Abstract
Background
In healthy individuals, voluntary modification of self-relevance has proven effective in regulating subjective emotional experience as well as physiologic responses evoked by emotive stimuli. As social anxiety disorder (SAD) is characterized by both altered emotional and self-related processing, we tested if emotion regulation through self-focused reappraisal is effective in individuals with SAD.
Methods
While undergoing 3 T functional magnetic resonance imaging, individuals with SAD and matched healthy controls either passively viewed neutral and aversive pictures or actively increased or decreased their negative emotional experience through the modification of self-relevance or personal distance to aversive pictures. Participants rated all pictures with regard to the intensity of elicited emotions and self-relatedness.
Results
We included 21 individuals with SAD and 23 controls in our study. Individuals with SAD reported significantly stronger emotional intensity across conditions and showed a nonsignificant tendency to judge pictures as more self-related than controls. Compared with controls, individuals with SAD showed an overactivation in bilateral temporoparietal regions and in the posterior midcingulate cortex during the passive viewing of aversive compared with neutral pictures. During instructed emotion regulation, activation patterns normalized and no significant group differences were detected.
Limitations
As no positive pictures were presented, results might be limited to the regulation of negative emotion.
Conclusion
During passive viewing of aversive images, individuals with SAD showed evidence of neural hyperreactivity that may be interpreted as increased bodily self-consciousness and heightened perspective-taking. During voluntary increase and decrease of negative emotional intensity, group differences disappeared, suggesting self-focused reappraisal as a successful emotion regulation strategy for individuals with SAD.
Introduction
Emotions are proposed to involve a “person–situation interaction that engages attention, has meaning to an individual, and causes a coordinated yet malleable multisystem response to the interaction,”1 including a subjectively experienced feeling component.2 Emotion regulation refers to processes that alter the nature, magnitude and duration of these multisystem responses and feelings.3 Once placed in an emotion-evoking situation, an individual can deploy attentional mechanisms for emotion regulation (e.g., through distraction or concentration) or use “cognitive reappraisal,” whereby he or she modifies the situation’s emotional importance by reinterpreting the outcomes and actions of the situation itself (situation-focused reappraisal) or by altering the situation’s personal relevance (self-focused reappraisal).
Emotion regulation by self-focused reappraisal has proven effective in decreasing negative emotional experience4 and altering physiologic responses in both the central3,5 and the autonomic nervous system.6
Social anxiety disorder
Social anxiety disorder (SAD) is a frequent anxiety disorder marked by an intense and persistent fear of interpersonal interactions, leading to their avoidance or endurance with distress. A central component of SAD is the perception of a scrutinizing audience.7 Individuals with SAD are especially afraid of doing or saying something embarrassing or of showing symptoms of their anxiety. Social anxiety disorder is classified as a phobic disorder in DSM-IV (300.23). It has a lifetime prevalence of up to 12%, with higher rates among girls and women and a mean symptom onset in late childhood or early adolescence.8
Anxiety disorders are marked by an attentional bias toward aversive stimuli or threat cues,9 and emotional hyper-reactivity or heightened saliency of negative emotional information has also been repeatedly observed in individuals with SAD.10 These alterations can be understood as stemming from facilitated bottom–up emotion processing and/or deficient top–down emotion regulation.11
In general, when not given explicit instructions on how to regulate their emotions, patients with anxiety disorders do not modulate their emotions as well as healthy individuals.12 However, there is evidence that, when instructed, individuals with SAD show the same degree of reduction in negative emotion as controls.13,14
Social anxiety disorder symptomatology is also marked by changes in self-related processing: individuals with SAD show atypical self-referential processing,15 manifesting as increased self-focused attention and a negativity bias concerning self-related beliefs and self-judgments.16 Self-related processes, therefore, are major components in explanatory models of SAD17–19 and are the focus of therapeutic interventions.20,21 However, it remains unclear how changes in self-related processing affect self-focused reappraisal performance.
On one hand, increased self-focused attention in individuals with SAD has been suggested to occur at the expense of external stimuli.17 While this is thought to contribute to SAD symptomatology by inhibiting the processing of nonnegative (e.g., positive) external stimuli, for aversive stimuli it could facilitate an active decrease of negative emotional experience through self-focused reappraisal. On the other hand, affective prioritization of negative stimuli in individuals with SAD would propose inverse effects, facilitating upregulation of negative intensity. It is therefore an open question how effective specifically self-focused reappraisal strategies are in individuals with SAD regarding both subjective emotional experience and neural activation patterns.
Neural correlates of aberrant emotion processing in individuals with SAD
Functional MRI studies of patients with SAD associated increased attention for and processing of social stimuli mainly with hyperactivation in brain areas subserving emotional experience, such as the amygdala and insula.22,23 It is important to note, however, that while self-reported anxiety and sensitivity to negative stimuli are robustly observed, several studies reported no group differences in insula and amygdala reac-tivity.24–26 This suggests that hyperactivation of emotion processing areas in patients with SAD depends on experimental details, such as stimulus type and modality25 or task complexity.26 In addition, increased activation during the processing of emotional faces has also been observed in other brain regions, such as the lateral and medial temporal and parietal cortices.27,28
Few fMRI experiments have explicitly studied emotion regulation using pictorial stimuli in patients with SAD: Brühl and colleagues29 instructed individuals with SAD to use the therapeutic emotion regulation strategy of “reality checking,” during which the participant tries to evaluate the “complete reality” like an outside observer. The authors showed that emotion regulation reduced activity not only in the insula, amygdala and medial thalamus, but also in widespread temporoparietal and frontal regions, whereas activation in prefrontal regions was not altered. The authors concluded that while emotion regulation influences emotion processing structures, patients with SAD show disturbed general emotion regulating circuits.
Goldin and colleagues13 used harsh faces (social threat), violent scenes (physical threat) and nonsocial neutral scenes (control condition) as stimuli. Participants were instructed to reinterpret the content of the picture through self-focused reappraisal for harsh faces (e.g., “This does not involve me”) and situation-focused reinterpretation for violent scenes (e.g., “The person will be okay”). Compared with healthy controls, patients with SAD showed greater brain activation in various areas involved in emotional, visual, facial and sensory processing during passive viewing of social threat stimuli compared with neutral scenes. Self-focused emotion regulation revealed an underactivation of a multitude of areas related to cognitive and attention regulation distributed over the entire cortex.
Blair and colleagues30 instructed participants to either passively view pictures, to make a picture’s content more positive (downregulating a negative and upregulating a positive image) or to make a picture’s content more negative (upregulating a negative and downregulating a positive image) without providing concrete instructions on how to achieve this. Using a separate task, the study functionally localized active areas during top–down attentional control. In both tasks and compared with controls, patients with anxiety disorders (SAD, generalized anxiety disorder or both conditions comorbidly) showed decreased activation in the dorsal anterior cingulate cortex and in parietal regions previously implicated in emotion regulation.
In summary, prior brain activation studies in individuals with SAD have found hyperreactivity of emotional structures,13 which can be effectively downregulated.29 In addition, cognitive control systems were found to be either generally disturbed13 or less efficiently activated29,30 during voluntary emotion regulation in individuals with SAD. These emotion regulation areas differed among studies and comprised regions located mainly in the lateral frontal,13,29 medial frontal13,30 and lateral parietal cortices.13,30
Hypotheses
Alterations in emotional and self-related processing are central to SAD symptomatology. It remains unclear how these processes interact and how accessible they are to voluntary modulation. We therefore tested how effectively individuals with SAD can use specifically self-focused (compared with situation-focused) reappraisal to up- and downregulate negative emotional intensity regarding both the patient’s subjective experience and brain activation patterns.
We expected anxiety to manifest as increased intensity of negative emotional experience in patients with SAD during passive viewing of aversive and potentially during neutral pictures. In the absence of sufficient prior empirical evidence regarding specifically self-focused reappraisal, no directed hypotheses were established for group differences in subjective experience during regulation. At the brain level, we hypothesized group differences during both unaltered emotional processing and emotion regulation. Despite differences in regulation strategies, we followed previous studies and expected hyperactivity in emotional structures together with decreased activation in control areas, such as the anterior cingulate and lateral frontoparietal regions, in individuals with SAD compared with controls.
Methods
Participants
We recruited patients with SAD over the Internet and through printed advertisements, and we recruited healthy controls through notices in public spaces and through university mailing lists. Groups were matched for sex, age, handedness (as assessed by the Edinburgh Handedness Inventory31), education and weight. Included patients had to meet DSM-IV criteria32 for current SAD. Diagnostic status was established in all participants using the structured clinical diagnostic interviews for DSM-IV Axis I (SCID-I33) and dissociative disorders (SCID-D34) as well as the International Personality Disorder Examination (IPDE35).
Exclusion criteria were any current mental disorders other than affective and anxiety disorders; lifetime psychotic, bipolar or neurologic disorders; MRI contraindication (e.g., magnetic or electronic implants); psychopharmacological treatment other than antidepressants; current or past abuse of alcohol/narcotics; pregnancy; claustrophobia; insufficient proficiency in German; and noise sensitivity or tinnitus. Our local ethics committee approved our study, and all participants gave their written informed consent.
Assessment
We assessed symptom severity using the German version of the self-report Liebowitz Social Anxiety Scale36 (Cronbach α = 0.985). For this questionnaire, 24 items are scored independently for frequency and duration on 4-point Likert-type scales. To fully characterize the 2 samples, participants completed the German versions of the Beck Depression Inventory (BDI-II37), the trait version of the State-Trait Anxiety Inventory (STAI-T38), the Toronto Alexithymia Scale (TAS-2039), the Trail-making Test (TMT40) parts A and B, the questionnaire for functional and dysfunctional self-focused attention (DFS41), the Kentucky Inventory of Mindfulness Skills (KIMS42), the Emotion Regulation Questionnaire (ERQ43), and the Interpersonal Reactivity Index (SPF-IRI44). As self-focused attention is an integral aspect of SAD symptomatology, we assessed both functional and dysfunctional forms of self-focused attention (KIMS and DFS). Similarly, we included a measure of executive functioning (TMT) to ensure comparability of our 2 samples.
Procedures
Emotion regulation task
In an extended variant of a previously published paradigm,5 participants were shown 54 aversive (valence: mean 3.02 ± 0.70; arousal: mean 6.00 ± 0.92) and 18 neutral (valence: mean 5.30 ± 0.57; arousal mean 3.37 ± 0.67) pictures from the International Affective Picture System (IAPS45). Normative ratings for IAPS pictures exist on 9-point scales of valence (1 = unpleasant, 9 = pleasant) and arousal (1 = calm, 9 = excited). Three sets consisting of 18 aversive and 6 neutral pictures each were matched for mean valence and mean arousal and for the depicted number of animals, humans, human faces, objects of threat and picture luminance. Stimuli were randomly assigned to 3 runs. Stimulus–condition pairing and the sequence of conditions were randomized with the constraint of a maximum of 2 consecutive trials of the same condition. In a slow event-related design, each picture was presented for 8 seconds after a 2-second instruction word. Picture presentation was followed by a rating period of 4 seconds during which participants indicated the intensity of their negative emotions by button press with their right hand on a 7-point Likert-type scale (1 = weak, 7 = strong). A 10.7-second fixation period completed the event.
There were 4 instruction conditions: participants were asked to passively view the pictures, leaving their emotional experience unaltered during neutral (permit neutral [PermN]) and aversive pictures (permit aversive [PermA]). In 2 further conditions, emotions were either intensified or downregulated. Importantly, emotion regulation was achieved exclusively through self-focused reappraisal, that is, through altering the personal relevance or subjective distance to the depicted situation. Instructions in the decrease or distancing condition (DistA) were to “take the role of a distanced observer, imagining that the depicted situation does not concern you.” Example tactics were “taking a professional distance or imagining the picture to shrink.” For the increase condition (IncA), participants were asked “to be personally affected by the situation” potentially by imagining “to be in the midst of the situation, perceiving smells, noises and movements” or “letting the image grow.”
Prior to scanning, participants completed a training session, including a careful assessment of the strategies used to ensure self-focused as opposed to situation-focused reappraisal. If necessary, the experimenter gave further verbal instruction to shape the emotion regulation strategy and the training session was repeated.
Functional MRI data acquisition
We obtained images using a Tim Trio 3 T MRI scanner (Siemens) with a 12-channel head coil at the Berlin Center for Advanced Neuroimaging. High-resolution T1-weighted anatomic images were acquired at the beginning of each session using a 3-dimensional magnetization-prepared rapid gradient-echo sequence with 192 sagittal slices of 1 mm thickness, repetition time (TR) 1900 ms, echo time (TE) 2.52 ms, flip angle 9°, field of view (FOV) 256 mm. During the task, including baseline scans at the beginning (10 volumes) and end (5 volumes) of each run, we recorded 3 × 277 (over 31 min 10 s) functional T2*-weighted volumes with an echo-planar gradient echo pulse sequence to measure blood oxygen level–dependent (BOLD) signal changes (TR 2250 ms, TE 25 ms, flip angle 80°, FOV 192 mm), and we acquired 37 slices of 3 mm thickness with 20% distance factor in descending order. We used cushions to minimize head motion during scanning. Visual stimuli inside the MRI scanner were delivered through a goggle system (VisualSystem by Nordic NeuroLab), which was attached to the head coil, carefully adjusted for the individual participant and set at a resolution of 800 × 600 pixels with 32-bit colour depth. Responses were collected using a button box (Current Designs Inc.).
Postscan rating of self-relatedness
After the scan, all pictures were presented again in a new sequence for a fixed duration of 5 seconds each. Participants rated the strength of self-relatedness or “personal association” of each picture on a 7-point Likert-type scale (1 = weak, 7 = strong).
Training, inscan and postscan experiments were presented using Presentation software v14.9 (Neurobehavioural Systems, www.neurobs.com).
Data analysis
Statistical analyses were conducted using SPSS software version 20.0 and R software (R Core Team, www.R-project.org/), applying a statistical threshold of p < 0.05. We tested the data for normality using the Kolmogorov–Smirnov test and for homoscedasticity using the Levene test. For group comparisons, 2-sided independent Student t tests and Welch t tests were used accordingly. We assessed behavioural effects using a 2 × 4 mixed-design analysis of variance (ANOVA) with group as a between-subjects factor and condition as a within-subjects factor, which was complemented by follow-up analyses. In particular, regulatory success was analyzed comparing differential ratings in the DistA and the IncA condition to the PermA baseline between (2-sample t test) and across groups (1-sample t test).
Functional MRI data analysis
We performed fMRI data analysis using SPM8 (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm/) on Matlab R2012a (MathWorks). The echo planar imaging (EPI) data were visualized for signal dropout and excessive head movement using ArtRepair (www.nitrc.org/projects/art_repair/), leading to the exclusion of 1 individual with SAD. Preprocessing involved realignment to the first image of each run, unwarping using the acquired fieldmap, coregistration of the structural T1 image onto mean EPI, segmentation of the T1 image into tissue classes (using “New Segment”), creation of an anatomic group template (from segmented grey matter, white matter and cerebrospinal fluid), normalization using individual flow fields and smoothing with a 6 mm full-width at half-maximum Gaussian kernel using DARTEL. For each participant, temporal information of the 4 conditions were convolved with a canonical hemodynamic response function to model the acquired BOLD signal. Similarly, instruction, fixation and rating periods (as 4-second motor blocks) across conditions as well as the 6 affine motion parameters were included in the model as regressors of no interest. Target contrasts (PermA > PermN, DistA > PermA, IncA > PermA) were created and transferred to the second level, where 2-sample t tests extracted group differences.
The fMRI results were thresholded at p < 0.001 and then corrected for multiple comparisons, resulting in a whole-brain correction threshold of p < 0.05 by determining individual cluster extent thresholds with the calculated intrinsic smoothness of the individual T value image, a cluster connection radius of 3 mm and a 1000-iteration Monte Carlo simulation, using AlphaSim as implemented in the REST Toolbox version 1.8 (www.restfmri.net/). Anatomic labels were determined using the AAL atlas46 and an amygdala region of interest (ROI) was created using the AAL implementation in the WFU PickAtlas version 3.0 (http://fmri.wfubmc.edu/).
Results
Participants
We recruited 23 patients with SAD and 23 controls to participate in our study. We had to discard fMRI data from 2 individuals with SAD: 1 patient reported having had visual difficulties, and 1 patient showed excessive, event-related head movement. Our fMRI analyses were therefore conducted on a total of 44 participants (21 patients with SAD and 23 controls). No ratings of self-relatedness were available from 3 individuals so that respective analyses comprised 41 participants (20 patients with SAD and 21 controls). The demographic and clinical characteristics of participants are summarized in Table 1). Patients with SAD had current DSM-IV comorbid diagnoses (major depression n = 2, dysthymia n = 3, panic disorder n = 2, obsessive–compulsive disorder n = 1), and 4 patients were taking antidepressants (selective serotonin re-uptake inhibitors [SSRI] n = 2, selective noradrenaline re-uptake inhibitors [SNRI] n = 1, tricyclicum n = 1).
Table 1.
Demographic, diagnostic and neuropsychological characteristics of study participants*
| Group; mean | ± SD† | ||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristic | SAD | Control | Statistics | p value | Cohen d |
| No. (female:male) | 21 (16:5) | 23 (18:5) | — | ||
| Age, yr | 30.5 ± 7.17 | 30.0 ± 7.99 | t42 = 0.23 | 0.82 | — |
| EHI | 0.88 ± 0.35 | 0.92 ± 0.15 | t42 = 0.59 | 0.56 | — |
| Education‡ | Dummy-coded | Dummy-coded | t42 = 1.11 | 0.27 | — |
| Weight, kg | 66.2 ± 13.3 | 67.3 ± 11.5 | t42 = 0.29 | 0.78 | — |
| TMT A, s | 27.7 ±10.5 | 24.9 ± 6.8 | t39 = 1.02 | 0.32 | — |
| TMT B, s | 62.3 ± 37.4 | 53.2 ± 18.6 | t39 = 0.99 | 0.33 | — |
| LSAS | 88.4 ± 18.2 | 17.4 ± 16.4 | t42 = 13.62 | < 0.001 | 4.2 |
| STAI-T | 57.7 ± 11.2 | 34.0 ± 11.4 | t42 = 6.94 | < 0.001 | 2.15 |
| BDI-II | 21.4 ± 16.5 | 2.48 ± 3.41 | t21.6 = 5.17 | < 0.001 | 1.66 |
| TAS-20 | 58.4 ± 9.7 | 46.6 ± 7.5 | t42 = 4.53 | < 0.001 | 1.4 |
| SPF-IRI, PersDist | 13.6 ± 2.73 | 9.74 ± 3.15 | t42 = 4.35 | < 0.001 | 1.34 |
| ERQ-app | 3.34 ± 0.81 | 4.83 ± 1.23 | t42 = 4.69 | < 0.001 | 1.45 |
| ERQ-supp | 4.18 ± 1.33 | 2.87 ± 1.26 | t42 = 3.36 | 0.002 | 1.04 |
| KIMS | 111.1 ± 16.1 | 142.2 ± 13.2 | t42 = 7.03 | < 0.001 | 2.17 |
| DFS | 74.2 ± 7.1 | 64.9 ± 9.3 | t42 = 3.71 | 0.001 | 1.02 |
BDI-II = Beck Depression Inventory; DFS = questionnaire of (dys)functional self-consciousness; EHI = Edinburgh Handedness Inventory; ERQ-app = Emotion Regulation Questionnaire — reappraisal subscale; ERQ-supp = Emotion Regulation Questionnaire — suppression subscale; KIMS = Kentucky Inventory of Mindfulness Skills; LSAS = Liebowitz Social Anxiety Scale; SAD = social anxiety disorder; SD = standard deviation; SPF-IRI, PersDist = Interpersonal Reactivity Index —personal distress subscale; STAI-T = State-Trait Anxiety Inventory, trait anxiety; TAS-20 = Toronto Alexithymia Scale; TMT = Trail Making Test, parts A and B.
All group comparisons are 2-sided independent t tests, either Student or Welch (in case of unequal variances).
Unless otherwise indicated.
Education was dummy-coded according to the German education system with increasing number codes corresponding to increasing mean years of education.
Self-report results
Self-reported symptom severity was significantly higher in patients with SAD than in controls (Table 1), and they reported significantly higher personal distress (SPF-IRI) and significantly higher trait anxiety (STAI-T), depressive (BDI-II) and alexithymic (TAS-20) tendencies. Groups did not differ in general information processing speed (TMT), but patients with SAD exhibited more functional and dysfunctional self-focused attention (DFS) and, convergently, less mindfulness skill (KIMS). In addition, patients with SAD reported habitual use of different emotion regulation strategies than controls (ERQ); they more commonly suppressed emotional expression and less often regulated their emotional experience through reappraisal.
Ratings of negative intensity and self-relatedness
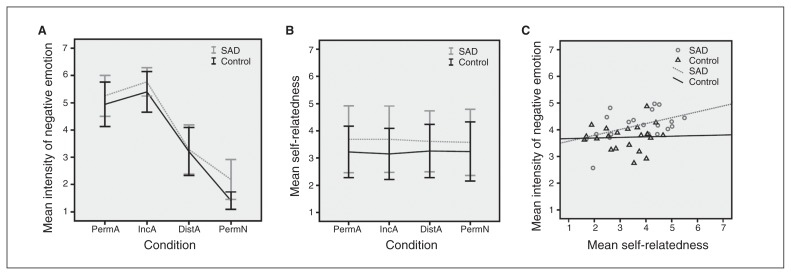
Ratings of intensity of negative emotion showed equal variance between groups for all conditions except for PermN (F = 8.74, p = 0.005) and all were normally distributed. The Mauchly test indicated a violation of the sphericity assumption (χ22 = 24.7, p < 0.001) so that degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ɛ = 0.79). For the intensity ratings (Fig. 1A and Table 2), there were significant main effects for the factors condition (F2.36, 99 = 388.2, ηp2 = 0.9, p < 0.001) and group (F1, 42 = 5.8, ηp2 = 0.12, p = 0.020), with patients with SAD rating stimuli as significantly more negative. Follow-up analyses of regulatory effects did not show group differences (DistA – PermA: t42 = 0.911, p = 0.37; IncA – PermA: t42 = 0.37, p = 0.72) but behaviourally indicated regulatory success across groups (DistA – PermA: t43 = 14, p < 0.001; IncA – PermA: t43 = 6.65, p < 0.001).
Fig. 1.
Group differences and correlations between ratings of the intensity of negative emotional experience and self-relatedness. Across all conditions (permit affective [PermA], increase affective [IncA], distance affective [DistA], and permit neutral [PermN]), and compared to healthy controls, individuals with social anxiety disorder (SAD) show (A) significantly increased intensity of negative emotion in the scanner (F1,42 = 5.8, ηp2 = 0.12, p = 0.020). The effect was driven by differential ratings in the PermN condition (t26,8 = 4.48, d = 1.43, p < 0.001) and there was a significant effect of condition (F2,36,99 = 388.2, ηp2 = 0.9, p < 0.001; Table 2). Follow-up analyses of regulatory effects did not show group differences but behaviourally indicated regulatory success across groups (DistA – PermA: t43 = 14, p < 0.001; IncA – PermA: t43 = 6.65, p < 0.001). (B) Patients with SAD showed a nonsignificant trend toward judging aversive and neutral images as more self-related than controls (F1,39 = 3.42, ηp2 = 0.081, p = 0.07; Table 2). (C) Ratings of self-relatedness, performed outside of the scanner, and of intensity of negative emotion across all conditions showed a significant positive correlation in the SAD group (r20 = 0.591, p = 0.006) but not in controls (r21 = 0.074, p = 0.75; Fisher z-transform: z = 1.79, p = 0.07). Error bars indicate standard deviation (SD).
Table 2.
Ratings of the intensity of negative emotional experience during passive viewing of aversive or neutral pictures or during the active increase or decrease of emotional experience through self-focused reappraisal while watching aversive images*
| Group; mean ± SD† | |||||
|---|---|---|---|---|---|
|
|
|||||
| Condition; rating | SAD | Control | Statistic | p value | Cohen d |
| Intensity of negative emotion | |||||
| No. of patients | 21 | 23 | |||
| PermA | 5.26 ± 0.75 | 4.94 ± 0.82 | t42 = 1.33 | 0.19 | — |
| IncA | 5.77 ± 0.52 | 5.4 ± 0.75 | t42 = 1.88 | 0.07 | 0.58 |
| DistA | 3.28 ± 0.9 | 3.21 ± 0.88 | t42 = 0.27 | 0.79 | — |
| PermN | 2.19 ± 0.73 | 1.41 ± 0.32 | t26.8 = 4.48 | < 0.001 | 1.44 |
| Self-relatedness | |||||
| No. of patients | 20 | 21 | |||
| PermA | 3.69 ± 1.22 | 3.22 ± 0.95 | t39 = 1.37 | 0.18 | — |
| IncA | 3.69 ± 1.22 | 3.15 ± 0.94 | t39 = 1.59 | 0.12 | — |
| DistA | 3.61 ± 1.12 | 3.26 ± 0.98 | t39 = 1.08 | 0.29 | — |
| PermN | 3.58 ± 1.22 | 3.24 ± 1.09 | t39 = 0.94 | 0.35 | — |
DistA = voluntary decrease of emotion to aversive images; IncA = voluntary increase of emotion to aversive images; PermA = passive viewing of aversive images; PermN = passive viewing of neutral images; SAD = social anxiety disorder; SD = standard deviation.
Participants additionally judged all pictures to the degree of self-relatedness. All group comparisons as 2-sided independent t tests, either Student or Welch (in case of unequal variances). Ratings (mean ± SD) were given on a Likert-type scale from 1 (weak) to 7 (strong). See also Figure 1.
Unless otherwise indicated.
In addition to the main effects, a trend toward an interaction emerged (F2.36, 99 = 2.73, ηp2 = 0.061, p = 0.06). Post hoc 2-sample t tests showed that this was mainly driven by PermN, where individuals with SAD reported significantly more negative emotional experience. Further division of neutral pictures into 13 social (showing humans and human faces) and 5 nonsocial (showing animals or objects) images and a post hoc 2 × 2 repeated-measures ANOVA with sociality as a within-subjects factor (nonsocial v. social) and group as the between-subjects factor (SAD v. control) revealed main effects for sociality (F1, 42 = 6.25, ηp2 = 0.13, p = 0.016) and group (F1, 42 = 15.75, ηp2 = 0.273, p < 0.001) as well as the 2 factors’ interaction (F1, 42 = 4.69, ηp2 = 0.1, p = 0.036; see the Appendix, Fig. S1, available at jpn.ca).
Ratings of self-relatedness (Fig. 1B) were homoscedastic and normally distributed. The main effect of group indicated that individuals with SAD showed a trend toward stronger self-relatedness (F1, 39 = 3.42, ηp2 = 0.081, p = 0.07). Under Greenhouse–Geisser correction (ɛ = 0.39), there was no effect of condition (F1.16, 45.4 = 0.019, p = 0.92) and no interaction (F1.16, 45.4 = 0.1, p = 0.79).
Across all 4 conditions, the mean intensity rating was correlated with the mean self-relatedness rating in the SAD group (r20 = 0.591, p = 0.006), but not in the control group (r21 = 0.074, p = 0.75; Fisher z transformation: z = 1.79, p = 0.074; Fig. 1C).
Brain activation
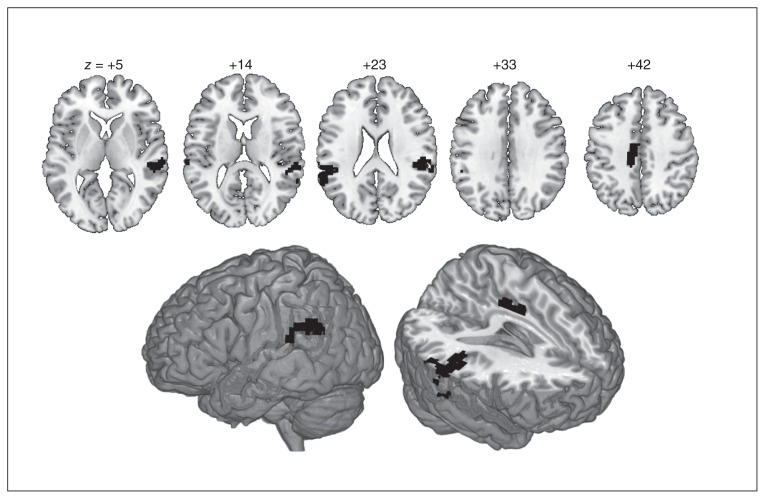
No significant group differences were detected during passive viewing of neutral pictures relative to the implicit baseline. During passive viewing of aversive versus neutral pictures (PermA > PermN), stronger activations of a large bilateral cluster in early and extrastriate visual cortices extending along the cortical midline to the left-lateralized frontal cortex emerged across all participants. For the same contrast, individuals with SAD showed significantly stronger activation than controls in bilateral superior temporal/inferior parietal cortices and in the posterior midcingulate cortex (pMCC; Table 3 and Fig. 2).
Table 3.
Coordinates and anatomic labels for overactivations in patients with social anxiety disorder relative to controls during passive viewing of aversive compared with neutral images
| MNI coordinates | Statistics | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hemisphere; anatomic label | x | y | z | t | k | p value* |
| Left hemisphere | ||||||
| Supramarginal | ||||||
| −54 | −39 | 27 | 5.57 | 116 | < 0.001 | |
| −63 | −48 | 24 | 4.65 | — | < 0.001 | |
| Superior temporal | −63 | −30 | 18 | 3.75 | — | < 0.001 |
| Middle cingulum | ||||||
| −15 | −18 | 42 | 4.56 | 66 | < 0.001 | |
| −12 | −30 | 39 | 4.46 | — | < 0.001 | |
| Right hemisphere | ||||||
| Rolandic operculum | ||||||
| 57 | −30 | 21 | 4.81 | 252 | < 0.001 | |
| 39 | −33 | 21 | 4.29 | — | < 0.001 | |
| Superior temporal | 57 | −30 | 3 | 4.47 | — | < 0.001 |
MNI = Montreal Neurological Institute.
Threshold: p < 0.001, uncorrected at the voxel level with cluster extent threshold of k > 60 to achieve whole-brain correction at p < 0.05 (calculated using AlphaSim, www.restfmri.net/). See Figure 2.
Fig. 2.
Overactivation of individuals with social anxiety disorder (SAD) compared to controls. For the contrast of passive viewing of aversive versus neutral pictures, individuals with SAD overactivated bilateral temporoparietal cortices, encompassing the superior temporal cortex and supramarginal gyrus/temporoparietal junction as well as the dorsal posterior cingulate/posterior midcingulate cortex. Numbers describe z coordinates (Montreal Neurological Institute space) of slices. For peak coordinates and individual thresholds (joint voxel activation and cluster extent), see Table 3.
No significant group differences at the brain level were observed during the voluntary increase or decrease of emotional intensity through self-focused reappraisal (IncA > PermA and DistA > PermA, respectively). Contrasts directly comparing regulatory strategies (IncA > DistA and DistA > IncA) did not show significant group differences either. Across groups, these contrasts revealed activation in regulation networks (see the Appendix, Figs. S1 and S2 and Table S1). Distancing compared with unaltered watching of affective pictures activated bilateral prefrontal and parietal clusters. Increasing emotional intensity was connected to a network comprising the bilateral insula, hippocampal-thalamic and medial clusters in the supplementary motor area/middle cingulum and precuneus but also the left-hemispheric lateral regions around the central fissure. In the same contrast (IncA > PermA), ROI analyses also showed that the bilateral amygdala was significantly more active (p < 0.05, family-wise error–corrected).
Notably, the amygdala was not significantly more active during unaltered watching of aversive compared with neutral pictures, and in all 3 contrasts, no regions were found in which activation was significantly stronger in controls than in patients with SAD.
Discussion
Self-reported characteristics
Reports of increased self-consciousness (DFS) and of altered emotion regulation (ERQ) in our sample are in line with the robust finding of heightened self-focused attention7 in patients with SAD and replicate patient reports of using reappraisal strategies less habitually and of using suppression to regulate their emotions more commonly.47 Increased alexithymic tendencies in our SAD sample are in line with reported difficulties of identifying and describing emotion in patients with SAD.48
Ratings of negative intensity and self-relatedness
Across all conditions, individuals with SAD judged their emotional experiences as more intensely negative than controls. There was a pronounced effect during passive viewing of neutral stimuli and a tendency during voluntary increase, but no group differences during passive viewing of and voluntary distancing from aversive pictures. This interpretation bias of perceiving ambiguous or neutral stimuli (particularly faces) as more negative and threatening has been previously reported in patients with SAD.7 Post hoc analysis suggests that the observed negativity bias for neutral stimuli in patients with SAD was differentially modulated by the presence of humans and human faces (see the Appendix, Fig. S3).
Contrary to our hypothesis, emotional hyperreactivity in patients with SAD did not manifest as significantly increased negative intensity of negative emotional experience during passive viewing of aversive pictures. This may be ascribed to the fact that static visual stimuli can only evoke a certain level of negative emotional intensity, leading to a ceiling effect that levelled out group differences.
The tendency toward generally increased self-relatedness in the patient group as well as the observation that perceived self-relatedness of the pictures increased as a function of emotional experience in the scanner in individuals with SAD but not in controls possibly reflect the general information-processing and interpretation bias toward negative content in patients with SAD.7
Functional MRI results
Across groups, the observed networks replicated results from comparable studies in healthy participants (see the Appendix, Fig. S2). Of note, amygdala activation was not selectively increased for passive viewing of aversive compared with neutral pictures as both groups showed amygdala activation in the latter condition. This may be due to instruction word ambiguity leading to preparatory activation or processing of uncertainty in the amygdala.49 Processing of neutral stimuli may have also been biased due to the restriction of the in-scan rating to (minimally “weak”) negative valence. As passive viewing of neutral stimuli usually serves as a control condition alone, studies rarely report specific brain activation. However, indirect evidence suggests amygdala activation during passive viewing of neutral pictures in comparable studies (e.g., Fig. 3 in the study by Kanske and colleagues50).
During passive viewing of aversive compared with neutral pictures, individuals with SAD showed significantly stronger activation in a network of lateral temporoparietal regions in both hemispheres as well as a medial cluster comprising the dorsal posterior (dPCC) or pMCC than controls. Temporoparietal overactivations included the posterior superior temporal sulcus (pSTS) and supramarginal gyrus (SMG)/temporoparietal junction (TPJ). These regions are assumed to form part of the “social brain,”51 and are activated during the judging of socioemotional stimuli.52 The SMG/TPJ integrates multisensory information for self-location in space, necessary for bodily self-consciousness,53 and for taking the perspective of another person to infer his or her goals and intentions.51
The pMCC/dPCC region has previously been implicated in emotion regulation through cognitive reappraisal.54 Other studies involving unregulated55 and regulated56 perception of socioemotional stimuli found this region specifically to be involved in the processing of aversive social stimuli. Together, these results broadly imply the pMCC/dPCC in the modulation of negative affective responses. Additional evidence for this involvement comes from findings of functional connectivity between the pMCC/dPCC and the amygdala during the resting state57 and affective face processing.58
Taken together, the overactivation of this temporoparietal–cingulate network may reflect increased spontaneous (i.e., uninstructed) perspective taking and increased bodily self-consciousness in patients with SAD when confronted with aversive pictures and not occupied with cognitive tasks. These 2 features resonate with the fear of potential evaluation by an audience or of showing anxiety symptoms, which lie at the core of SAD.
In contrast to the results of Goldin and colleagues,13 we found no neural evidence for altered emotion regulation in patients with SAD compared with controls. No significant group differences emerged at the brain level during the voluntary increase or decrease of the intensity of negative emotion through self-focused reappraisal (IncA > PermA and DistA > PermA, respectively). Our results therefore suggest that self-focused reappraisal eliminates differences between patients with SAD and controls and is thus a successful emotion regulation strategy for patients with SAD to normalize their neural activations.
Also contrary to our hypotheses, neither the insula nor the amygdala showed increased activation in our sample of individuals with SAD when completing a task in which emotion was voluntarily regulated or left unaltered during the viewing of aversive and neutral pictures. Although the insula and amygdala have been classically implied in emotional experience,2 recent evidence from neuropsychological patients suggests that feelings persist after bilateral insula lesions59 and that fear and panic still occur after damage to the bilateral amygdala.60 A recent study also found no increased amygdala and insula responses on 3 socioemotional tasks in a large sample of individuals with SAD.24
Limitations
To ensure ecological validity, we opted not to exclude patients with concurrent anxiety or affective disorders (other than bipolar depression) and we allowed the inclusion of 4 patients taking antidepressant medication. We cannot rule out that these comorbidities or pharmacological substances might have contributed to the observed effects. Control analyses excluding the 4 patients using antidepressants did not significantly alter the results of our study. In particular, the findings remained that there were no group differences in brain activation during emotion regulation and that individuals with SAD showed hyperreactivity in a temporoparietal–cingulate cluster during passive viewing of aversive pictures. Anxious and depressive tendencies, traits, or symptoms are not independent and thus share a lot of variance with social anxiety — the symptom and disorder of interest in our study. We believe that partialing out all variance explicable by symptom overlap would have weakened our analyses; therefore, we opted not to control for these factors. Future studies should compare our results to a sample of patients with SAD but no comorbidities to elucidate possible contribution of depressive disorders to our findings.
Post hoc classification of neutral pictures revealed a selective interpretation bias for socially neutral pictures (depicting humans and human faces) in patients with SAD. Comparing brain activation in the PermN condition between groups did not reveal significant differences, but owing to low stimulus numbers the power for this comparison was low. As the original randomization procedure was not devised for this post hoc distinction, we could not run a full analysis of brain data also classifying aversive pictures. As the behavioural results suggest a differentiation of social and nonsocial threat in patients with SAD, we will include the factor “sociality” in future studies to test its specificity.
As our task design already extended standard experiments of emotion regulation by including a condition of voluntary increase of emotional intensity, we included only negative and neutral visual stimuli. There is evidence that the attention bias in patients with SAD also manifests as diminished processing of positive (social) information.7 Future studies should extend the investigation of voluntary emotion regulation through self-focused reappraisal to positively valenced stimuli.
Conclusion
The strongest group differences emerged during passive viewing of neutral and aversive pictures, for which patients with SAD showed enhanced reactivity.
Patients with SAD rated neutral stimuli as eliciting significantly more negative affect than controls, replicating the well-known interpretation bias. On the brain level, however, there was no significant group difference during this condition. Conversely, during passive viewing of aversive pictures compared with neutral pictures, patients with SAD overactivated bilateral temporoparietal areas involved in judging socioemotional stimuli as well as a posterior midcingulate region implied in modulating negative affective responses, but showed only a trend toward higher intensity ratings.
While patients with SAD show exaggerated neural and behavioural reactivity during passive viewing, instructed emotion regulation evens out the differences from controls, suggesting that self-focused regulation is a successful behavioural and neural strategy for patients with SAD.
Acknowledgements
The work of M. Gaebler, J.K. Daniels and J.-P. Lamke was funded by the VW-foundation grant no. II/84051. We thank Dina Wittfoth-Schardt for providing source code for task presentation.
Footnotes
Competing interests: None declared.
Contributors: M. Gaebler, J.K. Daniels and H. Walter designed the study. M. Gaebler and J.-P. Lamke acquired the data, which M. Gaebler, J.K. Daniels, T. Fydrich and H. Walter analyzed. M. Gaebler and J.K. Daniels wrote the article, which all authors reviewed and approved for publication.
References
- 1.McRae K, Gross JJ. Emotion regulation. In: Sander D, Scherer KR, editors. Oxford companion to the affective sciences. New York (NY): Oxford University Press; 2007. [Google Scholar]
- 2.Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–52. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 3.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 5.Walter H, Von Kalckreuth A, Schardt DM, et al. The temporal dynamics of voluntary emotion regulation. PLoS ONE. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalisch R, Wiech K, Critchley H. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–83. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- 7.Morrison AS, Heimberg RG. Social anxiety and social anxiety disorder. Annu Rev Clin Psychol. 2013;9:249–74. doi: 10.1146/annurev-clinpsy-050212-185631. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Chiu WT. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Hattingh CJ, Ipser J, Tromp SA, et al. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front Hum Neurosci. 2012;6:347. doi: 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner K, Gross JJ. Emotion regulation and psychopathology: a conceptual framework. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: a transdiagnostic approach to etiology and treatment. New York (NY): Guildford; 2004. pp. 13–37. [Google Scholar]
- 12.Mennin DS, Heimberg RG, Turk CL, et al. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther. 2005;43:1281–310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Goldin PR, Manber T, Hakimi S, et al. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldin PR, Manber-Ball T, Werner K, et al. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009;66:1091–9. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair KS, Blair RJR. A cognitive neuroscience approach to generalized anxiety disorder and social phobia. Emotion Review. 2012;4:133–8. [Google Scholar]
- 16.Alden LE, Regambal MJ. Social anxiety, social anxiety disorder, and the self. In: Hofmann SG, Di Bartolo PM, editors. Social anxiety. 2nd ed. Waltham (MA): Academic Press; 2010. pp. 423–45. [Google Scholar]
- 17.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DR, et al., editors. Social phobia: diagnosis, assessment and treatment. New York (NY): Guilford; 1995. pp. 69–93. [Google Scholar]
- 18.Rapee R, Heimberg R. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 19.Stangier U, Fydrich T. Das störungskonzept der sozialen phobie oder der sozialen angststörung. In: Stangier U, Fydrich T, editors. Soziale phobie — soziale angststörungen. Göttingen (DE): Hogrefe; 2002. pp. 10–33. [Google Scholar]
- 20.Bögels SM. Task concentration training versus applied relaxation, in combination with cognitive therapy, for social phobia patients with fear of blushing, trembling, and sweating. Behav Res Ther. 2006;44:1199–210. doi: 10.1016/j.brat.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann SG, Moscovitch Da, Kim H-J, et al. Changes in self-perception during treatment of social phobia. J Consult Clin Psychol. 2004;72:588–96. doi: 10.1037/0022-006X.72.4.588. [DOI] [PubMed] [Google Scholar]
- 22.Shah SG, Klumpp H, Angstadt M, et al. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 23.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziv M, Goldin PR, Jazaieri H, et al. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3:5. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadflieg S, Mohr A, Mentzel H-J, et al. Modulation of the neural network involved in the processing of anger prosody: the role of task-relevance and social phobia. Biol Psychol. 2008;78:129–37. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Sripada CS, Angstadt M, Banks S, et al. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport. 2009;20:984–9. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amir N, Klumpp H, Elias J, et al. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57:975–81. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Gentili C, Gobbini MI, Ricciardi E, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with social phobia and healthy subjects. Brain Res Bull. 2008;77:286–92. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Brühl AB, Herwig U, Delsignore A, et al. General emotion processing in social anxiety disorder: neural issues of cognitive control. Psychiatry Res. 2013;212:108–15. doi: 10.1016/j.pscychresns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72:476–82. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): The Association; 1994. [Google Scholar]
- 33.Wittchen H-U, Wunderlich U, Gruschwitz S, et al. Strukturiertes klinisches interview für DSM-IV. Achse I: psychische störungen. Göttingen (DE): Hogrefe; 1997. [Google Scholar]
- 34.Gast U, Oswald P, Zündorf F, et al. Das strukturierte klinische interview für DSM-IV-dissoziative störungen. Interview und manual. Göttingen (DE): Hogrefe; 2000. [Google Scholar]
- 35.Mombour W, Zaudig M, Berger P, et al. International Personality Disorder Examination ICD-10 Modul. Deutschsprachige Ausgabe Im Auftrag der WHO. Göttingen (DE): Hogrefe; 1996. [Google Scholar]
- 36.Stangier U, Heidenreich T. Die Liebowitz Soziale Angst-Skala (LSAS) Göttingen (DE): Hogrefe; 2003. [Google Scholar]
- 37.Hautzinger M, Keller F, Kühner C. Beck Depressions-Inventar (BDI-II) Frankfurt (DE): Pearson Assessment; 2006. [Google Scholar]
- 38.Laux L, Glanzmann P, Schaffner P, et al. STAI–Das State-Trait-Angstinventar. Göttingen (DE): Beltz; 1981. [Google Scholar]
- 39.Franz M, Schneider C, Schäfer R, et al. [Factoral structure and psychometric properties of the German version of the Toronto Alexithymia Scale (TAS-20) in psychosomatic patients]. Psychother Psychosom Med Psychol. 2001;51:48–55. doi: 10.1055/s-2001-10755. [article in German] [DOI] [PubMed] [Google Scholar]
- 40.Reitan RM. The relation of the Trail Making Test to organic brain damage. J Consult Psychol. 1955;19:393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 41.Hoyer J. [Dysfunctional and Functional Self-Consciousness (DFS): theoretical concept, reliability, and validity]. Diagnostica. 2000;46:140–8. [article in German] [Google Scholar]
- 42.Ströhle G, Nachtigall C, Michalak J, et al. Die Erfassung von Achtsamkeit als mehrdimensionales Konstrukt. Zeitschrift für Klinische Psychologie und Psychotherapie. 2010;39:1–12. [Google Scholar]
- 43.Abler B, Kessler H. Emotion Regulation Questionnaire — Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica. 2009;55:144–52. [Google Scholar]
- 44.Paulus C. Der Saarbrücker Persönlichkeitsfragebogen SPF(IRI) zur Messung von Empathie — Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. Saarbrücken (DE): Universität des Saarlandes; 2009. [Google Scholar]
- 45.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Gainesville (FL): University of Florida; 2008. Report no.: A-8. [Google Scholar]
- 46.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 47.Brühl AB, Rufer M, Delsignore A, et al. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 48.Turk CL, Heimberg RG, Luterek JA, et al. Emotion dysregulation in generalized anxiety disorder: a comparison with social anxiety disorder. Cognit Ther Res. 2005;29:89–106. [Google Scholar]
- 49.Hsu M, Bhatt M, Adolphs R, et al. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–3. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 50.Kanske P, Heissler J, Schönfelder S, et al. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2010;21:1–10. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- 51.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 2012;13:556–71. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- 54.Diekhof EK, Geier K, Falkai P, et al. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 55.Müller VI, Habel U, Derntl B, et al. Incongruence effects in cross-modal emotional integration. Neuroimage. 2011;54:2257–66. doi: 10.1016/j.neuroimage.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koenigsberg HW, Fan J, Ochsner KN, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–22. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb Cortex. 2013;23:833–46. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feinstein JS, Buzza C, Hurlemann R, et al. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013;16:270–2. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]