Abstract
Rationale
Drugs can function as punishers. However, work on the study of drugs as punishers is limited, as are the range of compounds known to function as punishers. Kappa opioid agonists, which have received much experimental attention as potential therapeutics for drug abuse, reportedly produce aversive effects. However, kappa agonists have yet to be tested as punishers of behavior.
Objective
The goal of the current study was to determine if a kappa agonist could function as a punisher of drug self-administration.
Method
In separate experiments, monkeys were allowed to choose in a two-lever choice design between intravenous injections of equal doses of either cocaine (0.1 mg/kg/inj on each lever) or remifentanil (0.1 μg/kg/inj on each lever) when one of the two options was mixed with various doses of the kappa agonist, salvinorin A.
Results
Choice for the cocaine and remifentanil options that were combined with salvinorin A decreased as a function of salvinorin A dose in all monkeys. However, operant response rates were not systematically affected by salvinorin A administration.
Conclusion
The present findings demonstrate that the kappa agonist, salvinorin A, can punish self-administration of a mu opioid, remifentanil, and a psychostimulant, cocaine. In consideration of these findings, it may be possible to curtail the abuse of some drugs by contingently delivering kappa agonists (e.g., as combination formularies for prescription medications).
Keywords: cocaine, remifentanil, salvinorin A, punishment, kappa agonist, monkey, self-administration
INTRODUCTION
Punishment is operationally defined as a consequence of behavior that decreases the occurrence of that behavior (Azrin and Holz 1966). In the context of drug self-administration, a punisher is a response-contingent stimulus that, when coupled with the self-administration of a drug reinforcer, reduces responding for that drug. Researchers investigating the effects of punishment on drug self-administration have typically used exteroceptive stimuli such as electric shock to reduce operant responding for drug injections in non-humans (Deroche-Gamonet et al 2004; Grove and Schuster 1974; Johanson 1977). However, limited work has demonstrated that drugs can function as punishers, too. In the first systematic demonstration of punishment with a drug stimulus, Goldberg (1980) reported that intravenous histamine decreased responding for food in monkeys when it was contingently delivered with food pellets on a fixed schedule of reinforcement. Subsequent work extended these findings in monkeys (Katz and Goldberg 1986; Woolverton 2003, Negus 2005) and in rats (Podlesnik et al 2010). Recent work has also demonstrated that histamine, when delivered contingently with self-administered cocaine, can punish cocaine self-administration in monkeys (Freeman et al 2014; Negus 2005; Woolverton et al 2012) and in rats (Holtz et al 2013). These results demonstrate that a drug punisher can be used to reduce self-administration of a drug reinforcer, which suggests a possible use for drug punishers as deterrents for drug abuse. However, relatively little work has been done on the study of drugs as punishers, and little is known about the range of compounds that can function as punishers.
Numerous studies have demonstrated that kappa opioid agonists can, under certain conditions, attenuate the behavioral effects of drugs of abuse (Cosgrove and Carroll 2004; Glick et al 1995; Mello and Negus 1998; Morani et al 2009; Negus et al 1997; Prisinzano et al 2005; Ruedi-Bettshen et al 2010; Schenk et al 1999). However, kappa agonists have also been reported to produce aversive effects such as dysphoria and psychotomimesis, which has limited enthusiasm for their clinical potential (Chefer et al 2013; Pfeiffer et al 1986; Zhang et al 2005). In consideration of these aversive effects, kappa agonists may be able to function as punishers. In support of this, Negus et al (2007) reported that the kappa agonist, U69,593, when prepared as a mixture with fentanyl, reduced fentanyl self-administration in monkeys. This result is consistent with a punishment mechanism because the administration of U69,593 was contingent upon the same behavior that resulted in fentanyl administration. However, because this procedure used a single operant, fixed-schedule design, the putative punishing effects of U69,593 could not be clearly discerned from the rate-decreasing effects that have been reported to occur with the administration of kappa agonists (see Mello and Negus 2000).
Choice procedures have an advantage over single operant procedures in that their measure of reinforcing effect does not co-vary with response rate (see Banks and Negus 2012). This is due to the fact that reinforcement is measured in terms of the relative allocation of choice between two or more options. Choice procedures are therefore well suited to evaluate the punishing effects of kappa agonists and other drugs that have been reported to produce non-specific reductions in operant response rate. Accordingly, the current study used a choice procedure to determine if a kappa agonist could function as a punisher of drug self-administration. Specifically, using a two-lever operant design, monkeys were allowed to choose between two equal doses of the psychostimulant, cocaine (0.1 mg/kg/inj), or two equal doses of the short-acting mu opioid, remifentanil (0.1 μg/kg/inj). Various doses of the kappa agonist, salvinorin A, were mixed with one of the cocaine or remifentanil choice options. Salvinorin A was chosen as a kappa agonist probe because it is highly selective and efficacious at the kappa opioid receptor, and it has a relatively short half-life, which mitigated concern over drug accumulation across choice trials (Cunningham et al 2011). It was hypothesized that salvinorin A, when contingently administered with a reinforcer option, would decrease choice for that option in a dose-dependent manner.
METHOD
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (2011).
Animals and Apparatus
The subjects were three male rhesus monkeys (Macaca mulatta) weighing between 9.1 and 11.7 kg at the beginning of the study. All monkeys had histories of cocaine self-administration under conditions similar to those used here. In addition to cocaine, the most recent histories of drug self-administration were with remifentanil (DJ9J and R02050; unpublished data) or histamine self-administered as a mixture with cocaine (CJ82; Freeman et al 2014). All monkeys were provided with sufficient food to maintain stable body weights (approximately 200 g/day, Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI, USA) and had unlimited access to water. Fresh fruit was provided daily in addition to the standard diet, and a vitamin supplement was given three times per week. Lighting was cycled to maintain 16 h of light and 8 h of dark, with lights on at 0600 hours.
Each monkey was fitted with a mesh jacket (Lomir Biomedical, Malone, NY, USA) that was attached by a tether to the rear wall of the experimental cubicle (1.0 m3, Plaslabs, Lansing, MI, USA). The front door of the cubicle was made of transparent plastic, and the remaining walls were opaque. Two response levers (PRL-001, BRS/LVE, Beltsville, MD, USA) were mounted on the inside of the door. Four jeweled stimulus lights, two red and two white, were mounted above each lever. Drug injections were delivered by two peristaltic infusion pumps, one for each of the two levers (Cole-Parmer, Chicago, IL, USA). A Macintosh computer with custom interface and software controlled all events in an experimental session and recorded data.
Surgery
Each monkey had a double lumen i.v. catheter implanted according to the following protocol. The monkey was injected with a combination of atropine sulfate (0.04 mg/kg i.m.) and ketamine hydrochloride (10 mg/kg i.m.) followed 20-30 min later by inhaled isoflurane. When anesthesia was adequate, the catheter was surgically implanted into a major vein (jugular or femoral) with the tip terminating near the right atrium. The distal end of the catheter was passed subcutaneously to the mid- scapular region where it exited the subject’s back. After surgery, the monkey was returned to the experimental cubicle. The catheter was threaded through the tether to the back wall of the cubicle where it connected to a double-lumen swivel (Lomir). The individual lumens were then connected to the separate infusion pumps. An antibiotic (Kefzol; Eli Lilly & Company, Indianapolis, Indiana) was administered (22.2 mg/kg i.m.) twice daily for 7 days post-surgery to prevent infection. If a catheter became nonfunctional during the experiment, it was removed, and the monkey was removed from the experiment for a 1–2 week period to allow any infection to clear. After health was verified (i.e., no sign of systemic or local infection), a new catheter was implanted. The catheter was filled between sessions with a solution of 40 units/ml heparin to prevent clotting at the catheter tip.
Procedure
Experimental sessions were conducted daily and began at 11:00 a.m. each day. Each session consisted of four sampling and ten choice trials. During the sampling trials the white lights were illuminated above one lever, randomly determined, and five responses on that lever (i.e., fixed-ratio [FR] 5) resulted in the delivery of the drug injection condition associated with that lever. These responses had to be completed in consecutive order on a lever to receive an injection. That is, if the monkey operated the opposite lever before completing five consecutive responses on the other lever, the cumulated responses made towards the five needed for an injection were reset to zero. The injection was followed by a 10 min inter-trial interval (ITI) during which the lever lights were extinguished, and responses on either lever had no programmed consequence. At the conclusion of the ITI, white lights were illuminated on the opposite lever, signaling that it was active, and five responses on that lever resulted in the delivery of the injection condition associated with that lever. This alternating sequence was repeated once more to ensure that the subject had exposure to the contingencies programmed for each lever. When all sampling trials were complete, choice trials began. For choice trials, the white lights were illuminated over both levers and both consequences were available under FR5 schedules that were identical to the schedule in effect for the sampling period. Sessions ended when the monkey completed ten choice trials. For all sessions, the conditions associated with the two levers were a drug reinforcer alone on one lever (either 0.1 mg/kg/inj cocaine or 0.1 μg/kg/inj remifentanil) and that same drug reinforcer prepared as a mixture with a range of concentrations of salvinorin A on the other lever. There was one exception to the mixture approach: monkey R02050 received serial injections of cocaine and salvinorin A in separate lumens. Specifically, when the monkey pressed the salvinorin A associated lever, a 10 sec cocaine injection in one lumen was followed by an injection of salvinorin A in the other lumen, and the pump time for the salvinorin A infusion was adjusted to vary dose. This program was designed to allow for the manipulation of the delay to presentation of the drug punisher (see Woolverton et al 2012), and was used in the initial investigation of the punishing effects of SVA in R02050. However, we opted to run the remaining tests with the mixture approach described above because it more closely modeled the single formulary design in which drug punishers could be combined with prescription drugs that with established abuse potential (e.g., oxycodone). Therefore, the remifentanil condition in R02050 was run with the mixture approach used in the other monkeys (i.e., remifentanil alone in one lumen and the mixture in the other). Notably, both approaches produced comparable results in this monkey and across monkeys.
A given choice condition was in effect until choice was stable, defined as: 1) the number of choices of the drug alone was within 20% of the three-session mean for three consecutive sessions, and 2) there were no upward or downward trends over the three sessions. Once exception to the trend criterion was when the third session within a stable block ended at 0 or 100 % choice for that option (i.e., a floor or ceiling effect, respectively). In this case, which occurred in one monkey in a single condition (R02050 when choosing between equal doses of cocaine alone), the sessions were considered stable. Once stability was achieved, the injections associated with the two levers were reversed, and stable preference was re-determined. Testing with one drug reinforcer was completed in each monkey before starting testing with the other, with monkeys CJ82 and DJ9J completing testing with remifentanil first, and monkey R02050 completing testing with cocaine first.
Data Analysis
The dependent measure was percent choice for the salvinorin A-associated lever over the averaged trials, which included three stable sessions for a condition and its reversal. A dose of salvinorin A was considered to be an effective punisher if choice for the salvinorin A-associated lever was reduced to ≤ 25% of total choice trials for that condition. Response rates for all testing conditions were determined by dividing the number of responses emitted during the session by the total time of drug availability during the session (i.e., session length in seconds minus the sum of the seconds for all ITIs for the session).
Drugs
Cocaine hydrochloride was generously provided by the National Institute on Drug Abuse (Rockville, MD, USA), and remifentanil hydrochloride was purchased commercially (Shanghai ChemPartner). Salvinorin A was isolated from commercially available S. divinorum and supplied by the laboratory of Dr. Thomas E. Prisinzano (see Butelman et al 2007 for synthesis details). All drugs and drug combinations were dissolved in a vehicle of 1:1:20 ethanol:Tween 80:sterile water.
RESULTS
All monkeys completed all choice trials available for all sessions. When the choice was between identical reinforcers (i.e., equal doses of the same drug reinforcer with no salvinorin A) or the doses of salvinorin A mixed with one option were relatively low, all monkeys generally responded on one lever through the condition and its reversal. That is, a lever position preference appeared to control behavior (see Figures 1 and 2). Position preferences such as these are typical in monkeys when the differences between the alternatives are small to none (Iglauer and Woods 1974; Johanson and Schuster 1975; Woolverton et al 2012). Increasing the dose of salvinorin A reduced choice for the salvinorin A-associated lever for the cocaine and the remifentanil choice tests in all monkeys, and this effect was maintained through the reversals for the lever-response contingencies that included higher doses of salvinorin A (see Figures 1 and 2).
Fig 1.
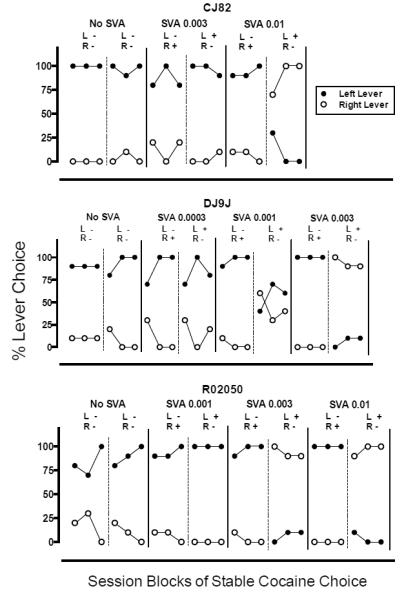
Session blocks of stable cocaine choice for each choice condition and its reversal in each of three monkeys (CJ82, DJ9J, and R02050). The ordinate represents percent choice for the conditions associated with responding on the left and right levers (solid circles and open circles, respectively). For each monkey, adjacent panels separated by solid lines represent various salvinorin A dose conditions (mg/kg/inj). Hatched lines within these panels represent reversals for the lever response contingencies within each condition. For each reversal within a condition, the lever on which responding results in the delivery of cocaine (0.1 mg/kg/inj) mixed with salvinorin A is indicated by a “+” sign. Alternatively, the “−” sign indicates that responding on the indicated lever results in an injection of cocaine alone (0.1 mg/kg/inj). SVA = salvinorin A; L = left lever; R = right lever
Fig 2.
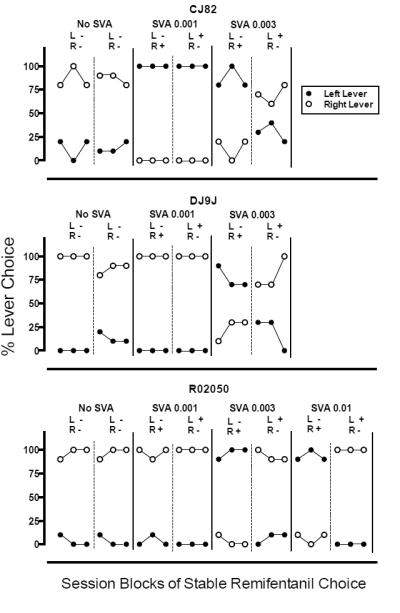
Session blocks of stable remifentanil choice for each choice condition and its reversal in each of three monkeys (CJ82, DJ9J, and R02050). The ordinate represents percent choice for the conditions associated with responding on the left and right levers (solid circles and open circles, respectively). For each monkey, adjacent panels separated by solid lines represent various salvinorin A dose conditions (mg/kg/inj). Hatched lines within these panels represent reversals for the lever response contingencies within each condition. For each reversal within a condition, the lever on which responding results in the delivery of remifentanil (0.1 μg/kg/inj) mixed with salvinorin A is indicated by a “+” sign. Alternatively, the “−” sign indicates that responding on the indicated lever results in an injection of remifentanil alone (0.1 μg/kg/inj). SVA = salvinorin A; L = left lever; R = right lever
The top two panels of Figure 3 illustrate the average percent choice for the salvinorin A-associated lever as a function of salvinorin A dose for all monkeys self-administering cocaine and remifentanil, respectively. Each data point represents the average of a condition and its reversal. There was variable sensitivity to salvinorin A’s punishing effects on cocaine self-administration across monkeys, with salvinorin A being most potent in DJ9J and least potent in CJ82 (Figure 3, top left panel). However, the potency of salvinorin A as a punisher of remifentanil self-administration was comparable in all monkeys as evidenced by the horizontal overlap of the dose-response functions (Figure 3, top right panel). The bottom two panels of Figure 3 illustrate average response rates for a condition and its reversal as a function of salvinorin A dose. Generally, salvinorin A did not produce systematic effects on response rate, although the highest dose of salvinorin A tested in one monkey (CJ82) did produce a marked reduction in response rate during remifentanil choice tests (Figure 3, bottom right panel).
Fig 3.
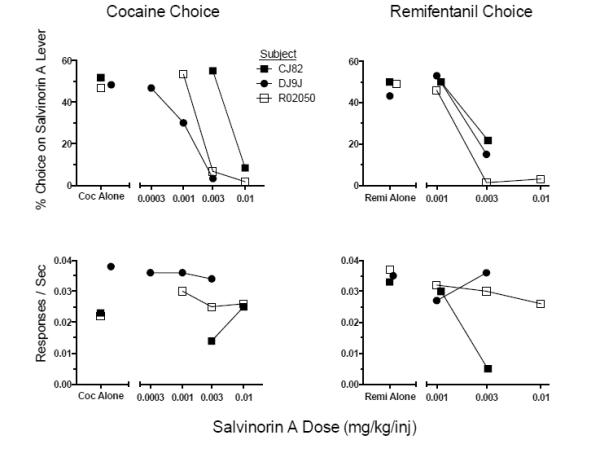
The top row illustrates the percentage of choice trials that monkeys chose a drug reinforcer (0.1 mg/kg cocaine, left; 0.1 μg/kg/inj remifentanil, right) mixed with various doses of salvinorin A when that same drug reinforcer was offered at the same dose without salvinorin A on the opposite lever. The bottom row illustrates response rates averaged across sessions for each choice condition. Symbols and legend codes represent individual monkeys. Remi = remifentanil; Coc = cocaine
DISCUSSION
The main finding of the present study was that the kappa agonist, salvinorin A, when paired contingently with cocaine or remifentanil, decreased choice for the drug option with which it was paired in a manner that was independent of its effects on response rate. This result is consistent with the effects of other drug and non-drug punishers that have been shown to decrease self-administration of drugs in operant choice procedures (Johanson 1977; Negus 2005; Woolverton 2003; Woolverton et al 2012) and demonstrates that a kappa agonist can function as a punisher under similar conditions. The current findings also demonstrate that a kappa agonist can punish self-administration of drug reinforcers from different classes, specifically cocaine, a psychostimulant, and remifentanil, a mu opioid agonist.
The finding that salvinorin A can punish drug choice suggests a potential use for kappa agonists in the development of abuse-deterrent formulations for prescription opioids and other medications. In support of this possibility, Negus et al (2007) demonstrated that mixtures of the kappa agonist, U69,593, and the mu agonist, fentanyl, were self-administered at lower rates than fentanyl alone. However U69,593 did not affect the antinociceptive effects of fentanyl in a thermal nociception test when the two were administered as mixtures. Taken together, the current results and the results of Negus et al (2007) indicate that kappa agonists, if delivered contingently as a mixture with prescription opioids, could punish intentional misuse and overuse of opioid medications without compromising their analgesic efficacy. A challenge will be identifying dose fractions for mu and kappa agonists that reduce abuse without causing aversion sufficient to discourage appropriate clinical use. Operant choice procedures like the one used herein can lay the groundwork for these assessments by quantifying the dose-effect relations for kappa agonists and other drug punishers.
Although the current results with salvinorin A are consistent with a punishment mechanism, an alternative account for salvinorin A’s ability to decrease drug choice could be attenuation of the dopaminergic effects of the cocaine and remifentanil injections with which it was paired. Salvinorin A and other kappa agonists have been shown to decrease extracellular brain dopamine levels (Carlezon et al 2006; Ebner et al 2010; Zhang et al 2005) and decrease cocaine-induced extracellular increases in dopamine in rodents (Maisonneuve et al 1994; Zhang et al 2004). Because dopaminergic activation is associated with the reinforcing effects of psychostimulants and opioids, it is possible that salvinorin A reduced the reinforcing effects for the drug injections with which it was paired. However, whether through punishment or amelioration of reward neurochemistry (or both), the current results demonstrate that combining salvinorin A with drug reinforcers decreases self-administration of these reinforcers independent of its non-specific effects on response rate.
Operant choice procedures can generally be categorized into two types: isomorphic choice, which involves choice between reinforcers that are qualitatively the same (e.g., cocaine vs. cocaine choice), and allomorphic choice, which involves the choice between qualitatively different reinforcers (e.g., cocaine vs. food choice). In the study of drug punishers, there are advantages to the approach used in the current report, which can be considered an equivalent isomorphic choice design (i.e., choice between equal magnitudes of the same reinforcer). First, it is relatively straight-forward to establish a baseline condition with which to test the effects of punishers on the modulation of reinforcer choice because the reinforcer options are identical. As such, there is no need to manipulate the relative magnitudes of the reinforcer options to establish a pre-test baseline condition, and dose-response functions for punishers can be determined relatively quickly. Second, because the reinforcer options are identical, long-term stability of baseline choice behavior is not affected by time, drug experience, or other environmental variables such as body weight or feeding status that might otherwise disproportionally modulate the reinforcing effects of qualitatively different options in an allomorphic choice design (e.g., Czoty and Nader 2012; Negus 2006). A potential disadvantage of the equivalent isomorphic approach is the appearance of lever position preferences, which we observed in all monkeys in the absence and at lower doses of salvinorin A. As stated above, such preferences have been amply reported in monkeys, particularly when the differences between the options are marginal (Iglauer and Woods 1974; Johanson and Schuster 1975; Woolverton et al 2012). However, salvinorin A eradicated the position preferences in all monkeys in a dose-dependent manner, which if anything strengthens the case that it functions as a punisher of drug self-administration behavior. Thus, at least for the study of punishment of drug choice, innate biases resulting in lever position preferences do not likely diminish the ability of the approach to detect punishing effects. It should be noted, however, that in the study of drug abuse, allomorphic choice approaches that include drug and non-drug options have the advantage of being more translational due to their closer approximation to the actual choice situation faced by drug abusers, i.e., the choice to take a drug at the expense of non-drug alternatives. Thorough characterizations of potential drug punishers might therefore use an isomorphic approach as a relatively quick screen for investigating a drug’s punishing effect, followed then by allomorphic choice assessments to determine the relative effectiveness of established punishers at shifting choice behavior from drug reinforcers to non-drug alternatives.
ACKNOWLEDGMENTS
This research was supported by National Institute on Drug Abuse grants DA019471 and DA026832 to W.L.W. and DA018151 to T.E.P. The authors thank Steven Ross, Jacob Smith, and Sarah Smith for their expert technical assistance.
REFERENCES
- Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant behavior: areas of research and application. Prentice-Hall Inc.; Englewood Cliffs: 1966. pp. 380–447. [Google Scholar]
- Negus Banks Mand. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. Effects of salvinorin A, a kappa opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa receptor homology in humans. J Pharmacol Exp Ther. 2007;320:300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Be guin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Backman CM, Gigante ED, Shippenberg TS. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharm. 2013:1–9. doi: 10.1038/npp.2013.171. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Differential effects of bremazocine on oral phencyclidine (PCP) self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:111–117. doi: 10.1037/1064-1297.12.2.111. [DOI] [PubMed] [Google Scholar]
- Cunningham CW, Rothman RB, Prisinzano TE. Neuropharmacology of the naturally occurring k-opioid hallucinogen salvinorin A. Pharmacol Rev. 2011;63:316–347. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Individual differences in the effects of environmental stimuli on cocaine choice in socially housed male cynomogus monkeys. Psychopharm. 2012;224:69–79. doi: 10.1007/s00213-011-2562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharm. 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, McMaster BM, Roma PG, Woolverton WL. Punishment with histamine reduces the reinforcing effectiveness of cocaine in monkeys. Psychopharm. 2014 doi: 10.1007/s00213-013-3396-y. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Histamine as a punisher in squirrel monkeys: effects of pentobarbital, chlordiazepoxide, and H1- and H2-receptor antagonists on behavior and cardiovascular responses. J Pharmacol Exp Ther. 1980;214:726–736. [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Anker JJ, Reiger PS, Claxton A, Carroll ME. Cocaine self-administration punished by i.v. histamine in rat models of high and low drug abuse vulnerability: Effects of saccharin preference, impulsivity, and sex. Physiol Behav. 2013;122:32–38. doi: 10.1016/j.physbeh.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglauer C, Woods JH. Concurrent performances: reinforcement by different doses of intravenous cocaine in rhesus monkeys. J Exp Anal Behav. 1974;22:179–196. doi: 10.1901/jeab.1974.22-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. The effects of electric shock on responding maintained by cocaine in a choice procedure in the rhesus monkey. Psychopharmacology. 1977;53:277–282. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. Effects of H1-receptor antagonists on responding punished by histamine injection or electric shock presentation in squirrel monkeys. Psychopharm. 1986;90:461–467. doi: 10.1007/BF00174061. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50488, a k-opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine. Preclinical Studies. Ann N Y Acad Sci. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69,593,U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;278:1282–1289. [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharm. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW. Mu/Kappa opioid interactions in rhesus monkeys: Implications for analgesia and abuse liability. Exp and Clin Psychopharm. 2007;16:386–399. doi: 10.1037/a0013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Jimez-Gomez C, Woods JH. A choice procedure to assess the aversive effects of drugs in rodents. J Exp Anal Behav. 2010;93:203–223. doi: 10.1901/jeab.2010.93-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE, Tidgewell K, Harding WW. k Opioids as potential treatments for stimulant dependence. AAPS Journal. 2005;7:E592–E599. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM. Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharm. 2010;210:169–177. doi: 10.1007/s00213-009-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk, et al. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharm. 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. A novel method for studying drugs as punishers. Pharmacol Biochem Behav. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self- administration in monkeys: effects of delayed punishment. Psychopharm. 2012;220:509–517. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharm. 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallcuinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharm. 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]


