Abstract
The prevalence of obesity is growing and now includes at least one-third of the adult population in the United States. As obesity and dementia rates reach epidemic proportions, an even greater interest in the effects of nutrition on the brain have become evident. This review discusses various mechanisms by which a high fat diet and/or obesity can alter the brain and cognition. It is well known that a poor diet and obesity can lead to certain disorders such as type II diabetes, metabolic syndrome, and heart disease. However, long-term effects of obesity on the brain need to be further examined. The contribution of insulin resistance and oxidative stress is briefly reviewed from studies in the current literature. The role of inflammation and vascular alterations are described in more detail due to our laboratory’s experience in evaluating these specific factors. It is very likely that each of these factors plays a role in diet-induced and/or obesity-induced cognitive decline.
Keywords: Cognition, Brain Health, Obesity, Inflammation, Cerebrovascularization
Obesity: a public health issue
The prevalence of obesity is growing and now includes at least one-third of the adult population in the United States. Another third of the population is characterized as overweight.1,2 Body mass index (BMI) is used to define overweight and obesity as between 25–30 kg/m2 and over 30 kg/m2, respectively.2 BMI is calculated by dividing weight in kilograms by height in meters squared (kilogram per square meter).3 With the current growth of this public health problem, it is projected that overweight and obesity rates will reach epidemic proportions in the United States during the next decade (as much as 75% of the population in 2015).2 Comparing these statistics to data collected in 1960 on the prevalence of obesity in the United States, the current population now includes almost triple the number of obese people (13.4% in 1960 compared to 35.7% in 2010).1,4 Worldwide, it is estimated that one billion people are overweight or obese.5
Obesity is a risk factor for many conditions including, but not limited to, diabetes, hypertension, dyslipidemia, stroke, heart disease, certain cancers, and arthritis.1,6 Although overall mortality rates continue to decline in our country due to medical and technological advancements, mortality linked to obesity-related disorders is increasing. It is clear that obesity is damaging to the health and wellness of our population, but biological mechanisms for its damaging effects are less explored. Future research must focus on the aspects of our current diet and lifestyle that lead to obesity, and the full extent of obesity-related effects on all organs of the body, including the brain.
‘The western diet’
One of the greatest factors contributing to the prevalence of obesity is choice of diet. A term to describe the unhealthy diet eaten by many Americans as well as other westernized populations is ‘the western diet’. Simply put, it is a diet that contains large amounts of red meat, refined sugars, high fat foods, and refined grains. This is in contrast to a healthier diet that is high in fruits, vegetables, lean protein, and fiber.7
Fat consumption has been found to be a key player in the obesity epidemic.7–9 The western diet often contains large amounts of saturated (SFA) and trans fatty acids (TFA) compared to a healthier diet containing more n–3 polyunsaturated fatty acids (PUFAs).10,11 The major sources of SFAs in the United States include fatty meats, baked goods, cheese, milk, margarine, and butter.8,12,13 Long-term consumption of the ‘western diet’ can lead to obesity and consequently damaging effects on general health. However, an area that has not yet been well evaluated is the damaging effects of the ‘western diet’ on the brain. This topic is the focus of the current review.
High-fat diets and cognition
Dementia by definition is a progressive deterioration in two or more modalities of cognitive performance. Diagnosis of dementia requires repeated analysis of the subject’s ability to perform complex tasks, activities of daily living, as well as changes in personality and mood. Within this review, we primarily refer to ‘cognitive decline’ or ‘cognitive impairment’ in order to examine a large spectrum of symptoms that may be affected by high-fat diets and/or obesity.
In the last decade, more scientific interest in nutrition-related effects on brain function has emerged. Rates of obesity, diabetes, and dementia continue to climb and both retrospective and prospective studies suggest that obesity and increased consumption of high-fat diets increases risk for development of dementia.14–20 As early as 1990, Greenwood and Winocur21 published one of the first studies revealing effects of a high SFA diet on learning and memory in rats. In this study, 1-month-old Long Evans rats were fed either a high SFA (lard-based diet, 40% calories from fat), a high PUFA diet (soybean oil-based, 40% calories from fat), or a standard rat chow diet (Purina, 4.5% w/w) for three months. Rats were evaluated on three different tasks: Olton’s radial arm maze, a variable interval delayed alternation task, and the Hebb-Williams maze series. Rats on the lard-based diet performed the worst on all three of these tasks, revealing damaging effects of this type of diet on the brain.21 However, the biological mechanisms involved to cause these effects were not evaluated at this time.
Following this study, eight more manuscripts were published by Greenwood and Winocur describing a link between a high fat diet and cognitive function.22–29 Later studies by this group explored the role of glucose and insulin resistance in the observed decline in cognitive function. In 2005, they published a review including results from both human epidemiological studies and rodent experiments that found insulin resistance to be at least one mechanism by which chronic consumption of a high fat diet is linked to cognitive decline and dementia.23 At this time, only a few other researchers were exploring the relationship between high-fat diets and cognition as well as the mechanisms involved. A manuscript from our research group demonstrated detrimental effects of a high-fat/high-cholesterol diet on performance in a radial arm maze in middle-aged rats, associated with reduced hippocampal dendritic integrity and activation of microglial cells in the hippocampus.30 All rodent studies exploring a correlation between a high-fat diet and cognitive impairment presented herein are summarized in Table 1.
Table 1.
Rodent studies: effect of diet on cognition
| Diet composition | Cognitive results | Postulated biological mechanism |
References |
|---|---|---|---|
| Lard-based diet (40% calories from fat) | Worse performance on working memory and retention |
Not discussed | 21 |
| Lard & corn oil (39% energy) | Worse performance on Morris water maze |
Oxidative stress, reduced BDNF levels |
31 |
| High fat diet (45% calories from fat) | Worse performance on operant- based delayed matching to position task |
Insulin resistance | 32 |
| High fat, high glucose diet supplemented with high fructose corn syrup |
Worse performance on a spatial learning task |
Insulin resistance, Reduced BDNF levels |
33 |
| High saturated fat and cholesterol | Worse performance on the Water Radial Arm Maze |
Inflammation, reduced dendritic integrity in the hippocampus |
34 |
| ‘Western diet’ (41% calories from fat) or Lard (60% calories from fat) |
Impaired retention on behavioral test for 60% fat but not ‘Western diet’ |
Oxidative stress | 35 |
| High fat diet (45% calories from fat) + Metformin |
Improved performance on operantbased task |
Insulin sensitivity | 36 |
| High-fat high-carbohydrate + Vitamin E | Improved performance on Water Radial Arm Maze |
Oxidative stress | 37 |
| High fat diet (45% calories from fat) | Impaired performance on Fear Conditioning Task |
Oxidative stress | 38 |
| High fat diet + Sugar | Impaired performance on serial feature negative task |
Vascular/adiposity | 39 |
In human epidemiological studies, it has been shown that intake of a high-fat diet that includes mostly omega-6 and SFAs is associated with worse performance on a cognitive task.14,40–43 Furthermore, studies have shown that a diet containing mostly SFAs and TFAs is associated with increased risk for Alzheimer’s disease (AD).15,40,44 On the other hand, a lower fat diet consisting of omega-3 fatty acids had a protective effect against cognitive decline in healthy older subjects.45 It has also been determined that high consumption of total fats, SFAs, and cholesterol is associated with increased cholesterolemia, risk of cardiovascular disease, and impaired intellectual function, suggesting that the circulating levels of cholesterol are closely associated with cognitive performance in humans.46 Ortega et al.41 and Greenwood and Winocur23 have also found that high-fat diets and those that lack proper vitamins and minerals consumed late in life can worsen the course of age-related cognitive decline. All human studies exploring a correlation between a high-fat diet and cognitive impairment presented herein are summarized in Table 2. During the last decade, more studies have focused on biological mechanisms for these observed cognitive effects of high-fat diets. The major proposed biological mechanisms include insulin resistance, developmental disturbances, altered membrane functioning, oxidative stress, inflammation, and altered vascularization.32,33,35,45,47,48 A summary of the proposed mechanisms for high-fat diet-induced cognitive decline is presented in Fig. 1, and will be discussed in detail in the next section of this review.
Table 2.
Human studies: effect of diet on cognition
| Diet composition | Cognitive results | Postulated biological mechanism |
References |
|---|---|---|---|
| High linoleic acid intake | Worse performance on Mini Mental State Exam | Oxidative stress | 14 |
| Poor diet resulting in impaired glucose tolerance |
Worse performance on Mini Mental State Exam | Disturbed glucose metabolism |
47 |
| Low intake of monounsaturated and saturated fat |
Best performance on the Mini-Mental State Examination and Pfeiffer’s Mental Status Questionnaire |
Oxidative Stress, lack of micronutrients such as vitamin C, folate, zinc |
41 |
| Higher intakes of saturated fat and trans-unsaturated fat |
Decline in performance on: East Boston Tests of Immediate and Delayed Recall, the Mini- Mental State Examination, and the Symbol Digit Modalities Test |
Cholesterol levels – atherogenic |
42 |
| High intake of n-3 polyunsaturated fatty acids and docosahexaenoic acid (22:6n-3) |
Reduced risk of Alzheimer’s disease | not discussed | 44 |
| Increased caloric intake and increased cholesterol intake |
Poorer performance on simple reaction time, symbol-digit substitution, and serial digit learning |
not discussed | 43 |
Figure 1.
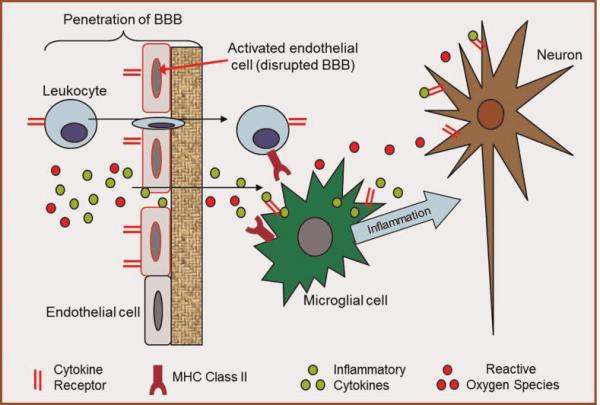
Possible mechanisms of diet-induced cognitive decline. Mechanisms described in this review likely act in concert to cause cognitive decline. These mechanisms include, but are not limited to, altered vascularization and BBB integrity, inflammation, and oxidative stress. In this diagram, we show activation of endothelial cells which increases BBB penetration allowing more inflammatory molecules and ROS to enter the brain. Then, microglial cells perpetuate the inflammatory cascade causing damage to neuronal health.
Insulin resistance
In a study by McNeilly et al.,32 rats were fed a high-fat diet (45% calories from fat) for 12 weeks which made the rats overweight and induced insulin resistance, as measured by elevated fasting plasma glucose and insulin levels. The rats consistently performed poorer than control animals on an operant-based delayed matching to position task.32 This study revealed a role for insulin resistance on behavioral flexibility. Furthermore, in a recent study by McNeilly et al.,36 the authors found that rats fed the high-fat diet (45% calories from fat) did not reveal any changes in insulin signaling-related proteins in the hypothalamus, hippocampus, striatum, or cortex. Rats that were treated with metformin had reduced weight gain and improved insulin sensitivity compared to those on the high-fat diet alone. However, metformin had no effect on behavioral performance suggesting the effects of insulin resistance on the brain and cognition include alternate or additional mechanisms. In another study utilizing diet-induced insulin resistance by Stranahan et al.,33 they fed rats a high fat, high glucose diet that was supplemented with high fructose corn syrup. The alterations to energy and lipid metabolism included elevated fasting glucose, cholesterol, and triglyceride levels which were similar to those described for clinical diabetes. After 8 months on this diet, the rats performed worse than controls on a spatial learning ability task, had reduced hippocampal dendritic spine density, reduced long-term potentiation (LTP) at Schaeffer collateral CA1 synapses, and reduced hippocampal brain-derived neurotrophic factor (BDNF) levels.33 With the increasing incidence of type II diabetes in the US population, the secondary effects of this disease including cognitive decline must be explored. In fact, recent work by Craft et al. describes an important link between insulin resistance and AD, with intranasal administration of insulin as a novel intervention candidate to improve cerebral glucose metabolism and cognitive ability.20,49–51 This work is new and growing; it may reveal important connections between diabetes and AD, leading to novel treatment options for this severe neurological disorder.
Oxidative stress
It has previously been determined that chronic elevation of oxidative stress by diet or by genetic alterations can lead to cognitive decline.52,53 In a study by our laboratory using a transgenic mouse model for Down syndrome, with a triplicated segment of murine chromosome 16, we discovered cognitive impairment in these mice associated with increased oxidative stress in brain54 further contributing to the literature that states oxidative stress plays a role in cognitive decline. In our study, vitamin E supplementation in the diet prevented age-related cognitive impairment, suggesting that antioxidant supplementation may prevent brain-related oxidative stress effects and enhance cognitive performance.54 Additional research has focused on the ability of antioxidant supplementation to reverse high levels of oxidative stress as well as declines in neuronal function and cognitive performance. For example, the Gomez-Pinilla laboratory investigated the interaction between increased oxidative stress from a high SFA diet (lard-based), BDNF levels, and cognition performance in a spatial task.31 It was found that high-fat diet-induced oxidative stress led to decreased levels of BDNF and impaired performance on the maze. Furthermore, treatment with vitamin E reversed these effects31 adding support to the hypothesis that oxidative stress causes diet-induced damage to the brain and cognition. This has also recently been shown following administration of a high-fat high-carbohydrate diet (HFCD). Rats that received the HFCD for 6 weeks had reduced levels of superoxide dismutase and catalase activity and increased thiobarbituric acid reactive substances and glutathione oxidase levels in the hippocampus. These animals were also impaired on the radial arm water maze revealing deficits in spatial learning and memory. However, rats given vitamin E concurrently with the HFCD had improved maze performance as well as reduced oxidative stress measures.37 In a study by Beltowski et al. in 2000,55 it was also found that a high-fat diet increases the tissue levels of free radicals. In a recent study by Morrison et al.,35 C57Bl/6 mice were fed either a ‘western diet’ containing 41% calories from fat, or a higher fat lard diet containing 60% calories from fat for 16 weeks. The very high-fat lard diet, but not the ‘western diet’ led to oxidative damage (as measured by protein carbonyls) in the hippocampus and impaired retention on a behavioral test (the 14-unit T-maze),35 therefore suggesting a type of ‘dose–response’ effect of the diets on oxidative stress measures in hippocampus. Lastly, we have recently shown elevated levels of total ROS in the brain due to diet-induced obese (DIO). Mice fed a high-fat diet (45% kcals from fat) had significantly higher levels of total ROS, superoxide, and peroxynitrite compared to mice fed a control diet (10% kcals from fat). The level of oxidative stress was highly related to the level of adiposity. The DIO animals also displayed impairments on a cognitive task.38 These studies present a role for oxidative stress in diet-induced cognitive impairment, and clearly suggest that oxidative stress is involved in cognitive impairment caused by high-fat diets.
Inflammation
IL-1, IL-6, and TNF-α are examples of pro-inflammatory cytokines orchestrating the inflammatory response to many stimuli, both systemically and in the brain. Most importantly, these cytokines have also been shown to cross the blood brain barrier (BBB). Pro-inflammatory cytokines can also be produced by cells within the brain parenchyma, specifically by microglial cells, astrocytes, and endothelial cells of the BBB.56–58 IL-1 and IL-6 receptors are located all over the brain, but they are especially enriched in the hippocampus,58 a critical component of the learning and memory circuitry. Pro-inflammatory cytokines have been shown to have direct detrimental effects on hippocampal circuitry and cognition. For instance, a systemic or intraventricular IL-1β injection gives rise to spatial memory impairments in rats,59,60 and significant effects of injected IL-1β on the win-shift paradigm of the radial arm maze have been reported.61 Bickford et al. have previously shown that an indirect IL-1 blockade, using a caspase ***-1 inhibitor, has significant improvement effects on memory in aged rats, suggesting that IL-1 is involved in impaired performance on memory tasks with aging.62 IL-1 has also been shown to be an important player in inflammation-induced memory impairments in rodents, following a chronic inflammation paradigm.63 Chronic inflammation due to an intra-hippocampal injection of heat-killed bacillus Calmette-Guérin gave rise to impaired performance in a hippocampal dependent task (the Y-maze), but was alleviated by the IL-1 receptor antagonist IL-1Ra.63 Pro-inflammatory cytokines have been found to impair hippocampal development, and alterations in their levels can also affect the hippocampus into adulthood.64 Specifically, IL-1 has been shown to inhibit N-Methyl-D-aspartate (NMDA)-mediated and non-NMDA mediated synaptic potentiation, LTP, and glutamate release in the hippocampus,65 providing a physiological explanation for inflammation-induced memory impairment in rodent models. Furthermore, IL-1 has been shown to affect learning and memory, BDNF expression, neurogenesis, and microglial activation,66 as indicated in the schematic drawing in Fig. 1. As outlined here, inflammation can cause damage to the brain, especially in the hippocampus. However, inflammation caused by consumption of a high fat diet has not yet been well studied.
A few recent studies from our laboratory and others have begun to investigate the role of a high fat diet on neuroinflammation and cognitive decline. Thirumangalakudi et al.67 fed a high fat/high cholesterol diet for 8 weeks to normal C57BL/6 mice and low density lipoprotein receptor (LDLR)-deficient mice (LDLR−/−). Mice fed the high fat/high cholesterol diet showed impaired working memory performance compared to controls and the LDLR−/− mice also had impaired working memory ability regardless of the diet they were fed. The LDLR−/− mice were used in this study because they naturally develop moderate hypercholesterolemia, a potential inducer of neuroinflammation and vascular damage. The high fat diet-fed and LDLR−/− mice revealed increased activated microglia and astrocytes in the hippocampus and increased mRNA expression of various pro-inflammatory cytokines/mediators such as TNF-α, IL-1-β, IL-6, nitric oxide synthase 2, and cyclooxygenase 2 in the hippocampus.67 Pistell et al.68 fed a high fat diet to C57BL/6 mice as well and found increased body weights, impaired cognition as measured by the Stone T-maze, increased brain inflammation, and decreased BDNF levels. Cytokine protein levels were measured in the cortex and revealed an increase in TNF-α, IL-6, and the chemokine monocyte chemotactic protein-1. Interestingly, these effects were only found in the high fat diet that consisted of 60% calories from fat (pork fat) but not the high fat diet that consisted of 41% calories from fat (butterfat and corn oil) with 29% sucrose.68 In multiple studies from our laboratory, we have shown morphological changes within the rat hippocampus following consumption of a high fat diet, mostly consisting of a combination of hydrogenated coconut oil (10% of diet) and 2% cholesterol.30,34,69,70 Consistent throughout our studies has been an increased number of activated microglia in rats fed the high fat diet compared to control-fed rats, which likely points to a role of neuroinflammaton in diet-induced neurodegeneration and cognitive disturbances.30,34,69,70 The activated microglia were labeled with an MHC Class II marker, OX-6, and were abundant particularly in the white matter overlying the hippocampal formation. In one of our studies, treatment of middle-aged rats with different sources of fat or increased cholesterol at equal concentrations to the combined high-fat diet were tested in order to better understand which component of the ‘Western Diet’ contributes to hippocampal morphological changes including the increased abundance of activated microglia. We determined that all components of this complex diet, including SFAs, TFAs, and cholesterol led to morphological alterations in hippocampal morphology and inflammatory activation, marked by increased activation of microglial cells, with SFAs having the greatest effect.34
However, microglia have a complex role in the brain. Although a set number of quiescent microglial cells are always present, and needed for normal function, activation of these inflammatory cells is typically correlated with the occurrence of an inflammatory event.56 For example, a single injection of the endotoxin lipopolysaccharide (LPS) results in a significant increase in activated microglia in the brain.71,72 It is well known that these cells function as macrophages in the brain, with the job of surveying the area and controlling any disturbance/foreign invader via phagocytosis.71 Microglia can release either pro- or anti-inflammatory cytokines and chemokines when stimulated.56 If they are exposed to a chronic stimulus, activated microglia remain ‘on’ and can also release toxic free radicals, as well as anti-inflammatory cytokines, including IL-10 and TGF-β.56,73,74 This suggests that microglial cells can be both ‘bad’ and ‘good’ for brain function depending on a set of triggers that are determined both by internal and external events. Microglia can switch between the classical phenotype (inflammatory), also called M1, and the alternative, neuroprotective, phenoptype, also called M2.74 Therefore, when activated microglia are visualized using immunohistochemical staining, it is difficult to discern which phenotype is expressed: M1 or M2. In support of the damaging role of microglia, the studies by Thirumangalakudi et al.67 and Pistell et al.68 reported increased levels of pro-inflammatory cytokines following high-fat diet treatment, suggesting a compensatory ramping up of the immune defense mechanisms. The source of inflammatory molecules is also an important factor that needs to be better understood. In a recent study, Buckman et al. demonstrated recruitment of peripheral immune cells into the CNS due to DIO. Using green-fluorescent protein (GFP) labeled peripheral immune cells, flow cytometry was utilized in order to quantify the number of immune cells present in the brain. Mice fed a high fat diet had a 30% increase in GFP+ cells compared to control mice. Additionally, the immune cells were further characterized and it was determined that they displayed characteristics of microglia/macrophages and were found in the parenchyma suggesting recruitment of immune cells into the CNS.75 However, it is still difficult to conclude the role of activated microglia in diet-induced neurodegeneration. This is a ‘chicken or egg’ –type question because we have not shown whether the activated microglia are the cause of neuronal damage or simply helping to remove cellular debris following neuronal loss. In future studies, the relationship between different microglial phenotypes should be examined more closely, in order to design better treatment paradigms during inflammatory insults to the brain. Nevertheless, inflammation as a key player in diet-induced and/or obesity-induced cognitive decline continues to be at the top of the list for mechanisms involved in this process.
Dysfunctional vascularization
Few studies have explored the correlation between a high fat diet or obesity and cerebral vascular changes. Studies have mostly examined the peripheral vasculature in these conditions or secondary effects of obesity/high fat diets such as metabolic syndrome and its effects on vasculature. The current studies that have explored the relationship between a high fat diet and altered cerebrovascularization include studies from our laboratory as well as a few others. For example, Constantinescu et al.76 fed a hyper-lipidemic diet to hamsters and reported not only fatty streaks in the carotid artery after 3 months on the diet and atherosclerotic plaques after 6 months, but altered micro-vascular pathology in the cerebral cortex as well. The changes to brain micro-vessels were reported to include: irregularly shaped vessels with large perivascular spaces, enlarged endothelial cells and some lumen filled with lipoprotein particles.76 In a study with LDLR−/− mice and C57BL/6J control mice fed either a high cholesterol diet or control diet, the LDLR−/− mice (regardless of diet) and control mice fed a high cholesterol diet revealed an increased microvessel diameter, vascular degeneration, and thicker basement membranes; features which were described to be similar to those found in an AD brain.77 In terms of diet-induced effects on the BBB, Kanoski et al. found decreased mRNA for claudin-5 and claudin-12, two tight junction proteins found at the BBB, in rats fed a high-energy diet compared to those fed a control diet. Furthermore, leakage of the BBB following the high-energy diet was determined with sodium fluorescein passage from the blood to the brain; interestingly, this was only found in the hippocampus.78 A follow-up study with rats fed the high-energy diet also found deficits in completing behavioral tasks. Rats were split into two groups: high-energy diet resistant (HE-DR) and high-energy diet-induced obese (HE-DIO). The HE-DIO revealed leakage of sodium fluorescein into the hippocampus and impairments on a hippocampal-dependent serial feature negative task. The HE-DR group did not exhibit BBB permeability or issues with the behavioral task and neither group was impaired on a hippocampal-independent simple discrimination problem.39 In a recent study from our laboratory, we also explored the effects of a high-fat diet on the BBB, with a focus on the hippocampus.69 First, no significant differences in glucose transporter 1 immunoreactivity (Glut-1; a transporter involved in moving glucose across the BBB and is therefore abundant on blood vessels in the brain) were found in the cornus ammonis 1 (CA1), cornus ammonis 3 (CA3), or dentate gyrus of the hippocampal formation. BBB integrity was measured using the antibody SMI-71 (an antibody specific to rat endothelial barrier protein, EBA), which has been shown in previous studies to accurately label an intact BBB.79,80 A significant decrease in SMI-71-ir was observed in the CA1 region of the hippocampus as well as parietal cortex of HFHC-fed rats. There were no significant differences observed in the CA3 region of the hippocampus, suggesting a high regional sensitivity to this type of diet. Results from the SMI-71 immunofluorescence experiment point to a possible disruption of the BBB for HFHC-treated animals. When BBB proteins such as the tight junction protein, occludin, and scaffold protein, ZO-181,82 were evaluated, decreased expression of occludin was found on blood vessels throughout the hippocampus. Interestingly, an up-regulation of occludin was found in neurons of the dentate gyrus and mossy fibers of the CA3 region (an area spared by BBB disruption according to our SMI-71 results), suggesting a possible compensation in neuronal occludin expression following the decrease observed in vascular occludin expression. These findings add to the hypothesis that a high-fat diet can alter vascular components of the brain, leading to BBB disruption and dysfunction of brain endothelial cells, but more studies are necessary to determine the direct mechanisms. A summary of plausible events following diet-induced changes in the brain vasculature is shown in Fig. 1. Taken together, these recent studies point to an alteration in cerebro-vascularization following a high-fat diet. Future studies should include exploration of the role of high-fat diets on cerebral blood flow and BBB integrity, and the subsequent effects on cognition as well as the interaction between inflammatory and vascular factors upon hippocampal function.
Contribution of the aging process
While high fat diet-induced neuroinflammation and cognitive decline have not been extensively explored, the role of neuroinflammation in aging and cognitive decline has been well studied. In fact, neuroinflammation has been proposed to be in the center of pathological alterations occurring in almost all age-related neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), AD, and Parkinson’s disease, as well as normal aging.83–85 During the aging process, there is a shift in the brain toward a pro-inflammatory state which leads to a chronic increase in activation of microglial cells. Studies have reported increased levels of TNF-α, IL-1, and IL-6 in brain tissue and serum of aged humans, as well as animal models.45,86,87 Levels of cyclooxygenase, lipoxygenase, prostanoids, and eicosanoids, all components of inflammatory pathways, have also been shown to be elevated in the brain with aging.45,88,89 Furthermore, it has been shown that there is a progressive deterioration of the immune response with increased aging, including time to build a response, level of activation, and speed in which the response is ended.90–92 This altered immune response occurs in the periphery as well as in the brain.93 It has also been shown that this process is coupled to a decrease in anti-inflammatory molecules which together create an environment for an exaggerated immune response.94 Age-induced neuroinflammation has been correlated with neurodegeneration and cognitive decline.95–97 Cytokines such as TNF-α and IL-6 have demonstrated a role in age-related neuroinflammation and neuronal dysfunction. However, IL-1 beta has been described as especially important for inflammatory changes occurring with aging.98–100 For example, Trompet et al.101 revealed better cognitive performance in an elderly population that had a genetic variation in the IL-1 beta converting enzyme (ICE) causing lower levels of IL-1 beta compared to those without the genetic variation.
Aging itself can also lead to disrupted cerebral blood flow and decreased angiogenesis.102 In fact, many human studies have revealed increased BBB permeability for elderly, healthy subjects compared with young, healthy subjects.103,104 Changes also occur during aging at the level of endothelial cells such as a decreased number of endothelial cell mitochondria, impaired endothelium dependent vasodilation, and a loss of elongation in endothelial cells.45,105–107 The mechanisms proposed to be involved in age-related BBB breakdown include increased oxidative stress,108 inflammation and hypertension.109–111 Further clinical evidence for vascular changes during aging includes visualization of white matter hyper-intensities (WMHs) which occur in 30% of healthy adults over 60 years old.112 WMHs are observed on T2-weighted magnetic resonance imaging scans as areas with increased signal. The reason for WMHs is controversial; however, they are believed to be involved in ischemia, hypoperfusion, BBB leakage, inflammation, and/or neurodegeneration.113–115 Neuropathological evaluations post-mortem have revealed various findings to explain WMHs and include arteriosclerosis, demyelination, and gliosis.116,117 While age is the strongest predictor of WMHs, hypertension, atherosclerosis, and decreased cortical blood vessel density have been found to be correlated as well.118–121 In vitro experiments have begun to explain at least one mechanism by which opening of the BBB occurs with aging. It is known that aging is associated with increased inflammation122,123 and that microglia and astrocytes can release pro-inflammatory cytokines such as IL-1, IL-6, and TNFα.124–126 These pro-inflammatory cytokines activate cerebral endothelial cells to produce eicosanoids which then open the BBB.127 It has also been determined that the type I IL-1 receptor is expressed directly on cerebral endothelial cells further explaining the mechanism by which increased inflammation can open the BBB127 (Fig. 1). Increased permeability of the BBB leads to migration of monocytes across the barrier, as well as infusion of other pro-inflammatory cytokines, such as TNFα, and further perpetuates an already increased neuroinflammatory environment caused by aging. This phenomenon has been observed in cerebral inflammatory diseases such as multiple sclerosis and bacterial meningitis.128,129 However, this has not been thoroughly evaluated in a model producing chronic inflammation from a poor diet or obesity. The only evidence to date that alludes to this connection include the following: (i) high-fat diet consumption and obesity increases risk of cerebral stroke130 possibly by altering cerebral perfusion45,131 and (ii) in a study by Osmond et al.,132 adult obese Zucker rats that exhibited moderate hypertension and severe insulin resistance also revealed increased cerebral vascular myogenic tone and inward cerebral vascular remodeling.132 The contributions of these age-related changes to inflammation and vascularization to obesity have important implications for the susceptibility and progression of cognitive decline.
Summary
As described above, a number of factors have been proposed to cause high-fat diet-induced damage to the brain, especially with aging, including oxidative stress, insulin resistance, inflammation, and changes to vascularization/BBB integrity. The contribution of insulin resistance, essential fatty acid consumption, and oxidative stress may be coordinated with inflammatory and vascular alterations to cause overall changes in brain function with consumption of high-fat and high-glycemic index-type diets. However, not enough studies have been conducted to fully understand the role of each of these cascades for high-fat-induced cognitive impairment. Based on the epidemic proportions of diabetes and obesity in the United States today, it is important to reveal these factors. A diagram illustrating our current thoughts regarding mechanisms involved, as outlined in this review is shown in Fig. 1. Our studies strongly suggest that aged individuals are more susceptible to damaging effects of high-fat diets than young subjects, making diet intervention and exercise programs even more valuable from the standpoint of preventing further cognitive decline in elderly patients.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA. The obesity epidemic in the united states–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA. Obesity: basic considerations and clinical approaches. Dis Mon. 1989;35:449–537. doi: 10.1016/0011-5029(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Runge CF. Economic consequences of the obese. Diabetes. 2007;56:2668–72. doi: 10.2337/db07-0633. [DOI] [PubMed] [Google Scholar]
- 6.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–79. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–7. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 9.Demigne C, Bloch-Faure M, Picard N, Sabboh H, Besson C, Remesy C, et al. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr. 2006;45:298–306. doi: 10.1007/s00394-006-0599-6. [DOI] [PubMed] [Google Scholar]
- 10.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 12.Subar AF, Krebs-Smith SM, Cook A, Kahle LL. Dietary sources of nutrients among us adults, 1989 to 1991. J Am Diet Assoc. 1998;98:537–47. doi: 10.1016/S0002-8223(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 13.Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med. 1988;84:739–49. doi: 10.1016/0002-9343(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 14.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the rotterdam study. Ann Neurol. 1997;42:776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 15.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–63. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 16.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hyper-tension: the framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–8. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 17.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the us population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 18.Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110:95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 19.Parrott MD, Greenwood CE. Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci. 2007;1114:389–97. doi: 10.1196/annals.1396.028. [DOI] [PubMed] [Google Scholar]
- 20.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–5. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- 22.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, et al. Memory impairment in obese zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–95. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26(Suppl 1):42–5. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–9. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutr. 2001;74:687–93. doi: 10.1093/ajcn/74.5.687. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol Learn Mem. 2001;75:179–89. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–36. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- 28.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101:153–61. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav Neurosci. 1996;110:451–9. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- 30.Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 32.McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res. 2011;217:134–41. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman LR, Haley-Zitlin V, Stevens C, Granholm AC. Diet-induced effects on neuronal and glial elements in the middle-aged rat hippocampus. Nutr Neurosci. 2011;14:32–44. doi: 10.1179/174313211X12966635733358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased nrf2 signaling. J Neurochem. 2010;114:1581–9. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55:3061–70. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- 37.Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin e prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiol Behav. 2013;119C:72–78. doi: 10.1016/j.physbeh.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Freeman LR, Zhang L, Nair A, Dasuri K, Francis J, Fernandez-Kim SO, et al. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic Biol Med. 2013;56:226–33. doi: 10.1016/j.freeradbiomed.2012.08.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 41.Ortega RM, Requejo AM, Andres P, Lopez-Sobaler AM, Quintas ME, Redondo MR, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66:803–9. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 42.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, McKeown RE, Muldoon MF, Tang S. Cognitive performance is associated with macronutrient intake in healthy young and middle-aged adults. Nutr Neurosci. 2006;9:179–87. doi: 10.1080/10284150600955172. [DOI] [PubMed] [Google Scholar]
- 44.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 45.Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, et al. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem. 2010;114:344–61. doi: 10.1111/j.1471-4159.2010.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Requejo AM, Ortega RM, Robles F, Navia B, Faci M, Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57(Suppl 1):S54–7. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 47.Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J Nutr Health Aging. 2000;4:202–7. [PubMed] [Google Scholar]
- 48.Uranga RM, Keller JN. Diet and age interactions with regards to cholesterol regulation and brain pathogenesis. Curr Gerontol Geriatr Res. 2010;14:219683. doi: 10.1155/2010/219683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schioth HB, Craft S, Brooks SJ, Frey WH, II, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol. 2012;46:4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–7. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–2. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 53.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 54.Lockrow J, Prakasam A, Huang P, Bimonte-Nelson H, Sambamurti K, Granholm AC. Cholinergic degeneration and memory loss delayed by vitamin e in a down syndrome mouse model. Exp Neurol. 2009;216:278–89. doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beltowski J, Wojcicka G, Gorny D, Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol. 2000;51:883–96. [PubMed] [Google Scholar]
- 56.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 57.Merrill JE, Murphy SP. Inflammatory events at the blood brain barrier: regulation of adhesion molecules, cytokines, and chemokines by reactive nitrogen and oxygen species. Brain Behav Immun. 1997;11:245–63. doi: 10.1006/brbi.1997.0496. [DOI] [PubMed] [Google Scholar]
- 58.Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 59.Gibertini M. Il1 beta impairs relational but not procedural rodent learning in a water maze task. Adv Exp Med Biol. 1996;402:207–17. doi: 10.1007/978-1-4613-0407-4_27. [DOI] [PubMed] [Google Scholar]
- 60.Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007;18:137–48. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- 61.Song C, Phillips AG, Leonard BE, Horrobin DF. Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of inter-leukin-1beta in rats. Mol Psychiatry. 2004;9:630–8. doi: 10.1038/sj.mp.4001462. [DOI] [PubMed] [Google Scholar]
- 62.Gemma C, Stellwagen H, Fister M, Coultrap SJ, Mesches MH, Browning MD, et al. Rosiglitazone improves contextual fear conditioning in aged rats. Neuroreport. 2004;15:2255–9. doi: 10.1097/00001756-200410050-00023. [DOI] [PubMed] [Google Scholar]
- 63.Palin K, Bluthe RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1beta mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus calmette-guerin in the rat hippocampus. Brain Behav Immun. 2004;18:223–30. doi: 10.1016/j.bbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22:854–62. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. Bdnf mrna expression in rat hippocampus following contextual learning is blocked by intrahippocampal il-1beta administration. J Neuroimmunol. 2004;155:119–26. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106:475–85. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freeman LR, Granholm AC. Vascular changes in rat hippo-campus following a high saturated fat and cholesterol diet. J Cereb Blood Flow Metab. 2012;32:643–53. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman LR, Small BJ, Bickford PC, Umphlet C, Granholm AC. A high fat/high cholesterol diet inhibits growth of fetal hippocampal transplants via increased inflammation. Cell Transplant. 2011;20:1499–514. doi: 10.3727/096368910X557281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakajima K, Kohsaka S, Tohyama Y, Kurihara T. Activation of microglia with lipopolysaccharide leads to the prolonged decrease of conventional protein kinase c activity. Brain Res Mol Brain Res. 2003;110:92–9. doi: 10.1016/s0169-328x(02)00644-7. [DOI] [PubMed] [Google Scholar]
- 72.Matsushita Y, Nakajima K, Tohyama Y, Kurihara T, Kohsaka S. Activation of microglia by endotoxin suppresses the secretion of glial cell line-derived neurotrophic factor (gdnf) through the action of protein kinase c alpha (pkcalpha) and mitogen-activated protein kinases (mapks) J Neurosci Res. 2008;86:1959–71. doi: 10.1002/jnr.21657. [DOI] [PubMed] [Google Scholar]
- 73.Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–53. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 74.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Constantinescu E, Safciuc F, Sima AV. A hyperlipidemic diet induces structural changes in cerebral blood vessels. Curr Neurovasc Res. 2011;8:131–44. doi: 10.2174/156720211795495330. [DOI] [PubMed] [Google Scholar]
- 77.Franciosi S, Gama Sosa MA, English DF, Oler E, Oung T, Janssen WG, et al. Novel cerebrovascular pathology in mice fed a high cholesterol diet. Mol Neurodegener. 2009;4:42. doi: 10.1186/1750-1326-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–19. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30:1939–50. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheen SH, Kim JE, Ryu HJ, Yang Y, Choi KC, Kang TC. Decrease in dystrophin expression prior to disruption of brain-blood barrier within the rat piriform cortex following status epilepticus. Brain Res. 2011;1369:173–83. doi: 10.1016/j.brainres.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 81.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 82.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 83.Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–72. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- 84.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 85.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–92. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 87.Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, et al. Immune response gene expression increases in the aging murine hippocampus. J Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- 88.Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. Faseb J. 1998;12:439–49. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- 89.Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. Faseb J. 2000;14:1464–9. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- 90.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty. Inflamm Res. 2008;57:558–63. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 91.Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, et al. Inflammaging as a prodrome to Alzheimer’s disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J Leukoc Biol. 2010;87:1001–9. doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perry VH, Bolton SJ, Anthony DC, Betmouni S. The contribution of inflammation to acute and chronic neurodegeneration. Res Immunol. 1998;149:721–5. doi: 10.1016/s0923-2494(99)80046-7. [DOI] [PubMed] [Google Scholar]
- 94.Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–65. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Craighead MW, Boutin H, Middlehurst KM, Allan SM, Brooks N, Kimber I, et al. Influence of corticotrophin releasing factor on neuronal cell death in vitro and in vivo. Brain Res. 2000;881:139–43. doi: 10.1016/s0006-8993(00)02759-1. [DOI] [PubMed] [Google Scholar]
- 96.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–44. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 97.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–66. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1 beta. Prog Neurobiol. 1998;56:571–89. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 99.Lynch AM, Lynch MA. The age-related increase in il-1 type i receptor in rat hippocampus is coupled with an increase in caspase-3 activation. Eur J Neurosci. 2002;15:1779–88. doi: 10.1046/j.1460-9568.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- 100.Lynch MA. Interleukin-1 beta exerts a myriad of effects in the brain and in particular in the hippocampus: analysis of some of these actions. Vitam Horm. 2002;64:185–219. doi: 10.1016/s0083-6729(02)64006-3. [DOI] [PubMed] [Google Scholar]
- 101.Trompet S, de Craen AJ, Slagboom P, Shepherd J, Blauw GJ, Murphy MB, et al. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The prosper study. Brain. 2008;131:1069–77. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- 102.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 103.Blennow K, Fredman P, Wallin A, Gottfries CG, Skoog I, Wikkelso C, et al. Protein analysis in cerebrospinal fluid. Iii. Relation to blood-cerebrospinal fluid barrier function for formulas for quantitative determination of intrathecal igg production. Eur Neurol. 1993;33:134–42. doi: 10.1159/000116920. [DOI] [PubMed] [Google Scholar]
- 104.Toornvliet R, van Berckel BN, Luurtsema G, Lubberink M, Geldof AA, Bosch TM, et al. Effect of age on functional p-glycoprotein in the blood-brain barrier measured by use of (r)-[(11)c]verapamil and positron emission tomography. Clin Pharmacol Ther. 2006;79:540–8. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Alessio P. Aging and the endothelium. Exp Gerontol. 2004;39:165–71. doi: 10.1016/j.exger.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 106.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, et al. Impaired l-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 107.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–94. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 108.Yasui F, Ishibashi M, Matsugo S, Kojo S, Oomura Y, Sasaki K. Brain lipid hydroperoxide level increases in senescence-accelerated mice at an early age. Neurosci Lett. 2003;350:66–8. doi: 10.1016/s0304-3940(03)00827-9. [DOI] [PubMed] [Google Scholar]
- 109.Baumbach GL, Dobrin PB, Hart MN, Heistad DD. Mechanics of cerebral arterioles in hypertensive rats. Circ Res. 1988;62:56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- 110.Nag S, Takahashi JL, Kilty DW. Role of vascular endothelial growth factor in blood-brain barrier breakdown and angiogenesis in brain trauma. J Neuropathol Exp Neurol. 1997;56:912–21. doi: 10.1097/00005072-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Nag S, Kilty DW. Cerebrovascular changes in chronic hyper-tension. Protective effects of enalapril in rats. Stroke. 1997;28:1028–34. doi: 10.1161/01.str.28.5.1028. [DOI] [PubMed] [Google Scholar]
- 112.Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- 113.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental mri white matter signal hyperintensities. Neurology. 1993;43:1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 114.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham study. Stroke. 2004;35:1831–5. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 115.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the framingham study. Stroke. 2004;35:1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 116.Gouw AA, van der Flier WM, Pantoni L, Inzitari D, Erkinjuntti T, Wahlund LO, et al. On the etiology of incident brain lacunes: longitudinal observations from the ladis study. Stroke. 2008;39:3083–5. doi: 10.1161/STROKEAHA.108.521807. [DOI] [PubMed] [Google Scholar]
- 117.Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, et al. Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–98. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- 118.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the rotterdam scan study. Neurology. 2001;56:1539–45. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 119.Pico F, Dufouil C, Levy C, Besancon V, de Kersaint-Gilly A, Bonithon-Kopp C, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the eva-mri cohort. Cerebrovasc Dis. 2002;14:109–15. doi: 10.1159/000064741. [DOI] [PubMed] [Google Scholar]
- 120.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–90. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 121.Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian stroke prevention study. Stroke. 2005;36:1410–4. doi: 10.1161/01.STR.0000169924.60783.d4. [DOI] [PubMed] [Google Scholar]
- 122.Agrawal A, Agrawal S, Gupta S. Dendritic cells in human aging. Exp Gerontol. 2007;42:421–6. doi: 10.1016/j.exger.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. 2012;44:206–17. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chao CC, Hu S, Close K, Choi CS, Molitor TW, Novick WJ, et al. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992;166:847–53. doi: 10.1093/infdis/166.4.847. [DOI] [PubMed] [Google Scholar]
- 125.Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci. 1994;16:128–36. doi: 10.1159/000112099. [DOI] [PubMed] [Google Scholar]
- 126.Giulian D, Li J, Leara B, Keenen C. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int. 1994;25:227–33. doi: 10.1016/0197-0186(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 127.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 128.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988;82:1339–46. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moor AC, de Vries HE, de Boer AG, Breimer DD. The blood-brain barrier and multiple sclerosis. Biochem Pharmacol. 1994;47:1717–24. doi: 10.1016/0006-2952(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 130.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics–2008 update: a report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 131.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–13. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 132.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the zucker rat. Hypertension. 2009;53:381–6. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]


