Abstract
Rationale
Cognitive dysfunctions, including deficits in hippocampus-mediated learning and memory, are core features of the psychopathology of schizophrenia (SZ). Increased levels of kynurenic acid (KYNA), an astrocyte-derived tryptophan metabolite and antagonist of α7 nicotinic acetylcholine and N-methyl-D-aspartate receptors, have been implicated in these cognitive impairments.
Objectives
Following recent suggestive evidence, the present study was designed to narrow the critical time period for KYNA elevation to induce subsequent cognitive deficits.
Methods
KYNA levels were experimentally increased in rats (1) prenatally (embryonic day [ED] 15 to ED 22) or (2) during adolescence (postnatal day [PD] 42 to PD 49). The KYNA precursor kynurenine was added daily to wet mash fed to (1) dams (100 mg/day; control: ECon; kynurenine-treated: EKyn) or (2) adolescent rats (300 mg/kg/day; control: AdCon; kynurenine-treated: AdKyn). Upon termination of the treatment, all animals were fed normal chow until biochemical analysis and behavioral testing in adulthood.
Results
On the last day of continuous kynurenine treatment, forebrain KYNA levels were significantly elevated (EKyn: +472%; AdKyn: +470%). KYNA levels remained increased in the hippocampus of adult EKyn animals (+54%), but were unchanged in adult AdKyn rats. Prenatal, but not adolescent, kynurenine treatment caused significant impairments in two hippocampus-mediated behavioral tasks, passive avoidance and Morris water maze.
Conclusions
Collectively, these studies provide evidence that a continuous increase in brain KYNA levels during the late prenatal period, but not during adolescence, induces hippocampus-related cognitive dysfunctions later in life. Such increases may play a significant role in illnesses with known hippocampal pathophysiology, including SZ.
Keywords: Astrocyte, Cognition, Hippocampus, Kynurenic acid, Schizophrenia
INTRODUCTION
Cognitive dysfunctions, including deficits in hippocampus-mediated learning and memory, are a core domain of the psychopathology of schizophrenia (SZ). These impairments are associated with structural abnormalities and volumetric reductions of the hippocampus (Heckers et al. 1998; Steen et al. 2006). This brain area is richly endowed with both N-methyl-D-aspartate (NMDA) and α7 nicotinic acetylcholine (α7nACh) receptors from an early age (Ben-Ari et al. 1997; Dwyer et al. 2009), and a considerable body of evidence suggests that abnormal neurotransmission at these receptors is causally related to the cognitive impairments in individuals with SZ (Timofeeva and Levin 2011).
Kynurenic acid (KYNA), an endogenous neuroactive metabolite of the kynurenine pathway (KP) of tryptophan degradation, acts as an antagonist of both NMDA and α7nACh receptors. Elevated KYNA has been implicated in the pathology of SZ. An increase in the levels of both KYNA and its direct bioprecursor kynurenine, as well as disruptions in KP enzymes, have been documented in postmortem brain tissue and cerebrospinal fluid of individuals with SZ (Erhardt et al. 2001; Linderholm et al. 2012; Miller et al. 2006; Nilsson et al. 2005; Sathyasaikumar et al. 2011; Schwarcz et al. 2001).
Excessive antagonism of NMDA and α7nACh receptors by KYNA in the brain may be especially related to the cognitive impairments seen in SZ. Thus, in animals, an acute elevation of brain KYNA induces cognitive dysfunctions reminiscent of those seen in patients with SZ. This includes deficits in pre-pulse inhibition of the acoustic startle reflex (Shepard et al. 2003), sensorimotor gating (Erhardt et al. 2004), working memory (Chess et al. 2007), contextual learning (Chess et al. 2009; Pocivavsek et al. 2011) and cognitive flexibility (Alexander et al. 2012). Additionally, in humans, polymorphisms in the gene of a pivotal KP enzyme, kynurenine 3-monooxygenase (KMO), have been associated with SZ (Aoyama et al. 2006; Holtze et al. 2011) and, specifically, with an impairment in smooth pursuit eye movement and visuospatial working memory (Wonodi et al. 2011). Thus, both animal studies and genetic evidence in humans suggest a pathophysiologically significant association between KYNA and cognitive dysfunctions in SZ.
Epidemiological and neuropathological studies suggest that SZ is a neurodevelopmental disorder, wherein brain abnormalities are either inherited or sustained early in life, but not fully expressed until early adulthood (Murray and Lewis 1987; Weinberger 1987). In fact, the clinical onset of SZ typically occurs after puberty, and the long latency between the presumed neurodevelopmental insult and the overt manifestation of the disorder later in life is a key feature of the disorder (Castle et al. 1998; DeLisi 2008; Kinney et al. 2010; Lieberman et al. 2001; Meyer and Feldon 2010). Interestingly, the connection between KYNA and SZ may also have a developmental dimension. Thus, several of the perinatal risk factors associated with SZ, including stress and infections (Brown and Derkits 2010; Meyer and Feldon 2010; van Os and Selten 1998), result in the activation of indoleamine 2,3-dioxygenase (IDO), a cytokine-responsive enzyme that catalyzes the formation of the key KP metabolite kynurenine (Kiank et al. 2010; Saito et al. 1991; Widner et al. 2000). Kynurenine subsequently enters the brain from the circulation and is then irreversibly transaminated to KYNA. The formation of KYNA takes place preferentially in astrocytes, which release the metabolite into the extracellular milieu where it is ideally positioned to inhibit neuronal NMDA and α7nACh receptors and thus influence cognitive processes (Schwarcz et al. 2012). Notably, KYNA concentrations in the mammalian brain are exceptionally high during the prenatal period (Beal et al. 1992; Ceresoli-Borroni and Schwarcz 2000).
The current study is based on our recent demonstration that elevating brain KYNA from embryonic day (ED) 15 to postnatal day (PD) 21 results in impaired hippocampal-based learning and memory in the adult offspring (Pocivavsek et al. 2012). In an effort to better define a critical developmental phase for this effect, we limited the experimental elevation of brain KYNA to the late prenatal period (ED 15 to ED 22), and compared this manipulation to an elevation of brain KYNA during the late adolescent period (“adolescence”), i.e. from PD 42 to PD 49. As in our previous studies (Alexander et al. 2013; Pocivavsek et al. 2012), KYNA levels were increased by lacing rodent chow daily with kynurenine. Our results provide evidence that prenatal KYNA elevation alone is sufficient to induce behavioral dysfunctions later in life, and that essentially identical increases in brain KYNA during adolescence do not induce delayed deficits in hippocampus-mediated cognitive behaviors.
MATERIALS AND METHODS
Animals
Adult, pregnant Wistar rats (gestational age: 2 days) were obtained from Charles River Laboratories for all embryonic kynurenine (EKyn) treatments. To normalize the size of the litters and to maximize the number of males, each litter was culled to 10 pups on PD 2. Special attention was paid to assure that kynurenine administration did not disturb maternal behavior. Male offspring were used on ED 22, PD 2 or PD 56–85 (Fig. 1). To minimize the contribution of individual litters, the average of 2–3 fetuses or pups per litter was considered as one subject in all biochemical experiments. For the behavioral experiments, subjects were the progeny from 7 litters per group, with no more than 3 pups from any given litter.
Figure 1. Experimental design and kynurenine treatment paradigms.
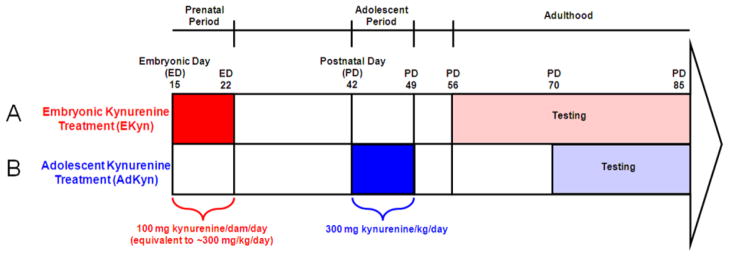
(A) Embryonic kynurenine treatment (EKyn): Kynurenine-treated dams were fed a diet of wet rodent chow mash laced with 100 mg kynurenine per dam per day from embryonic day (ED) 15 until ED 22. Upon birth, normal rodent chow was fed to all offspring. Biochemical analysis was performed on ED 22, postnatal day (PD) 2, and PD 56–85. Behavioral testing was performed in adult animals between PD 56–85. (B) Adolescent kynurenine treatment (AdKyn): Kynurenine-treated adolescent rats were fed a diet of wet rodent chow mash laced with 300 mg kynurenine per kg body weight per day from PD 42 to PD 49. Upon completion of treatment, normal rodent chow was fed to all animals. Biochemical analysis was performed on PD 49 and PD 70–85. Behavioral testing was performed in adult animals between PD 70–85.
For adolescent kynurenine (AdKyn) treatment experiments, PD 35 male Wistar rats were obtained from Charles River Laboratories and pair-housed. Animals were tested experimentally on PD 49 and PD 70–85 (Fig. 1).
All experimental animals were housed in a temperature-controlled facility, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), at the Maryland Psychiatric Research Center. The rats were kept on a 12h/12h light dark cycle and had free access to food and water. Experimental protocols followed the ‘Principles of Laboratory Animal Care’ (NIH publication No. 86-23, 1996) and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine.
Treatment
Rodent chow was ground finely in a food processor, and each dam or adolescent rat ate approximately 30 grams per day. Rather than adjusting the amount of kynurenine administered to pregnant dams whose body weight rapidly increases during the last gestational week, a steady amount of kynurenine (100 mg) was administered for the EKyn treatment. This amount is equivalent to approximately 300 mg of kynurenine per kg body weight per day. Kynurenine was thoroughly mixed into the food daily for the kynurenine-treated dams, while controls received wet mash alone. Dams were fed the diet daily from ED 15 to ED 22. After giving birth, each dam received normal rodent chow pellets ad libitum. Male offspring were weaned on PD 21, pair-housed and given normal rodent chow pellets ad libitum. For AdKyn treatment, beginning on PD 42, 300 mg of kynurenine per kg body weight per day was thoroughly mixed into the food daily for kynurenine-treated animals, while controls received wet mash alone. Animals were fed the diet daily from PD 42 to PD 49. Upon completion of treatment, animals received normal rodent chow pellets ad libitum.
Chemicals
KYNA was purchased from Sigma (St. Louis, MO, USA). L-Kynurenine sulfate (“kynurenine”; purity: 99.4%) was obtained from Sai Advantium (Hyderabad, India). Other chemicals were obtained from a variety of suppliers and were of the highest commercially available purity.
Chemical analyses
Kynurenine determination in serum
Animals were euthanized using CO2, and whole trunk blood from dams and adolescent rats was collected in individual tubes containing 25 μl K3-EDTA (0.15 %) as an anticoagulant. Using the same anticoagulant, trunk blood, pooled from 4 fetuses per litter, was collected in (1.5 ml) Eppendorf tubes. The blood was gently centrifuged (300 × g, 15 min) to separate serum and blood cells. The supernatant serum was transferred to new Eppendorf tubes, frozen on dry ice and stored at −80°C. On the day of the assays, the samples were thawed, the serum was diluted 1:2, and 100 μl of diluted serum were acidified with 25 μl of 6% perchloric acid. After centrifugation (12 000 × g, 10 min), 20 μl of the supernatant were subjected to high performance liquid chromatography (HPLC). Kynurenine was isocratically eluted from a 3-μm C18 reverse-phase column (80 mm × 4.6; ESA, Chelmsford, MA, USA), using a mobile phase containing 250 mM zinc acetate, 50 mM sodium acetate, and 3% acetonitrile (pH adjusted to 6.2 with glacial acetic acid), using a flow rate of 1.0 ml/min. In the eluate, kynurenine was detected fluorimetrically (excitation: 365 nm, emission: 480 nm; Perkin Elmer Series 200 fluorescence detector, Waltham, MA) at a retention time of approximately 6 min.
KYNA determination in tissue
All animals were euthanized using CO2, followed by rapid removal of the brain. The forebrain (brain minus cerebellum and brainstem) (EKyn: ED 22 and PD 2) or hippocampus (EKyn: PD 56–85; AdKyn: PD 49 and PD 70–85) were dissected out, rapidly frozen on dry ice, and stored at −80°C. On the day of the assays, tissues were thawed and sonicated in ultrapure water (1:5, w/v, for PD 2 brains, 1:10, w/v for all other brains). One hundred μl of the homogenate were acidified with 25 μl of 6% perchloric acid. After centrifugation (12 000 × g, 10 min), 20 μl of the supernatant were analyzed by HPLC as described above for kynurenine. In the eluate, KYNA was detected fluorimetrically (excitation: 344 nm, emission: 398 nm; Perkin-Elmer Series 200) at a retention time of approximately 7 min.
Behavioral testing
An experimenter who was unaware of the treatment condition performed all behavioral experiments.
Contextual memory
The passive avoidance apparatus had two compartments of equal size (each 22 cm high, 18 cm wide and 16 cm deep) separated by a guillotine door. One compartment was illuminated and the other remained dark,. On the training day (acquisition trial), the animal was first placed in the illuminated compartment. The guillotine door was then opened, prompting the rat to naturally move quickly into the preferred dark compartment. The latency to enter the dark compartment was recorded as the ‘approach latency’. After the door was closed, an inescapable foot shock (0.56 mA for 1 sec) was delivered through metal rods of the floor. The retention of the newly formed aversive foot shock memory was tested 24 hours later (retention trial). The rat was again placed in the light compartment, the guillotine door was opened, and the ‘avoidance latency’, i.e. the time from opening the guillotine door to the time of entering the dark compartment, was recorded.
Spatial navigation and reference memory
The Morris water maze (MWM) (pool dimension: 180 cm in diameter) was used to study spatial navigation and reference memory as previously described (Morris 1984; Pocivavsek et al. 2012). Briefly, each animal was habituated to the procedure and environment by navigating the opaque pool (non-toxic tempera paint) for 120 sec, at least 24 hours prior to training, to locate a hidden platform (10 cm in diameter, approximately 2 cm below water level). Then, each animal was trained for 4 trials, given up to 120 sec to locate the hidden platform on each of 6 consecutive days. The inter-trial interval was 120 sec. The platform remained in the same hidden location across the 6 days of training. Rats were placed into the maze at different locations in a counterbalanced order across trials, days, and animals. On the seventh day, retention of the newly learned task and spatial navigation strategy were assessed in a single ‘probe trial’, wherein the hidden platform was removed. Each animal was then given 120 sec to navigate the pool, allowing the experimenter to examine retention and spatial navigation strategy. Immediately following the probe trial, each animal was tested again, with the platform made visible by a red flag and raised slightly above water level (‘visible trial’). This latter trial was used to monitor visual acuity and general sensorimotor capabilities. Behavior during all trials was recorded by Noldus EthoVision tracking system (Leesburg, VA, USA).
Statistical analysis
For all biochemical analyses, comparisons were made using an unpaired Student’s t-test. Results from the passive avoidance paradigm were also analyzed using an unpaired Student’s t-test. MWM escape latency across days was analyzed using a two-way repeated measures Analysis of Variance (ANOVA) with treatment group as a between-subject factor and days as a within-subject factor. Post-hoc analyses for the analysis of MWM latencies were performed using a Bonferroni t-test correction with an adjusted alpha = P < 0.008 per comparison. Swim speed, probe trial and visual platform trial comparisons were made using an unpaired Student’s t-test. Statistical significance in the test was defined as P < 0.05. All statistical analyses were performed using GraphPad Prism 5.0 software or SPSS 12.0 software.
RESULTS
Kynurenine feeding increases serum kynurenine levels on the last day of treatment
We first determined the effect of the kynurenine diet on serum kynurenine levels in both treatment paradigms. Kynurenine-treated (EKyn) dams, control (ECon) dams and their respective fetuses were killed on ED 22. At that time, kynurenine-treated dams had significantly higher levels of serum kynurenine compared to control dams (ECon: 2.4 ± 0.4 μM, N = 6; EKyn: 13.4 ± 3.5 μM, N = 7; P < 0.05). Kynurenine levels in the serum of fetuses from kynurenine-treated dams were also significantly elevated compared to fetuses from control dams (ECon: 3.3 ± 0.4 μM, N = 5 litters; EKyn: 16.4 ± 4.4 μM, N = 6 litters; P < 0.05) (Fig. 2A). In contrast, on PD 2, kynurenine levels in the serum were no longer significantly different between pups from kynurenine-treated litters compared to control litters (ECon: 2.3 ± 0.1 μM, N = 5 litters; EKyn: 1.7 ± 0.5 μM, N = 4 litters; P = 0.43), suggesting that the elevation in fetal blood kynurenine levels on ED 22 had been dependent on the presence of the kynurenine in the dam’s diet.
Figure 2. Both prenatal (EKyn) and adolescent (AdKyn) kynurenine treatment increases serum kynurenine levels.
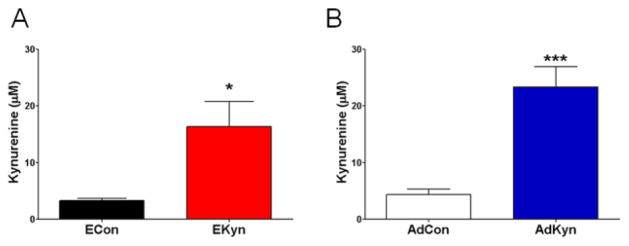
(A) Exposure to kynurenine (100 mg/day; EKyn) from ED 15 to ED 22 raised kynurenine levels in the serum of fetuses on ED 22. Data are the mean ± SEM (ECon: n = 5 litters; EKyn: n = 6 litters). * P < 0.05 vs. control (ECon). (B) Exposure to kynurenine (300 mg/kg/day; AdKyn) from PD 42 to PD 49 raised kynurenine levels in the serum of treated animals on PD 49. Data are the mean ± SEM (n = 7 per group). *** P < 0.001 vs. control (AdCon).
On PD 49, serum kynurenine levels in adolescent rats (AdKyn) that had been treated with kynurenine for one week were significantly elevated compared to control (AdCon) adolescent rats (AdCon: 4.4 ± 1.0 μM; AdKyn: 23.4 ± 3.6 μM; N = 7 per group; P < 0.001) (Fig. 2B). Notably, the percent increase in serum kynurenine was not different in the EKyn offspring on ED 22 and in AdKyn animals on PD 49 (EKyn: 397%, AdKyn: 432%), indicating equivalence of the two treatment paradigms.
Brain KYNA levels are elevated with kynurenine treatment in both fetal (EKyn) and adolescent (AdKyn) animals
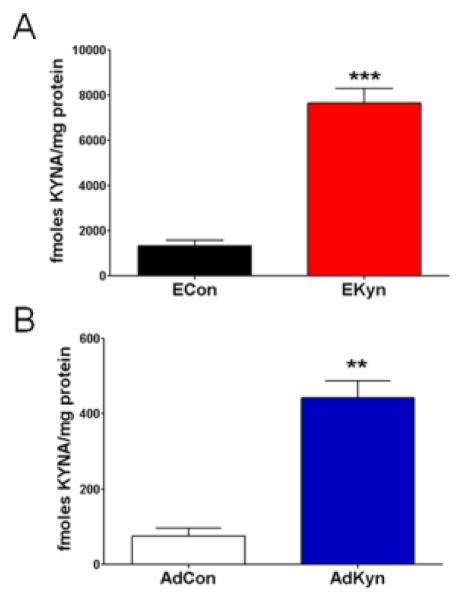
Fetuses of kynurenine-treated dams showed substantial increases in KYNA levels in the brain. On ED 22, KYNA in the forebrain of fetuses from treated litters was significantly higher than in controls (ECon: 1344 ± 233 fmoles/mg protein, N = 6 litters; EKyn: 7654 ± 656 fmoles/mg protein, N = 7 litters; P < 0.0001) (Fig. 3A). However, on PD 2, consistent with the serum kynurenine data, there was no difference between forebrain KYNA content in the offspring from treated litters compared to control litters (ECon: 80.7 ± 18.8 fmoles/mg protein, N = 5 litters; EKyn: 107.0 ± 22.5 fmoles/mg protein, N = 4 litters; P = 0.40).
Figure 3. Both prenatal (EKyn) and adolescent (AdKyn) kynurenine treatment increases kynurenic acid (KYNA) levels in the brain.
(A) Exposure to kynurenine (100 mg/day; EKyn) from ED 15 to ED 22 raised KYNA in the forebrain of fetuses on ED 22. Data are the mean ± SEM (ECon: n = 6 litters; EKyn: n = 7 litters). *** P < 0.001 vs. control (ECon). (B) Treatment with kynurenine (300 mg/kg/day; AdKyn) from PD 42 to PD 49 increased KYNA levels in the hippocampus of treated animals on PD 49. Data are the mean ± SEM (n = 7 per group). ** P < 0.01 vs. control (AdCon).
On the last day of AdKyn manipulation, i.e. PD 49, a significant elevation of hippocampal KYNA was seen in kynurenine-treated animals compared to controls (AdCon: 75.9 ± 20.6 fmoles/mg protein; AdKyn: 442.7 ± 44.4 fmoles/mg protein; N = 7 per group; P < 0.0001) (Fig. 3B). Brain KYNA levels were similarly elevated after kynurenine treatments at both ages (EKyn: 470%, AdKyn: 482%), again confirming equivalence of the two experimental protocols in raising KYNA levels.
Prenatal, but not adolescent, kynurenine treatment increases KYNA in the adult rat brain
After treatment was terminated on either ED 22 (EKyn) or PD 49 (AdKyn), all rats received normal rodent chow until the completion of the study. When the animals reached adulthood and were at least three weeks off kynurenine treatment (EKyn: PD 56–85; AdKyn: PD 70–85), the levels of KYNA were assessed in the hippocampus. KYNA levels were significantly higher in EKyn (104.1 ± 23.5 fmoles/mg protein, N = 4 litters) than in ECon (47.4 ± 3.5 fmoles/mg protein, N = 6 litters; P < 0.05) rats (Fig. 4A). In contrast, there was no difference in KYNA levels in the hippocampus of rats treated with kynurenine or regular chow during the adolescent period (AdCon: 59.7 ± 8.6 fmoles/mg protein; AdKyn: 51.5 ± 4.8 fmoles/mg protein; N = 8 per group; P = 0.42) (Fig. 4B).
Figure 4. Effects of prenatal (EKyn) and adolescent (AdKyn) kynurenine treatment on tissue levels of kynurenic acid (KYNA) in adulthood.
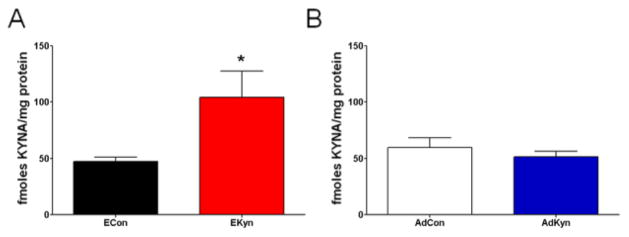
(A) Treatment with kynurenine (100 mg/day; EKyn) from ED 15 to ED 22 raised KYNA in the hippocampus of adult offspring (PD 56–85). Data are the mean ± SEM (ECon: n = 6 litters; EKyn n = 4 litters). * P < 0.05 vs. control (ECon). (B) Exposure to kynurenine (300 mg/kd/day; AdKyn) from PD 42 to PD 49 did not impact KYNA levels in the hippocampus in adulthood (PD 70–85). Data are the mean ± SEM (n = 8 per group).
Deficits in hippocampal learning and memory in adult EKyn, but not AdKyn, animals
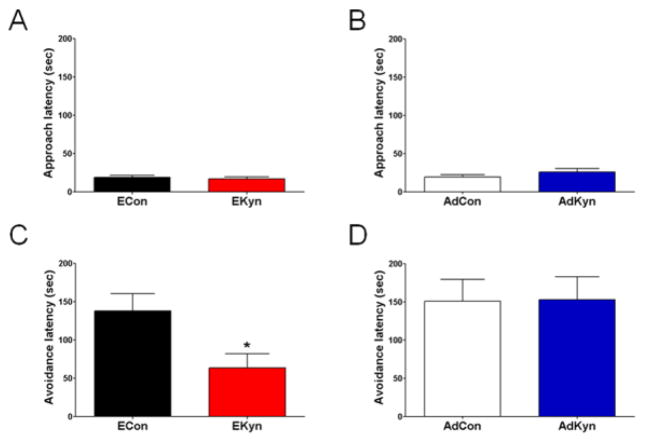
Next, we investigated the impact of continuous prenatal or adolescent kynurenine treatment on performance in two hippocampus-mediated behaviors, i.e. contextual memory in the passive avoidance paradigm, and spatial learning and memory in the MWM. Adult animals were examined on both tasks in a counterbalanced order, with at least 4 days between tasks. On the first day of passive avoidance testing, the acquisition trial, rats from control and kynurenine-treated groups did not differ significantly in approach latencies (ECon: 19.5 ± 3.3 sec; EKyn: 22.9 ± 4.6 sec; N = 15 each, 7 litters represented in both groups; P = 0.58) (AdCon: 19.3 ± 3.1 sec; AdKyn 25.9 ± 4.6 sec; N = 15 per group; P = 0.25) (Figs. 5A and 5B). When tested 24 hours later in the retention trial, the avoidance latencies of animals treated prenatally with kynurenine were significantly shorter than those of control animals (ECon: 138.2 ± 22.5 sec; EKyn: 63.6 ± 18.4 sec; P < 0.05) (Fig. 5C). However, there was no difference in the avoidance latencies of animals receiving kynurenine during adolescence and their respective controls (AdCon: 150.7 ± 28.7 sec; AdKyn: 152.6 ± 30.2 sec; P = 0.96) (Fig. 5D).
Figure 5. Only animals exposed to kynurenine during the prenatal period (EKyn) show contextual memory impairment in adulthood.
Prenatal treated rats were exposed to kynurenine (100 mg/day; EKyn) from ED 15 to ED 22 and adolescent treated rats were exposed to kynurenine (300 mg/kg/day; AdKyn) from PD 42 to PD 49. Adult animals were tested in the passive avoidance paradigm (EKyn: PD 56–85; AdKyn: PD 70–85; n = 15 per group). No differences in approach latency were observed on the training day for both EKyn (A) and AdKyn (B) animals compared to respective controls (ECon or AdCon). Avoidance latency differed significantly between the EKyn and ECon group (C), but did not differ between the AdKyn and AdCon group (D). All data are the mean ± SEM. * P < 0.05 vs. control (ECon).
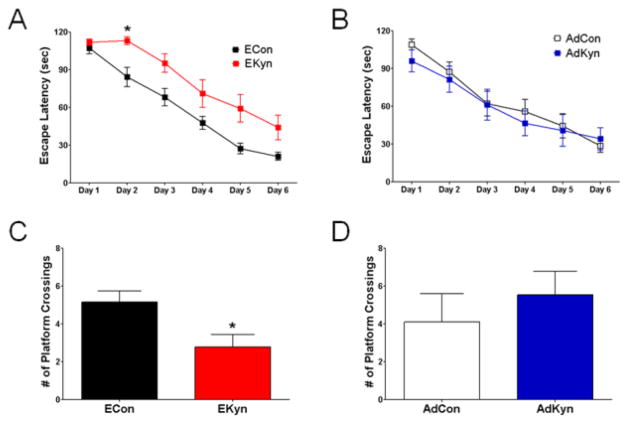
When tested in the MWM, adult rats treated prenatally with kynurenine showed an overall significant effect on escape latency compared to controls (F1,28 = 10.16, P < 0.01). There was no significant interaction between treatment and escape latency across days (F5,140 = 1.71, P = 0.135). A series of one-way ANOVAs comparing escape latency of ECon vs. EKyn on each day revealed that the two groups differed significantly on day 2 (P < 0.008) (N = 15 each, 7 litters represented in both groups) (Fig. 6A). These data demonstrated that kynurenine treatment during a sensitive period of prenatal development slows the rate of acquisition in the spatial learning task in adulthood. In contrast, adult rats that were treated with kynurenine during adolescence showed no difference in escape latency across days compared to controls (F1,19 = 0.18, P = 0.69) (AdCon: N = 10; AdKyn: N = 11), suggesting no deficit in learning the task (Fig. 6B).
Figure 6. Spatial learning and reference memory is only impaired in animals receiving kynurenine during the prenatal period (EKyn).
Prenatal treated rats were exposed to kynurenine (100 mg/day; EKyn) from ED 15 to ED 22, and adolescent treated rats were exposed to kynurenine (300 mg/kg/day; AdKyn) from PD 42 to PD 49. Animals were tested in the MWM during adulthood (EKyn: PD 56–85; AdKyn: PD 70–85). (A & B) Test across 6 days of training; (C & D) number of platform crossings during the probe trial (video tracking analysis). All data are the mean. (ECon: n = 15; EKyn: n = 15) (AdCon: n = 10; AdKyn: n = 11) * P < 0.05 vs. control (ECon).
Retention of the newly learned task and spatial navigation strategy were assessed in one single probe trial 24 hours after the last training session. Animals treated prenatally with kynurenine crossed into the area formerly occupied by the platform less frequently than control animals (ECon: 5.2 ± 0.6 crosses; EKyn: 2.8 ± 0.7 crosses; P < 0.05) (Fig. 6C), whereas no such difference was seen between animals treated with kynurenine during adolescence and their respective controls (AdCon: 4.1 ± 1.5 crosses; AdKyn: 5.5 ± 1.2 crosses; P = 0.46) (Fig. 6D). Moreover, swim speeds did not differ significantly between the comparable groups (ECon: 30.3 ± 1.0 cm/sec, EKyn: 32.6 ± 0.8 cm/sec; P = 0.09; AdCon: 38.9 ± 1.9 cm/sec, AdCon: 40.2 ± 2.1 cm/sec; P = 0.65) during the probe trial. Finally, a visible trial was conducted immediately after the probe trial. There were no differences in the escape latencies between each of the two experimental groups (ECon: 15.0 ± 6.0 sec, EKyn 20.4 ± 3.5 sec; P = 0.47; AdCon: 14.7 ± 3.2 sec, AdKyn: 16.4 ± 3.4 sec; P = 0.73), indicating that kynurenine treatment, either prenatally or during adolescence, did not cause gross visual deficits later in life.
DISCUSSION
The present study explored and compared the effects of continuous prenatal (EKyn) and adolescent (AdKyn) exposure to kynurenine on brain KYNA levels and on delayed hippocampus-dependent learning and memory in rats. First, we confirmed that the lacing of chow with kynurenine resulted in elevated brain KYNA levels on the last day of treatment in both groups. Notably, the magnitude of this biotransformation was quantitatively similar in EKyn and AdKyn rats. Upon completion of treatment, both EKyn and AdKyn animals received normal non-laced chow until biochemical and behavioral testing in adulthood, a period defined as either PD 56 or later (EKyn) or at least three weeks after the last day of treatment (AdKyn). In adulthood, animals that had received kynurenine during the prenatal period displayed a significant increase in hippocampus KYNA when compared to respective controls, but no such difference was seen in adult AdKyn rats.
In separate animals, we investigated performance in two hippocampus-dependent behavioral tasks, the passive avoidance paradigm and the MWM test. Adult EKyn rats, which showed increased KYNA levels in the brain, displayed abnormalities in both tasks, signifying dysfunction of learning and memory. In contrast, adult AdKyn animals, which have normal brain KYNA, were not impaired. These findings indicate that prenatal exposure to kynurenine, leading to long-lasting elevations in brain KYNA levels, is sufficient to cause biochemical and behavioral effects in adulthood, whereas the same treatment during adolescence does not produce these enduring changes.
The present set of experiments was based on our previous studies, where dams were continuously treated with kynurenine from ED 15 to PD 21, and where brain KYNA levels in the offspring remained chronically elevated until weaning (Alexander et al. 2013; Pocivavsek et al. 2012). Here we showed that a rise in brain KYNA levels during a relatively narrow embryonic period alone, i.e. from ED 15 to ED 22, was sufficient to induce biochemical and behavioral abnormalities in adulthood. These results complement several reports on long-lasting behavioral impairments following various experimental manipulations during the last week of prenatal development (Kapoor et al. 2009; Koenig et al. 2005; Lodge and Grace, 2009; Meyer 2013; Meyer and Feldon 2010). While several of the insults used in these studies, including stress or immune activation, increase the formation of KP metabolites as a consequence of IDO activation (Asp et al. 2010; Miura et al. 2011; Raison et al. 2010; Widner et al. 2000), the present study demonstrates more directly that a prenatal increase in brain KYNA is associated with long-lasting biochemical and behavioral abnormalities. In line with the concept of “early-life priming of adult diseases” (Bale et al. 2010), we therefore suggest that exposure of the brain to abnormally elevated amounts of KYNA during a critical period of prenatal development disrupts normal brain maturation and subsequently curtails the ability of the adult brain to learn novel tasks and form contextual memories (as assessed by MWM and passive avoidance). Notably, these results and conclusions are in excellent agreement with a recent series of experiments by Stone and collaborators, who used a pharmacological approach, namely systemic application of the specific KMO inhibitor Ro 61-8048 (Röver et al. 1997), to raise brain kynurenine and KYNA levels during the late gestational period. In these studies, repeated prenatal administration of Ro 61-8048 led to long-lasting deficits in long-term potentiation, measured in the CA1 region of hippocampal slices generated from adult offspring (PD 60), and was associated with a significant decrease in the expression of GluN2A glutamate receptors (Forrest et al. 2013a,b).
In recent years, the effects of early postnatal or adolescent KP manipulations on behavioral performance in adult animals have been explored in several laboratories. This included intermittent systemic kynurenine injections between PD 27 and PD 53, and behavioral testing on PD 61 (Akagbosu et al. 2010; Trecartin and Bucci 2011), repeated kynurenine injections on PD 7–16 (Holtze et al. 2010) or PD 7–10 (Iaccarino et al. 2013) with testing in adulthood, and our own perinatal kynurenine administration paradigm (Alexander et al. 2013; Pocivavsek et al. 2012). In all these studies, as well as after transient elevation of brain KYNA levels following early postnatal viral infection (Asp et al. 2010), adult animals exhibited impairments in a variety of cognitive or social behaviors.
In the present study, we failed to demonstrate long-term effects of a one-week exposure to kynurenine when the animals were treated during the adolescent period (AdKyn rats; PD 42 to PD 49). Potential explanations for the contrasting positive findings of Akagbosu et al. (2010) and Trecartin and Bucci (2011) may be related to the respective treatment paradigms (intermittent vs. continuous application of kynurenine), the mode and dose of kynurenine administration (100 mg/kg given i.p. vs. 300 mg/kg laced into daily chow), the amount of time elapsed between the final exposure to kynurenine and behavioral testing (8 vs. 21 days), the behavioral tests selected, and the age of the animals when the treatment was initiated (PD 27 vs. PD 42). Elucidation of these differences will likely provide important insights regarding the mechanism(s) linking early KP abnormalities to delayed behavioral deficits.
Two preferential targets of endogenous KYNA, NMDA and α7nACh receptors (Albuquerque and Schwarcz 2013; Moroni et al. 2012; Stone et al. 2013), play central roles in brain maturation in the perinatal period by participating in neuronal migration, cellular integration, and hippocampal neurogenesis (Hensch 2005; Huntley et al. 1994; Lozada et al. 2012; Russo and Taly 2012; Ultanir et al. 2007). It therefore seems reasonable to speculate that a prenatal elevation in KYNA levels, which will increase inhibition of both of these receptors, may have an adverse impact on developmental processes. This effect may be especially pronounced in the hippocampus, which is richly endowed with both receptors from an early stage in development (Ben-Ari et al. 1997; Dwyer et al. 2009; Falk et al. 2002; Jantzie et al. 2013). In addition, as both α7nACh and NMDA receptors are critically involved in learning and memory formation throughout life (Levin et al. 2006; Robbins and Murphy 2006), excessive inhibition of these receptors by elevated hippocampal KYNA levels (cf. Fig. 4A) may also be causally related to the behavioral deficits shown here in adult EKyn rats. Conversely, the normal KYNA levels in the brain of adult AdKyn rats (Fig. 4B) may explain why these animals performed normally in tests of contextual memory, and spatial learning and memory.
In adult rodents, elevation of brain KYNA causes a prompt reduction in extracellular glutamate levels in several regions including the hippocampus (Carpenedo et al. 2001; Pocivavsek et al. 2011; Wu et al. 2010). This effect, which appears to be mediated by inhibition of glutamate release via presynaptic α7nACh receptors (Alexander et al. 2012), has been proposed to be causally related to the array of cognitive and other behavioral changes that are observed when kynurenine or KYNA are applied acutely to adult animals (Alexander et al. 2012; Chess et al. 2007, 2009; Erhardt et al. 2004; Pocivavsek et al. 2011; Shepard et al. 2003). As several of these effects resemble phenomena that are observed in individuals with SZ, and as KYNA levels are elevated in brain and cerebrospinal fluid of patients (Erhardt et al. 2001; Schwarcz et al. 2001), KYNA may play a pathophysiologically significant part in the disease (cf. Introduction). The present demonstration of relevant long-term effects in EKyn, but not AdKyn, rats suggests that experimental KYNA elevation during a sensitive period of prenatal development can be used to examine the role of KP dysfunction in the etiology of SZ.
Ongoing studies in our laboratories use the new experimental paradigm to test adult EKyn rats for additional neurochemical, structural and behavioral abnormalities (Bortz et al. 2013; Pershing et al. 2013) and to study the trajectory of molecular and cellular changes that must occur in the brain of EKyn animals between birth and adulthood. We are especially intrigued by the mechanism(s) underlying the elevation in hippocampal KYNA levels in adult EKyn rats. This increase is also seen in the prefrontal cortex (Bruno et al. 2013) and, though less pronounced and so far only observed in microdialysis samples, in animals that are continuously treated with kynurenine between GD15 and PD21 (Pocivavsek et al. 2012). Preliminary studies indicate that this second surge in KYNA levels may be causally related to a delayed reduction in cerebral KMO activity, which is seen in adult EKyn, but not in adult AdKyn, rats (Pocivavsek et al. 2013).
Finally, we are also attempting to prevent the early accumulation of KYNA in the brain by administering kynurenine together with BFF-816, which selectively inhibits kynurenine aminotransferase II (KAT II), the major biosynthetic enzyme of KYNA in the rat brain (Guidetti et al. 2007; Wu et al. 2013). Together, these and complementary studies may have significant translational value for the treatment of SZ and other psychiatric disorders (Schwarcz et al. 2012; Wonodi and Schwarcz 2010).
Acknowledgments
This work was supported by USPHS grant MH83729 to JPB and RS.
Footnotes
No conflicts of interest to report
References
- Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr Bull. 2012;38:769–78. doi: 10.1093/schbul/sbq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol. 2013;85:1027–32. doi: 10.1016/j.bcp.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28. doi: 10.1016/j.neuroscience.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2012;220:627–37. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama N, Takahashi N, Saito S, Maeno N, Ishihara R, Ji X, Miura H, Ikeda M, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Yoshida K, Iwata N, Inada T, Ozaki N. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes Brain Behav. 2006;5:364–8. doi: 10.1111/j.1601-183X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1−/− mice. Int J Neuropsychopharmacol. 2010;13:475–85. doi: 10.1017/S1461145709990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Swartz KJ, Isacson O. Developmental changes in brain kynurenic acid concentrations. Brain Res Dev Brain Res. 1992;68:136–9. doi: 10.1016/0165-3806(92)90256-v. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–9. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bortz DM, Schwarcz R, Bruno JP. Mesolimbic regulation of prefrontal glutamate release is blocked by local kynurenic acid and restored with oral administration of a KAT II inhibitor. Soc Neurosci Abstr. 2013;38:255.12. [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JP, Vunck S, Pershing M, Pocivavsek A, Bortz D, Jorgensen C, Fredericks P, Leuner B, Schwarcz R. Elevations of brain kynurenic acid in prenatal rats result in long-lasting impairments in cortical development and cognitive flexibility: implications for schizophrenia. Neuropsychopharmacology. 2013;38:S108–09. [Google Scholar]
- Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–7. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- Castle D, Sham P, Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res. 1998;33:179–83. doi: 10.1016/s0920-9964(98)00070-x. [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, Schwarcz R. Perinatal kynurenine pathway metabolism in the normal and asphyctic rat brain. Amino Acids. 2000;19:311–23. doi: 10.1007/s007260070062. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325–31. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–21. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–39. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–60. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellstrom-Lindahl E. The alpha7 nicotinic receptors in human fetal brain and spinal cord. J Neurochem. 2002;80:457–65. doi: 10.1046/j.0022-3042.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Darlington LG, Stone TW. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013a;1504:1–15. doi: 10.1016/j.brainres.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, Stone TW. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience. 2013b doi: 10.1016/j.neuroscience.2013.09.034. pii:S0306-4522(13)00806-3. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–23. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Holtze M, Asp L, Karlsson H, Engberg G, Erhardt S. Behavioral disturbances in adult mice following neonatal influenza infection - possibly induced by a transient elevation of brain kynurenic acid levels. Soc Neurosci Abstr. 2010;35:363.6. [Google Scholar]
- Holtze M, Saetre P, Erhardt S, Schwieler L, Werge T, Hansen T, Nielsen J, Djurovic S, Melle I, Andreassen OA, Hall H, Terenius L, Agartz I, Engberg G, Jönsson EG, Schalling M. Kynurenine 3-monooxygenase (KMO) polymorphisms in schizophrenia: an association study. Schizophr Res. 2011;127:270–2. doi: 10.1016/j.schres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Vickers JC, Morrison JH. Cellular and synaptic localization of NMDA and non-NMDA receptor subunits in neocortex: organizational features related to cortical circuitry, function and disease. Trends Neurosci. 1994;17:536–43. doi: 10.1016/0166-2236(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Suckow RF, Xie S, Bucci DJ. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia. Schizophr Res. 2013 doi: 10.1016/j.schres.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. Developmental expression of N-methyl-D-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb Cortex. 2013 doi: 10.1093/cercor/bht246. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res. 2009;197:144–9. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, Schuett C. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One. 2010;5:e11825. doi: 10.1371/journal.pone.0011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Hintz K, Shearer EM, Barch DH, Riffin C, Whitley K, Butler R. A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med Hypotheses. 2010;74:555–63. doi: 10.1016/j.mehy.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–61. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–99. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204:306–12. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci. 2012;32:7651–61. doi: 10.1523/JNEUROSCI.6246-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.011. pii:S0006-3223(13)00638-0. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Miura H, Ando Y, Noda Y, Isobe K, Ozaki N. Long-lasting effects of inescapable-predator stress on brain tryptophan metabolism and the behavior of juvenile mice. Stress. 2011;14:262–72. doi: 10.3109/10253890.2010.541539. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119:133–9. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295:681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, Nordin C, Karanti A, Persson P, Erhardt S. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Pershing ML, Bortz D, Pocivavsek A, Fredericks PJ, Leuner B, Jørgensen CV, Schwarcz R, Bruno JP. Prenatal kynurenic acid elevation alters cortical development and prefrontal glutamate release, corresponding to cognitive inflexibility in adults. Soc Neurosci Abstr. 2013;38:255.11. [Google Scholar]
- Pocivavsek A, Thomas MAR, Elmer GI, Bruno JP, Schwarcz R. Chronic prenatal kynurenine elevation in rats: A naturalistic model of schizophrenia with biochemical abnormalities and deficits in hippocampal-mediated learning and memory. Soc Neurosci Abstr. 2013;38:255.10. [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–67. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–48. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röver S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem. 1997;40:4378–85. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- Russo P, Taly A. alpha7-Nicotinic acetylcholine receptors: an old actor for new different roles. Curr Drug Targets. 2012;13:574–8. doi: 10.2174/138945012800398874. [DOI] [PubMed] [Google Scholar]
- Saito K, Markey SP, Heyes MP. Chronic effects of gamma-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res. 1991;546:151–4. doi: 10.1016/0006-8993(91)91171-v. [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–62. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–43. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Levin ED. Glutamate and nicotinic receptor interactions in working memory: importance for the cognitive impairment of schizophrenia. Neuroscience. 2011;195:21–36. doi: 10.1016/j.neuroscience.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Trecartin KV, Bucci DJ. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr Res. 2011;133:156–8. doi: 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA. 2007;104:19553–8. doi: 10.1073/pnas.0704031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–6. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Widner B, Ledochowski M, Fuchs D. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr Drug Metab. 2000;1:193–204. doi: 10.2174/1389200003339063. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36:211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665–74. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–10. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]