Introduction
Mucormycoses are rare life-threatening fungal infections caused by fungi of the order Mucorales [1]. These infections usually afflict patients with immunosuppressed systems due to neutropenia, hematologic malignancies, corticosteroid treatment, diabetes or trauma [1, 2]. Owing to the rising prevalence of diabetes, cancer and transplantation in aging populations in the developed world, the number of mucormycosis infections are rising [3].
Rhizopus spp. are the most common cause of mucormycosis [1]. Despite disfiguring surgical debridement and adjunctive antifungal therapy, the overall mortality of mucormycosis remains ~50%. In the absence of surgical removal of the infected focus, antifungal therapy alone is rarely curative, resulting in ~100% mortality for patients with hematogenously disseminated disease [1]. Thus, there is a critical need to identify new prophylactic measures or therapeutic targets against this lethal disease.
Previous studies showed that vaccination with the heat-killed yeast (HKY) Saccharomyces cerevisiae resulted in a cross-protective effect against systemic aspergillosis [4, 5], coccidioidomycosis [6], cryptococcosis [7], and candidiasis [8]. Because HKY elicited cross-protective activities against several fungal pathogens, we studied the effect of the HKY vaccine in protecting DKA mice from pulmonary mucormycosis infection caused by R. oryzae. These mice were infected intratracheally to recapitulate the mode of infection in the second most common form of mucormycosis in diabetics (i.e. pulmonary mucormycosis [14–16%] vs. rhinocerebral disease [43–52%]) [1, 9].
Methods
Vaccine preparation, organisms and culture conditions
Heat-killed, endotoxin-free S. cerevisiae clinical strain 96-108 (HKY) vaccine preparations were made as previously described [6] and kept at 4°C at 4 × 108 cells/ml. R. oryzae 99-880, a clinical isolate [10], was grown on potato dextrose agar (PDA) plates for 3–5 days at 37°C and the inoculum prepared as previously described in phosphate-buffered saline (PBS) [10].
Mice vaccination and infection
The HKY vaccine was administered subcutaneously (SC) to ICR mice using two dorsal sites (0.075 ml each). The total number of HKY per dose was 6 × 107 or 1.2 × 108 cells. HKY vaccine was given either on days 28, 21, and 14; or days 35, 28, 21, and 14 prior to fungal challenge. Control mice were given PBS SC instead of HKY.
Mice were rendered diabetic using streptozotocin ten days before infection [10] Diabetic. ketoacidotic (DKA) mice were also dosed with cortisone acetate on day −2 and +3, relative to infection [10]. Sera samples (from ~100 μl blood) for ELISA testing were collected two days prior to infection by nicking the tail.
Mice were infected intratracheally with R. oryzae spores after sedation with ketamine/xylazine [10]. To confirm the delivered inoculum three mice were euthanatized immediately after the infection and lungs were dissected, homogenized, and quantitatively cultured on PDA plates plus 0.1% Triton.
The primary endpoint of efficacy was time to moribundity through day 21 post-infection. As a secondary endpoint, tissue fungal burdens in the lungs and brains (the primary and secondary target organs, respectively) were determined by quantitative PCR (qPCR) [10].
ELISAs
ELISA [10] was adapted for detection of antibodies against R. oryzae cell surface antigens extracted by high-salt treatment overnight [11]. Negative control wells received sera from PBS-vaccinated mice. Other wells received an irrelevant isotype control monoclonal antibody as an internal control. The antibody titer was taken as the reciprocal of the last serum dilution with an OD reading ≥ mean OD of negative control samples.
Statistical analysis
The non-parametric Log-Rank and Wilcoxon Rank Sum tests were used to determine differences in survival times and in antibody titers and tissue fungal burdens, respectively. Comparisons with P values of <0.05 were considered significant.
Results
Vaccination with HKY protects DKA/steroid treated mice from R. oryzae infection
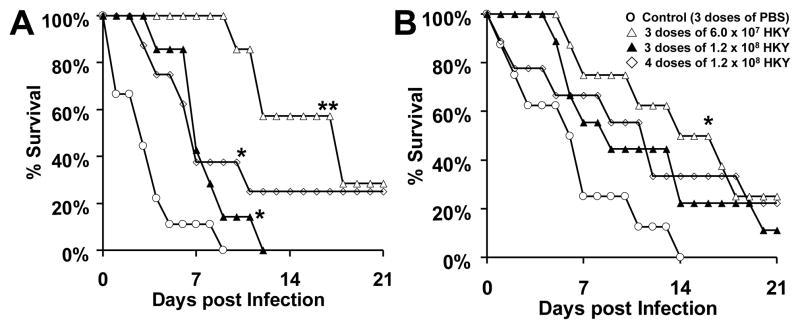
Mice vaccinated with HKY survived significantly longer than those vaccinated with PBS, regardless of the HKY dose and the schedule of administration of the vaccine regimen (Figure 1A) (P<0.02, all comparisons). Furthermore, administration of the HKY vaccine in three doses of 6.0 × 107 cells at weekly intervals significantly prolonged the survival of DKA/cortisone acetate mice when compared to administering the vaccine at the higher dose of 1.2 × 108 cells given on the same schedule (P = 0.001). In a repeat study, similar results were obtained with HKY vaccine in that three doses of 6.0 × 107 cells at weekly intervals significantly prolonged the survival of mice (P = 0.009 vs. control mice) and the other two regimens of the higher dose of 1.2 × 108 HKY trended to enhance survival vs. control PBS-vaccinated mice (Figure 1B) (P = 0.09). Upon combining both experiments all vaccination regimens with HKY enhanced survival of DKA/cortisone acetate mice vs. those vaccinated with PBS (P <0.006).
Figure 1. HKY vaccine significantly prolonged survival in DKA/cortisone acetate-treated mice infected with R. oryzae.
Mice were vaccinated with different doses of HKY. Control mice were given PBS alone. Mice were then treated to produce DKA and dosed with cortisone acetate prior to intratracheal infection with R. oryzae 99-880 isolate at 2.5 × 105 spores. In the first experiment (delivered inoculum = 7.8 × 103 spore per mouse) (A), vaccinating mice (n= 7–9 per arm) with any HKY regimen prolonged survival compared to PBS-vaccinated mice (*P < 0.02). The lower dose of HKY, 6.0 × 107 spores, administered three times also demonstrated better efficacy than a higher dose of 1.2 × 108 HKY administered three (**P = 0.001) or four times. In the second experiment (delivered inoculum = 3.3 × 103 spores) (B), all HKY regimens prolonged survival compared to PBS-vaccinated mice, but only the lower dose regimen of 6.0 × 107 HKY prolonged survival significantly (* P=0.009) (n=8–9 mice per arm).
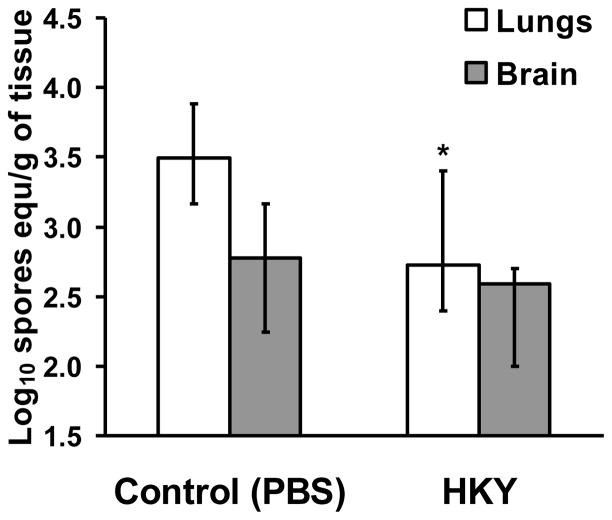
To determine the effect of HKY on the tissue fungal burden, DKA/cortisone acetate mice were vaccinated with the most protective regimen (i.e. three doses of 6.0 × 107), and then infected intratracheally. Three days post-infection, lungs and brains were harvested from euthanized mice and processed for tissue fungal burden by qPCR. Vaccination with the HKY reduced the tissue fungal burden of the lungs by ~10-fold when compared to lungs harvested from control, PBS-vaccinated mice (Figure 2) (P = 0.048).
Figure 2. HKY vaccine significantly reduced lung fungal burden in DKA/cortisone acetate-treated mice infected with R. oryzae.
Mice were vaccinated with 3 doses of 6.0 × 107 of HKY. Control mice were given PBS alone. Mice were then treated to produce DKA and treated with cortisone acetate prior to intratracheal infection with R. oryzae 99-880 isolate at 2.5 × 105 spores (delivered inoculum = 2.0 × 103 spores per mouse). Mice (n=10 per arm) were euthanatized at day 3 postinfection for determination of fungal burden in lungs and brains by quantitative PCR. *P <0.05 vs. Control by Wilcoxon Rank Sum test.
Vaccination with HKY enhanced the mouse immune response against R. oryzae
Blood samples obtained from uninfected mice vaccinated with three doses of 6 × 107 HKY cells showed enhanced serum antibody titers against R. oryzae by more than 250-fold when compared to control mice vaccinated with PBS, as determined by ELISA plates coated with R. oryzae cell surface material (Figure 3) (P = 0.00001). These data clearly demonstrate the presence of shared antigens between S. cerevisiae and R. oryzae.
Figure 3. HKY vaccination significantly increased the mouse immune response as determined by detection of increased anti-R. oryzae cell surface antibody titers.
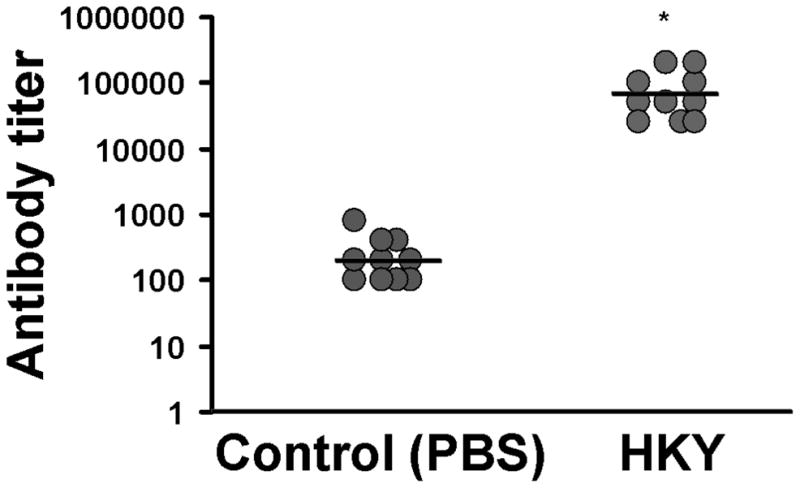
Mice (n=10 per group) were vaccinated with three doses (at weekly intervals) 6 × 107 HKY cells or PBS alone as control. Serum was collected and anti-R. oryzae antibodies were evaluated by ELISA. * P= 0.00001 vs. PBS vaccinated control mice by Wilcoxon Rank Sum test.
Discussion
In this study we demonstrate the activity of HKY vaccine against murine mucormycosis due to R. oryzae infection. Our results also show for the first time that the HKY vaccine can protect against a fungal infection in a model with a pulmonary route of infection. Moreover, results presented in this study are the first demonstration of HKY efficacy in a model employing classical immunosuppression, such as corticosteroids. In addition, our model had a second form of immunocompromise elicited by DKA. The most compromised animals previously showing protection with HKY were complement-deficient animals, with protection against aspergillosis [5]. It is important to note that these results lend support to the concept of vaccinating patients prior to therapeutic manipulations that result in severe immunosuppression (e.g., induction of neutropenia, chemotherapy, or uncontrolled diabetes etc.) where the vaccine induced protection carries over through the period the patient is at high risk of developing mucormycosis. However, our data also suggest additional experiments are required to optimize the vaccination regimens, since higher doses were not as protective as lower doses. Furthermore, the DKA/cortisone acetate mice were not completely protected, however, the challenge was severe (e.g. highly lethal, with 100% mortality in controls in ≤2 weeks).
The demonstration of cross reacting antibodies engendered by HKY vaccination is consistent with previous work indicating the presence of shared antigens among fungi, including key cell wall glycan components (i.e. glucan and mannan), and homologous proteins [4, 5, 8, 12]. Although HKY induced a robust immune response against R. oryzae, as determined by anti-R. oryzae antibodies, the mechanism of protection remains to be determined. In studies examining the immune response of mice to HKY, we found vaccination induced proliferation of spleen and lymph node cells, enhanced cytokine levels for interferon-γ (IFN-γ), interleukin (IL)-6, and IL-17A from stimulated lymphocytes, and TNF-α and IFN-γ in bronchoalveolar lavage fluid [13]. In addition, HKY vaccination resulted in stimulation of specific antibodies to mannan and glucan. Thus, the mechanism of HKY-induced protection against R. oryzae may be a result of antibody and an adaptive T cell response. In this respect, antibodies targeting the cell surface CotH invasin of R. oryzae were recently shown to protect against experimental pulmonary mucormycosis when administered prophylactically or therapeutically [10], thereby confirming the protective role of antibodies against mucormycosis. It is also possible that the mechanism of protection could rely largely on stimulation of innate immunity by HKY, and/or on epigenetic reprogramming (“trained immunity”) [14]. More than one arm of immunity resulting from vaccination could be responsible for protection [15]. Therefore, future studies of lymphocytes of immunized animals, for cytokine profiling, before and after Rhizopus challenge, and of monocyte function after vaccination [14], would be of great interest.
Yeast-based vaccines are safe in man [16], and initial studies with heat-killed Saccharomyces have shown tolerance by cancer patients to this vaccine [17, 18]. Our results in combination with those from studies showing efficacy against aspergillosis, coccidioidomycosis, candidiasis, and cryptococcosis [4–8] warrant the further development of HKY as a pan-fungal vaccine candidate, perhaps by generating a derivative of HKY, particularly a more purified derivative that mimics or exceeds HKY in its efficacy, and presumably with a similar mechanism of action. Such a vaccine could have a glycan(s) and/or proteins(s) that are shared among fungal species. In this respect, we have shown that purified mannan, or glucan, with their respective conjugates, can reproduce the protection shown with whole killed Saccharomyces against some lethal mycoses [19, 20].
Highlights.
HKY vaccine protects against DKA/steroid treated mice with pulmonary mucormycosis
This is the first illustration of HKY efficacy in classically immunosuppressed mice
HKY induces antibodies that react with R. oryzae cell wall antigens
Our results broaden the HKY efficacy spectrum to a fifth mycosis
Our data warrant the future development of HKY as a pan-fungal vaccine
Acknowledgments
Funding: This work was supported by Public Health Service grant R01 AI063503 to A.S.I.
Footnotes
Conflict of Interest: None to declare.
Author Contributions: DAS, KVC and ASI conceived and designed the experiments. GL, TG, and ASI performed the experiments. GL, TG and ASI analyzed the data. KVC and DAS contributed reagents. GL and ASI wrote the paper. KVC and DAS revised the paper. All authors have approved the final article.
This work was presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Abstract #M-792. Denver. CO. Research described in this manuscript was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, a non-profit 501c research institute. Los Angeles Biomedical Research Institute had no input on study design, conduct, or analysis, or the drafting of this manuscript.
All procedures involving mice were approved by the institutional animal care and use committee and followed the National Institutes of Health guidelines for animal housing and care.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005 Sep 1;41(5):634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 2.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne JR, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012 Dec;55(11):1441–9. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009 Nov;85(1009):573–81. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DA, Clemons KV, Liu M. Developing a vaccine against aspergillosis. Med Mycol. 2011 Apr;49( Suppl 1):S170–6. doi: 10.3109/13693786.2010.497775. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Capilla J, Johansen ME, Alvarado D, Martinez M, Chen V, et al. Saccharomyces as a vaccine against systemic aspergillosis: ‘the friend of man’ a friend again? J Med Microbiol. 2011 Oct;60(Pt 10):1423–32. doi: 10.1099/jmm.0.033290-0. [DOI] [PubMed] [Google Scholar]
- 6.Capilla J, Clemons KV, Liu M, Levine HB, Stevens DA. Saccharomyces cerevisiae as a vaccine against coccidioidomycosis. Vaccine. 2009 Jun 2;27(27):3662–8. doi: 10.1016/j.vaccine.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Majumder T, Liu M, Chen V, Martinez M, Alvarado D, Clemons KV, et al. Killed Saccharomyces cerevisiae protects against lethal challenge with Cryptococcus grubii. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011; Chicago, IL: American Society for Microbiology; 2011. [Google Scholar]
- 8.Liu M, Clemons KV, Johansen ME, Martinez M, Chen V, Stevens DA. Saccharomyces as a vaccine against systemic candidiasis. Immunol Invest. 2011;41(8):847–55. doi: 10.3109/08820139.2012.692418. [DOI] [PubMed] [Google Scholar]
- 9.Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011 Dec;17(12):1859–67. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 10.Gebremariam T, Liu M, Luo G, Bruno V, Phan QT, Waring AJ, et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014 Jan 2;124(1):237–50. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellenc D, Schmitt E, Gallet O. Purification of a plant cell wall fibronectin-like adhesion protein involved in plant response to salt stress. Protein Expr Purif. 2004 Apr;34(2):208–14. doi: 10.1016/j.pep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Champer J, Diaz-Arevalo D, Champer M, Hong TB, Wong M, Shannahoff M, et al. Protein targets for broad-spectrum mycosis vaccines: quantitative proteomic analysis of Aspergillus and Coccidioides and comparisons with other fungal pathogens. Ann N Y Acad Sci. 2012 Dec;1273:44–51. doi: 10.1111/j.1749-6632.2012.06761.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Clemons KV, Bigos M, Medovarska I, Brummer E, Stevens DA. Immune responses induced by heat killed Saccharomyces cerevisiae: a vaccine against fungal infection. Vaccine. 2011 Feb 17;29(9):1745–53. doi: 10.1016/j.vaccine.2010.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens DA. Vaccinate against aspergillosis! A call to arms of the immune system. Clin Infect Dis. 2004 Apr 15;38(8):1131–6. doi: 10.1086/382882. [DOI] [PubMed] [Google Scholar]
- 16.DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS) Vaccine. 2006 Feb 6;24(6):703–7. doi: 10.1016/j.vaccine.2005.07.069. [DOI] [PubMed] [Google Scholar]
- 17.Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumour antigens in cancer immunotherapy. Expert Opin Biol Ther. 2005 Apr;5(4):565–75. doi: 10.1517/14712598.5.4.565. [DOI] [PubMed] [Google Scholar]
- 18.Munson S, Parker J, King T, Lu Y, Kelly V, Guo Z, et al. Coupling innate nad adaptive immunity with yeast-based cancer immunotherapy. In: Orentas R, Hodge JW, Johnson BD, editors. Cancer Vaccines and Tumor Immunity. Hoboken, N.J: John Wiley & Sons; 2008. pp. 131–49. [Google Scholar]
- 19.Liu M, Machova E, Nescakova Z, Medovarska I, Clemons KV, Martinez M, et al. Vaccination with mannan protects mice against systemic aspergillosis. Med Mycol. 2012 Nov;50(8):818–28. doi: 10.3109/13693786.2012.683539. [DOI] [PubMed] [Google Scholar]
- 20.Clemons KV, Danielson M, Michel KS, Liu M, Martinez M, Chen V, et al. Whole glucan particles as a vaccine against murine aspergillosis. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2013; Denver, Colorado: American Society for Microbiology; [Google Scholar]