMed] [Google Scholar]
- Hakkarainen K, Turunen H, Miettinen A, Karppelin M, Kaitila K, Jansson E. Mycoplasmas and arthritis. Ann Rheum Dis. 1992;51:1170–1172. doi: 10.1136/ard.51.10.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald GW, Collier AM, Clyde WA. Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics. 1975;55:327–335. [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H, Taguchi H, Watanabe H, Kawai S, Fujioka Y, Goto H, Kobayashi H, Kamiya S. Immunological analysis and pathological examination of gnotobiotic mice monoassociated with Mycoplasma pneumoniae. J Med Microbiol. 2009;58:697–705. doi: 10.1099/jmm.0.007872-0. [DOI] [PubMed] [Google Scholar]
- Kurata S, Taguchi H, Sasaki T, Fujioka Y, Kamiya S. Antimicrobial and immunomodulatory effect of clarithromycin on macrolide-resistant Mycoplasma pneumoniae. J Med Microbiol. 2010;59:693–701. doi: 10.1099/jmm.0.014191-0. [DOI] [PubMed] [Google Scholar]
- Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland LE, Mages J, Loddenkemper C, Hoerauf A, Wagner H, Lang R, da Costa CU. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J Immunol. 2010;184(2):713–724. doi: 10.4049/jimmunol.0901435. [DOI] [PubMed] [Google Scholar]
- Mohanty SK, Ivantes CA, Mourya R, Pacheco C, Bezerra JA. Macrophages are targeted by rotavirus in experimental biliary atresia and induce neutrophil chemotaxis by Mip2/Cxcl2. Pediatr Res. 2010;67(4):345–351. doi: 10.1203/PDR.0b013e3181d22a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Ishikawa S, Matsui Y, Tamesada M, Harashima N, Harada M. Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci. 2011;102(3):516–521. doi: 10.1111/j.1349-7006.2010.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–185. [PubMed] [Google Scholar]
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Grool TA, van Dullemen H, Meenan J, Koster F, ten Kate FJ, Lebeaut A, Tytgat GN, van Deventer SJ. Anti-inflammatory effect of interleukin-10 in rabbit immune complex-induced colitis. Scand J Gastroenterol. 1998;33:754–758. doi: 10.1080/00365529850171710. [DOI] [PubMed] [Google Scholar]
- Tomoyose M, Mitsuyama K, Ishida H, Toyonaga A, Tanikawa K. Role of interleukin-10 in a murine model of dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 1998;33:435–440. doi: 10.1080/00365529850171080. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Pillars article: immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol. 2011;186:3808–3821. [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Wing K, Fehérvári Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol. 2006;18:991–1000. doi: 10.1093/intimm/dxl044. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Rubins JB, Duane PG, Clawson D, Charboneau D, Young J, Niewoehner DE. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61:1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BR, Sharma VD, Chandra R. Purification and characterization of Klebsiella pneumoniae cytotoxins. Indian J Exp Biol. 1999;37:681–690. [PubMed] [Google Scholar]
- Sekowska A, Gospodarek E, Janickca G, Jachna-Sawicka K, Sawicki M. Hydrolytic and haemolytic activity of Klebsiella pneumoniae and Klebsiella oxytoca. Med Dosw Mikrobiol. 2006;58:135–141. [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Hashimoto K, Takagi R, Mizuno Y, Okazaki Y, Tanaka Y, Matsushita S. Curdlan induces DC-mediated Th17 polarization via Jagged1 activation in human dendritic cells. Allergol Int. 2010;59:161–166. doi: 10.2332/allergolint.09-OA-0103. [DOI] [PubMed] [Google Scholar]


