SUMMARY
A fundamental challenge facing physiological ecologists is to understand how variation in life history at the whole-organism level might be linked to cellular function. Thus, because tropical birds have higher annual survival and lower rates of metabolism, we hypothesized that cells from tropical species would have greater cellular resistance to chemical injury than cells from temperate species. We cultured dermal fibroblasts from 26 tropical and 26 temperate species of birds and examined cellular resistance to cadmium, H2O2, paraquat, thapsigargin, tunicamycium, methane methylsulfonate (MMS) and UV light. Using ANCOVA, we found that the values for the dose that killed 50% of cells (LD50) from tropical birds were significantly higher for H2O2 and MMS. When we tested for significance using a generalized least squares approach accounting for phylogenetic relationships among species to model LD50, we found that cells from tropical birds had greater tolerance for Cd, H2O2, paraquat, tunicamycin and MMS than cells from temperate birds. In contrast, tropical birds showed either lower or no difference in tolerance to thapsigargin and UV light in comparison with temperate birds. These findings are consistent with the idea that natural selection has uniquely fashioned cells of long-lived tropical bird species to be more resistant to forms of oxidative and non-oxidative stress than cells from shorter-lived temperate species.
KEY WORDS: tropical birds, temperate birds, life-history variation, cellular resistance
INTRODUCTION
Life-history theory posits that key events during an organism's life, such as the rate of juvenile development, age of first reproduction, number of offspring produced and the rate of senescence (Mitteldorf and Pepper, 2009; Longo et al., 2005), are shaped by natural selection to produce the largest possible number of surviving offspring (Stearns, 1992). Variation in life-history events is thought to reflect the differential allocation of resources, time and/or energy to competing life functions, largely growth, body maintenance and reproduction (Charnov, 1993; Ghalambor and Martin, 2001). Life-history variables are constrained within limited ecological space, lying along a ‘slow–fast’ life-history axis: animals that invest large quantities of resources in reproduction early in life generally die young, whereas animals that invest more resources in bodily maintenance tend to have fewer offspring and live longer (Saether, 1988; Promislow and Harvey, 1990; Ricklefs, 2000).
With low annual reproductive output and high annual survival rate, tropical birds lie at the slow end of the life-history continuum (Francis et al., 1998; Ricklefs, 1997; Brawn et al., 1998), whereas temperate birds tend to cluster more at the fast end of the spectrum, with large clutch sizes and high rates of mortality. For example, tropical house wrens (Troglodytes musculus) invest fewer resources in reproduction, and have lower basal and field metabolic rates and higher survival rates than temperate house wrens (Troglodytes aedon), consistent with the idea that tropical birds have a slow pace of life (Tieleman et al., 2006). Likewise, the yellow warbler (Dendroica petechia), whose range encompasses Alaska to Central America, one of the largest breeding distributions of any New World passerine, shows considerable variation in life history along its latitudinal gradient. Further, tropical mangrove warblers (Dendroica erythracoides), exhibited significantly smaller clutch sizes, longer incubation and nestling periods, and higher annual adult survival rates than temperate yellow warblers (Salgado-Ortiz et al., 2008).
Although it is thought that physiological processes underlie many life-history trade-offs (Ricklefs and Wikelski, 2002), the precise linkages between an organism's life history and the function of its organs, tissues and cells remain obscure (Stearns, 1992; Speakman, 2005; Williams et al., 2010). Evidence of a linkage between slow pace of life and low rate of metabolism in tropical birds came to light when it was shown that they had a significantly lower whole-animal basal metabolic rate (Wiersma et al., 2007a) and peak metabolic rate as measured by cold-exposure or by exercise (Wiersma et al., 2007b). Later it was discovered that a contributing factor to the reduced rate of metabolism in tropical birds was their smaller metabolically active organs, such as the heart, liver, kidneys and pectoral muscles, compared with similar-sized temperate species (Wiersma et al., 2012). These findings offer evidence of a connection between the life history of tropical birds and their physiology, at least at the organismal and organ levels. How the actual cells of tropical bird species might differ from temperate species as a result of their life history remains unknown.
A fundamental challenge facing physiological ecologists is an understanding of how variation in life history at the whole-organism level might be linked to cellular function (Williams et al., 2010). As a result of normal oxidative phosphorylation in the mitochondria, cells produce free-radicals such as O2•, and other reactive molecules such as H2O2 (Harman, 2001), which can attack DNA, proteins and lipids, causing impairment of function and ultimately cell death if the damage cannot be repaired. Over time, damage from these reactive oxygen species (ROS) is thought to be an important aspect of the aging process, encapsulated in the free-radical theory of aging (Huang and Manton, 2004; Fukagawa, 1999; Finkel and Holbrook, 2000; Golden et al., 2002; Dufour and Larsson, 2004). Some level of oxidants, particularly H2O2, is thought to be essential for cell survival because of their role in gene regulation, cell signaling and apoptosis (Sohal and Orr, 2012). However, cells also have a number of mechanisms to combat oxidative stress and scavenge free radicals, including antioxidants such as glutathione peroxidase, catalase and super oxide dismutase (Finkel and Holbrook, 2000; Sohal and Orr, 2012). It is thought that the production of potentially harmful ROS and the concentration of antioxidants exist in a balance, which is important in the aging process (Sohal and Orr, 2012).
A corollary of the free-radical theory of aging is the idea that the cells of long-lived animals should be constructed in such a way as to resist ROS better than cells from short-lived animals. To test this idea, Kapahi et al. (Kapahi et al., 1999) examined the resistance of primary fibroblasts of eight species of mammals, ranging in body mass from 0.1 to 450 kg, to chemicals that imposed oxidative and non-oxidative stress. Even with such a small sample size, they found that cells from large, long-lived mammals resisted chemical stress agents better than cells from small short-lived mammals, a finding later supported by results from skin-derived fibroblasts of both rodents (Harper et al., 2007) and birds (Harper et al., 2011). Furthermore, cells from renal epithelium isolated from long-lived birds have also been found to have higher resistance to oxidative stressors (Ogburn et al., 1998). Interpretation of the above studies on mammals is often confounded by the fact that large animals tend to live longer than small ones, and that large animals also have cells with a low mass-specific rate of metabolism, thereby influencing exposure to stress agents. In the present study, we overcome these two problems and address whether differences in life-history trade-offs influence cellular resistance to chemical insult, such as oxidative stress, in tropical and temperate birds of similar body sizes. Furthermore, we explicitly account for the phylogenetic relationships among bird taxa in our statistical models.
We hypothesized that primary cell lines derived from tropical species of birds would be more resistant to chemical stress agents than cell lines derived from their temperate counterparts. Using conventional and phylogenetically adjusted statistical analyses, we found that cells from 26 species of tropical birds were generally more resistant to multiple forms of oxidative and non-oxidative stress compared with 26 species of temperate birds. These results suggest that tropical birds with their slow pace of life have evolved cells with a chemical make-up that resists forms of chemical injury more effectively that cells from temperate birds.
MATERIALS AND METHODS
Collection of birds
Tropical birds were collected by mist net in the lowland forest around Gamboa, Panama (9.12°N, 79.72°W), and temperate birds were collected by mist net, or salvaged as dead by catch, in or around the state of Ohio, USA. All birds that we collected were adults of unknown age. All procedures were approved by the Institutional Animal Care and Use Committee of The Ohio State University (protocol IACUC2004A0093) and capture of birds in Panama was accomplished under permit from the Panamanian Autoridad Nacional del Ambiente (no. SEX/A-22-12) and Autoridad del Canal de Panamá. Tissues were exported from Panama under USDA permit 118465.
Establishment of cell lines
We evaluated primary cell lines from 26 temperate and 26 tropical bird species for their resistance to a variety of chemicals that induce cell injury or metabolic stress: Cd, H2O2, paraquat, thapsigargin, tunicamycium, methane methylsulfonate (MMS) and UV light. For all species, primary cell cultures were established from the skin of free-living adult birds. Immediately after birds were killed, their feathers were plucked and the exposed skin was washed with antimicrobial soap. We excised a 5×5 mm2 piece of skin and placed it into cold complete bird cell culture medium [Dulbecco's modified Eagle medium (DMEM), high-glucose variant (4.5 mg ml−1), with sodium pyruvate (110 mg l−1), supplemented with 10% heat-inactivated fetal bovine serum, 2% heat-inactivated chicken serum and antibiotics (100 U ml−1 pen/strep), containing 10 mmol l−1 HEPES].
We established primary fibroblast cell cultures after the skin was exposed to 1.5% Collagenase B solution overnight (Harper et al., 2007). Cells were grown in culture flasks at 37°C in an atmosphere of 3% O2 (Harper et al., 2011). When cells reached 90% confluence, they were trypsinized (0.25%) and passaged. Seventy-five percent of the medium was replaced with fresh complete medium after day 3 in all flasks, and we split cells into new 75 cm2 flasks at day 7–10. After cells grew for another 7 to 10 days, they were harvested and cryopreserved at 106 cells ml−1 in DMEM supplemented with 40% fetal bovine serum and DMSO at a final concentration of 10%. We stored cells in liquid N2 for up to 12 months prior to assessment of their resistance to stress agents. All stress tests were conducted using cells at passage 4.
Assessment of fibroblast resistance to lethal stress
We tested cellular resistance to multiple agents using one to four cell lines for a given species. We seeded 3×104 cells in 100 μl of complete media into each well of a 96-well microtiter plate, allowing cells to attach for 24 h, and then exposed cells to DMEM lacking serum and sodium pyruvate but containing 2% bovine serum albumin for an additional 24 h. This step was added because the presence of fetal calf serum alters resistance to chemical insult from 3 to 20-fold (Harper et al., 2007). Thereafter, we exposed cells to graded doses of Cd, H2O2, paraquat, MMS, tunicamycin or thapsigargin for 6 h, or to graded doses of UV radiation. Cells were then washed with phosphate-buffered saline, and incubated in serum-free medium for an additional 18 h. Cell survival was evaluated using conversion of the extracellular tetrazolium dye WST-1 to its colored formazan product; inspection of the plates revealed a large number of dead cells at the higher concentrations of stress agents. Reference cells, incubated without any stress agent and representing 100% viability, were included in each day's set of assays and for each given cell line. All tests were carried out at 37°C in a humidified incubator with 5% CO2 in 3% O2.
We evaluated cellular resistance to a variety of chemicals that induce cell injury or metabolic stress. H2O2 causes OH− radical formation in the presence of metal ions (Stohs and Bagchi, 1995), paraquat is a herbicide that induces O2• formation (Bus et al., 1976), thapsigargin inhibits Ca2+ pumping in the endoplasmic reticulum (Thastrup, 1990), tunicamycin is an antibiotic that interferes with protein processing in the endoplasmic reticulum (Elbein, 1987) and MMS is a DNA alkylating agent (Harper et al., 2007). The heavy metal Cd indirectly generates the superoxide radical and hydroxyl radical, although the mechanism remains unclear (Galán et al., 2001). Some experiments suggest that Cd generates in cells hydrogen peroxide which may be a significant source of radicals via Fenton chemistry (Valko et al., 2005), and UV light provokes DNA strands to break causing misreading and mis-replication of genes (Griffiths et al., 1998).
Calculation of LD50 values
The resistance of each cell line to chemical stressors was calculated for duplicate wells run in parallel on the sample plate, with one plate per stressor. The concentration needed to obtain 50% survival of the cells, the LD50, was calculated using the FORECAST function in Excel. Each of our statistical tests used mean LD50 values for a given measure as a representative for each species, even in cases when multiple cell lines were independently assessed.
Statistics
We used both conventional and phylogenetically controlled statistical analyses. First, to account for body mass effects on mean LD50, per species and environment, all LD50 data were analyzed using an analysis of covariance (ANCOVA) with mean LD50 as the dependent variable and body mass as the covariate. Second, to determine whether tropical birds had greater cellular resistance to chemical insults relative to cells from temperate birds, we modeled mean LD50 per species for each stressor treatment as a function of environment (tropical or temperate) using generalized least squares (gls function in the R package nlme), including body mass as a covariate.
To account for the evolutionary relationships among species, we compared our basic model against four models in which the structure of the error term incorporated the phylogeny of the species by adjusting the variance–covariance matrix, thus taking into account the non-independence of data points as a result of evolutionary history (Paradis, 2006; Rohlf, 2001). Covariances can be manipulated into different models of trait evolution, each of which incorporates varying degrees of phylogenetic signal. Many models assume trait evolution that follows Brownian motion, where differences in traits between species are proportional to time since divergence (supplementary material Table S1) (Felsenstein, 1985). For our analyses, we also used Pagel's, Martins' and Grafen's models. Pagel's model modifies covariances between species by multiplying by a constant, λ; when traits are phylogenetically uncorrelated, λ=0, and when there is strong phylogenetic signal within the data, λ=1. For the latter, evolution is assumed to follow Brownian motion. Martin's model incorporates stabilizing selection, where trait covariances decrease exponentially with increasing time since divergence and where the strength of the directional selective force is controlled by the parameter α (Martins and Hansen, 1997). This model allows trait evolution to vary between non-directional Brownian motion (α=0) and strong directional selection (α=1). Grafen's model incorporates a calculation of branch lengths based on number of descendants (Grafen, 1989). The tree is scaled so that the root has a depth of 1, and branch lengths are raised to the power ρ. When ρ=1, a strong phylogenetic signal is implied.
For all five models of evolution (one model assuming no evolutionary relationships and four others assuming a variety of hypotheses), we tested a suite of five nested candidate models using an information theoretic approach (Burnham and Anderson, 2002). Rather than using P-values to assign significance to each parameter in the model, this approach uses likelihood methods to determine the best model, given the data, from a suite of candidate models. Our five candidate models included a full interaction model (environment × body mass, assuming a different relationship between LD50 and body mass for tropical and temperate birds), an additive model (environment + body mass, assuming different intercepts but identical slopes for tropical and temperate birds), environment as a single predictor (thus assuming only different mean LD50 values between tropical and temperate birds), body mass as a single predictor (assuming no difference between tropical and temperate birds) and the simplest null model assuming no relationship of LD50 to body mass or environment. We used the corrected Akaike's information criterion (AICc; a modification of AIC for small sample sizes) to differentiate among the five models, the model with the lowest AICc value being the best model that fits the data. The AICc values also allow us to compare across models of evolutionary hypotheses and determine whether adding a phylogenetically modified variance–covariance matrix to the basic model improves our understanding of the data.
All analyses were performed on untransformed LD50 values in R version 2.12 (R Development Core Team, 2011) using the package ape v. 2.6 (Paradis et al., 2004) to calculate phylogenetic correlation structures, the package nlme v. 3.1 (Pinheiro et al., 2012) for generalized least squares analyses and the package AICcmodavg v.1.24 (Mazerolle, 2011) for calculating AICc.
Phylogenies
For all species in our data set, we used a species-level tree (supplementary material Fig. S1). We constructed tree branch lengths from Sibley and Ahlquist (Sibley and Ahlquist, 1990) and modified the tree accordingly to recent literature (Johnson and Sorenson, 1999; Klicka et al., 2000; Yuri and Mindell, 2002; Boyd, 2011). Trees were manipulated using Mesquite (Maddison and Maddison, 2010).
RESULTS
Body mass for our sample of 52 species of tropical and temperate birds ranged from 2.9 to 613.0 g, a 200-fold range (Table 1). There was no relationship between LD50 values and body mass in all but one chemical stressor (UV; see Table 2, Fig. 1). Body mass had little effect on the majority of our results.
Table 1.
Common names, species names and body masses for each species of temperate and tropical birds in this study
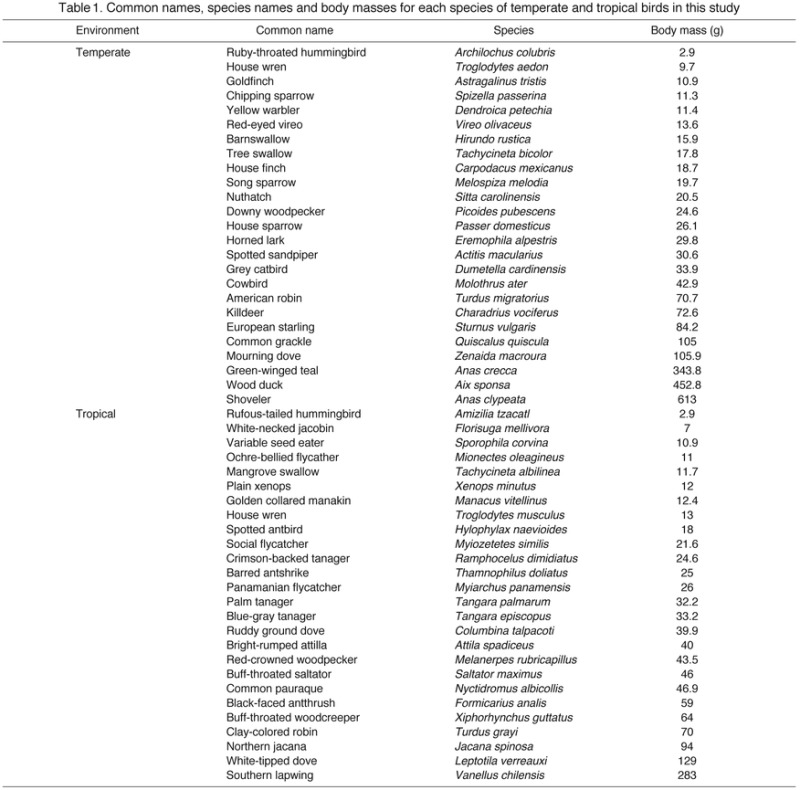
Table 2.
Results of phylogenetic generalized least squares models describing mean LD50 per chemical stressor treatment as a function of body mass and environment (tropical or temperate)
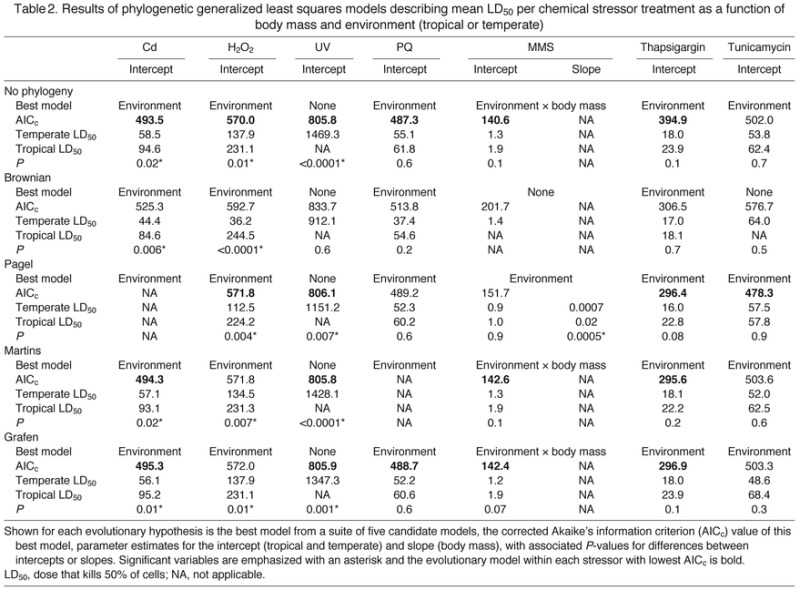
Fig. 1.
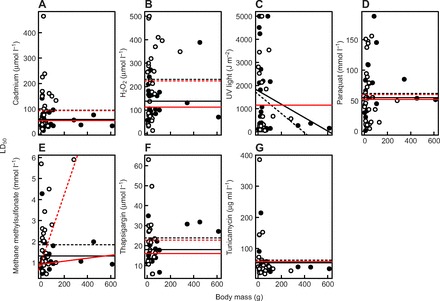
The association between body mass and mean LD50 (the dose that killed 50% of cells) for each species in tropical (○) and temperate (●) birds. The lines show the outcome of a least square regression, where the dashed line represents the regression for tropical birds and the solid line the regression for temperate birds. We show fitted lines for the basic non-phylogenetic model in black and those for a model including Pagel's evolutionary hypothesis in red.
LD50 values showed wide variation, ranging from 14.7 to 464.8 μmol l−1 for cadmium-treated cells, 13 to 500 μmol l−1 for peroxide, 57 to 5000 J m−2 for UV light, 1.2 to 188.5 mmol l−1 for paraquat, 0.46 to 5.90 mmol l−1 for MMS, 5.83 to 63.00 μmol l−1 for thapsigargin and 18 to 385 μg ml−1 for tunicamycin.
We found a significant effect of environment on mean LD50 values, with tropical birds having significantly higher values for a number of chemical stressors, although these results varied depending on the type of statistical analyses used. An ANCOVA, which takes into account body mass, revealed that LD50 for H2O2 (P=0.013) and MMS (P=0.01) were significantly higher in tropical birds.
Using generalized least squares, we selected the best model from a suite of five nested candidate models, ranging from a full model consisting of an interaction between environment (tropical versus temperate) and body mass, to the null model with no explanatory variables (supplementary material Table S1). Without accounting for phylogeny, the best model for six of the seven stressors (Cd, H2O2, paraquat, MMS, thapsigargin and tunicamycin) included only environment as a single predictor variable. In all of these six cases, tropical birds had higher LD50 estimates than temperate birds (Table 2, Fig. 1). When exposed to MMS, the null model was within two AICc units of the environment-only model, suggesting that environment played a weaker role than for the other stressors. When exposed to UV, the best model was the full interaction model, but here tropical birds had slightly lower LD50 estimates than temperate birds.
When we accounted for the phylogenetic relationships among species by modifying the variance–covariance matrix in four different ways, there was little qualitative difference to the results from the basic models that assumed complete independence among species, although specific coefficient estimates differed in most cases (Table 2, Fig. 1). For models with variance–covariance matrices modified according to Pagel's, Martins' and Grafen's evolutionary hypotheses, the results were qualitatively identical in all but two cases. Tropical birds had higher LD50 values than temperate birds in response to Cd, H2O2, paraquat, MMS, thapsigargin and tunicamycin. When exposed to UV, for all phylogenetic models, the best model was the null model. Models assuming a Brownian motion model of evolution were not considered because they performed very poorly, usually with AICc values over 20 units greater than all other candidate models, indicating a far worse fit than models with no evolutionary hypothesis. Thus, statistical models accounting for the evolutionary relationships among bird species concurred with models assuming no evolutionary relationships: cells of tropical birds had higher tolerance to chemical stressors than cells of temperate birds.
We should also note, however, that the AICc values of models incorporating phylogeny were never lower (i.e. better) than the model without phylogeny. In many cases, models accounting for phylogeny were within two AICc units (higher) of the best non-phylogeny model. Thus, accounting for the phylogenetic relationships among species did little to improve the fit of the models.
The particular species of tropical bird that had higher tolerance to chemical stressors varied with treatment, but some species uniformly had high tolerance to oxidative stress. The white-tipped dove, jacana, clay colored robin and mangrove swallow were the most tolerant when exposed to Cd or H2O2. When exposed to MMS and H2O2, the clay colored robin and the jacana were still two of the most tolerant species for this treatment.
DISCUSSION
We hypothesized that because tropical birds live a slow pace of life, their cells would be more resistant to chemical stress agents than cells from their temperate equivalents. Our data indicate that cells from tropical species have a higher LD50 after exposure to several forms of oxidative and non-oxidative stress. Thus, our study offers compelling evidence that the life history of a species influences not only whole-organism physiology, but also cellular attributes. Cells from tropical birds were more resistant to chemical injury than were cells from temperate birds.
The free radical theory of aging proposed by Harman (Harman, 1956) states that metabolic rate and longevity are linked by the amount of free radicals produced during aerobic respiration. Aging results from the accumulation of biological damage caused by the production of free radicals (Harman, 2001). To deal with these stresses, cells possess a network of defenses that include antioxidants, ion transporters and metal chelators, as well as repair systems for damaged cellular macromolecules such as DNA, lipids and proteins (Pacifici and Davies, 1991). One might suspect that the higher the rate of metabolism, the more free-radical production, but the production of free radicals in mitochondria is more complicated (Ricklefs and Wikelski, 2002; Harman, 2001; Barja, 2007). Birds have higher metabolic rates and longer lifespans, but have lower rates of ROS generation, than similarly sized mammals (Barja, 2007; Strecker et al., 2010). Thus, birds provide a unique study system to address how ROS production affects aging and cellular stress resistance processes (Pamploma et al., 1999).
Birds accumulate less ROS-induced damage than mammals, which, according to the free radical theory of aging, should lead to a longer lifespan (Ogburn et al., 1998; Harman, 2001; Strecker et al., 2010). However, how ROS production and the accumulation of damage to macromolecules are related to life-history differences in tropical and temperate birds remains unknown. ROS production by mitochondria is largely dependent on respiration rate, such that highly active mitochondria have high concentrations of ADP and high rates of ATP synthesis, which results in a lower potential across the inner mitochondrial membrane (IMM); and when the potential across the IMM is low, almost no ROS are produced (Chance et al., 1979; Barja, 2007). In contrast, and controlling for the number of mitochondria, when cellular metabolism is low, the high potential across the IMM results in moderate ROS production (Chance et al., 1979; Cadenas and Boveris, 1980; Van Voorhies, 2004).
Tropical birds have a significantly lower basal and peak metabolic rate than temperate birds (Wiersma et al., 2007a; Wiersma et al., 2007b), which may translate into a higher production of damaging free radicals, because ROS production is highest when respiration rate is low. However, because tropical birds have high rates of survival (Ricklefs, 1997; Tieleman et al., 2006), they may have evolved efficient cellular mechanisms to regulate ROS-induced damage. By extension, the increased production of ROS in tropical birds should lead to increased oxidative stress defense mechanisms. Montgomery et al. found that the lifespan of birds was positively correlated with the antioxidants glutathione peroxidases and glutathione-S-transferases (Montgomery et al., 2012). These high antioxidant activities were hypothesized to protect against the production of hydroxyl radicals from hydrogen peroxide via the Fenton reaction. Thus, long-lived bird species, such as tropical birds, may utilize this mechanism to guard against the production of hydroxyl radicals, or it may be an adaptive response to inherently high levels of oxidative stress associated with high production rates of hydrogen peroxide and lipid hydroperoxides (Montgomery et al., 2012).
The fact that temperate birds have a higher basal metabolism than do tropical birds may suggest that the former have higher ROS production by their mitochondria (Perez-Campo et al., 1998); the prevailing view is that ROS production is higher in short-lived species (such as temperate birds) and that these species should show high levels of tissue antioxidants in order to combat their high rates of ROS production (Barja et al., 1994; Herrero and Barja, 1997; Lopez-Torres et al., 1993; Perez-Campo et al., 1994). At least in birds, it has recently been shown that the antioxidant capacity has a complex pattern relative to longevity (Cohen et al., 2008), whereby higher antioxidant levels were generally characteristic of ‘fast-paced’ life-history traits such as rapid development, low survival, smaller body size, larger clutch size and higher mass-specific metabolic rate. Thus, convincing a priori predictions about how cells of tropical and temperate birds might resist oxidative and non-oxidative stress are difficult to make.
ROS production and antioxidant defenses alone are not the only factors that could alter oxidative stress resistance and lifespan. The level of susceptibility of lipids in membranes to oxidative damage may also be important. The ‘membrane pacemaker theory of aging’ suggests that phospholipids vary in their susceptibility to peroxidation, leading to the production of secondary lipid-based ROS (Hulbert et al., 2007). A possible physiological mechanism that would allow birds to have high metabolic rates and long lifespans is a decrease in the number of double bonds in membrane phospholipids. Unsaturated fatty acids are sensitive to free radical damage, thus low double bond content should be advantageous by decreasing the sensitivity of tissues to lipid peroxidation; this would be crucial in mitochondrial membranes as they are the major source of ROS (Pamplona et al., 1999). Pamplona et al. noted that the degree of unsaturation of fatty acids and the sensitivity of lipid peroxidation of liver and heart mitochondria are lower in the long-lived pigeon than in the short-lived rat, although they have similar metabolic rates (Pamplona et al., 1996; Pamplona et al., 1999). The susceptibility of fatty acids to free radical damage increases exponentially as a function of the number of double bonds per fatty acid molecule. Also, lipid peroxidation products are known to damage nearby macromolecules including proteins, a process that has consequences for aging (Pamplona et al., 1999). These ideas suggest that tropical birds have a low degree of fatty acid unsaturation in their membranes, which might protect them from oxidative damage (Pamplona et al., 1999; Montgomery et al., 2011).
In summary, we found that primary cell lines derived from tropical birds are more resistant to some cellular stressors compared with cells from their temperate counterparts. The physiological mechanism that allows tropical birds to be more resistant to these stressors is unclear; however, an increased resistance to ROS damage seems to be an important pathway to allow these birds the capacity to occupy the ‘slow pace of life’ part of the spectrum.
Supplementary Material
ACKNOWLEDGEMENTS
Our sincere appreciation to Dr Popko Wiersma and Alex Champagne for sharing their phylogenetic expertise, and Dr John Klicka for his feedback on our phylogenetic tree. John Simpson and staff from Winous Point Duck Club graciously provided skin samples from ducks. Also thanks to Dr Jim Van Brocklyn for his critical evaluation of the manuscript. We would also like to thank Dr Raineldo Urriola and the Autoridad Nacional del Ambiente for permission to collect birds in Panama, and the Smithsonian Tropical Research Institute for hosting us.
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/216/8/1373/DC1
COMPETING INTERESTS
No competing interests declared.
FUNDING
This study was funded by the National Science Foundation [IBN 0212587 to J.B.W.], the Comparative Biology Core of the Nathan Shock Center, University of Michigan [Parent Grant AG013283], and a Smithsonian Tropical Research Institute post-doctoral fellowship [to A.G.J.].
REFERENCES
- Barja G. (2007). Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 10, 215-224 [DOI] [PubMed] [Google Scholar]
- Barja G., Cadenas S., Rojas C., López-Torres M., Pérez-Campo R. (1994). A decrease of free radical production near critical targets as a cause of maximum longevity in animals. Comp. Biochem. Physiol. 108B, 501-512 [DOI] [PubMed] [Google Scholar]
- Boyd J. H. (2011). Aves – a taxonomy in flux. Version 2.5. Available at http://jboyd.net/Taxo/taxo1.html
- Brawn J. D., Karr J. R., Nichols J. D., Robinson W. D. (1998). Demography of tropical forest birds in Panama: how do transients affect estimates of survival rates? In Proceedings 22nd International Ornithological Congress (ed. Adams N. J., Slotow R. H.), pp. 297-305 Johannesburg: BirdLife South Africa; [Google Scholar]
- Burnham K. P., Anderson D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-theoretic Approach. New York: Springer; [Google Scholar]
- Bus J. S., Cagen S. Z., Olgaard M., Gibson J. E. (1976). A mechanism of paraquat toxicity in mice and rats. Toxicol. Appl. Pharmacol. 35, 501-513 [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A. (1980). Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem. J. 188, 31-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. (1979). Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527-605 [DOI] [PubMed] [Google Scholar]
- Charnov E. L. (1993). Life History Invariants. Oxford: Oxford University Press; [Google Scholar]
- Cohen A. A., McGraw K. J., Wiersma P., Williams J. B., Robinson W. D., Robinson T. R., Brawn J. D., Ricklefs R. E. (2008). Interspecific associations between circulating antioxidant levels and life-history variation in birds. Am. Nat. 172, 178-193 [DOI] [PubMed] [Google Scholar]
- Dufour E., Larsson N. G. (2004). Understanding aging, revealing order out of chaos. Biochim. Biophys. Acta. 1658, 122–132 [DOI] [PubMed] [Google Scholar]
- Elbein A. D. (1987). Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu. Rev. Biochem. 56, 497-534 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Phylogenies and the comparative method. Am. Nat. 125, 1-15 [Google Scholar]
- Finkel T., Holbrook N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408, 239-247 [DOI] [PubMed] [Google Scholar]
- Francis C. M., Terborgh J. S., Fitzpatrick J. W. (1998). Survival rates of understorey forest birds in Peru. In Proceedings 22nd International Ornithological Congress (ed. Adams N. J., Slotow R. H.), pp. 326-335 Johannesburg: BirdLife South Africa; [Google Scholar]
- Fukagawa N. K. (1999). Aging: is oxidative stress a marker or is it causal? Proc. Soc. Exp. Biol. Med. 222, 293-298 [DOI] [PubMed] [Google Scholar]
- Galán A., Troyano A., Vilaboa N. E., Fernández C., de Blas E., Aller P. (2001). Modulation of the stress response during apoptosis and necrosis induction in cadmium-treated U-937 human promonocytic cells. Biochim. Biophys. Acta 1538, 38-46 [DOI] [PubMed] [Google Scholar]
- Ghalambor C. K., Martin T. E. (2001). Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494-497 [DOI] [PubMed] [Google Scholar]
- Golden T. R., Hinerfeld D. A., Melov S. (2002). Oxidative stress and aging: beyond correlation. Aging Cell 1, 117-123 [DOI] [PubMed] [Google Scholar]
- Grafen A. (1989). The phylogenetic regression. Philos. Trans. R. Soc. B 326, 119-157 [DOI] [PubMed] [Google Scholar]
- Griffiths H. R., Mistry P., Herbert K. E., Lunec J. (1998). Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit. Rev. Clin. Lab. Sci. 35, 189-237 [DOI] [PubMed] [Google Scholar]
- Harman D. (1956). Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298-300 [DOI] [PubMed] [Google Scholar]
- Harman D. (2001). Aging: overview. Ann. N. Y. Acad. Sci. 928, 1-21 [DOI] [PubMed] [Google Scholar]
- Harper J. M., Salmon A. B., Leiser S. F., Galecki A. T., Miller R. A. (2007). Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell 6, 1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. M., Wang M., Galecki A. T., Ro J., Williams J. B., Miller R. A. (2011). Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J. Exp. Biol. 214, 1902-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A., Barja G. (1997). Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech. Ageing Dev. 98, 95-111 [DOI] [PubMed] [Google Scholar]
- Huang H., Manton K. G. (2004). The role of oxidative damage in mitochondria during aging: a review. Front. Biosci. 9, 1100-1117 [DOI] [PubMed] [Google Scholar]
- Hulbert A. J., Pamplona R., Buffenstein R., Buttemer W. A. (2007). Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175-1213 [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Sorenson M. D. (1999). Phylogeny and biogeography of dabbling ducks (Genus: Anas): a comparison of molecular and morphological evidence. Auk 116, 792-805 [Google Scholar]
- Kapahi P., Boulton M. E., Kirkwood T. B. (1999). Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med. 26, 495-500 [DOI] [PubMed] [Google Scholar]
- Klicka J., Johnson K. P., Lanyon S. M. (2000). New world nine-primaried oscine relationships: constructing a mitochondrial DNA framework. Auk 117, 321-336 [Google Scholar]
- Longo V. D., Mitteldorf J., Skulachev V. P. (2005). Programmed and altruistic ageing. Nat. Rev. Genet. 6, 866-872 [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M., Perez-Campo R., Rojas C., Cadenas S., Barja G. (1993). Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech. Ageing Dev. 70, 177-199 [DOI] [PubMed] [Google Scholar]
- Maddison W. P., Maddison D. R. (2010). Mesquite: a modular system for evolutionary analysis. Available at http://mesquiteproject.org
- Martins E. P., Hansen T. F. (1997). Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646-667 [Google Scholar]
- Mazerolle M. J. (2011). AICcmodavg: model selection and multimodel inference based on (Q)AIC©. R package version 1.17 ed2011 Available at http://CRAN.R-project.org/package=AICcmodavg
- Mitteldorf J., Pepper J. (2009). Senescence as an adaptation to limit the spread of disease. J. Theor. Biol. 260, 186-195 [DOI] [PubMed] [Google Scholar]
- Montgomery M. K., Hulbert A. J., Buttemer W. A. (2011). The long life of birds: the rat-pigeon comparison revisited. PLoS ONE 6, e24138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery M. K., Buttemer W. A., Hulbert A. J. (2012). Does the oxidative stress theory of aging explain longevity differences in birds? II. Antioxidant systems and oxidative damage. Exp. Gerontol. 47, 211-222 [DOI] [PubMed] [Google Scholar]
- Ogburn C. E., Austad S. N., Holmes D. J., Kiklevich J. V., Gollahon K., Rabinovitch P. S., Martin G. M. (1998). Cultured renal epithelial cells from birds and mice: enhanced resistance of avian cells to oxidative stress and DNA damage. J. Gerontol. 53, B287-B292 [DOI] [PubMed] [Google Scholar]
- Pacifici R. E., Davies K. J. A. (1991). Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology 37, 166-180 [DOI] [PubMed] [Google Scholar]
- Pamplona R., Prat J., Cadenas S., Rojas C., Pérez-Campo R., López Torres M., Barja G. (1996). Low fatty acid unsaturation protects against lipid peroxidation in liver mitochondria from long-lived species: the pigeon and human case. Mech. Ageing Dev. 86, 53-66 [DOI] [PubMed] [Google Scholar]
- Pamplona R., Portero-Otin M., Requena J.R., Thorpe S.R., Herrero A., Barja G. (1999). A low degree of fatty acid unsaturation leads to lower lipid peroxidation and lipoxidation-derived protein modification in heart mitochondria of the longevous pigeon than in the short-lives rat. Mech. Ageing Dev. 106, 283-296 [DOI] [PubMed] [Google Scholar]
- Paradis E. (2006). Analysis of Phylogenetics and Evolution with R. New York: Springer Science and Business Media; [Google Scholar]
- Paradis E., Claude J., Strimmer K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289-290 [DOI] [PubMed] [Google Scholar]
- Perez-Campo R., Lopez-Torres M., Rojas C., Cadenas S., Barja G. (1994). Longevity and antioxidant enzymes, non-enzymatic antioxidants and oxidative stress in the vertebrate lung: a comparative study J. Comp. Physiol. B 163, 682-689 [DOI] [PubMed] [Google Scholar]
- Perez-Campo R., López-Torres M., Cadenas S., Rojas C., Barja G. (1998). The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J. Comp. Physiol. B 168, 149-158 [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., R Development Core Team (2012). Nlme: Linear and nonlinear mixed effects models. R package version 3.1-103. Available at http://cran.rproject.org/web/packages/nlme/index.html
- Promislow D. E. L., Harvey P. H. (1990). Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 220, 417-437 [Google Scholar]
- R Development Core Team (2011). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Ricklefs R. E. (1997). Comparative demography of New World populations of thrushes (Turdus spp.). Ecol. Monogr. 67, 23-43 [Google Scholar]
- Ricklefs R. E. (2000). Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 102, 9-22 [Google Scholar]
- Ricklefs R. E., Wikelski M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462-468 [Google Scholar]
- Rohlf F. J. (2001). Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution 55, 2143-2160 [DOI] [PubMed] [Google Scholar]
- Saether B. E. (1988). Pattern of covariation between life-history traits of European birds. Nature 331, 616-617 [DOI] [PubMed] [Google Scholar]
- Salgado-Ortiz J., Marra P. P., Sillett T. S., Robertson R. J. (2008). Breeding ecology of the mangrove warbler (Dendroica petechia bryanti) and comparative life history of the yellow warbler subspecies complex. Auk 125, 402-410 [Google Scholar]
- Sibley C. G., Ahlquist J. E. (1990). Phylogeny and Classification of Birds. New Haven, CT: Yale University Press; [Google Scholar]
- Sohal R. S., Orr W. C. (2012). The redox stress hypothesis of aging. Free Radic. Biol. Med. 52, 539-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. R. (2005). Body size, energy metabolism and lifespan. J. Exp. Biol. 208, 1717-1730 [DOI] [PubMed] [Google Scholar]
- Stearns S. C. (1992). The Evolution of Life Histories. Oxford: Oxford University Press; [Google Scholar]
- Stohs S. J., Bagchi D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18, 321-336 [DOI] [PubMed] [Google Scholar]
- Strecker V., Mai S., Muster B., Beneke S., Bürkle A., Bereiter-Hahn J., Jendrach M. (2010). Aging of different avian cultured cells: lack of ROS-induced damage and quality control mechanisms. Mech. Ageing Dev. 131, 48-59 [DOI] [PubMed] [Google Scholar]
- Thastrup O. (1990). Role of Ca2+-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2+-ATPase inhibitor, thapsigargin. Agents Actions 29, 8-15 [DOI] [PubMed] [Google Scholar]
- Tieleman B. I., Dijkstra T. H., Lasky J. R., Mauck R. A., Visser G. H., Williams J. B. (2006). Physiological and behavioural correlates of life-history variation: a comparison between tropical and temperate zone house wrens. Funct. Ecol. 20, 491-499 [Google Scholar]
- Valko M., Morris H., Cronin M. T. D. (2005). Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161-1208 [DOI] [PubMed] [Google Scholar]
- Van Voorhies W. A. (2004). Live fast – live long? A commentary on a recent paper by Speakman et al. Aging Cell 3, 327-330 [DOI] [PubMed] [Google Scholar]
- Wiersma P., Chappell M. A., Williams J. B. (2007a). Cold- and exercise-induced peak metabolic rates in tropical birds. Proc. Natl. Acad. Sci. USA 104, 20866-20871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma P., Muñoz-Garcia A., Walker A., Williams J. B. (2007b). Tropical birds have a slow pace of life. Proc. Natl. Acad. Sci. USA 104, 9340-9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma P., Nowak B., Williams J. B. (2012). Small organ size contributes to the slow pace of life in tropical birds. J. Exp. Biol. 215, 1662-1669 [DOI] [PubMed] [Google Scholar]
- Williams J. B., Miller R. A., Harper J. M., Wiersma P. (2010). Functional linkages for the pace of life, life-history, and environment in birds. Integr. Comp. Biol. 50, 855-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuri T., Mindell D. P. (2002). Molecular phylogenetic analysis of Fringillidae, ‘New World nine-primaried oscines’ (Aves: Passeriformes). Mol. Phylogenet. Evol. 23, 229-243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


