Abstract
Inflammasomes continue to generate interest in an increasing number of disciplines owing to their unique ability to integrate a myriad of signals from pathogen- and damage-associated molecular patterns into a proinflammatory response. This potent caspase-1–dependent process is capable of activating the innate immune system, initiating pyroptosis (an inflammatory form of programmed cell death), and shaping adaptive immunity. The NLRP3 inflammasome is the most thoroughly studied of the inflammasome complexes that have been described thus far, perhaps owing to its disparate assortment of agonists. This review will highlight our current understanding of the mechanisms of both priming and activation of the NLRP3 inflammasome.
Keywords: NLRP3, inflammasome, caspase-1, mitochondria, calcium
Introduction
The maintenance of homeostasis is a primary function of the innate immune system, achieved in part through immune surveillance by germline-encoded pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding domain leucine-rich repeat–containing receptors (NLRs), predominantly expressed by cells of the myeloid lineage. Through the prompt detection of markers of stress, damage, and infection, PRRs serve as an early warning system to initiate an immune response in order to induce the return to the steady state. The cytosolic NLR family of PRRs functions to sense and respond to threats in the intracellular compartment. NLR members are classified based on their shared three-domain structural homology. The N-terminal effector domain can include a pyrin domain (PYD), a caspase-recruitment domain (CARD), or a baculovirus inhibitory-repeat (BIR) domain, and is the basis by which NLRs may be further subcategorized. The central nucleotide-binding oligomerization domain (NACHT) is a common feature of all NLR proteins, followed by a leucine-rich repeat domain (LRR) at the C-terminus.1
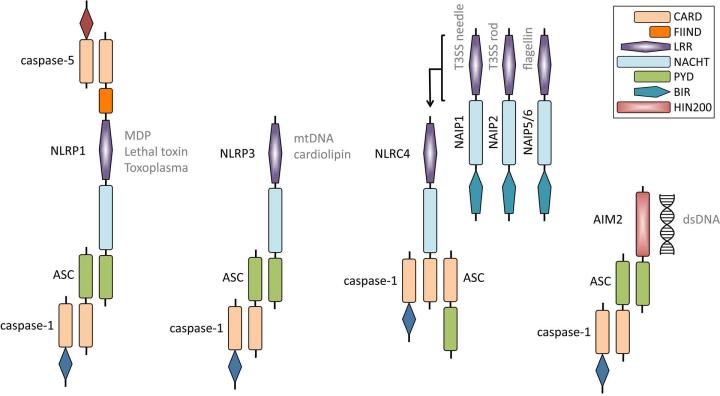
Three of the 22 NLR members described in humans (NLRP1, NLRP3, and NLRC4),2–10 along with the PYHIN family member AIM2 (absent in melanoma 2),11–14 can be induced to associate with other proteins in large multimeric complexes termed an inflammasome (Fig. 1) . Consisting of an NLR or PYHIN, in some cases the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD domain), and the cysteine protease procaspase-1, inflammasome formation ultimately results in the autocatalysis and activation of caspase-1.5,15 Once enzymatically active, caspase-1 can go on to process the cytokines pro-IL-1β and pro-IL-18 into their mature secreted forms.16,17 These markedly pro-inflammatory cytokines initiate an inflammatory cascade that leads to the recruitment of innate immune cells and can also determine the character of the subsequent adaptive immune response.18 Inflammasome-mediated caspase-1 activity has also been shown to result in a proinflammatory form of programmed cell death known as pyroptosis.19 Reports describing the potential for additional innate immune sensing molecules such as NLRP6, NLRP7, RIG-I, Pyrin, and IFI16 to form inflammasome complexes exist but require further characterization.20–24
Figure 1.
Schematic of NLRP1, NLRP3, NLRC4, and AIM2 inflammasomes. Human NLRP1 can interact with ASC and caspase-1 via an N-terminal PYD and also bind caspase-5 to the complex via the C-terminal CARD. Muramyl dipeptide (MDP), Bacillus anthracis lethal toxin, and Toxoplasma gondii can induce the activation of the NLRP1 inflammasome. Mouse Nlrp1b does not possess a functional N-terminal PYD, hence caspase-1 may interact with its C-terminal CARD. NLRP3 interacts with ASC through an N-terminal PYD domain, which then recruits caspase-1. Mitochondrial DNA (mtDNA) and cardiolipin have been postulated to bind to NLRP3 and induce its activation. The NLRC4 inflammasome is activated by the intermediary molecules NAIP1, NAIP2, and NAIP5/6, which have been shown to bind to the type three secretion system (T3SS) needle and rod proteins and bacterial flagellin, respectively. It remains unclear how ASC interacts with the NLRC4 inflammasome complex. The AIM2 inflammasome can directly bind double-stranded DNA (dsDNA) via its HIN200 domain. AIM2 recruits ASC and caspase-1 through its N-terminal PYD domain. CARD, caspase recruitment domain; FIIND, domain with function to find; NACHT, nucleotide-binding and oligomerization domain; PYD, pyrin domain; LRR, leucine-rich repeats; BIR, baculovirus IAP repeat domain; HIN200; HIN-200 domain.
NLRP3 inflammasome
The NLRP3 inflammasome is the most extensively investigated of the inflammasomes identified, yet many questions remain to be addressed conclusively. The NLRP3 protein (also referred to as cryopyrin and NALP3) was first identified due to gain-of-function mutations in the encoding gene that are associated with the autoinflammatory cryopyrin-associated periodic syndromes (CAPS).25–27 In addition to the central NACHT and C-terminal LRR, NLRP3 is characterized by its N-terminal PYD, which allows NLRP3 to recruit the adaptor molecule ASC through PYD– PYD interactions, thus facilitating the recruitment of procaspase-1 to form the inflammasome complex.6,28 In contrast to the other known inflammasomes, activation of the NLRP3 inflammasome can be achieved by a wide range of structurally dissimilar agonists, including pathogens, pore-forming toxins, environmental irritants, and endogenous damage-associated molecular patterns (DAMPs) (reviewed in Ref. 29). The diversity of molecules capable of triggering NLRP3 inflammasome formation seems to preclude a scenario in which direct binding of these ligands by NLRP3 is plausible, thus confounding efforts to reach a consensus regarding the actual mechanism of inflammasome activation. However, a two-step model in which both priming and activating signals are required to produce a functional inflammasome is generally accepted. Herein, we focus on recent advances in our understanding of the mechanisms of NLRP3 inflammasome priming and activation.
Signal 1: priming
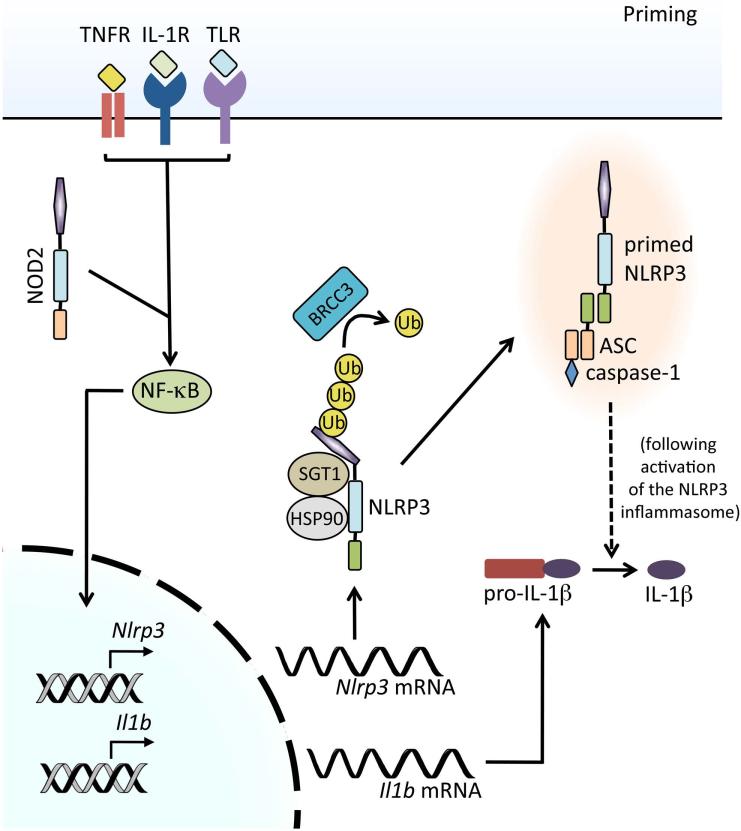
Considering the potency of the products of inflammasome activation, the requirement for two signals to achieve activation represents an important regulatory checkpoint to avoid specious immune responses capable of harming the host. Indeed, the consequences of such aberrant inflammasome activation can be seen in patients with NLRP3 inflammasome–mediated autoinflammatory disorders. The initial inflammasome priming step upstream of activation affects NLRP3 at the transcriptional level and also serves to trigger posttranslational modifications of inflammasome components that allow for oligomerization (Fig. 2). Paramount is the preparation of NLRP3 that receipt of inflammasome-activating signals in the absence of priming results in either a response of a nominal magnitude or the complete failure to generate an inflammasome.
Figure 2.
Signals mediating NLRP3 inflammasome priming. Upon engagement, pattern recognition receptors (PRR), such as TLR4 and NOD2, or cytokine receptors, such as TNFR and IL-1R, activate NF-κB, leading to the transcription and translation of NLRP3 and pro-IL-1β. Dissociation of HSP90 and SGT1 from NLRP3 is required for NLRP3 inflammasome activation. Additionally, NLRP3 undergoes deubiquitylation by the JAMM domain–containing Zn2+ metalloprotease deubiquitinating enzyme BRCC3, which is crucial for subsequent NLRP3 inflammasome activation. Upon activation of the NLRP3 inflammasome (see Fig. 4), active caspase-1 can process pro-IL-1β (and pro-IL-18) into their mature secreted forms.
In general, priming stimuli can include any whose receptor signaling results in the activation of the transcription factor NF-κB, such as ligands for IL-1R1, TLRs, NLRs, and the cytokine receptors TNFR1 and TNFR2.30,31 The activation of NF-κB is critical for upregulating the transcription of both pro-IL-1β and NLRP3, as pro-IL-1β is not constitutively expressed and basal levels of NLRP3 are inadequate for efficient inflammasome formation. In contrast, transcriptional modulation is not required to license the inflammasome components, ASC and procaspase-1, for inflammasome activation, nor the caspase-1 substrate pro-IL-18, as these are found at adequate concentrations in the steady state.30–32 Although NLRP3 inflammasome activation is possible if priming and activation signals are provided simultaneously, the kinetics and extent of inflammasome activation are greatly enhanced with increased availability of NLRP3 and pro-IL-1β.33,34 It is important to note that the threshold of inflammasome activation can differ depending on the cell type and NLRP3 agonist used, for example dendritic cells appear to have a lower threshold for inflammasome activation compared to macrophages. Unsurprisingly, the signaling events between PRR engagement and NF-κB activation are more complex than initially appreciated. Signaling immediately downstream of the IL-1R family proceeds through either MyD88 or TRIF, followed by certain IL-1R–associated kinase (IRAK) family members, and studies using mice deficient in different combinations of these molecules have demonstrated a role for both MyD88 and TRIF in priming; however, it is increasingly evident that their mechanisms of priming differ. That is, MyD88, along with IRAK1 and IRAK4, is suggested to be responsible for NF-κB–dependent transcriptional priming, whereas TRIF and IRAK1 have been implicated in a form of transcription-independent priming.30,35,36
The mechanistic description of an additional priming event that relies on PRR signaling but is independent of de novo protein synthesis stemmed from the observation that NLRP3 inflammasome activation could be achieved after just 10 min of priming, as opposed to the 2-h priming step needed to observe upregulated NLRP3 expression.34 Such comparatively dynamic priming was found to act on the posttranslational modifications of inflammasome components that function as an additional layer of control. Specifically, free cytosolic NLRP3 is maintained in an inactive ubiquitinated state incapable of oligomerization until priming signals stimulate the deubiquitination of its LRR domain, which is mediated by the deubiquitinase BRCC3.34,37 Interference with the deubiquitination enzyme by knockdown or the use of small-molecule inhibitors blocks inflammasome activation, which both underscores the critical requirement for this priming pathway and further distinguishes it from transcriptional priming, which remains intact.34,37,38 Although the involvement of mitochondrial reactive oxygen species (ROS) in the priming versus activation of the NLRP3 inflammasome continues to be a matter of debate,39 there is evidence to suggest that priming via NLRP3 deubiquitination, but not transcription-dependent priming, may be antioxidant sensitive.34 Interestingly, regulation of inflammasome assembly by deubiquitination is not unique to NLRP3, as deubiquitinases also govern ASC oligomerization, which represents a checkpoint common to both the NLRP3 and AIM2 inflammasomes38 and makes this priming mechanism a highly attractive target for therapeutic intervention. Between transcriptional upregulation and posttranslational modifications of inflammasome components, PRR signal-dependent priming establishes a high threshold that must be reached to achieve activation of the NLRP3 inflammasome.
There also exist a collection of lesser-known molecules whose functions are important for the maintenance of the priming threshold and without which NLRP3 inflammasome activation cannot occur. Two such proteins include the molecular chaperone heat shock protein 90 (HSP90) and its co-chaperone, the ubiquitin ligase–associated protein SGT1. These highly evolutionarily conserved molecules bind to one another and associate with the NACHT and LRR domains of free NLRP3 to aid in the stability of the mature protein, effectively sustaining it in an inactive yet signal-receptive state. HSP90 and SGT1 are then thought to dissociate from NLRP3 under priming conditions to allow for inflammasome oligomerization.40 Of note, inhibition of HSP90 affects the stability of both free NLRP3 and pro-IL-1β proteins, as well as interferes with inflammasome activity, whereas knockdown of SGT1 only affects inflammasome activity, implying separate functions for these cooperative molecules.
The cellular inhibitors of apoptosis proteins (cIAPs) are another group of molecules that control priming of the NLRP3 inflammasome. Typically thought of in terms of programmed cell death, cIAP1 and cIAP2 have also been shown to play a role in the inflammasome pathway by acting on caspase-1. Working in a preformed complex, cIAP1 and cIAP2, together with the adaptor molecule TRAF2, bind and exert their E3 ubiquitin ligase activity on caspase-1, resulting in the non-degradative polyubiquitination of caspase-1. Although the exact significance of this modification is unclear, it is speculated to either aid in the recruitment and oligomerization of the other NLRP3 inflammasome components or to facilitate the conformational changes necessary for optimal catalytic activity of caspase-1.41 The latter scenario is supported by the fact that while the presence of cIAPs greatly enhances caspase-1 catalysis, their absence does not completely abrogate inflammasome function.41 Indeed, each step of the NLRP3 inflammasome pathway is so highly regulated that fresh insights on its molecular orchestration continue to emerge, often involving previously unknown roles of well-described cellular constituents.
Inhibitors of NLRP3 inflammasome activation
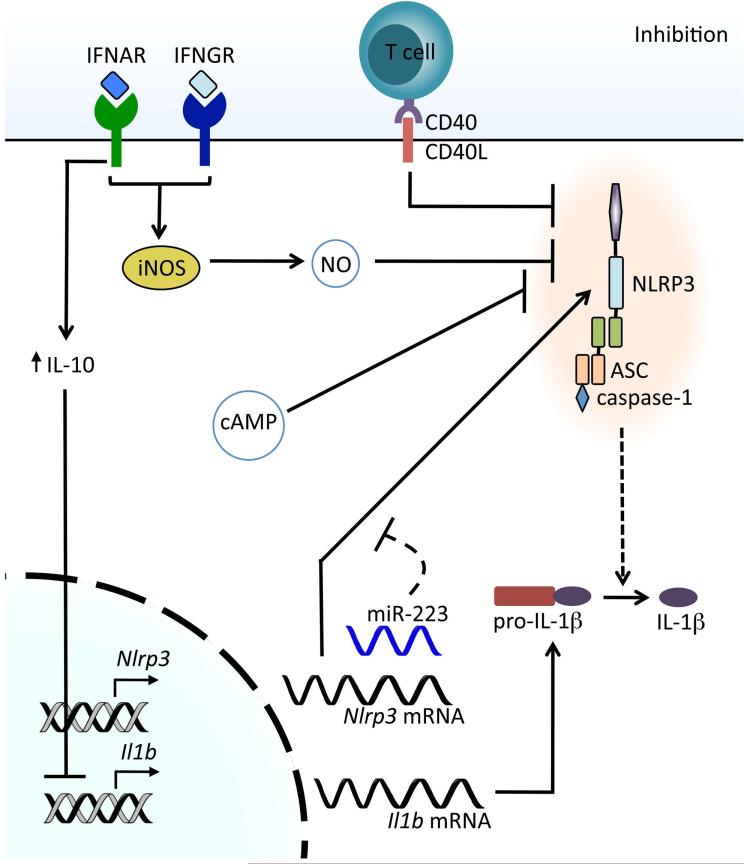
The negative regulation of NLRP3 inflammasome priming parallels the importance of the successful integration of the priming elements described above (Fig. 3). Potent inflammatory responses must abate once their primary purpose has been fulfilled or risk causing more harm to the host than the initiating insult. It follows that NLRP3 transcription would be a point of negative control owing to the contribution of NLRP3 expression levels to inflammasome priming. The endogenous microRNA miR-223 was found to exert control of NLRP3 gene expression by binding to the 3′-untranslated region of NLRP3 mRNA, thereby targeting it for degradation. miR-223 expression levels are unaffected by priming signals, but rather remain constitutively high in cells of the myeloid lineage. Interestingly, differential miR-223 expression exists within subsets of myeloid cells, suggesting a sort of fine-tuning mechanism to bias certain cell types toward NLRP3 inflammasome activation or restraint.42,43 So effective is this posttranscriptional regulatory checkpoint that miR-223–deficient mice show signs of NLRP3 inflammasome hyperactivation,44 and Epstein-Barr virus (EBV) takes advantage of it as a means of host evasion by generating its own miRNA against the same binding site on NLRP3 as miR-223.42
Figure 3.
Inhibition of NLRP3 inflammasome activation. Type I IFNs acting through IFNAR inhibit the transcription of pro-IL-1β through the upregulation of the anti-inflammatory cytokine IL-10. Type I IFNs and IFN-γ inhibit NLRP3 through the production of nitric oxide (NO) via the inducible nitric oxide synthase (iNOS), resulting in nitrosylation of NLRP3. Interaction of mature or memory T cells with macrophages via CD40–CD40L results in inhibition of the NLRP3 inflammasome. Elevation of cellular cAMP levels also results in the inhibition of NLRP3. The microRNA mir-223 regulates the amount of NLRP3 mRNA and, consequently, NLRP3 expression.
An additional source of negative regulation comes from type I interferons (IFN-α and IFN-β), which is perhaps unsurprising given their established immunomodulatory effects. Type I IFNs inhibit the NLRP3 inflammasome by two disparate means, only one of which affects priming, while the other influences the activation step. To limit NLRP3 inflammasome priming, type I IFNs influence the availability of the precursor forms of IL-1α and IL-1β by reducing their expression levels through the induction of IL-10 secretion and subsequent activation of STAT3.45
Another mechanism by which NLRP3 inflammasome activation can be downregulated is through the actions of the adaptive immune system. Activation of the NLRP3 inflammasome was inhibited when macrophages were stimulated with NLRP3 agonists in the presence of stimulated T cells.46 Interestingly, while the inhibition extended to the NLRP1 inflammasome, there was no effect on activation of the NLRC4 inflammasome or non-caspase-1–dependent cytokines. The authors were able to show that this downregulation was dependent upon contact between the macrophages and T cells and was driven by a number of activation-specific T cell surface receptors.46
Two studies have recently described inhibition of NLRP3 inflammasome activation by nitric oxide (NO), but by two discrete mechanisms. IFN-γ and type I IFNs are involved in the activation of inducible nitric oxide synthase (iNOS), which in turn generates NO. While Mishra et al. report NO-induced nitrosylation of NLRP3 blocks activation by preventing assembly of the inflammasome, the study by Mao et al. suggests that NO downregulates NLRP3 activation through enhancing the removal of the dysfunctional mitochondria that activate NLRP3.47,48 Considered together, the components both negatively modulating and contributing to NLRP3 inflammasome priming serve as the custodians of the signal 1 threshold by ensuring the validity of a perceived threat and preparing a response of the appropriate magnitude. NLRP3 inflammasome activation will be described next and, in contrast to priming, the additional levels of control implied by the requirement for a second signal involve tailoring the inflammatory response to specific threats as well as managing the duration of inflammasome activation.
Signal 2: activation
As described above, the second step in activation of the NLRP3 inflammasome is provided by one of a diverse group of agonists that triggers the specific activation of NLRP3, assembly of the inflammasome complex, and finally culminates in the activation of caspase-1. The activators include both exogenous and endogenous molecules such as crystalline molecules (alum, silica, asbestos, monosodium urate) that require phagocytosis for activation, ATP acting through its cell surface receptor P2X7R, and pore-forming toxins such as nigericin. It is important to note that the involvement of P2X7R in NLRP3 inflammasome activation is specific to the agonist ATP, as other agonists can efficiently activate the NLRP3 inflammasome in the absence of P2X7R. As the activators are structurally dissimilar and act upon the cell in discrete ways, it has been suggested that the final activation signal, the binding of a ligand directly to NLRP3, must occur downstream from these unrelated upstream activators. Thus, much of the research aimed at defining the mechanism of NLRP3 inflammasome activation has focused on tracing the common pathways activated by these disparate stimuli in the expectation of uncovering the cognate ligand that directly activates NLRP3. Common pathways necessary for NLRP3 inflammasome activation include cationic fluxes both to and from extracellular spaces as well as from within the cell, and, additionally, the involvement of discrete subcellular organelles including lysosomes, mitochondria, and the endoplasmic reticulum (Fig. 4). Intriguingly, in the course of attempting to define the canonical signaling pathway leading to NLRP3 inflammasome activation, a number of studies have revealed significant similarities between NLRP3 inflammasome activation and apoptotic cell death pathways mediated by APAF-1 and the apoptosome. The definitive mechanism for NLRP3 inflammasome activation remains elusive, but in this section we will summarize the current literature and present a model in an attempt to unite these discrete findings.
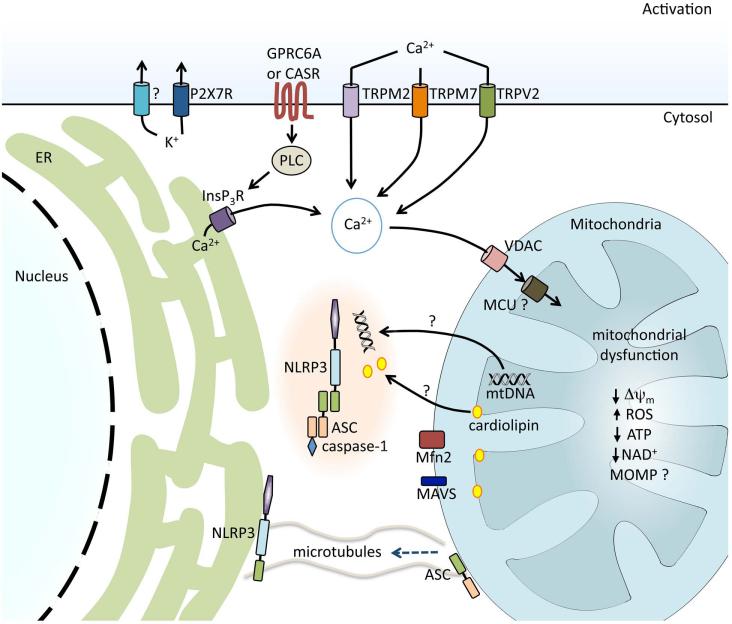
Figure 4.
Regulation of NLRP3 inflammasome activation in response to ion fluxes and mitochondrial dysfunction. K+ efflux is required for NLRP3 inflammasome activation and is achieved either directly by bacterial pore-forming toxins, such as nigericin, or indirectly via receptors such as the purinergic receptor for ATP, P2X7R. During the regulatory volume decrease response to cell swelling, Ca2+ fluxes can be regulated by the transient receptor potential receptors TRPM7 and TRPV2. Ca2+ influx can also be mediated via the ROS-sensitive TRPM2. G protein–coupled receptors CASR and GPRC6A are activated by sensing extracellular Ca2+ and trigger phospholipase C (PLC), generating inositol-1,4,5-triphosphate (InsP3) and triggering the release of Ca2+ from the endoplasmic reticulum (ER). Ca2+ acts on the mitochondria via voltage-dependent anion channels (VDAC) and the mitochondrial Ca2+ uniporter (MCU), resulting in mitochondrial dysfunction. Decrease in mitochondrial membrane potential (Δψm), generation of mitochondrial ROS, decrease in ATP and NAD generation, and, possibly, mitochondrial outer membrane permeabilization (MOMP) are associated with NLRP3 inflammasome activation. Mitochondrial dysfunction and a reduction in NAD+ result in microtubule-driven apposition of ASC on the mitochondria to NLRP3 on the ER. Mitochondrial DNA (mtDNA) and the inner mitochondrial membrane lipid cardiolipin have been shown to bind to NLRP3 and induce its activation. Association between NLRP3 and mitochondrial antiviral signaling protein (MAVS) and NLRP3 and mitofusin 2 (Mfn2) have also been implicated in effective NLRP3 inflammasome activation.
Ion fluxes in NLRP3 inflammasome activation
That potassium efflux is required for the activation of the NLRP3 was demonstrated in studies where elevating the extracellular potassium concentration prevented potassium from migrating down its concentration gradient into the cytosol and inhibited NLRP3 inflammasome activation.49 Confirmation that potassium migration out of the cell is required for NLRP3 inflammasome activation was shown by loss of activation in the presence of potassium channel blockade.50 More recently, a role for calcium has been shown for NLRP3 inflammasome activation, both acting as a discrete activator at elevated extracellular concentrations51,52 and representing a common pathway as extracellular calcium flux into the cell, and shifts of intracellular calcium stores into the cytosol have been shown to be pivotal for NLRP3 inflammasome activation by numerous activators.52–57 The G protein–coupled receptors, calcium-sensing receptor (CASR), and GPRC6A sense extracellular calcium and trigger the release of calcium from the endoplasmic reticulum (ER) via a PLC–InsP3–InsP3R pathway.51,52 Other studies have demonstrated that calcium fluxes can be regulated by the transient receptor potential cation channels TRPM7 and TRPV2 during the volume-decrease response to cell swelling.53 The ROS-sensitive TRPM2 channel has also been shown to mediate calcium influx required for NLRP3 inflammasome activation.55
Whether movement of both potassium and calcium are required is not yet clear. One possibility is that only one of these cationic fluxes is truly acting to trigger NLRP3 inflammasome activation, while the other represents a reciprocal movement to balance ionic forces. This was suggested by both the studies of Murakami et al. where calcium shifts into the cell induced by the NLRP3 inflammasome activator ATP were lost in the presence of elevated extracellular potassium, and the studies by Muñoz-Planillo et al. where activation was only induced by particulate extracellular calcium, and this activation was blocked by elevated extracellular potassium.54,58 However, this is incongruent with other reports where prevention of potassium efflux had no appreciable impact on calcium influx into the cytosol.55,59
The mechanism by which cation movement impacts NLRP3 inflammasome activation remains unclear. One study suggested a shift of intracellular calcium into the cytosol followed an extracellular calcium influx and resulted in the phosphorylation of transforming growth factor β-activated kinase 1 (TAK1).53 Further, it was shown that inhibition of TAK1 phosphorylation, as well as siRNA knockdown of TAK1, was associated with loss of IL-1β release in response to hypotonic stress.53 Whether this requirement for TAK1 is seen in NLRP3 inflammasome activation by other activators, whether it is specific to the NLRP3 inflammasome, and whether it reflects interference with signal 1 or signal 2 remain to be defined.
An intriguing model proposed that NLRP3 inflammasome activation is a function of the balance between intracellular levels of calcium and cAMP.52 It was shown that calcium flux sensed through CASR was induced by a variety of NLRP3 inflammasome activators and resulted in inhibition of adenylate cyclase and a subsequent decrease in cAMP. A decrease in cytosolic cAMP or an increase in intracellular calcium concentrations triggers the activation of the NLRP3 inflammasome.52 However, a separate study found that modulation of cAMP levels by either activating or inhibiting adenylate cyclase did not affect extracellular calcium–induced IL-1β release.51 The reason for these differences is not clear, but may be reflective of different experimental protocols as well as readouts representing NLRP3 inflammasome activation.
Some studies suggest ion fluxes induce mitochondrial dysfunction, which has been proposed to be required for NLRP3 inflammasome activation. It has been shown that calcium flux is associated with ER stress and results in mitochondrial damage including a reduction of the normal negative mitochondrial membrane potential (Δψm) and the generation of mitochondrial ROS.54 Calcium flux has also been linked directly to the mitochondria, as an elevation in intracellular calcium resulted in uptake of calcium into the mitochondrial matrix via the mitochondrial calcium uniporter (MCU), and this uptake was followed by mitochondrial dysfunction.56 Elevated calcium has also been tied to the colocalization of NLRP3 with the mitochondria, as this was prevented in the presence of calcium chelators.56 While this is an intriguing connection, interpretation is limited, as these studies were performed with epithelial cells and it remains to be seen whether similar results will be seen in myeloid cells.
In contrast to the above studies that suggest a necessary interaction between cation movement and the mitochondria, another study found no conserved role for the mitochondria in NLRP3 inflammasome activation.58 In particular, it was shown that while mitochondrial ROS production and mitochondrial dysfunction occur during NLRP3 inflammasome activation, their inhibition does not prevent NLRP3 inflammasome activation. Rather, these authors posit the key event in driving NLRP3 inflammasome activation is potassium efflux, although the precise mechanism by which this occurs is not yet known.58 The reason for the difference in results between these studies is not clear.
The mitochondria in NLRP3 inflammasome activation
A role for subcellular organelles in NLRP3 inflammasome activation was initially suggested by studies revealing a link between lysosomal damage and activation (reviewed in Ref, 60). An additional association with organelles was shown in a study from Jürg Tschopp's group.61 This study revealed that the ROS required for NLRP3 inflammasome activation was generated by the mitochondria (reviewed in Ref. 62) . This groundbreaking study also showed that mitochondria are pivotal in NLRP3 inflammasome activation for more than the generation of ROS. NLRP3 inflammasome activation was associated with mitochondrial dysfunction, as evidenced by a reduction in the normal negative potential within the mitochondria (δψm).61 NLRP3 inflammasome activation was prevented by the knockdown of voltage-dependent anion channel (VDAC) isotypes 1 or 3, the predominant channels for metabolites and ions present in the outer mitochondrial membrane. VDAC knockdown also prevented the generation of mitochondrial ROS in response to NLRP3 agonists. To further evaluate the role of VDAC in NLRP3 inflammasome activation, the authors overexpressed the anti-apoptosis protein Bcl-2, which resulted in closure of VDAC, which in turn lowered mitochondrial calcium and ROS generation. This overexpression of Bcl-2 was associated with an inhibition of NLRP3 inflammasome activation. While the exact effect of Bcl-2 family members on VDAC and mitochondrial calcium is controversial,63 these data distinctly establish an association for NLRP3 inflammasome activation with the mitochondria, and more precisely that both activation and physical association of NLRP3 with the mitochondria are dependent upon the state of ion channels in the outer mitochondrial membrane.
A separate study demonstrated that dysfunction of mitochondria is associated with NLRP3 inflammasome activation and suggested a novel requirement for mitochondrial DNA (mtDNA) in the activation of NLRP3.64 Release of mtDNA from dysfunctional mitochondria occurs in response to LPS plus ATP and drives NLRP3 inflammasome activation.64 The precise pathway that is involved in this model of activation is less easily understood, as both the release of mtDNA and the loss of Δψm were impaired in NLRP3-deficient macrophages while the ability of these NLRP3-deficient cells to generate ROS remained intact. By extrapolation, these data predict a model wherein NLRP3 acts downstream of ROS but is required upstream of mitochondrial dysfunction. mtDNA has also been shown to directly bind to NLRP3 in order to induce inflammasome activation, and this association depended upon the mtDNA being oxidized.65
NLRP3 has also been shown to be associated with the ER at rest, while the adaptor molecule ASC was found associated with the mitochondria and the nucleus and within the cytosol.66 Colocalization of NLRP3 with the mitochondria has been shown to be critical for inflammasome activation;61,66 however, the roles of potassium efflux and ROS generation in this process are unclear.61,66 A recent study by Misawa et al. suggests that mitochondrial damage reduces nicotinamide adenine dinucleotide (NAD) levels, which in turn inactivate sirtuin 2, leading to an abundance of acetylated α-tubulin that drives the microtubules to bring the mitochondria to the perinuclear area. This brings NLRP3 and the adaptor molecule ASC into close proximity and facilitates NLRP3 inflammasome activation.
Studies performed by our group have also found a central role for mitochondrial dysfunction in NLRP3 inflammasome activation. The oxazolidinone antibiotic linezolid was found to induce NLRP3 inflammasome activation in a manner that is independent of mitochondrial ROS production but dependent on mitochondrial dysfunction.67 This unique separation between mitochondrial dysfunction and ROS production in linezolid-induced NLRP3 inflammasome activation is significant, as it provides evidence that ROS generation is not an absolute requirement for inflammasome activation. A study defining the mechanism by which viruses activate NLRP3 independently reported a disconnect between the requirement for mitochondrial ROS and mitochondrial dysfunction.68 Iyer et al. observed an association between NLRP3 and the inner mitochondrial membrane–specific lipid cardiolipin following activation of macrophages with NLRP3 agonists.67 This binding was dependent upon the LRR domain of NLRP3, and interference with cardiolipin synthesis prevented both the colocalization of NLRP3 to the mitochondria and NLRP3 inflammasome activation. In a broken-cell system, the addition of cardiolipin liposomes was found to induce caspase-1 activation, suggesting cardiolipin can directly activate the inflammasome. How this interaction occurs is not known, but previous studies have shown that, during extrinsic apoptosis, the activation of caspase-8 is dependent on its binding to cardiolipin on the outer mitochondrial membrane.69 In the apoptotic pathway, cardiolipin moves from the inner to the outer membrane following activation of phospholipid scramblase 3 (PLS3) downstream of the generation of ROS.70,71 Whether a similar movement of cardiolipin to the outer mitochondrial membrane occurs in NLRP3 inflammasome activation is not known; a separate possibility remains that NLRP3, perhaps related to its transport with the ER and mitochondrial-associated ER membranes (MAM), might be translocated into the intermembrane space or within the inner mitochondrial membrane.
Investigations into the mechanism of NLRP3 inflammasome activation by RNA viruses revealed that the extent of mitochondrial dysfunction was relevant to whether the NLRP3 inflammasome was activated, as the complete loss of Δψm was associated with impaired activation of the NLRP3 inflammasome.68 An important finding that will be discussed below is that the loss of Δψm induced by NLRP3 activators can be transient rather than permanent.68 The relevance of the extent of loss of Δψm was suggested to occur via effects on the outer mitochondrial membrane proteins mitofusin (Mfn) 1 and 2, as immunoprecipitation experiments showed an association of NLRP3 with Mfn1 and Mfn2 that was dependent upon Δψm. Finally, the authors not only found that Mfn2 binds to NLRP3 but also that Mfn2 is required for NLRP3 inflammasome activation, as release of IL-1β was attenuated in cells in which Mfn2 levels were knocked down.68 As these studies focused on the mechanism of NLRP3 inflammasome activation induced by RNA viruses, further studies will be necessary to assess whether similar pathways are required for NLRP3 inflammasome activation by other agonists.
Two additional studies have defined a role for the mitochondria in NLRP3 inflammasome activation that is unique to non-crystalline activators. In these studies, the mitochondrial outer membrane protein mitochondrial antiviral signaling protein (MAVS) is required for effective NLRP3 inflammasome activation by non-crystalline activators, and MAVS facilitates the association of NLRP3 with the mitochondria.72,73
The recycling of cellular contents via autophagy and the specific removal of mitochondria via mitophagy have also been linked to NLRP3 inflammasome activation. This was first suggested by an increase in IL-1β production in response to NLRP3 inflammasome stimulation in cells that lacked the autophagy regulator Atg16L1.74 Additional studies have supported this association by showing similar results from cells lacking other pivotal autophagy genes.61,64 The mechanism by which autophagy or mitophagy impacts NLRP3 inflammasome activation is not yet clear; one hypothesis is that autophagy is part of the pathway by which inflammasomes are removed.75 Another hypothesis is that the removal of damaged mitochondria by mitophagy limits the availability of activating signals required for NLRP3 inflammasome activation.61,64
Similarities between APAF-1 apoptosome and NLRP3 inflammasome activation
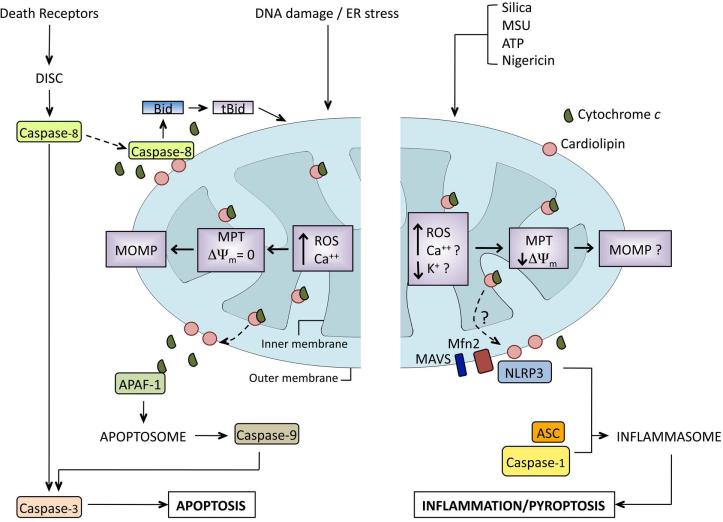
Apoptotic cell death can be triggered by death signals from outside the cell via the extrinsic pathway, from internal danger signals that drive intrinsic apoptosis, or through a combination of the two (Fig. 5). In activation of extrinsic apoptosis, cell surface death receptor stimulation is sufficient to activate caspase-8, which in turn can cleave and activate caspase-3, the final executioner caspase responsible for apoptosis. In intrinsic apoptosis, disruption of cellular homeostasis, such as increases in cytosolic calcium, result in specific changes to the mitochondria as the mitochondria takes up the excess calcium. Classically, this results in a change in the permeability of the inner mitochondrial membrane, called mitochondrial permeability transition (MPT). MPT is associated with a loss of Δψm that is followed by failure of electron transport, generation of mitochondrial ROS, matrix swelling, and mitochondrial outer membrane permeabilization (MOMP), resulting in release of pro-apoptotic compounds such as cytochrome c (reviewed in Ref. 76). Release of cytochrome c into the cytosol is actually a two-step process, with the first step being its release from its tether to the inner mitochondrial membrane lipid cardiolipin by ROS-induced oxidation. This release makes cytochrome c available to diffuse into the cytosol if the mitochondrial dysfunction progresses to loss of integrity of the outer mitochondrial membrane.77 If the release of cytochrome c is above a threshold level, it will bind to apoptotic protease-activating factor 1 (APAF-1) to form an apoptosome that in turn activates pro-caspase-9, which finally cleaves and activates caspase-3.78,79 As noted above, in some cells external death signals are insufficient to activate caspase-8 and a second, internal danger signal is required for activation of apoptosis through a combination of extrinsic and modified intrinsic apoptosis.80 In this pathway, mitochondrial damage and ROS trigger the dissociation of cardiolipin and cytochrome c, after which cardiolipin is translocated to the outer mitochondrial membrane where it binds caspase-8 and is insufficiently activated by death receptor signaling. Cardiolipin binds and triggers the activation of caspase-8, which, in turn, cleaves the pro-apoptotic Bcl-2 family member Bid to truncated Bid (tBid).69,81 tBid then interacts with other pro-apoptotic Bcl-2 family members on the surface of the mitochondria that permeabilize the outer mitochondrial membrane, releasing cytochrome c and triggering caspase-9 and caspase-3, as above. There are a number of striking similarities in the events leading up to apoptosome formation that mirror NLRP3 inflammasome activation (Fig. 5). Activation of caspase-1 through NLRP3 inflammasome activation can trigger an inflammatory death pathway known as pyroptosis (reviewed in Ref. 82). While on one hand, it seems odd that two pathways with such divergent inflammatory outcomes would have overlap, advances in our understanding of the pathways leading to NLRP3 inflammasome activation suggest that multiple overlaps among apoptotic pathways exist.
Figure 5.
Comparison of apoptotic pathways and NLRP3 inflammasome activation. The left panel represents the extrinsic pathway of apoptosis. Death receptors trigger the movement of pro-caspase-8 to the mitochondria, where its activation is dependent upon the translocation of cardiolipin from the inner to the outer mitochondrial membrane. This translocation follows the generation of ROS and is accompanied by the oxidation of cardiolipin by cytochrome c (cyt c), resulting in the loss of cardiolipin–cyt c association, with cyt c remaining in the intermembrane space. Active caspase-8 cleaves Bid to tBid, which in turn triggers mitochondrial outer membrane permeabilization (MOMP). Cyt c diffuses into the cytosol through the MOMP, where it binds to APAF-1, resulting in assembly of the apoptosome and inducing cell death. The right panel depicts a proposed model for NLRP3 inflammasome activation. NLRP3 agonists cause mitochondrial dysfunction, resulting in the generation of mitochondrial ROS. It is postulated that increases in mitochondrial Ca2+ occur through voltage-dependent anion channels (VDAC) and the mitochondrial Ca2+ uniporter (MCU). This results in a transient loss of mitochondrial membrane potential (Δψm). It is unclear if the mitochondrial dysfunction induced by NLRP3 agonists proceeds to MOMP. Cardiolipin, miofusin 2 (Mfn2), and mitochondrial antiviral signaling protein (MAVS) have been implicated in the association of NLRP3 with the mitochondria and are required for efficient inflammasome activation.
Controversy surrounds the exact state of the mitochondria during NLRP3 inflammasome activation. It has been reported that NLRP3 inflammasome activation correlates with a partial loss of mitochondrial dysfunction, reflected by an incomplete loss of Δψm within the mitochondria, while other studies suggest that the dysfunction induced in the mitochondria was profound and long lasting.61,65 A complete loss of Δψm induced by the respiratory chain uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) resulted in minimal mitochondrial ROS generation and ineffective NLRP3 inflammasome activation.61,65,68 In contrast, treatment of cells with a low dose of CCCP triggered a partial loss of Δψm; this resulted in the generation of ROS and was associated with IL-1β processing and release.61,65 Importantly, a partial loss of Δψm induced by either low-dose CCCP or viral infection was not necessarily a terminal event, as removal of stimuli was associated with restoration of Δψm.68 These findings suggest a partial and perhaps transient mitochondrial impairment drives NLRP3 inflammasome activation while a complete or permanent loss of mitochondrial function results in cell death. This idea of transient versus irreversible mitochondrial dysfunction is not unique to the field of NLRP3 inflammasome activation, but rather has been suggested as a mechanism by which distinct cell death pathways can be activated (reviewed in Ref. 76).
Another possible divergence point between apoptosis and NLRP3 inflammasome activation may be found at the priming stage. It has been shown the extrinsic/intrinsic apoptosis inducer staurosporine can activate NLRP3, but only if macrophages have first been primed.65,67 This supports the supposition that the activation steps of these two pathways have substantial overlap and further suggests that they may differ more at the initial priming steps. It is certainly attractive to imagine that, in the context of a preceding inflammatory trigger, a pro-inflammatory response such as NLRP3 inflammasome activation may be more beneficial to a cell than an immunologically benign apoptotic one. Although these findings are interesting, their biological relevance has yet to be confirmed. Particular questions that remain include whether NLRP3 activators can activate apoptotic pathways in the absence of priming and how the apoptotic pathways are initially licensed.
Conclusions
There have been many recent studies implicating calcium fluxes, ER stress, and mitochondrial dysfunction in activation of the NLRP3 inflammasome. However, despite these advances, there are many questions that remain and new questions that have arisen. For example, while it is clear that NLRP3 inflammasome activation is a remarkably complex and nuanced event, we still cannot say exactly where and how it occurs. Future studies will need to define the specific role mitochondria play in NLRP3 inflammasome activation. In particular, it will need to be shown whether mitochondria are simply a platform upon which assembly and activation occur or whether they play a more active role in triggering the activation of this inflammatory pathway. Separately, the role of cation flux remains muddied, both as discrete pathways and how it may or may not relate to the putative function of mitochondria. Despite the conclusions of a number of well-controlled studies, the direct activating ligand for NLRP3 also remains unclear and awaits definition. Finally, additional investigations are needed to determine what regulates NLRP3 inflammasome activation leading to pyroptotic cell death versus the maintenance of cell viability and the processing and secretion of IL-1β.
Acknowledgments
NIH Grants R01 AI087630 (F.S.S.), R01 AI104706 (S.L.C.), T32 AI007511 (S.H.), and an Asthma and Allergy Foundation of America fellowship (S.L.C.) supported this work. The Inflammation Program (S.L.C. and F.S.S) is supported by resources and use of facilities at the Veterans Affairs Medical Center, Iowa City, IA.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ting JP, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 3.Bruey JM, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Witola WH, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 6.Agostini L, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 7.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 8.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 10.Rayamajhi M, et al. Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J Immunol. 2013;191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 12.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 15.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 16.Thornberry NA, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 19.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 20.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khare S, et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 23.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae JJ, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman HM, et al. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratsimandresy RA, Dorfleutner A, Stehlik C. An Update on PYRIN Domain-Containing Pattern Recognition Receptors: From Immunity to Pathology. Front Immunol. 2013;4:440. doi: 10.3389/fimmu.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroder K, et al. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology. 2012;217:1325–1329. doi: 10.1016/j.imbio.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes-Alnemri T, et al. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin KM, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Py BF, et al. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Molecular cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Castejon G, et al. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. The Journal of biological chemistry. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauernfeind F, et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayor A, et al. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 41.Labbe K, et al. Cellular Inhibitors of Apoptosis Proteins cIAP1 and cIAP2 Are Required for Efficient Caspase-1 Activation by the Inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Haneklaus M, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. Journal of immunology. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 43.Bauernfeind F, et al. NLRP3 inflammasome activity is negatively controlled by miR-223. Journal of immunology. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 44.Johnnidis JB, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 45.Guarda G, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Guarda G, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 47.Mishra BB, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao K, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23:201–212. doi: 10.1038/cr.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 50.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossol M, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Compan V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong Z, et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triantafilou K, et al. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 57.Abdul-Sater AA, et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013;14:900–906. doi: 10.1038/embor.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munoz-Planillo R, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross O, et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 62.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 63.Huang H, et al. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem. 2013;288:19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimada K, et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 67.Iyer SS, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichinohe T, et al. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A. 2013;110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalvez F, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, et al. Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-alpha-induced apoptosis. Biochemistry. 2008;47:4518–4529. doi: 10.1021/bi701962c. [DOI] [PubMed] [Google Scholar]
- 71.Garcia Fernandez M, et al. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002;13:449–455. [PubMed] [Google Scholar]
- 72.Subramanian N, et al. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park S, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191:4358–4366. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 75.Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Ott M, et al. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clayton R, Clark JB, Sharpe M. Cytochrome c release from rat brain mitochondria is proportional to the mitochondrial functional deficit: implications for apoptosis and neurodegenerative disease. J Neurochem. 2005;92:840–849. doi: 10.1111/j.1471-4159.2004.02918.x. [DOI] [PubMed] [Google Scholar]
- 79.Kinnally KW, et al. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schug ZT, et al. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011;18:538–548. doi: 10.1038/cdd.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]