Abstract
Background
It is uncertain if endovascular stroke therapy leads to improved clinical outcomes due to a paucity of data from randomized placebo-controlled trials. The aim of this study was to determine if MRI can be used to identify patients who are most likely to benefit from endovascular reperfusion.
Methods
Consecutive patients, scheduled to undergo endovascular therapy within 12 hours of stroke onset, were enrolled in a multi-center prospective cohort study. Aided by an automated image analysis software program, investigators interpreted the baseline MRI. They determined, prior to endovascular treatment, if the patient had an MRI profile (Target Mismatch) that suggested salvageable tissue was present. Reperfusion was assessed on an early follow-up MRI and defined as a >50% reduction in the volume of the baseline perfusion lesion. A favorable clinical response was defined as a ≥8 point improvement on the NIH Stroke Scale (NIHSS) between baseline and day 30 or an NIHSS score of 0–1 at 30 days.
Findings
Following endovascular therapy reperfusion occurred in 46 of 78 (59%) Target Mismatch patients and in 12 of 21 (57%) No Target Mismatch patients. The adjusted odds ratio for favorable clinical response associated with reperfusion was 8·5 (95% CI 2·6 – 28) in the Target Mismatch group and 0·2 (95% CI 0·0 – 1·6) in the No Target Mismatch group (p=0·003 for difference between odds ratios). Reperfusion was associated with an increased odds of good functional outcome at 90 days (OR is 5.2, 95% CI 1.4–19) and attenuation of infarct growth at 5 days (30 ml of median growth with reperfusion vs. 73 ml without reperfusion, p=0·01) in the Target Mismatch group but not in patients without Target Mismatch.
Interpretation
Target Mismatch patients who achieved early reperfusion following endovascular stroke therapy had more favorable clinical outcomes and less infarct growth. No association between reperfusion and favorable outcomes was present in patients without Target Mismatch. These data support a randomized controlled trial of endovascular treatment in patients with the Target Mismatch profile.
INTRODUCTION
The goal of acute stroke therapy is to restore perfusion to ischemic brain tissue prior to irreversible injury. Detection and quantification of salvageable tissue is particularly pertinent to endovascular therapy because intra-arterial recanalization techniques are typically initiated many hours after stroke onset. Imaging criteria for patient selection for reperfusion therapies has typically been limited to the data available from non-contrast head CT. MR diffusion and perfusion imaging has higher sensitivity for identification of the ischemic core and can potentially estimate the volume of salvageable ischemic tissue. However, MRI has not been demonstrated to be superior to routine CT for selection of candidates for endovascular reperfusion therapy.
Utilization of endovascular therapy for acute stroke increased substantially over the last decade following the introduction of mechanical clot retrieval devices.1, 2 Cohort studies of endovascular therapies have shown recanalization rates up to 82%, but the rates of good clinical outcome have typically been around 30%.3–5 Because of these modest rates of good outcome and the lack of data from placebo controlled trials, there is considerable controversy regarding the clinical effectiveness and optimal patient selection for endovascular therapy.6–8
Endovascular therapy that restores perfusion to irreversibly injured tissue is not beneficial and may be harmful if it leads to edema or hemorrhage.9, 10 Magnetic resonance imaging (MRI) has the potential to differentiate unsalvageable from salvageable tissue. Tissue with hypoperfusion that is severe enough to eventually result in cell death can be identified with perfusion weighted MRI (PWI). Acute ischemic tissue that is highly likely to have already suffered irreversible injury displays abnormal signal on diffusion weighted MRI (DWI).11, 12 The Target Mismatch profile is a term that has been used to describe patients with a substantial volume of salvageable tissue (small DWI lesions relative to their PWI lesions).13 The DEFUSE and EPITHET studies found that only patients with the Target Mismatch profile experienced clinical benefits and attenuation of infarct growth following intravenous tPA-associated reperfusion.14
Identification of MRI profiles in the acute setting has been challenging because it requires cumbersome and time consuming post-processing of MRI data. To mitigate this limitation, we developed an image reconstruction software program (RAPID) that automatically outlines and calculates DWI and PWI lesion volumes.15 We hypothesized that patients with Target Mismatch would have more favorable clinical and radiographic responses following endovascular reperfusion therapy than patients without Target Mismatch. Furthermore, we predicted that, with the aid of this automated image analysis software, clinicians could prospectively identify which patients had the Target Mismatch profile.
METHODS
Study design
The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) Study was a multi-center prospective cohort study of acute stroke patients who underwent serial MR imaging before and after endovascular therapy. Patients were enrolled at eight sites in the US and one European site. DEFUSE 2 began as a single center study at Stanford in July 2008; additional sites were activated after NIH funding for the study was obtained in September 2009. Enrollment was completed in September 2011. Approval for the study was obtained from local institutional review boards.
Study patients and protocol
All patients who met local site criteria for acute endovascular stroke therapy were screened. Written informed consent from the patient or a relative was required for participation in the study. Patients were eligible for enrollment if their endovascular procedure was anticipated to begin within 12 hours of symptom onset, they had a National Institutes of Health Stroke Scale (NIHSS) score of ≥ 5, were ≥ 18 years old, and able to undergo MRI imaging prior to the endovascular procedure. Patients who had been treated with intravenous tPA were eligible. Pregnancy or a modified Rankin Scale score of ≥3 prior to stroke onset were exclusions.
All imaging was performed on 1·5T or 3T MRI systems. A standardized MRI protocol, designed to acquire all images within 15 minutes, was installed at each site (see on-line appendix). Image reconstruction software (RAPID), which generated quantitative DWI and PWI lesion maps with 4–7 minutes of processing time, was also installed.15
Each patient underwent three MRIs. A baseline MRI, obtained within 90 minutes prior to the start of the endovascular procedure, included gradient recalled echo (GRE), intracranial MRA, DWI and PWI sequences. An early follow-up scan with the same sequences was obtained within 12 hours after the endovascular procedure. A late follow-up MRI was obtained on day 5 or at discharge from the hospital (whichever was sooner) and included GRE, DWI and FLAIR.
Stroke severity was assessed with the NIHSS by a trained investigator at the time of the baseline MRI, at each subsequent imaging time point, and at 30 and 90-day follow-up visits. Functional outcome was assessed at days 30 and 90 on the modified Rankin Scale. Clinical endpoints at 30 and 90 days were assessed blinded to the patients’ baseline clinical and radiographic data. If clinical endpoint data was missing for the 30 or 90-day endpoints, the last observation was carried forward.
According to local clinical practice, the standard results of the baseline MRI scan (prior to RAPID processing) could be used to influence the decision to proceed with endovascular therapy. Following this decision but prior to endovascular treatment, the local investigator reviewed the RAPID output to determine the patient’s Target Mismatch status. The RAPID output included 1) a DWI map with an outline and volume estimate of the ischemic core (tissue considered irreversibly injured) based on an apparent diffusion coefficient (ADC) threshold of <600 s/mm2 and 2) a PWI map with a volume estimate of critically hypoperfused tissue based on a time to maximum of the tissue residue function (Tmax) threshold of >6 sec (Figure 1).15, 16 The pre-specified criteria for the Target Mismatch profile were based on prior studies.17, 18 They included 1) a ratio between the volumes of critically hypoperfused tissue and the ischemic core ≥1·8, with an absolute difference ≥ 15 mL; 2) ischemic core volume <70 mL and; 3) volume of tissue with a severe delay in bolus arrival (Tmax>10 sec) <100 mL. The local investigators’ assignments of patients’ Target Mismatch status was used for all analyses that compared the response to reperfusion in patients with and without Target Mismatch.
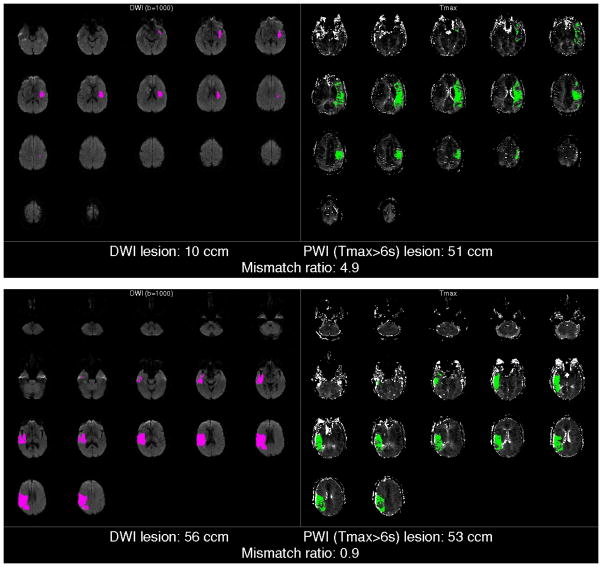
Figure 1. Examples of RAPID output.
An example of the output of the automated MR image processing software (RAPID) for a patient with the Target Mismatch Profile (above); in this patient the volume of irreversibly injured ischemic tissue is estimated on the DWI map to be 10 ccm which is considerably smaller than the volume of critically hypoperfused tissue (estimated to be 51 ccm on the PWI map) yielding a mismatch ratio of 4·9. The example below demonstrates the No Mismatch profile; the estimated volume of irreversibly injured tissue (56 ccm) is approximately the same as the volume of critically hypoperfused tissue (53 ccm) yielding a mismatch ratio of 0·9.
Core Lab Assessments
A core imaging laboratory reviewed all MRI and cerebral angiography studies blinded to clinical data. Baseline DWI lesion volume was determined from the output of RAPID and manually adjusted if it was determined that RAPID had underestimated or overestimated the DWI lesion volume. Baseline PWI volumes were also based on automated segmentation by RAPID with manual removal of artifacts when indicated. The volume of the ischemic lesion on day 5 was determined by manual outlining of the lesion on FLAIR.
Lesion volumes assessed by the core lab were used to calculate infarct growth between baseline DWI and 5-day FLAIR, and establish whether early reperfusion was achieved. Reperfusion was defined as a >50% reduction in the volume of the PWI (Tmax>6sec) lesion between the baseline and the early follow-up MRI obtained within 12 hours after the endovascular procedure. If the early follow-up study was of insufficient quality, not obtained, or obtained >18 hours from end of the endovascular procedure, then reperfusion was based on angiographic criteria: perfusion reestablished to >50% of the territory supplied by the occluded vessel at completion of the angiographic procedure.
Data monitoring and endpoint definitions
Case report forms were audited by a study monitor. The primary clinical endpoint was “favorable clinical response” defined as an improvement in the NIHSS of 8 or more points between baseline and day 30 or an NIHSS score ≤ 1 at day 30. This endpoint was chosen to match the primary endpoint of the DEFUSE 1 study.13 Secondary clinical endpoints were good functional outcome, defined as a modified Rankin Scale ≤2 at day 90, and infarct growth defined as the change in infarct volume between baseline and day 5. Major symptomatic intracranial hemorrhage was defined as any hemorrhage associated with a ≥4 point worsening on the NIHSS within 36 hours of symptom onset. Brain hemorrhages, detected on any CT or MRI performed within 7 days of stroke onset, were classified as hemorrhagic infarction (HI) or parenchymal hematoma (PH).19
Statistical analysis
The primary pre-specified hypothesis of the study was that the association between reperfusion and favorable clinical response would be stronger in patients with the Target Mismatch profile than in patients without Target Mismatch. This was tested statistically using a linear logistic regression model with “favorable clinical response” as the dependent variable. Binary terms for Target Mismatch assessed by the local investigator, successful reperfusion, and an interaction term were included as explanatory variables. A significant interaction term signaled a statistical difference between the odds ratios. The pre-specified analysis plan required adjustment of the model for imbalances in significant baseline predictors of outcome. Potential predictors that were examined individually included DWI volume, PWI volume, NIHSS, age, time to initiation of endovascular treatment, glucose, pre-treatment with IV-tPA, and gender. Baseline predictors were entered in a multivariable model if their p-value from the univariate model was less than 0·1. They were retained in the model if their p-value was less than 0·05.
The frequency of attaining good functional outcome, defined as a mRS score of two or less at 90 days, was compared between patients with and without reperfusion using the Fisher exact test. Differences in median lesion growth were assessed using the Mann-Whitney-U test. All analyses were conducted with SAS/STAT software.
Sample size
The sample size was based on the following assumptions: power of 90% to test the primary hypothesis; 50% of patients with a Target Mismatch profile; reperfusion rate of 75% in both Target Mismatch and No Target Mismatch; 37% difference in the favorable clinical response rates between Target Mismatch patients with and without reperfusion (67% vs. 30%); no difference in the rate of favorable clinical response in No Target Mismatch patients with and without reperfusion. Based on these assumptions we estimated 100 patients would be required in the MRI profile cohort. Given the assumption that approximately 25% of the enrolled patients would be excluded from the MRI Profile cohort, a sample size of 130 enrolled patients was chosen.
Role of the funding source
The study sponsor had no role in study design; the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit the report for publication.
RESULTS
Patients
One hundred and thirty-eight consecutively encountered eligible patients signed written informed consent for the study and were enrolled. Twenty-eight did not undergo catheter angiography (Figure 2). Ninety-eight of the 110 patients who underwent catheter angiography had an endovascular intervention. Ten of the twelve patients who did not have an intervention had lesions that were deemed unsuitable for endovascular therapy. Excessive bleeding at the femoral insertion site and inability to access the carotid artery led to termination of the procedure before intervention in the other two patients. Fifty-nine (54%) of the 110 patients who underwent catheter angiography were pretreated with intravenous tPA. Intravenous tPA was administered at the study site in 19 patients and at an outside hospital in 40 patients. Full dose (0.9 mg/kg) intravenous tPA was used in all but one patient who received 35% of the full dose. Median time from symptom onset to the beginning of the MRI examination was 4½ hours (IQR 2.8 – 6.0).
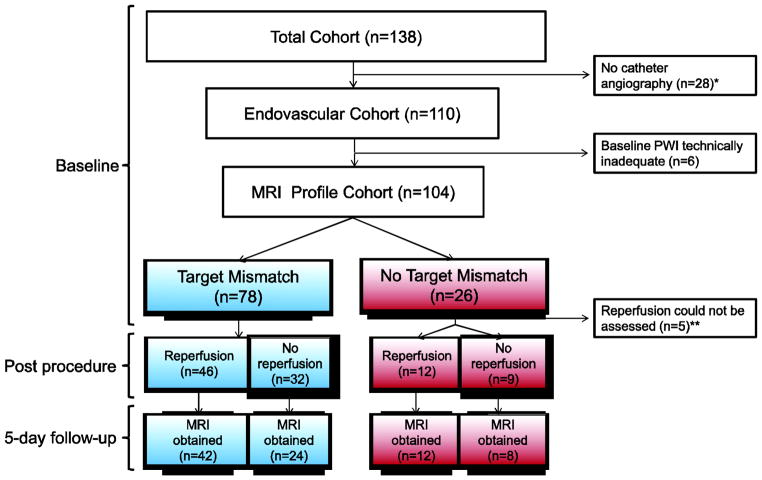
Figure 2. Disposition of patients.
The Total Cohort consists of 138 patients who were considered for endovascular therapy and consented to participation in the study. All 138 patients underwent a baseline MRI. Following the baseline MRI examination the decision was made not to proceed with the endovascular procedure in twenty-eight patients. Among the remaining 110 patients who underwent catheter angiography (Endovascular Cohort), the local investigator deemed the baseline PWI to be of sufficient quality for assessment of Target Mismatch in 104 patients (MRI profile cohort). In 5 of these 104 patients reperfusion could not be assessed (see ** below) and these 5 cases were excluded from the primary clinical analysis which required an assessment of reperfusion status to calculate the odds ratio of favorable clinical response in patients with reperfusion compared to patients without reperfusion. Thirteen of the 99 patients who were included in the primary clinical analyses did not have a 5-day follow-up MRI and were excluded from the radiographic outcome analysis which compared infarct growth between patients with and without reperfusion.
*Reasons that patients did not undergo an endovascular procedure are absence of large vessel occlusion on MR angiography (n=8); large DWI lesion (n= 12); clinical improvement (n= 4); and other (n=4). Of the 28 patients who did not undergo an endovascular procedure, 24 had the No Target Mismatch profile.
**In five patients who underwent catheter angiography, reperfusion could not be assessed because these patients did not have a vessel occlusion on cerebral angiography and they had a small (<10 ml) perfusion lesion at baseline.
The local investigator judged the RAPID summary maps to be of sufficient quality to determine the MRI profile in 104 of the 110 patients who underwent catheter angiography (Table 1). Investigators classified 78 (75%) of the 104 patients as Target Mismatch and 26 (25%) as No Target Mismatch. The analyses reported in the paragraphs below are all based on the investigators’ classification of Target Mismatch status.
Table 1.
Baseline characteristics and endovascular therapies employed in patients included in the MRI profile cohort.
| Characteristic | Target Mismatch (N=78) | No Target Mismatch (n=26) |
|---|---|---|
| Mean (SD) age – yr | 66 (16) | 63 (13) |
| Female sex – no. (%)* | 42 (54) | 8 (31) |
| Hypertension – no. (%) | 55 (70) | 17 (65) |
| Diabetes mellitus – no. (%) | 16 (20) | 6 (23) |
| Hyperlipidemia – no. (%) | 44 (56) | 9 (35) |
| Atrial fibrillation – no. (%) | 27 (35) | 9 (35) |
| Prior stroke/TIA – no. (%) | 17 (22) | 8 (31) |
| Median NIHSS score (IQR)† | 14 (11–20) | 18 (12–19) |
| Intravenous tPA pretreatment – no. (%)* | 36 (46) | 19 (73) |
| Median (IQR) time from symptom onset to start of MRI – hrs* | 4·6 (3·0–6·7) | 3·6 (2·5–4·9) |
| Median (IQR) time from symptom onset to femoral puncture – hrs* | 6·2 (4·9–8·1) | 4·7 (3·7–6·4) |
| Median (IQR) time from arrival at study site to endovascular therapy – hrs | 2·4 (1·7–3·2) | 1.9 (1·3–3·1) |
| Median (IQR) volume of infarct core – ml* | 13 (5–26) | 45 (2–68) |
| Median (IQR) volume of perfusion lesion – ml | 80 (50–108) | 74 (10–166) |
| Vessel occlusion on angiogram* – no. (%) | ||
| Internal carotid artery, proximal | 14 (18) | 1 (4) |
| Internal carotid artery, distal | 7 (9) | 2 (8) |
| Middle cerebral artery, proximal | 48 (62) | 9 (35) |
| Middle cerebral artery, distal | 8 (10) | 8 (31) |
| Other | 0 (0) | 1 (4) |
| None | 1 (1) | 5 (19) |
| Endovascular therapy employed‡ – no. (%) | ||
| Merci device | 37 (47) | 8 (31) |
| Penumbra system | 31 (40) | 10 (39) |
| Intra-arterial tPA | 32 (41) | 8 (31) |
| Other | 24 (31) | 4 (15) |
The Target Mismatch group had a higher percentage of women (p=0·05), a lower percentage of patients who had received intravenous tPA prior to catheter angiography (p=0·02), longer time intervals between symptom onset and femoral puncture (p=0·03), smaller infarct cores (p <0·02) and a higher percentage of patients with occlusions of the internal carotid and proximal middle cerebral artery (p<0·001) than the No Target Mismatch group.
Scores on the National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42 with higher scores indicating more severe neurologic deficits.
Combinations of more than one endovascular therapy were used in 45%. Other mechanical devices included: Acculink stent (n=5), Solitaire (N=4), Trevo (N=2), and Enterprise stent (n=2).
In 97% (101/104) of the patients the core imaging lab agreed with the local investigator regarding the MRI profile (kappa 0·92; 95% CI 0·83 – 1·0). In all three cases of disagreement, the core lab assigned Target Mismatch status whereas the local investigators classified the patients as No Target Mismatch. Core lab adjustments were made to the baseline DWI lesion volume generated by RAPID in 4 patients (median change in lesion size 7.5 mL) and the baseline PWI lesion volume in 18 patients; median (IQR) change in lesion size 7.6 mL (5.7–22.2). These adjustments led to a change in mismatch profile in only one patient and accounted for 1 of the 3 disagreements between the local investigator and the core lab.
Primary outcome
Reperfusion status could be determined in 99 of the 104 patients in the MRI profile cohort (Figure 2). None of these 99 patients were lost to follow-up. Reperfusion was assessed based on PWI criteria in 87 patients. In the remaining 12 patients in whom the early follow-up PWI was not obtained or of insufficient quality to assess reperfusion status, reperfusion was assessed based on catheter angiography criteria. Table 2 displays the key clinical and radiographic outcomes of the 99 patients in whom reperfusion was assessed. Among these 99 patients, reperfusion was associated with an increased rate of favorable clinical response (OR 2·8, 1·2 – 6·2). This overall increase was driven by the Target Mismatch group (n=78) that had an odds ratio of 5·0 (95% CI 1·9 – 13, p=0·001) for favorable response following reperfusion. In contrast, patients without the Target Mismatch profile (n=21) had a trend toward less favorable outcomes with reperfusion (OR=0·2; 95% CI 0·0 – 1·4). The difference between the odds ratios for the Target Mismatch vs. No Target Mismatch groups was significant (p=0·004). Age and baseline DWI lesion volume were significant predictors of favorable clinical response that were retained in the multivariable regression model; with these variables in the model, the odds ratios for favorable clinical response in patients with reperfusion were 8·5 (95% CI 2·6 – 28) for the Target Mismatch profile and 0·2 (95% CI 0·0 – 1·6) for the No Target Mismatch profile; the difference between these odds ratios is significant (p=0·003 for the interaction of reperfusion by Target Mismatch status).
Table 2.
Clinical and radiologic outcomes
| Outcome | - - -Target Mismatch - - - | - - -No Target Mismatch - - - | ||||
|---|---|---|---|---|---|---|
| Reperfusion (N=46) | No Reperfusion (N=32) | P value | Reperfusion (N=12) | No Reperfusion (N=9) | P value | |
| Favorable clinical response* 30 days – no. (%) | 32 (70%) | 10 (31%) | 0·001 | 5 (42%) | 7 (78%) | 0·18 |
|
| ||||||
| Median (IQR) lesion growth** - mL | 30 (5–67) | 73 (32–127) | 0·005 | 96 (68–159) | 34 (4–147) | 0·21 |
|
| ||||||
| Good functional outcome*** 90 days – no. (%) | 26 (57%) | 10 (31%) | 0·04 | 3 (25%) | 2 (22%) | 1·0 |
|
| ||||||
| Major symptomatic ICH§ – no. (%) | 3 (7%) | 6 (19%) | 0·15 | 1 (8%) | 1 (11%) | 1·0 |
|
| ||||||
| Parenchymal hematoma§§ – no. (%) | 10 (22%) | 9 (29%) | 0·59 | 4 (33%) | 2 (22%) | 0·66 |
P values for the comparison between patients with reperfusion and patients without reperfusion are listed separately for the Target Mismatch and the No Target Mismatch cohorts. Groups were compared using the Fisher exact test for categorical variables and the Mann-Whitney-U test for absolute lesion growth.
Favorable clinical response is defined as an improvement of ≥8 points on the National Institutes of Health Stroke Scale (NIHSS) between baseline and day 30, or a score of 0–1 points on the NIHSS at day 30.
Lesion growth between baseline and 5-day follow-up was assessed in all patients who had a 5 day MRI scan (see Figure 2 for number of patients in each group who completed the 5 day scan)
Good functional outcome was defined as a modified Rankin Score of 0–2
Major symptomatic intracranial hemorrhage (ICH) is defined as any intracranial hemorrhage associated with a worsening of 4 or more points on the NIHSS, within 36 hours after the baseline NIHSS assessment.
Parenchymal Hemorrhage was defined as a PH 1 or 2 per ECASS criteria19 on a CT or MRI obtained within 5 days of the endovascular procedure. One Target Mismatch patient who did not reperfuse did not have any CT or MRI scans performed after the endovascular procedure. Therefore, N=31 for assessment of parenchymal hematoma in this subgroup.
Secondary outcomes
Reperfusion was associated with a higher percentage of good functional outcomes at 90 days in the Target Mismatch group (Table 2 and Figure 3). The adjusted odds ratio for the association between reperfusion and good functional outcome at 90 days was 5·2 (95% CI 1·4–19) in the Target Mismatch group; no significant association was present in the No Target Mismatch group.
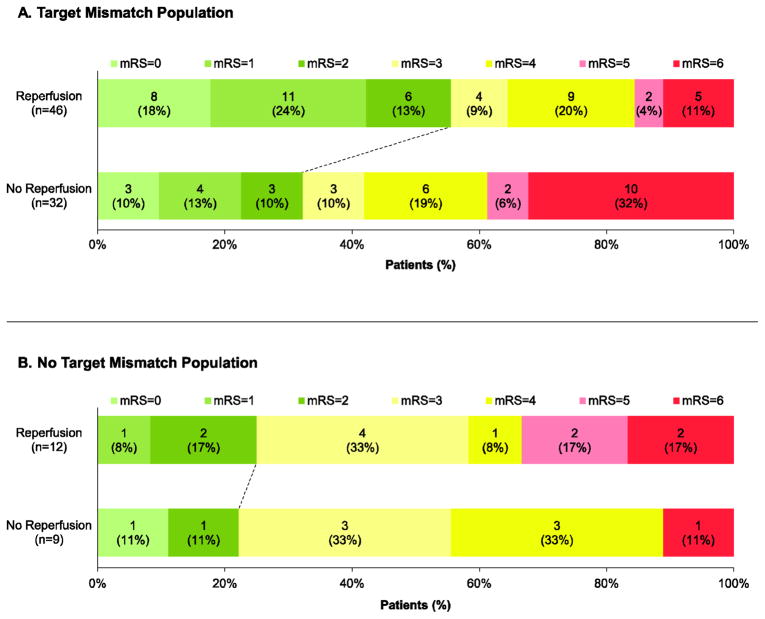
Figure 3. Functional outcome (modified Rankin Scale) at 90 days for patients with and without Target Mismatch.
Scores on the modified Rankin Scale are categorized as good outcome with no or minimal disability (mRS 0–2, green bars), disabled and dependent on others for activities of daily living (mRS 3–4, yellow bars), and bedridden or dead (mRS 5–6, red bars). p=0·04 for the comparison of good functional outcome (mRS 0–2 at 90 days) between patients with and without reperfusion in the Target Mismatch group (Panel A). There is no significant difference in good outcome between patients with and without reperfusion in the No Target Mismatch cohort (Panel B).
In patients with the Target Mismatch profile, reperfusion was associated with a reduction in infarct growth (Table 2). In contrast, there was a trend toward more lesion growth following reperfusion in the No Target Mismatch group.
Additional outcomes and post-hoc analyses
Eleven (10%) of the 110 patients in the endovascular cohort suffered a major symptomatic intracerebral hemorrhage (see Table 2). These all occurred prior to the early follow-up MRI scan and occurred at similar rates in patients with and without Target Mismatch (Table 1) and in patients who were pretreated with IV tPA (6 of 59; 10%) and those who were not (5 of 51; 10%). Major endovascular complications occurred in 5 (4.5%) of the 110 patients in the endovascular cohort including three patients with a major symptomatic intracerebral hemorrhage. All five major endovascular complications occurred in patients with the Target Mismatch profile.
Two post-hoc analyses were conducted. First, the association between the degree of reperfusion, defined as the relative difference between the baseline and the early follow-up PWI (Tmax>6sec) lesion, and clinical outcome was evaluated in the Target Mismatch cohort. In this cohort, the proportion of patients with a favorable clinical response at day 30 increased from 5 of 17 (29%) for patients in the lowest reperfusion quartile (<25% reduction in PWI lesion volume), to 8 of 18 (44%) in the second quartile, to 11 of 17 (65%) in the third quartile, and to 16 of 17 (94%) in the top quartile (≥96% reduction in PWI lesion volume) (p for trend < 0·001).
Second, the association between reperfusion and favorable clinical response was compared in Target Mismatch patients in whom endovascular therapy was begun early (≤6 hrs from symptom onset, median 4·8 hrs, n=36) vs. late (>6 hrs, median 7·9 hrs, n=42). Reperfusion was associated with an increased odds of favorable clinical response at 30 days regardless of treatment delay (OR=2·9, 95% CI 0·7–11·4 in early treated vs. OR=8·5, 95% CI 2·1–35 in late treated Target Mismatch patients; p for difference between the odds ratios = 0·28).
DISCUSSION
DEFUSE 2 demonstrates that outcomes following endovascular reperfusion differ between patient subgroups. Target Mismatch patients have an increased chance of favorable clinical and radiographic outcomes following reperfusion, whereas no association between reperfusion and favorable outcomes is apparent in the No Target Mismatch group. Furthermore, local investigators in the acute stroke setting, with the aid of automated MRI analysis software, can rapidly and accurately identify Target Mismatch patients.
There are no data from randomized trials to clarify which types of stroke patients are most likely to benefit from endovascular therapy. Randomized trials have been challenging to perform because many clinicians feel that randomizing an acute stroke patient with a large vessel occlusion to medical treatment alone is not ethical when mechanical devices that can restore of blood flow are available.20, 21 Although DEFUSE 2 was not a randomized trial, the high rate of favorable clinical outcomes in Target mismatch patients who achieved reperfusion following endovascular therapy is encouraging and suggests that this group may be optimal to include in future randomized trials. In contrast, the lack of association between reperfusion and favorable outcome in the No Target mismatch group suggests these patients are potentially less likely to respond favorably to endovascular therapies and excluding them from a randomized trial may improve the power of the study to detect reperfusion-related clinical benefits.
The degree of reperfusion achieved corresponded with clinical recovery in Target Mismatch patients. This finding suggests that endovascular approaches that can produce high rates of complete tissue reperfusion, such as the recently introduced stent-retrievers22, will lead to enhanced clinical outcomes in patients with Target Mismatch.
The association between reperfusion and favorable clinical and imaging outcomes did not diminish over time in Target Mismatch patients. This finding supports the concept that salvageable tissue can persist many hours after stroke onset in selected stroke patients and that time–independent factors, such as the degree of collateral circulation23, play an important role in the progression from reversible to irreversible ischemic injury.
The No Target Mismatch patients differed from the Target Mismatch patients; they were more likely to receive intravenous tPA prior to endovascular therapy, had larger baseline DWI lesion volumes and received endovascular therapy sooner after symptom onset. Despite earlier treatment, reperfusion was not associated with favorable outcomes in the No Target Mismatch patients, potentially because these patients have minimal or no salvageable tissue. The trend towards more lesion growth in the non-Target mismatch patients who experienced reperfusion may reflect reperfusion-related edema and/or the slightly higher rate of parenchymal hematoma that was observed in these patients.
The DEFUSE 2 study was not designed to demonstrate the efficacy of endovascular therapy. The absence of a control group that did not receive endovascular therapy precludes definitive conclusions regarding the benefits or risks of endovascular therapy. For example, we cannot exclude the possibility that the endovascular procedures performed contributed to the less favorable 90 day outcomes that occurred in the No Target mismatch group. The fact that a variety of endovascular treatment strategies were employed in a non-randomized manner limits any conclusions about specific endovascular approaches. The median time from symptom onset to MRI was 4 ½ hours with an interquartile range from 2.8 to 6 hours. The DEFUSE 2 results may therefore not apply to patients who are imaged outside of this time-window.
Door-to-femoral puncture times were longer than optimal, in part related to the protocol requirement to obtain an MRI before treatment as well as the fact that approximately half of the patients received a one-hour intravenous infusion of tPA prior to enrollment. In the future, door-to-femoral puncture times can be shortened if the MRI is started while the patient is receiving the tPA infusion. Some stroke patients have contraindications to MRI (such as a pacemaker) and excessive patient motion makes perfusion imaging technically unsatisfactory in a small proportion of patients. This limits the applicability of MRI for patient selection in the general population. Other imaging techniques or analysis methods, such as CT perfusion or structured assessment of early hypodensity (such as ASPECTS24), might be as effective as MRI techniques for identifying optimal candidates for reperfusion therapies. Post-hoc analyses of the DEFUSE 2 data to explore the utility of CT criteria for patient selection will be reported separately.
Forty-nine patients (36%) of the total cohort had the non-Target Mismatch profile, including 26 patients who underwent endovascular therapy and an additional 23 patients who did not go onto endovascular therapy because of findings on the standard baseline MRI. This suggests that non-Target Mismatch patients account for an important percentage of all patients who are currently considered for endovascular therapy. The relatively small number of non-Target Mismatch patients that underwent endovascular therapy in DEFUSE 2 restricts our ability to precisely characterize the response to reperfusion in this subgroup. However, demonstration of a differential clinical and radiographic response following endovascular reperfusion in patients with the Target Mismatch profile reinforces the concept that advanced imaging techniques may improve patient selection for reperfusion therapies. These findings support the conduct of a randomized controlled trial of endovascular stroke therapy in Target Mismatch patients.
Research in Context
Systematic review
We searched Medline (1950 to July, 2012) with the search terms ((“stroke”[MeSH Terms] OR “stroke”[All Fields]) AND endovascular[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields]) AND (“magnetic resonance imaging”[MeSH Terms] OR (“magnetic”[All Fields] AND “resonance”[All Fields] AND “imaging”[All Fields]) OR “magnetic resonance imaging”[All Fields] OR “mri”[All Fields])) AND “selection”[All Fields]. Searches were restricted to human studies. All types of trial design were included. We also hand-searched relevant journals and the reference lists of included papers.
Interpretation
The DEFUSE 2 study shows that by using baseline MRI data in conjunction with an automated image analysis software program, physicians can categorize stroke patients into two distinct groups that respond differently to endovascular reperfusion: Target Mismatch patients have favorable clinical outcomes and reduced infarct growth whereas patients without Target Mismatch do not. These results are consistent with previous studies which established that pre-treatment MRI profiles are associated with a differential response to reperfusion following treatment with intravenous tPA25, 26 and support a randomized trial of endovascular therapy in Target Mismatch patients.
Acknowledgments
Funding: The study was funded by grants from the National Institute for Neurological Disorders and Stroke (NINDS). R01 NS03932505 (G. Albers), K23 NS051372 (M. Lansberg) and 1ZIANS003043 (S. Warach, Division of Intramural Research)
Appendix
List of investigators
Data and safety monitoring board: J. Saver, Los Angeles, CA (chair); P. Fayad; G. Howard; T. Tomsick
Clinical Centers: T. Jovin, L. Wechsler and S. DeCesare, University of Pittsburgh Medical Center, Pittsburgh, PA; G. Albers, M. Lansberg, S Kemp and D. Thai, Stanford Medical Center, Stanford, CA; M. Wilder, A. Tricot and A. Sherr, University of Utah Health Sciences Center, Salt Lake City, UT; H. Lutsep, Logan McDaneld and D. Larsen, Oregon Health & Science University, Portland, OR; T. Czartoski, B. Keogh, A.M. Malik and A. Brown, Swedish Neuroscience Institute, Seattle, WA; R. Bernstein and K. Muskovich, Northwestern Memorial Hospital, Chicago, IL; C. Chang and T. Stern, Queens Medical Center, Honolulu, HI; S. Warach and L. Davis, National Institutes of Health, Bethesda, MD; F. Fazekas and Thomas Seifert-Held, University Hospital Graz, Austria.
Core imaging lab: G.W. Albers (chair), G. Zaharchuk (measurement of 5-day FLAIR lesion volumes), M.P. Marks (assessment of catheter angiography results), A. Tipirneni and M. Mlynash (removal of artifacts from PWI and DWI lesions).
Footnotes
Authors’ contributions
The study was designed by Drs Albers, Bammer, Hamilton, Ms. Kemp, Drs Lansberg, Marks and Mlynash. Data were collected by Drs Albers, Lansberg, Wechsler, Jovin, Wilder, Lutsep, Czaroski, Bernstein, Chang, Warach, and Fazekas. Data were analyzed and interpreted by Drs Albers, Lansberg, Straka, Mlynash, Inoue, Tipirneni, Hamilton, Zaharchuk and Marks. The manuscript was drafted by Drs Albers and Lansberg and critical revisions were made by all authors.
Conflicts of Interest
G. Albers has received consulting fees and expenses from Lundbeck for Steering Committee work and consulting fees from Concentric for serving on a Data Safely and Monitory Board. G Albers and R Bammer are equity shareholders in iSchemaView. G. Zaharchuk is a member of the Neuroradiology Advisory Board for GE Healthcare, and receives modest research funding support from GE Healthcare. Helmi Lutsep has received consulting fees and expenses from Concentric Medical for serving on the Executive Committee of the TREVO2 trial, from Co-Axia for serving on the DSMB of the SENTIS trial and from AGA Medical for serving on the Neurology Executive Committee of the RESPECT trial. All other authors report no conflicts of interest.
References
- 1.Hirsch JA, Yoo AJ, Nogueira RG, Verduzco LA, Schwamm LH, Pryor JC, et al. Case volumes of intra-arterial and intravenous treatment of ischemic stroke in the USA. Journal of NeuroInterventional Surgery. 2009;1(1):27–31. doi: 10.1136/jnis.2009.000166. [DOI] [PubMed] [Google Scholar]
- 2.Hassan A, Chaudhry S, Suri F, Qureshi A. National Trends in Utilization and Outcomes of Endovascular Treatment in Acute Ischemic Stroke Patients. International Stroke Conference; 2012; New Orleans, Louisiana. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical Thrombectomy for Acute Ischemic Stroke: Final Results of the Multi MERCI Trial. Stroke. 2008;39(4):1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 4.The Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: Safety and Effectiveness of a New Generation of Mechanical Devices for Clot Removal in Intracranial Large Vessel Occlusive Disease. Stroke. 2009;40(8):2761–8. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 5.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and Efficacy of Mechanical Embolectomy in Acute Ischemic Stroke: Results of the MERCI Trial. Stroke. 2005;36(7):1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 6.Lansberg M, O’Donnell M, Khatri P, Lang E, Nguyen-Huynh M, Schwartz N, et al. Chest. 9. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis; p. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albers G, Goldstein L, Hess D, Wechsler L, Furie K, Gorelick P, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke; a journal of cerebral circulation. 2011;42:2645–95. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 8.Baker WL, Colby JA, Tongbram V, Talati R, Silverman IE, White CM, et al. Neurothrombectomy Devices for the Treatment of Acute Ischemic Stroke: State of the Evidence. Annals of Internal Medicine. 2011;154(4):243–52. doi: 10.7326/0003-4819-154-4-201102150-00306. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Fisher M. Improving the accuracy of perfusion imaging in acute ischemic stroke. Annals of Neurology. 2011;70(3):347–9. doi: 10.1002/ana.22524. [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke; a journal of cerebral circulation. 2011;42(1):93–7. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 11.Campbell B, Purushotham A, Christensen S, Desmond P, Nagakane Y, Parsons M, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:50–6. doi: 10.1038/jcbfm.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40(2):469–75. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Annals of Neurology. 2006;60(5):508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42(6):1608–14. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32(5):1024–37. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke; a journal of cerebral circulation. 2010;41(6):1169–74. doi: 10.1161/STROKEAHA.110.580670. [DOI] [PubMed] [Google Scholar]
- 17.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke; a journal of cerebral circulation. 2011;42(5):1270–5. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28(5):887–91. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) Jama. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 20.Furlan AJ. Ethics and Feasibility of Placebo-Controlled Interventional Acute Stroke Trials. Stroke. 2009;40(9):e533–e4. doi: 10.1161/STROKEAHA.109.553214. [DOI] [PubMed] [Google Scholar]
- 21.Furlan AJ. Clot Retrieval for Stroke Should Be Restricted to Clinical Trials. Stroke. 2010;41(1):194–5. doi: 10.1161/STROKEAHA.109.569913. [DOI] [PubMed] [Google Scholar]
- 22.Rohde S, Bösel J, Hacke W, Bendszus M. Stent retriever technology: concept, application and initial results. Journal of NeuroInterventional Surgery. 2011 doi: 10.1136/neurintsurg-2011-010160. [DOI] [PubMed] [Google Scholar]
- 23.Bang OY, Saver JL, Kim SJ, Kim G-M, Chung C-S, Ovbiagele B, et al. Collateral Flow Predicts Response to Endovascular Therapy for Acute Ischemic Stroke. Stroke. 2011;42(3):693–9. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670–4. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 25.Donnan GA, Baron J-C, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurology. 2009;8(3):261–9. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- 26.Yoo AJ, Pulli B, Gonzalez RG. Imaging-based treatment selection for intravenous and intra-arterial stroke therapies: a comprehensive review. Expert Review of Cardiovascular Therapy. 2011;9(7):857–76. doi: 10.1586/erc.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]