Abstract
Preclinical studies suggest that prior treatment with escalating doses of methamphetamine (METH) attenuates the persistent deficits in hippocampal serotonin (5-hydroxytryptamine; 5HT) transporter (SERT) function resulting from a subsequent “binge” METH exposure. Previous work also demonstrates that brain derived neurotrophic factor (BDNF) exposure increases SERT function. The current study investigated changes in hippocampal BDNF protein and SERT function in rats exposed to saline or METH self-administration prior to a binge exposure to METH or saline. Results revealed that METH self-administration increased hippocampal mature BDNF (mBDNF) immunoreactivity compared to saline-treated rats as assessed 24 h after the start of the last session. Further, mBDNF immunoreactivity was increased and SERT function was not altered at this timepoint in rats that self-administered METH prior to the binge METH exposure. These results suggest that prior exposure to contingent METH increases hippocampal mBDNF, and this may contribute to attenuated deficits in SERT function.
Keywords: Methamphetamine, Self-administration, Serotonin Transporter, Brain Derived Neurotrophic Factor, Hippocampus
Introduction
Methamphetamine (METH) abuse is a serious worldwide health problem. METH abusers often display structural and neurochemical changes within the brain and higher rates of psychiatric disorders and cognitive deficits (for review, see Chang et al., 2007; Panenka et al., 2013). Specifically, positron emission tomography has indicated decreased serotonin (5-hydroxytryptamine; 5HT) transporter (SERT) densities in several brain regions in abstinent METH users which were associated with an increased magnitude of aggression (Sekine et al., 2006). However, postmortem studies found no significant decreases in SERT immunoreactivity in the hippocampus of METH users (Kish et al., 2009). These studies emphasize the need for study of the neurochemical changes in the hippocampus following METH exposure.
Recent studies have suggested that prolonged escalating-dose pretreatments by either noncontingent or contingent administration more closely models some aspects of human METH abuse (Lacan et al., 2013; Krasnova et al., 2013). Of note these dosing paradigms often lead to attenuated persistent deficits compared to those animals only given METH in a “binge-like” pattern (e.g., 3–6 injections, 7.5–50 mg/kg/injection, 2–8-h intervals; Johnson-Davis et al., 2003; Belcher et al., 2008; Cadet et al., 2009; McFadden et al., 2012A,B). Specifically, the protection afforded by the escalating doses of METH results in attenuated persistent deficits in 5HT content and SERT binding in the hippocampus (Johnson-Davis et al., 2003; Belcher et al., 2008). Prior self-administration of METH that leads to escalating METH intake also attenuates the persistent deficits induced by a binge METH exposure in the hippocampus and striatum (McFadden et al., 2012A,B). Despite these findings, the mechanism underlying this protection is not clearly understood.
Brain-derived neurotrophic factor (BDNF) has been shown to regulate SERT function (Benmansour et al., 2008). Infusions of BDNF increase SERT function in the hippocampus as measured by in vivo chronoamperometry and microdialysis (Benmansour et al., 2008). Further, BDNF can increase terminal sprouting in serotonergic neurons following a serotonergic lesion (Mamounas et al., 2000). These findings suggest that BDNF can influence the serotonergic system.
BDNF is upregulated following insults to the central nervous system (Braun et al., 2011). Whereas the mature form of BDNF (mBDNF) promotes the growth, development, differentiation and maintenance of neuronal systems, neuronal plasticity, synaptic activity and neurotransmitter-mediated activities, the precursor of BDNF (proBDNF) when bonded to the p75 neurotrophin receptor promotes apoptosis (Koshimizu et al., 2010). Rodent studies have found METH alters BDNF mRNA in various regions of the brain (Cadet et al., 2009; Braun et al., 2011; Cadet et al., 2011). Recently, time dependent changes in BDNF proteins were found in the dorsal striatum following METH self-administration (Krasnova et al., 2013). Similarly, human METH users often have elevated blood serum levels of BDNF (Kim et al., 2005). Of note, BDNF levels decreased with prolonged abstinence in human psychostimulant users (Hilburn et al., 2011). These findings suggest that BDNF may play a role in the neurochemical changes following METH use. Therefore, the purpose of the current study was to investigate the changes in BDNF as they related to changes in serotonergic function in hippocampus following METH self-administration and a binge exposure to the drug.
Methods
Animals
Adult male Sprague-Dawley rats (275–300 g; Charles River Laboratories, Portage, MI) were housed four rats/cage. Following surgery, each rat was individually housed in a transparent plastic cage. Water was available in their home cage ad libitum. During food training, rats were food restricted such that no rat dropped below 90% of their body weight at the time the experiment was initiated. Rats were maintained under the same 14:10 h light/dark cycle in the animal facility and in the operant chambers. Animals were sacrificed by decapitation. All experiments were approved by the University of Utah’s Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Self-Administration & METH Challenge
Food training and self-administration occurred in an operant chamber (Coulbourn Instruments, Whitehall, PA USA) as described in McFadden et al., 2012A,B. Rats underwent 7 d of self-administration (8 h/session; FR1; 0.12 mg/infusion racemic-METH expressed as free-base or saline; generously supplied by the National Institute on Drug Abuse, National Institute of Health, Bethesda, MD) during the light cycle as previously described (McFadden et al., 2012A,B). For each active lever press, an infusion pump delivered 10 ul of METH or saline over a 5-s duration. During this period, both levers were retracted. Following the infusion, the levers remained retracted for an additional 20 s. Pressing the inactive lever resulted in no programmed consequences although it was recorded. Animals were sacrificed 24 h after the start of the last self-administration session or received a binge of METH or saline. Twenty-four h after the start of the last self-administration session, rats were challenged with 4 injections of METH (7.5 mg/kg/injection; 2-h interval) or saline (1 ml/kg/injection) as described previously (McFadden et al., 2012A,B). Animals were sacrificed 24 h after the binge exposure.
Synaptosomal [3H]5HT uptake
[3H]5HT uptake was determined using a rat hippocampal synaptosomal preparation as previously described (McFadden et al., 2012,B). In brief, synaptosomes were prepared by homogenizing freshly dissected hippocampal tissue in ice-cold 0.32 M sucrose pH 7.4, and centrifuged (800×g, 12 min; 4°C). The supernatants were centrifuged (22,000×g, 15 min; 4°C) and the resulting pellets were resuspended in ice-cold assay buffer (in mM: 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 glucose and 1 ascorbic acid; pH 7.4) and 1 µM pargyline. Samples were incubated for 10 min at 37 °C and the assays initiated by the addition of [3H]5HT (5 nM final concentration). Following incubation for 3 min, samples were placed on ice to stop the reaction. Samples were then filtered through GF/B filters (Whatman, Florham Park, NJ) soaked previously in 0.05% polyethylenimine. Filters were rapidly washed three times with 3 ml of ice-cold 0.32M sucrose buffer using a filtering manifold (Brandel, Gaithersburg, MD). Nonspecific values were determined in the presence of 10 µM fluoxetine. Radioactivity trapped in filters was counted using a liquid scintillation counter. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories Inc., Hercules, CA).
Western Blotting
Equal quantities of protein (10 µg) of hippocampal synaptosomes were loaded into each well of a 4 to 12% NuPAGE Novex Bis-Tris Midi gradient gel (Invitrogen, Carlsbad, CA) and electrophoresed using a XCell4 Surelock Midi-cell (Invitrogen). Membranes were blocked for 45 min with Starting Block Blocking Buffer (Pierce Chemical, Rockford, IL) and incubated for 1 h at room temperature with an anti-BDNF polyclonal antibody (N-20; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The polyvinylidene difluoride membrane was then washed five times in Tris-buffered saline with Tween (250 mM NaCl, 50 mM Tris, pH 7.4, and 0.05% Tween 20). The membranes were then incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody (BioSource International, Camarillo, CA). After five washes in Tris-buffered saline with Tween, the bands (mBDNF- 14 kDa and its precursor proBDNF- 32 kDa; Molteni et al., 2009) were visualized using Western Lightning Chemiluminescence Reagents Plus (PerkinElmer Life and Analytical Sciences) and were quantified by densitometry using a FluorChem SP Imaging System (Alpha Innotech, San Leandro, CA). Membranes were then stripped with Restore Western Blot Stripping Buffer (Thermo Scientific, Pittsburgh, PA), blocked for 45 min with Starting Block Blocking Buffer (Pierce Chemical, Rockford, IL), and incubated for 1 h at room temperature with an anti-β-Actin primary antibody (13E5, Cell Signaling Technology, Danvers, MA). Membranes were then washed, incubated in secondary antibody, washed, and visualized (β-Actin-45 kDa) as described above. Protein concentrations were determined using the Bradford Protein Assay.
Statistical Analysis
Statistical analysis was conducted in GraphPad Prism (La Jolla, CA). Statistical analyses among groups were conducted using a t-test or analysis of variance (ANOVA) followed by Newman-Keuls posthoc analyses. The data represent means ± standard error of the mean (S.E.M.) of 6–14 rats/group.
Results
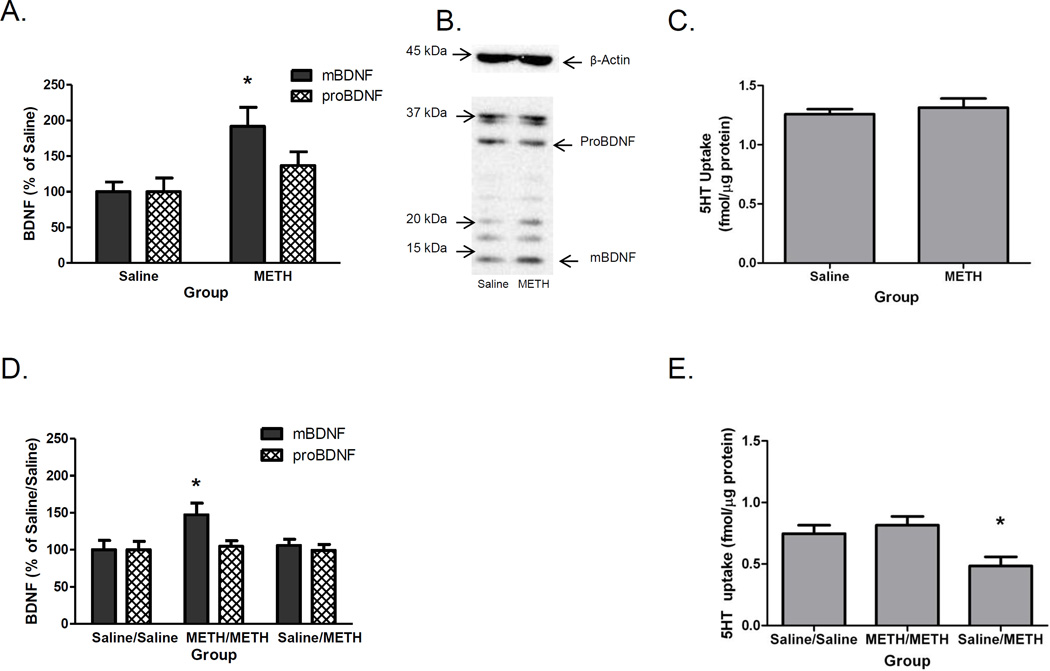
Results presented in Figure 1A and B indicate that METH self-administration increased hippocampal mBDNF (t(13)=3.197, p<0.05), but not proBDNF (t(13)=1.343, ns) immunoreactivity or β-Actin immunoreactivity (t(13)=0.78, ns; Saline: 1062±102.20 arbitrary units; METH: 1168±89.12 arbitrary units) as assessed 24 h after the last self-administration session. Previous reports have shown SERT function was unaltered at this time point in METH self-administering rats ((t(12) = 0.62, ns; Figure 1C; from McFadden et al., 2012B with permission). This time point corresponded with the first injection of METH during the multiple METH exposure. During self-administration, rats self-administered 2.38±0.17 mg METH on D1 and increased intake to 3.67±0.31 mg METH on D7.
Figure 1.
Prior METH self-administration increases mBDNF immunoreactivity 24 h after the start of the last self-administration session (Panels A and B) and results in no change in SERT function (Panel C), and increases mBDNF immunoreactivity (Panel D) and SERT function (Panel E) 24 h after the binge exposure to METH. Rats self-administered METH (0.12 mg/infusion) or saline (10 µl/infusion) for 7 d (8 h/d). Twenty-four hours after the beginning of the final self-administration session, rats were sacrificed (Panel A, B, and C) or received METH (4 × 7.5 mg/kg/injection, s.c., 2-h intervals) or saline (1 ml/kg/injection, s.c., 2-h intervals) and were sacrificed 24 h later (Panels D and E), and BDNF immunoreactivity or SERT function was assessed in the hippocampus. Panel B: Representative β-Actin (~45 kDa), proBDNF immunoreactivity (~32 kDa) and mBDNF immunoreactivity (~14 kDa) in Saline (Left) and METH (Right) self-administering rats shown in Panel A. *p < 0.05 differs from all other groups
Similarly, when animals were sacrificed 24 h after the last injection of the binge exposure, no decreases in SERT function (Figure 1E) and an increase in mBDNF (Figure 1D) occurred in METH/METH animals compared to the saline control animals. Only rats that had previously self-administered saline prior to the binge METH exposure had a significant decrease in SERT function (F(2,17)=5.792, p<0.05) compared to the Saline/Saline group. The lack of significant decreases in SERT function 24 h after the binge exposure in the METH/METH group was associated with a significant increase in mBDNF immunoreactivity (F(2,16)=4.405, p<0.05; Figure 1D), but no significant change in proBDNF immunoreactivity (F(2,16)=0.116, ns; Figure 1D) or β-Actin immunoreactivity (F(2,16)=0.58, ns; Saline/Saline: 963.50±95.46 arbitrary units; METH/METH: 906.30±39.60 arbitrary units; Saline/METH: 867.20±41.40 arbitrary units). During self-administration, rats self-administered 2.13±0.22 mg METH on D1 and increased to 3.44±0.26 mg METH by D7.
Discussion
Previous reports have shown prior METH exposure can attenuate persistent serotonergic deficits in the hippocampus following binge exposure to the drug (Johnson-Davis et al., 2003; Belcher et al., 2008; McFadden et al., 2012B). No significant decrease in SERT function was observed 24 h following the binge METH exposure in the animals that were allowed to self-administer METH prior to the binge. The increase in SERT function 24 h after the binge METH exposure in the METH/METH group compared to the Saline/METH may contribute to attenuations of the persistent neurotoxic deficits in hippocampal SERT function or binding and 5HT content afforded by prior escalating dose METH exposures (Johnson-Davis et al., 2003; Belcher et al., 2008; McFadden et al., 2012B).
It is possible that an increase in mBDNF contributes to the elevated SERT function 24 h after the binge exposure. Both intracerebroventricular and local infusions of BDNF increase SERT function in the hippocampus as measured by in vivo chronoamperometry and microdialysis (Benmansour et al., 2008). Of note, these changes were seen within 30–120 min following BDNF infusions and were not associated with increases in SERT binding densities suggesting that these are acute changes in SERT function (Benmansour et al., 2008). Further evidence for the role of BDNF in regulating the SERT function comes from heterozygous BDNF mice that have lower BDNF protein levels (Daws et al., 2007). Adult 5 and 10 month old BDNF(+/−) have reduced rates of 5HT clearance in the hippocampus compared to wild-type mice but have similar SERT binding (Daws et al., 2007). Similarly, 3–4 month old BDNF(+/−) mice have reduced synaptosomal SERT uptake compared to wild-type mice in the hippocampus (Guiard et al., 2008). These findings suggest that BDNF can affect SERT functioning.
In light of the previous research, the results of the present study suggest that increases in mBDNF protein by METH self-administration can contribute to the regulation of SERT function. Of interest, previous studies have reported no significant difference in SERT function 24 h or 8 d after the start of the last self-administration session (McFadden et al., 2012B), but slight decreases in SERT immunoreactivity following METH self-administration (Reichel et al., 2012). The increase in mBDNF following METH self-administration may have increased SERT function thus resulting in no differences in SERT function (McFadden et al., 2012B) but differences in SERT immunoreactivity (Reichel et al., 2012). Further, the significant increase in mBDNF in the animals that received a binge of METH following METH self-administration may contribute to the lack of significant decreases of SERT function 24 h after the binge exposure to METH. It can be speculated that the observed changes in mBDNF following contingent METH exposure may be a contributing factor underlying METH-induced tolerance to serotonergic deficits.
Previous studies have shown trophic factors can increase terminal sprouting in serotonergic neurons (Mamounas et al., 2000). For example, the administration of BDNF into the hippocampus promoted the regenerative sprouting of serotonergic axons with a pattern of reinnervation reminiscent of normal 5-HT innervation when given 1 week following a neurotoxic para-chloroamphetamine (PCA) exposure (Mamounas et al, 2000). It was suggested that the sprouting of these terminals was due in part to a retrograde signaling mechanism which takes between 4–7 d to initiate sprouting (Mamounas et al., 2000). It is unknown if the increases in mBDNF found in the METH/METH group 24 h following the binge exposure persists and therefore may help promote sprouting of serotonergic terminals to attenuate persistent deficits. Future studies are required to investigate this.
These findings are consistent with reports by Marshall and colleagues in that wheel running can attenuate persistent serotonergic deficits as a result of binge exposure to METH (O’Dell et al., 2012). Voluntary exercise has been previously shown to increase trophic factors in the hippocampus including BDNF (Griesbach et al., 2008). Wheel running prior to and following the binge exposure to METH may increase trophic factors including mBDNF similar to those afforded by METH self-administration and result in decreased persistent serotonergic deficits including losses in SERT function/binding and 5HT content following binge exposure to METH, similar to what was observed in the current study.
In summary, prior METH exposure through escalating doses or self-administration decreases persistent serotonergic deficits within the hippocampus (Johnson-Davis et al., 2003; Belcher et al., 2008; McFadden et al., 2012B), however the mechanism underlying this phenomenon is unknown. The findings of the current study suggest that prior METH self-administration increases mBDNF in the hippocampus, perhaps as a compensatory mechanism, which may in turn influence SERT function and the response of serotonergic systems to a neurotoxic METH administration. Because previous studies suggested that BDNF can increase SERT function, it can be speculated that BDNF may be responsible for the increased in SERT function in the METH/METH group compared to the Saline/METH group. The observed increase in mBDNF in the METH/METH group may have also lead to an increase in serotonergic axonal sprouting at later time points and thus resulted in the attenuated decreases in persistent serotonergic deficits induced by the binge exposure to METH as previously reported (McFadden et al., 2012B). The putative role of key BDNF-associated pathways including tyrosine receptor kinase B, phosphorilated extracellular signal-regulated kinases (ERK)/total ERK ratios, and phospho-AKT/total AKT ratios (Koshimizu et al., 2010) in these phenomena remains to be explored. No changes were observed in proBDNF immunoreactivity. Of interest are findings that pro-BDNF levels were unchanged by the treatments under study, perhaps reflective of findings that neurons have a “limited capacity to process pro-BDNF” (Matsumoto et al., 2008). Notwithstanding, the current study suggests that BDNF within the hippocampus may play an important role SERT function following a binge exposure to METH. Future studies are needed to demonstrate the potential role of BDNF as a mechanism underlying METH induced tolerance.
Acknowledgements
Funding for this study was provided by NIH grants: DA033097, 011389, 019447, 000378, 013367, 031883.
Footnotes
Statement of Interests METH was generously supplied by the National Institute on Drug Abuse, National Institute of Health, Bethesda, MD.
References
- 1.Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33(6):1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 2.Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587(1–3):90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 3.Braun AA, Herring NR, Schaefer TL, Hemmerle AM, Dickerson JW, Seroogy KB, Vorhees CV, Williams MT. Neurotoxic (+)-methamphetamine treatment in rats increases brain-derived neurotrophic factor and tropomyosin receptor kinase B expression in multiple brain regions. Neuroscience. 2011;184:164–171. doi: 10.1016/j.neuroscience.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadet JL, Brannock C, Ladenheim B, McCoy MT, Beauvais G, Hodges AB, Lehrmann E, Wood WH 3rd, Becker KG, Krasnova IN. Methamphetamine preconditioning causes differential changes in striatal transcriptional responses to large doses of the drug. Dose Response. 2011;9(2):165–181. doi: 10.2203/dose-response.10-011.Cadet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadet JL, Krasnova IN, Ladenheim B, Cai NS, McCoy MT, Atianjoh FE. Methamphetamine preconditioning: differential protective effects on monoaminergic systems in the rat brain. Neurotox Res. 2009;15(3):252–259. doi: 10.1007/s12640-009-9026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl. 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 7.Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101(3):641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 8.Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154(2):530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guiard BP, David DJ, Deltheil T, Chenu F, Le Maître E, Renoir T, Leroux-Nicollet I, Sokoloff P, Lanfumey L, Hamon M, Andrews AM, Hen R, Gardier AM. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11(1):79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- 10.Hilburn C, Nejtek VA, Underwood WA, Singh M, Patel G, Gangwani P, Forster MJ. Is serum brain-derived neurotrophic factor related to craving for or use of alcohol, cocaine, or methamphetamine? Neuropsychiatr Dis Treat. 2011;7:357–364. doi: 10.2147/NDT.S18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482(1–3):151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, Kim YK. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 2005;388(2):112–115. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 13.Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202(4):649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- 14.Koshimizu H, Hazama S, Hara T, Ogura A, Kojima M. Distinct signaling pathways of Precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neurosci Lett. 2010;473(3):229–232. doi: 10.1016/j.neulet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 15.Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL. CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis. 2013;58:132–143. doi: 10.1016/j.nbd.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laćan G, Hadamitzky M, Kuczenski R, Melega WP. Alterations in the striatal dopamine system during intravenous methamphetamine exposure: Effects of contingent and noncontingent administration. Synapse. 2013;67(8):476–488. doi: 10.1002/syn.21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20(2):771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11(2):131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 19.McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012A;340(2):295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFadden LM, Hunt MM, Vieira-Brock PL, Muehle J, Nielsen SM, Allen SC, Hanson GR, Fleckenstein AE. Prior methamphetamine self-administration attenuates serotonergic deficits induced by subsequent high-dose methamphetamine administrations. Drug Alcohol Depend. 2012B;126(1–2):87–94. doi: 10.1016/j.drugalcdep.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molteni R, Calabrese F, Cattaneo A, Mancini M, Gennarelli M, Racagni G, Riva MA. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology. 2009;34(6):1523–1532. doi: 10.1038/npp.2008.208. [DOI] [PubMed] [Google Scholar]
- 22.O'Dell SJ, Galvez BA, Ball AJ, Marshall JF. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse. 2012;66(1):71–80. doi: 10.1002/syn.20989. [DOI] [PubMed] [Google Scholar]
- 23.Panenka WJ, Procyshyn RM, Lecomte T, Macewan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: A comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62(2):1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63(1):90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]