SUMMARY
Northern elephant seal pups naturally endure a 2–3 month post-weaning fast that is associated with activation of systemic renin–angiotensin system (RAS), a decrease in plasma adiponectin (Acrp30), and insulin resistance (IR)-like conditions. Angiotensin II (Ang II) and tumor necrosis factor-alpha (TNF-α) are potential causal factors of IR, while Acrp30 may improve insulin signaling. However, the effects of fasting-induced activation of RAS on IR-like conditions in seals are not well described. To assess the effects of prolonged food deprivation on systemic and local RAS, and their potential contribution to TNF-α as they relate to an IR condition, the mRNA expressions of adipose and muscle RAS components and immuno-relevant molecules were measured along with plasma RAS components. Mean plasma renin activity and Ang II concentrations increased by 89 and 1658%, respectively, while plasma angiotensinogen (AGT) decreased by 49% over the fast, indicative of systemic RAS activation. Prolonged fasting was associated with decreases in adipose and muscle AGT mRNA expressions of 69 and 68%, respectively, corresponding with decreases in tissue protein content, suggesting suppression of local AGT production. Muscle TNF-α mRNA and protein increased by 239 and 314%, whereas those of adipose Acrp30 decreased by 32 and 98%, respectively. Collectively, this study suggests that prolonged fasting activates a systemic RAS, which contributes to an increase in muscle TNF-α and suppression of adipose Acrp30. This targeted and tissue-specific regulation of TNF-α and Acrp30 is likely coordinated to synergistically contribute to the development of an IR-like condition, independent of local RAS activity. These data enhance our understanding of the adaptive mechanisms evolved by elephant seals to tolerate potentially detrimental conditions.
KEY WORDS: adiponectin, food deprivation, insulin resistance, obesity, renin–angiotensin system, tumor necrosis factor-alpha
INTRODUCTION
Northern elephant seals repeatedly endure spontaneous, extended (2–3 months) fasting on land as part of their life history. Pups are suckled for 4 weeks before an abrupt weaning that initiates an 8–12 week fast (Le Boeuf et al., 1972; Reiter et al., 1978). In preparation for fasting, pups consume extremely high-fat milk (~50%) that increases their body fat to up to 50% of their mass (Reiter et al., 1978), which is referred to as ‘natural adiposity’ (Kirby and Ortiz, 1994; Ortiz et al., 1978; Ortiz et al., 1984).
For the maintenance of circulating glucose levels, fasting pups rely on lipid oxidation and efficient glucose recycling (Champagne et al., 2005; Champagne et al., 2012; Costa and Ortiz, 1982; Tavoni et al., 2013), coupled with insulin resistance (IR)-like conditions (Fowler et al., 2008; Viscarra et al., 2011a; Viscarra et al., 2011b). These adapted mechanisms allow pups to meet their energetic burdens during prolonged fasting. Prolonged fasting in elephant seals is also characterized by activation of systemic renin–angiotensin system (RAS) (Ortiz et al., 2000; Ortiz et al., 2006), decreased plasma adiponectin (Acrp30) (Viscarra et al., 2011a) and an increasing trend in plasma tumor necrosis factor-alpha (TNF-α) (Vázquez-Medina et al., 2010), suggesting that increases in systemic RAS may contribute to the changes in Acrp30 and TNF-α.
Inappropriate activation of systemic and local RAS, when associated with obesity, contributes to insulin resistance through cytokine expression (Dandona et al., 2007; Henriksen, 2007; Kalupahana and Moustaid-Moussa, 2012; Karlsson et al., 1998; Olivares-Reyes et al., 2009; Shiuchi et al., 2004). Angiotensin (Ang) II activates tissue-derived cytokines such as TNF-α in adipose and muscle tissue (Kurata et al., 2006; Wei et al., 2008b). Recent data suggest that AT1 activation by Ang II binding in adipose and muscle tissue impairs glucose uptake by inhibiting Akt activation and the subsequent translocation of GLUT4 (Shiuchi et al., 2004; Wei et al., 2008a). The over-production of ROS and upregulation of TNF-α are implicated in the impairment of glucose uptake (Shiuchi et al., 2004; Wei et al., 2008a). The RAS components, angiotensinogen (AGT), angiotensinogen converting enzyme (ACE) and angiotensin II type 1 receptor (AT1), are expressed in adipose tissue (Cassis et al., 2008; Engeli et al., 2000; Kalupahana and Moustaid-Moussa, 2012; Yasue et al., 2010) and skeletal muscle (Johnston et al., 2011; Jones and Woods, 2003; Kadowaki et al., 2006; Vermes et al., 2003) suggesting that an imbalance between systemic and local RAS has the potential to exacerbate metabolic disorders.
Obesity may induce the expression of inflammatory cytokines [TNF-α and monocyte chemoattractant protein-1 (MCP-1)] in peripheral tissues that may contribute to the development of IR (Plomgaard et al., 2005; Saghizadeh et al., 1996). TNF-α has been demonstrated to be a causative factor of obesity-related IR by directly inhibiting tyrosine phosphorylation of insulin receptor substrate (IRS), leading to impaired insulin signaling (Plomgaard et al., 2005; Steinberg et al., 2006). Increased local cytokine production induced by activation of RAS can worsen systemic insulin sensitivity (Yvan-Charvet and Quignard-Boulangé, 2011). Conversely, secretion of adipose-derived Acrp30 may counter IR by activating IRS-1-associated PI3-kinase and glucose uptake, accelerating free fatty acid clearance and fatty acid oxidation in muscle, and suppressing TNF-α production (Maeda et al., 2002; Stefan et al., 2002; Wang et al., 2007). However, Ang II reduces plasma Acrp30 levels in an AT1-dependent manner (Ran et al., 2006), suggesting that RAS activation may induce local cytokine by suppressing adipose Acrp30. Unfortunately, the relationships between systemic and local RAS, and tissue cytokines and Acrp30 during prolonged food deprivation in mammals are not well defined.
While seal pups experience prolonged fasting in a state of ‘natural adiposity’, the effects of protracted bouts of food deprivation on local RAS and its contribution to increased potential for a local TNF-α-associated response during IR-like conditions in mammals are not well defined. In addition, activation of systemic and local RAS can induce IR, but data on the contribution of local versus systemic activation of RAS during prolonged fasting are scarce. To assess these relationships, we measured the expression of local (adipose and muscle) RAS components and IR-related immuno-relevant molecules, TNF-α, MCP-1 and Acrp30, over 7 weeks of absolute fasting in northern elephant seal pups. We hypothesized that increased activation of systemic RAS during prolonged fasting may increase local TNF-α production.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California Merced and Sonoma State University. All work was realized under National Marine Fisheries Service marine mammal permit no. 87-1743.
Sample collection
Adipose tissue and muscle biopsies, and plasma samples were collected from northern elephant seal [Mirounga angustirostris (Gill 1866)] pups at Año Nuevo State Park, CA, at three periods during their natural, post-weaning fast: (1) 1 week (week 1, N=5), (2) 3 weeks (week 3, N=6) and (3) 7 weeks (week 7, N=5). Sampling procedures were performed as described previously (Vázquez-Medina et al., 2011). Briefly, animals were sedated with 1 mg kg−1 tiletamine hydrochloride and zolazepam hydrochloride (Telazol, Fort Dodge Animal Health, Fort Dodge, IA, USA) administrated intramuscularly. Immobilization was maintained with injection of ketamine (Fort Dodge Animal Health) as needed. After immobilization, a spinal needle was inserted into the extradural spinal vein and blood samples were collected and placed on ice until plasma separation. Tissue samples were rinsed with ice-cold sterile saline solution and placed in cryogenic vials. Plasma and tissue samples were frozen by immersion in liquid nitrogen immediately after collection and stored at −80°C until analyzed.
Measurement of PRA and Ang II concentrations
Plasma renin activity (PRA) was measured using a commercially available radioimmunoassay kit (Diasorin, Saluggia, Italy), previously validated for elephant seals (Ortiz et al., 2000; Ortiz et al., 2006). Plasma Ang II concentrations were measured using a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals, Burlingame, CA, USA), previously validated for elephant seals (Ortiz et al., 2000; Ortiz et al., 2006), following methanol extraction.
Real-time PCR
Extraction of RNA from adipose and muscle tissue was completed with TRIzol according to the manufacturer's instructions (Life Technologies, Grand Island, NY, USA). Contamination of genomic DNA in total RNA was eliminated by digestion with DNase I (Roche, Indianapolis, IN, USA). Synthesis of cDNA was accomplished with 1 μg of RNA and oligo (dT) primer (Table 1) using the QuantiTect reverse transcription kit (Qiagen, Valencia, CA, USA). PCR reactions were performed for ACE, AGT, renin, AT1a, TNF-α, MCP1, Acrp30 and GAPDH, and the PCR fragments were sequenced. Real-time PCR was performed with 1 μl of the cDNA for each gene except for renin. Primer sequences used are shown in Table 1.
Table 1.
Primers used for PCR
Rapid amplification of cDNA ends or amplification of full-length translated region
Following qPCR, rapid amplification of cDNA ends (RACE) PCR or amplification of full-length translated region was conducted to obtain full-length sequences of the genes for which mRNA expression was significantly changed during prolonged fasting (AGT, Acrp30 and TNF-α). Five micrograms of extracted RNA were used to synthesize cDNAs for RACE using the GeneRacer Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's manual. RACE PCR was conducted using each gene-specific primer (AGT: FW2 or RV2; Acrp30: FW or RV2; Table 1) and the 3′ or 5′ primer provided in the kit. The PCR condition was 94°C for 2 min, five cycles of 94°C for 30 s and 72°C for 1 min, five cycles of 94°C for 30 s and 70°C for 1 min, 25 cycles of 94°C for 30 s, 60–65°C for 30 s and 72°C for 1 min, and 68°C for 10 min. Amplification of the full-length translated region of cDNA was performed for TNF-α using the provided set of primers (FW2 and RV2; Table 1).
Western blots
The amount of tissues obtained from the biopsies prohibited us from performing western blot analyses on all proteins for which PCR data were also obtained. Thus, western blot analyses were prioritized to those proteins for which the most robust changes in mRNA expression were detected. Protein expression in adipose and muscle AGT and TNF-α, and adipose Acrp30 were quantified by standard western blotting as previously described (Vázquez-Medina et al., 2010; Vázquez-Medina et al., 2011; Viscarra et al., 2011a). Primary antibodies were diluted 1:1000 (sheep anti-dog AGT, a kind of gift from Dr C. Sernia, University of Queensland, Australia; and goat anti-Acrp30, Santa Cruz Biotechnologies, Dallas, TX, USA, catalog no. sc-26497) or 1:500 [goat anti-TNF-α (L1-19), Santa Cruz Biotechnologies, catalog no. sc-1351]. The HRP-conjugated secondary antibodies were diluted 1:10,000 to 1:20,000 (donkey anti-sheep IgG for AGT; Abcam, Cambridge, MA, USA, catalog no. ab97125; donkey anti-goat IgG for TNF-α and Acrp30, Santa Cruz Biotechnologies, catalog no. sc-2042). Membranes were developed using the Immun-Star Western C kit (Bio-Rad Laboratories, Hercules, CA, USA). Blots were visualized using a Kodak Image Station 440 (Kodak, Rochester, NY, USA) and quantified using Kodak 1D 3.6 Image Analysis Software. In addition to consistently loading the same amount of total protein per well (20 μg), densitometry values were further normalized by correcting for the densitometry values of β-actin (Viscarra et al., 2011a). Because commercial AGT kits were not sufficiently robust to detect plasma AGT concentration, we performed standard western blotting to measure relative changes in plasma AGT content. Plasma samples were diluted to normalize the total protein of samples to 1 μg well−1. The same primary antibody used for tissue AGT measurements was used for plasma AGT at a 1:10,000 dilution.
Statistics
Means (±s.e.m.) were compared among groups by Kruskal–Wallis ANOVA. In the case where differences were detected by ANOVA, the difference between week 1 and another week was tested by the Mann–Whitney U-test. Differences were considered significant at P<0.05. Statistical analyses were performed with Statplus professional software (AnalystSoft, Alexandria, VA, USA).
RESULTS
Systemic RAS components
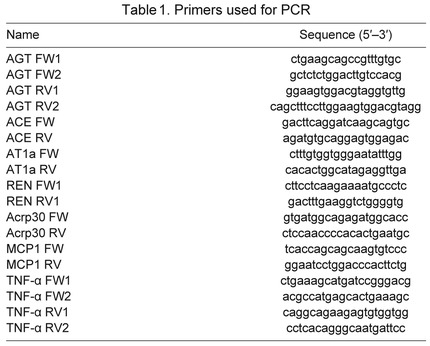
To evaluate the status of systemic RAS during fasting, PRA, plasma AGT content and plasma Ang II concentrations were measured. After 7 weeks of fasting, mean PRA increased by 89%, mean plasma AGT content decreased by 49% and mean plasma Ang II increased by 1658% (Fig. 1). These results indicate that prolonged fasting is associated with activation of systemic RAS in elephant seals.
Fig. 1.
Prolonged fasting increased mean (±s.e.m.) plasma renin activity (PRA; A) and plasma angiotensin II (Ang II) concentration (inset, representative western blot; B), and decreased mean (±s.e.m.) plasma angiotensinogen (AGT) content (C) of northern elephant seal pups. Asterisk denotes significant difference from week 1 (P<0.05).
Sequencing of qRT-PCR fragments and full-length cDNA
To confirm the validity of qRT-PCR data, we sequenced fragments obtained by qRT-PCR. Fragment lengths obtained by qRT-PCR were 190 bp for ACE, 197 bp for Acrp30, 202 bp for AGT, 235 bp for AT1a, 98 bp for MCP-1 and 143 bp for TNF-α. The nucleotide sequences and deduced amino acid sequences are highly conserved in relation to those of other carnivores such as the dog and the panda. To confirm mRNA expression, PCR fragments of 3′- and 5′-RACE or amplification of full-length translated regions were obtained for AGT, TNF-α and Acrp30 using adipose and muscle tissue samples. Those full-length cDNAs were sequenced for AGT (GenBank accession number KC013289), TNF-α (KC013291) and Acrp30 (KC013290). The molecular weights estimated by deduced amino acid sequences were 51.5 kDa for AGT, 25.6 kDa for TNF-α and 28.4 kDa for Acrp30, which are comparable to the molecular weights deduced for other mammals.
Local expression of RAS components
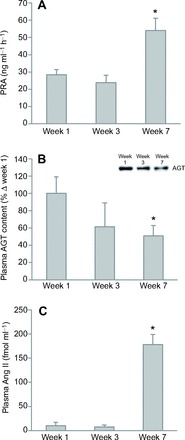
To evaluate the status of local RAS during fasting, mRNA expression and protein content of RAS components in adipose and muscle tissue were measured by qRT-PCR and western blotting. In both muscle and adipose tissue, AGT mRNA expression decreased by over 50% after 7 weeks (Fig. 2A,B). Similarly, muscle AGT protein at 52 kDa decreased by 98% after 7 weeks (Fig. 2C), while adipose AGT protein tended (P<0.10) to decrease after weeks 3 and 7 (Fig. 2D), as demonstrated by the representative western blot (Fig. 2D, inset). Neither ACE nor AT1a in tissues were significantly altered with fasting (Table 2).
Fig. 2.
Prolonged fasting decreased mean (±s.e.m.) angiotensinogen (AGT) mRNA in (A) muscle and (B) adipose tissue, and mean (±s.e.m.) protein content (detected at 52 kDa) in (C) muscle (inset, representative western blot) and (D) adipose tissue of northern elephant seal pups. Asterisks denote significant difference from week 1 (*P<0.05, **P<0.01).
Table 2.
Changes from week 1 in mean (%, normalized relative to week 1 values; ±s.e.m.) RNA expression of angiotensin converting enzyme (ACE), angiotensin II receptor-type 1a (AT1a), adiponectin (Acrp30), monocyte chemotactic protein-1 (MCP1), and tumor necrosis factor-α (TNF-α)
Expression of adipose and muscle adiponectin and immuno-relevant molecules
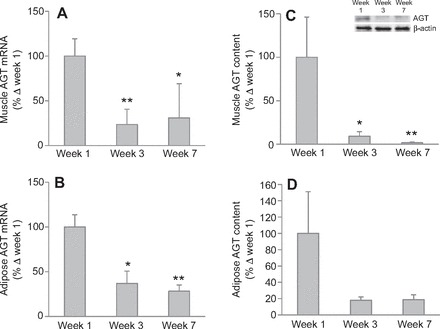
In addition to the RAS components, local inflammation status was assessed by measuring mRNA and protein of TNF-α and MCP-1, in addition to Acrp30. Adipose Acrp30 mRNA decreased (P<0.05) by 40% after 3 weeks, and remained decreased by 32% after 7 weeks, although this decrease was not statistically significant (P<0.10; Fig. 3A). Adipose Acrp30 protein decreased by 98% after 7 weeks (Fig. 3B), although we could not detect a statistical increase in adipose TNF-α and MCP-1 mRNA expressions, despite strong tendencies to increase after 7 weeks (Table 2). Muscle TNF-α mRNA expression increased by 239% after 7 weeks (Fig. 4A), but Acrp30 and MCP-1 mRNA expressions were not changed with fasting (Table 2). Similarly, muscle TNF-α protein increased by 314% after 7 weeks (Fig. 4B).
Fig. 3.
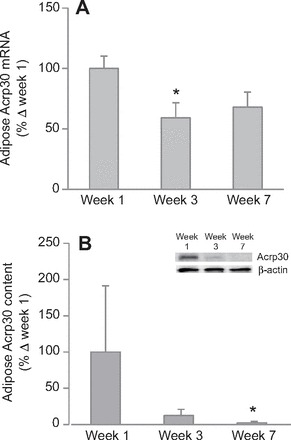
Prolonged fasting decreased mean (±s.e.m.) adiponectin (Acrp30) (A) mRNA and (B) protein content (inset, representative western blot) in adipose tissue of northern elephant seal pups. Asterisk denotes significant difference from week 1 (P<0.05).
Fig. 4.
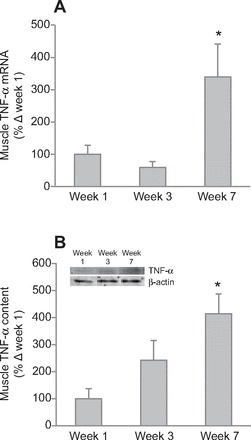
Prolonged fasting increased mean (±s.e.m.) tumor necrosis factor-alpha (TNF-α) (A) mRNA and (B) protein content (inset, representative western blot) in muscle of northern elephant seal pups. Asterisks denote significant difference from week 1 (*P<0.05, **P<0.01).
DISCUSSION
A principal finding of the present study is that prolonged fasting decreases the expression of adipose and muscle AGT, while circulating RAS is activated, suggesting that local RAS is regulated independently from systemic RAS during extended bouts of absolute food deprivation. The decreases in adipose and muscle AGT production along with the lack of a change in tissue ACE suggests that local RAS is suppressed with prolonged food deprivation. Conversely, the increase in PRA, the decrease in plasma AGT (increased conversion of AGT to Ang I) and the subsequent elevation in plasma Ang II indicate that circulating RAS is activated after 7 weeks of fasting. While activation of systemic RAS may result from food deprivation to help regulate osmotic homeostasis (Ortiz et al., 2000; Ortiz et al., 2002; Ortiz et al., 2006), the effects of this increase in RAS on cellular functions in seals have not been examined. Given that these animals present with an IR-like condition during prolonged fasting (Fowler et al., 2008; Viscarra et al., 2011a; Viscarra et al., 2011b), this study suggests that the activation of systemic RAS, not local RAS, is most likely contributing to the IR-like condition observed in fasting seal pups.
In humans and rodents, excess TNF-α in muscle can disrupt the tyrosine-phosphorylation of IRS (Plomgaard et al., 2005) and suppress fatty acid oxidation by suppressing AMPK signaling (Steinberg et al., 2006), which can contribute to impaired whole-body insulin sensitivity because muscle is responsible for the majority of glucose uptake in vivo. In humans, muscle of diabetic patients expressed fourfold more TNF-α than muscle from non-diabetic subjects (Saghizadeh et al., 1996). Moreover, a significant, inverse linear relationship between maximal glucose disposal rate and muscle TNF-α content is detected (Saghizadeh et al., 1996), providing evidence for the impact of local TNF-α on insulin signaling during diabetic conditions. In the present study, muscle TNF-α mRNA and protein expression increased with fasting duration. Furthermore, the increase in local TNF-α expression coincides with the increase in plasma Ang II late in the fast, whereas local AGT production declined with fasting. The Ang II-mediated upregulation of muscle TNF-α expression in terrestrial mammals is well established (Togashi et al., 2000; Shiuchi et al., 2004; Wei et al., 2008a; as reviewed by Kalupahana and Moustaid-Moussa, 2012), providing substantial evidence for the contribution of increased Ang II to the upregulation of TNF-α expression in the present study. However, despite the increase in plasma Ang II, plasma TNF-α was not significantly increased and these levels are relatively low (Vázquez-Medina et al., 2010), suggesting that the Ang II-mediated increase in local TNF-α is not sufficient to significantly increase circulating levels of TNF-α over the fasting period measured. We have previously reported a lack of increased plasma TNF-α and C-reactive protein during fasting in the presence of increased activities and protein content of antioxidant enzymes, which likely alleviate the development of a systemic inflammatory response (Vázquez-Medina et al., 2010; Vázquez-Medina et al., 2011).
Nonetheless, this does not preclude increased plasma Ang II from exerting effects on insulin signaling in muscle via stimulation of local TNF-α. As insulin or insulin-like growth factor-stimulated glucose uptake into skeletal muscle is necessary for growth and differentiation (Duan et al., 2010; Florini, 1987), an IR-like condition in developing pups could be disadvantageous. Thus, in addition to systemic RAS activation, Ang II-induced IR-like condition via TNF-α in the pups could be a contributing factor to initiating the termination of the fast.
While increased Ang II can activate adipose cytokines such as TNF-α and IL-6 (Olivares-Reyes et al., 2009; Skurk et al., 2004), it can simultaneously decrease plasma Acrp30 concentrations in rats (Ran et al., 2006). In the present study, prolonged fasting decreased adipose Acrp30 mRNA expression, which coincides with the previously demonstrated decrease in plasma Acrp30 after 8 weeks of fasting in elephant seals (Viscarra et al., 2011a). The present study suggests that the IR-like condition observed in late-fasted seals may be the consequence of the simultaneous and coordinated increase in muscle TNF-α and decrease in adipose Acrp30. The lack of a change in muscle Acrp30 should not be surprising as muscle is not a significant source of Acrp30 and suppression of muscle Acrp30 would not be expected to contribute significantly to the decrease in circulating levels. The differential response in tissue TNF-α is much more intriguing. While the increased expression in muscle TNF-α was not sufficient to translate into increased circulating levels, the increase in muscle levels may be sufficient to contribute to local impairment of insulin signaling, especially because muscle is the primary sink of peripheral glucose utilization. Thus, the lack of an increase in adipose TNF-α may be adaptive to allow this relatively large mass of tissue to utilize glucose as needed to facilitate lipid metabolism to support the protracted fasting duration (Viscarra et al., 2012). Hence, the result is differential responsiveness of tissue TNF-α to increased plasma Ang II that is dependent on the metabolic contributions of the two tissues, adipose versus muscle.
The production of MCP-1 in adipocytes can be induced by Ang II (Asamizu et al., 2009). Conversely, in humans, Acrp30 can protect tissues from Ang II-mediated TNF-α-induced inflammation (Xu et al., 2008). However, in the present study, MCP-1 mRNA expression in either adipose or muscle tissue was not significantly altered with fasting, suggesting that: (1) a typical inflammatory response does not occur in seal tissues and/or (2) the increase in Ang II was not sufficient to induce an MCP-1-mediated inflammatory response in seal pups.
The present study may be the first to compare systemic and local RAS dynamics during prolonged fasting in mammals. The study also revealed that adipose and muscle tissue in seals express the genes encoding most RAS components with the exception of renin. We obtained PCR fragments of renin with the same sets of primers when using genomic DNA of the seal, indicating a lack of mRNA expression in those tissues. Similarly, in humans and rodents, renin expression or its activity is absent or very low in the adipose (Engeli et al., 2003; Giacchetti et al., 2002; Saye et al., 1993) and muscle tissue (Johnston et al., 2011), suggesting that, despite the large fat depots in seals, their adipose tissue does not significantly contribute to circulating renin levels. While several enzymes, such as cathepsin D and pepsin, can release Ang I from AGT (Johnston et al., 2011), renin may not be the functionally relevant converting enzyme in adipose tissue of seals. Nonetheless, adipose tissue does not likely contribute significantly to circulating Ang II concentrations in elephant seals.
Perspectives
In northern elephant seal pups, prolonged fasting decreases local AGT production while circulating Ang II is elevated and associated with increased muscle TNF-α and decreased adipose Acrp30, suggesting that: (1) increased plasma Ang II could contribute to the stimulation of tissue TNF-α and to the decrease in adipose Acrp30, (2) the IR-like condition observed in pups during prolonged fasting might be the consequence of the simultaneous increase in muscle TNF-α and decrease in adipose Acrp30, and (3) ‘natural adiposity’ may not induce local activation of RAS or inflammation in elephant seals. Elucidating the mechanisms that prevent local RAS activation and inflammation during a condition of ‘natural adiposity’, despite the presence of systemic RAS activation, and comparing this condition with obesity-related pathological conditions in humans has the potential to contribute to the development of effective therapeutic approaches for metabolic syndromes. The present study provides a better understanding of the adaptive mechanisms protecting these animals from potentially detrimental conditions.
ACKNOWLEDGEMENTS
We thank J. T. Sharick (Sonoma State University) for his help sedating the seals. We appreciate M. Kitazawa, J. Minas and B. Martinez (University of California Merced) for their technical assistance.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This research was funded by Nihon University [a grant for Overseas Researcher to M.S.], the Japan Society for the Promotion of Science [Grant-in-Aid for Scientific Research (C) 23580265 to M.S.] and the National Heart Lung and Blood Institute [NIH NHLBI HL091767 to R.M.O. and D.E.C.; NIH NHLBI HL091767-S1 to R.M.O.; and NIH NHLBI K02HL103787 to R.M.O.]. J.P.V.-M. was supported by fellowships from The University of California Institute for Mexico and The United States (UC MEXUS), Mexico's Consejo Nacional de Ciencia y Tecnología (CONACYT) and The University of California (Miguel Velez Fellowship, University of California Merced Graduate and Research Council). J.G.S.-O. was supported by a postdoctoral fellowship from UC MEXUS-CONACYT. Deposited in PMC for release after 12 months.
REFERENCES
- Asamizu S., Urakaze M., Kobashi C., Ishiki M., Norel Din A. K., Fujisaka S., Kanatani Y., Bukahari A., Senda S., Suzuki H., et al. (2009). Angiotensin II enhances the increase in monocyte chemoattractant protein-1 production induced by tumor necrosis factor-α from 3T3-L1 preadipocytes. J. Endocrinol. 202, 199-205 [DOI] [PubMed] [Google Scholar]
- Cassis L. A., Police S. B., Yiannikouris F., Thatcher S. E. (2008). Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 10, 93-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne C. D., Houser D. S., Crocker D. E. (2005). Glucose production and substrate cycle activity in a fasting adapted animal, the northern elephant seal. J. Exp. Biol. 208, 859-868 [DOI] [PubMed] [Google Scholar]
- Champagne C. D., Houser D. S., Fowler M. A., Costa D. P., Crocker D. E. (2012). Gluconeogenesis is associated with high rates of tricarboxylic acid and pyruvate cycling in fasting northern elephant seals. Am. J. Physiol. 303, R340-R352 [DOI] [PubMed] [Google Scholar]
- Costa D. P., Ortiz C. L. (1982). Blood chemistry homeostasis during prolonged fasting in the northern elephant seal. Am. J. Physiol. 242, R591-R595 [DOI] [PubMed] [Google Scholar]
- Dandona P., Dhindsa S., Ghanim H., Chaudhuri A. (2007). Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J. Hum. Hypertens. 21, 20-27 [DOI] [PubMed] [Google Scholar]
- Duan C., Ren H., Gao S. (2010). Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 167, 344-351 [DOI] [PubMed] [Google Scholar]
- Engeli S., Negrel R., Sharma A. M. (2000). Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 35, 1270-1277 [DOI] [PubMed] [Google Scholar]
- Engeli S., Schling P., Gorzelniak K., Boschmann M., Janke J., Ailhaud G., Teboul M., Massiéra F., Sharma A. M. (2003). The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int. J. Biochem. Cell Biol. 35, 807-825 [DOI] [PubMed] [Google Scholar]
- Florini J. R. (1987). Hormonal control of muscle growth. Muscle Nerve 10, 577-598 [DOI] [PubMed] [Google Scholar]
- Fowler M. A., Champagne C. D., Houser D. S., Crocker D. E. (2008). Hormonal regulation of glucose clearance in lactating northern elephant seals (Mirounga angustirostris). J. Exp. Biol. 211, 2943-2949 [DOI] [PubMed] [Google Scholar]
- Giacchetti G., Faloia E., Mariniello B., Sardu C., Gatti C., Camilloni M. A., Guerrieri M., Mantero F. (2002). Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am. J. Hypertens. 15, 381-388 [DOI] [PubMed] [Google Scholar]
- Henriksen E. J. (2007). Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am. J. Physiol. 293, R974-R980 [DOI] [PubMed] [Google Scholar]
- Johnston A. P., Baker J., De Lisio M., Parise G. (2011). Skeletal muscle myoblasts possess a stretch-responsive local angiotensin signalling system. J. Renin Angiotensin Aldosterone Syst. 12, 75-84 [DOI] [PubMed] [Google Scholar]
- Jones A., Woods D. R. (2003). Skeletal muscle RAS and exercise performance. Int. J. Biochem. Cell Biol. 35, 855-866 [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. (2006). Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784-1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalupahana N. S., Moustaid-Moussa N. (2012). The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes. Rev. 13, 136-149 [DOI] [PubMed] [Google Scholar]
- Karlsson C., Lindell K., Ottosson M., Sjöström L., Carlsson B., Carlsson L. M. S. (1998). Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J. Clin. Endocrinol. Metab. 83, 3925-3929 [DOI] [PubMed] [Google Scholar]
- Kirby V. L., Ortiz C. L. (1994). Hormones and fuel regulation in fasting elephant seals. In Elephant Seals: Population Ecology, Behavior, and Physiology (ed. Le Boeuf B. J., Laws R. M.). Berkeley, CA: University of California Press; [Google Scholar]
- Kurata A., Nishizawa H., Kihara S., Maeda N., Sonoda M., Okada T., Ohashi K., Hibuse T., Fujita K., Yasui A., et al. (2006). Blockade of Angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int. 70, 1717-1724 [DOI] [PubMed] [Google Scholar]
- Le Boeuf B. J., Whiting R. J., Gantt R. F. (1972). Perinatal behavior of northern elephant seal females and their young. Behaviour 43, 121-156 [DOI] [PubMed] [Google Scholar]
- Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., et al. (2002). Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731-737 [DOI] [PubMed] [Google Scholar]
- Olivares-Reyes J. A., Arellano-Plancarte A., Castillo-Hernandez J. R. (2009). Angiotensin II and the development of insulin resistance: implications for diabetes. Mol. Cell. Endocrinol. 302, 128-139 [DOI] [PubMed] [Google Scholar]
- Ortiz C. L., Costa D. P., Burney J., Le Boeuf B. J. (1978). Water and energy flux in elephant seal pups fasting under natural conditions. Physiol. Zool. 51, 166-178 [Google Scholar]
- Ortiz C. L., Burney J., Le Boeuf B. J., Costa D. P. (1984). Milk intake of elephant seal pups: an index of parental investment. Am. Nat. 124, 416-422 [Google Scholar]
- Ortiz R. M., Wade C. E., Ortiz C. L. (2000). Prolonged fasting increases the response of the renin-angiotensin-aldosterone system, but not vasopressin levels, in postweaned northern elephant seal pups. Gen. Comp. Endocrinol. 119, 217-223 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Wade C. E., Costa D. P., Ortiz C. L. (2002). Renal responses to plasma volume expansion and hyperosmolality in fasting seal pups. Am. J. Physiol. 282, R805-R817 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Crocker D. E., Houser D. S., Webb P. M. (2006). Angiotensin II and aldosterone increase with fasting in breeding adult male northern elephant seals (Mirounga angustirostris). Physiol. Biochem. Zool. 79, 1106-1112 [DOI] [PubMed] [Google Scholar]
- Plomgaard P., Bouzakri K., Krogh-Madsen R., Mittendorfer B., Zierath J. R., Pedersen B. K. (2005). Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54, 2939-2945 [DOI] [PubMed] [Google Scholar]
- Ran J., Hirano T., Fukui T., Saito K., Kageyama H., Okada K., Adachi M. (2006). Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism 55, 478-488 [DOI] [PubMed] [Google Scholar]
- Reiter J., Stinson N. L., Le Boeuf B. J. (1978). Northern elephant seal development: the transition from weaning to nutritional independence. Behav. Ecol. Sociobiol. 3, 337-367 [Google Scholar]
- Saghizadeh M., Ong J. M., Garvey W. T., Henry R. R., Kern P. A. (1996). The expression of TNF α by human muscle. Relationship to insulin resistance. J. Clin. Invest. 97, 1111-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saye J. A., Ragsdale N. V., Carey R. M., Peach M. J. (1993). Localization of angiotensin peptide-forming enzymes of 3T3-F442A adipocytes. Am. J. Physiol. 264, C1570-C1576 [DOI] [PubMed] [Google Scholar]
- Shiuchi T., Iwai M., Li H. S., Wu L., Min L. J., Li J. M., Okumura M., Cui T. X., Horiuchi M. (2004). Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 43, 1003-1010 [DOI] [PubMed] [Google Scholar]
- Skurk T., van Harmelen V., Hauner H. (2004). Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-κB. Arterioscler. Thromb. Vasc. Biol. 24, 1199-1203 [DOI] [PubMed] [Google Scholar]
- Stefan N., Vozarova B., Funahashi T., Matsuzawa Y., Weyer C., Lindsay R. S., Youngren J. F., Havel P. J., Pratley R. E., Bogardus C., et al. (2002). Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes 51, 1884-1888 [DOI] [PubMed] [Google Scholar]
- Steinberg G. R., Michell B. J., van Denderen B. J. W., Watt M. J., Carey A. L., Fam B. C., Andrikopoulos S., Proietto J., Görgün C. Z., Carling D., et al. (2006). Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 4, 465-474 [DOI] [PubMed] [Google Scholar]
- Tavoni S. K., Champagne C. D., Dorian S., Houser D. S., Crocker D. E. (2013). Lactate flux and gluconeogenesis in fasting, weaned northern elephant seals (Mirounga angustirostris). J. Comp. Physiol. B 183, 537-546 [DOI] [PubMed] [Google Scholar]
- Togashi N., Ura N., Higashiura K., Murakami H., Shimamoto K. (2000). The contribution of skeletal muscle tumor necrosis factor-alpha to insulin resistance and hypertension in fructose-fed rats. J. Hypertens. 18, 1605-1610 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Crocker D. E., Forman H. J., Ortiz R. M. (2010). Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J. Exp. Biol. 213, 2524-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Forman H. J., Crocker D. E., Ortiz R. M. (2011). Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J. Exp. Biol. 214, 1294-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes E., Ducharme A., Bourassa M. G., Lessard M., White M., Tardif J. C., Studies Of Left Ventricular Dysfunction (2003). Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the studies of left ventricular dysfunction (SOLVD). Circulation 107, 1291-1296 [DOI] [PubMed] [Google Scholar]
- Viscarra J. A., Vázquez-Medina J. P., Crocker D. E., Ortiz R. M. (2011a). Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am. J. Physiol. 300, R150-R154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra J. A., Champagne C. D., Crocker D. E., Ortiz R. M. (2011b). 5′AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J. Endocrinol. 209, 317-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra J. A., Vázquez-Medina J. P., Rodriguez R., Champagne C. D., Adams S. H., Crocker D. E., Ortiz R. M. (2012). Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris). J. Exp. Biol. 215, 2455-2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Mao X., Wang L., Liu M., Wetzel M. D., Guan K. L., Dong L. Q., Liu F. (2007). Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J. Biol. Chem. 282, 7991-7996 [DOI] [PubMed] [Google Scholar]
- Wei Y., Chen K., Whaley-Connell A. T., Stump C. S., Ibdah J. A., Sowers J. R. (2008a). Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. 294, R673-R680 [DOI] [PubMed] [Google Scholar]
- Wei Y., Sowers J. R., Clark S. E., Li W., Ferrario C. M., Stump C. S. (2008b). Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-κB activation via NADPH oxidase. Am. J. Physiol. 294, E345-E351 [DOI] [PubMed] [Google Scholar]
- Xu S. Q., Mahadev K., Wu X., Fuchsel L., Donnelly S., Scalia R. G., Goldstein B. J. (2008). Adiponectin protects against angiotensin II or tumor necrosis factor α-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler. Thromb. Vasc. Biol. 28, 899-905 [DOI] [PubMed] [Google Scholar]
- Yasue S., Masuzaki H., Okada S., Ishii T., Kozuka C., Tanaka T., Fujikura J., Ebihara K., Hosoda K., Katsurada A., et al. (2010). Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am. J. Hypertens. 23, 425-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L., Quignard-Boulangé A. (2011). Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 79, 162-168 [DOI] [PubMed] [Google Scholar]


