Abstract
Background
A significant factor in impaired movement caused by stroke is the inability to activate muscles independently. While the pathophysiology behind this abnormal co-activation is not clear, reducing the co-activation could improve overall arm function. A myoelectric computer interface (MCI), which maps EMG signals to cursor movement, could be used as a treatment to help retrain muscle activation patterns.
Objective
To investigate the use of MCI training to reduce abnormal muscle co-activation in chronic stroke survivors.
Methods
Five healthy subjects and five stroke survivors with hemiparesis participated in multiple sessions of MCI training. The level of arm impairment in stroke survivors was assessed using the upper extremity portion of Fugl-Meyer Motor Assessment (FMA-UE). Subjects performed isometric activations of up to five muscles. Activation of each muscle was mapped to different directions of cursor movement. The MCI specifically targeted one pair of muscles in each subject for reduction of co-activation.
Results
Both healthy subjects and stroke survivors learned to reduce abnormal co-activation of the targeted muscles with MCI training. Three out of five stroke survivors exhibited objective reduction in arm impairment as well (improvement in FMA-UE of 3 points in each of these subjects).
Conclusions
These results suggest that the MCI was an effective tool in directly retraining muscle activation patterns following stroke.
Keywords: stroke, rehabilitation, arm, co-activation, muscles, EMG, synergies
Introduction
More than 3.2 million people in the U.S. suffer chronically-impaired upper limb function due to a stroke.1 Impairment of voluntary arm movement can be due not only to weakness and loss of sensation, but also to abnormal co-activation.2-5 In contrast to spasticity, which is increased tone during externally-imposed limb movement, abnormal co-activation, sometimes called “abnormal muscle synergy,”6,7 consists of increased tone during active or attempted voluntary movement by the patient. Stroke survivors often experience co-activation of anterior deltoid with biceps (flexor synergy), and posterior deltoid with triceps (extensor synergy). This constrains their movement to stereotypical patterns.7 By reducing abnormal co-activation and restoring more normal patterns of activation, it may be possible to improve function.
While some studies questioned the amount of impairment caused by abnormal co-activation,8,9 other evidence strongly suggests that its role is significant.10 Moreover, the clearest way to determine the amount of significance is to prospectively treat the co-activation and assess the effects on movement.10 Abnormal co-activation can also be defined as abnormal coupling between joint torques.3 Ellis et al.11 recently demonstrated that abnormal arm joint torque couplings in stroke survivors could be reduced by training the subjects to isolate individual joint torques with the use of visual feedback. This intervention also led to an increase in strength, demonstrating the significant role of abnormal co-activation in impaired function. However, this paradigm is not suitable for widespread use outside specialized clinics, due to the size and expense of the necessary robotic equipment.
This study attempted to reduce co-activation directly by using a myoelectric computer interface (MCI). In an MCI, surface electromyographic (EMG) signals are mapped to different directions of cursor movement on a monitor.12 Here, we tested whether an MCI specifically designed to target muscle co-activation could allow stroke subjects to reduce abnormal muscle co-activation.
We first tested the MCI on healthy subjects to determine whether they could learn to decouple two normally co-activating muscles (biceps and brachioradialis). Then we tested the extent to which stroke survivors could learn to decouple two abnormally co-activating muscles (biceps and anterior deltoid). Our results suggest that MCI training did allow stroke survivors to reduce abnormal co-activation and improve upper limb function.
Methods
Subjects
Five right-handed subjects (4 men, 1 woman, ages 23-27) free from neurological and musculoskeletal disorders and five subjects (1 man, 4 women, ages 50-58) whose stroke occurred 1.5-25 years prior to enrollment gave informed consent to participate in this study, which was approved by the Northwestern University Institutional Review Board. We included stroke survivors who had 1) hemiparesis with moderate to severe impairment of the affected arm (score of 12-40 on the upper-extremity portion of the Fugl-Meyer Motor Assessment, FMA-UE13), 2) exhibited co-activation of the biceps and anterior deltoid muscles determined by clinical observations during FMA-UE and by subjects' performance using the MCI during the initial screening process, and 3) a single, unilateral, ischemic stroke at least 1 year prior to enrollment. We excluded those who had 1) significant pain in the upper limbs or spine, 2) substantial sensory loss in the affected upper arm, 3) significant vision loss, 4) cognitive impairment severe enough to affect digit span and understanding of task-related instructions, or 5) participated in another arm-motor study within 30 days of the start of this study. Subjects were allowed to continue their usual exercise or physical therapy regimens, but not allowed to start a new regimen during the study. All stroke subjects had right-sided hemiparesis (FMA-UE score 19 ± 3, mean ± SD).
Behavioral Task
Subjects sat in a chair with their right arm in an armrest. Healthy subjects' arms were held in a semi-pronated position (to make it more difficult to decouple biceps and brachioradialis) and immobilized with cushioned restraints at the hand, wrist and upper forearm. Stroke subjects' impaired arms were held in a prone position.
Subjects performed isometric contractions of multiple muscles to move the cursor (circle, radius 1.5 cm, Figure 1A) from a square target in the center of a monitor to a randomly-presented target near the outer edge of the monitor—a modified center-out task14 (Figure 1B). Activation of each muscle was mapped to one of four directions within the 2-D cursor space (see Muscle Mapping). The center target corresponded to zero net muscle activation (resting position). Each trial started after holding the cursor in the center target for 512 ms, when an outer target appeared and the center target disappeared. Outer targets were located at a distance of 12 cm from the center target in all sessions except the first 5 and first 2 sessions for subjects 1 and 2, respectively; in these first few sessions they were located 7 cm from the center. (Distance was increased to make the task more challenging after it was noted to be too easy for these subjects.) When the cursor reached the outer target it changed color, and subjects were required to hold the cursor there for at least 33 ms (and up to 512 ms) to achieve success.
Figure 1.
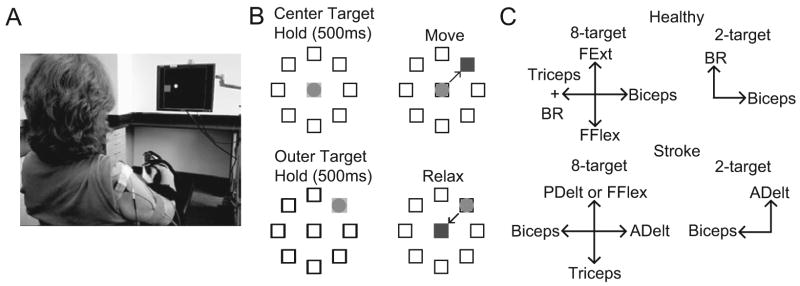
MCI task overview. (A) Experimental setup with a stroke subject viewing a circular cursor and square target on the monitor. (B) Schematic of MCI task and muscle mapping directions. Subjects moved the cursor to one of either 2 or 8 (shown here) outer targets. Relaxing all muscles moved the cursor to the center. (C) Muscle mapping directions in both the 8-target task (left) and 2-target task (right) for both healthy (top) and stroke (bottom) subject groups. BR, brachioradialis; FFlex, flexor digitorum superficialis; FExt, extensor digitorum; ADelt, anterior deltoid; PDelt, posterior deltoid.
Cursor Control Signal
We derived cursor position in real time using EMGs from multiple arm muscles. Surface EMG was recorded with bipolar electrodes spaced 1 cm apart, amplified with a gain of 1000 (Delsys Bagnoli), digitally sampled at 1 kHz (National Instruments USB-6229) and collected using a customized program in BCI2000.15 The electrode positions were marked at the end of the session with a henna marker to ensure the same location in the next session.
The control signals in each direction were derived from EMGs by low-pass filtering at 500 Hz, rectifying, high-pass filtering at 20 Hz, and then convolving with a 400-ms rectangular window. The vector sum of the control signals determined the 2-D cursor position.
At the start of each session, subjects were instructed to produce two maximum voluntary contractions (MVCs) of each muscle independently. To avoid fatigue, the control signals were scaled by a factor that allowed cursor movement to the targets at a relatively comfortable level of contraction (∼42% of the MVC). Since EMG activation levels sometimes varied due to slight changes in electrode placement or skin impedance, we sometimes altered the gains across sessions, to make the effort level similar across sessions. However, the gains remained the same across all trials within each session.
Muscle Mapping
Each subject performed two tasks in each session: a training task that used 2 outer targets and a “generalization” task that used 8 outer targets. In the training task, the two co-activating muscles (biceps brachii and brachioradialis for healthy subjects, anterior deltoid and biceps brachii for stroke survivors) were mapped to orthogonal directions and only these two muscle signals were summed to form the control signal (Figure 1C). Thus, subjects could only succeed in the task if they learned to decouple the co-activating muscles. We also designed an 8-target task to attempt to assess the extent to which subjects could generalize the learned decoupling to a different task. In the 8-target task, the two co-activating muscles were mapped to opposite horizontal directions. In healthy subjects, an independent muscle (triceps) was added in the direction of brachioradialis to make the task more challenging. Two other muscles not typically co-activated with these muscles (flexor and extensor digitorum superficialis for healthy subjects, triceps and either posterior deltoid or flexor digitorum superficialis in stroke subjects) were mapped in opposite vertical directions. Subjects were informed of the specific directions corresponding to activation of each recorded muscle (mapping direction, Figure 1C).
In some stroke subjects, the affected muscles retained some spontaneous muscle activity (mainly in biceps and flexor digitorum) when trying to relax,16,17 which prevented the cursor from reaching the center target. Therefore we subtracted the baseline activity of each muscle (0.5-s window starting 1 s after the completion of a trial) from the corresponding control signal for the next trial.
Experimental Paradigm
Healthy subjects participated in three sessions separated up to one week. In each session, they performed 10 minutes of the 8-target task (pre-training), 20 minutes of the training task, and 10 minutes of the 8-target task (post-training). Stroke subjects participated in three sessions per week for 6 weeks. In each session, they performed 10 minutes of the pre-training task, 30 minutes of the training task, and another 10 minutes of the post-training task. For analysis, the first and last 2.5 minutes (for healthy) or 5 minutes (for stroke) of training were denoted early and late training, respectively.
Performance and outcome measures
We quantified subjects' performance in controlling the MCI via three different metrics: success rate (percentage of targets successfully acquired), time to target (TT, time between the outer target appearance and the cursor entering the target), and path length (PL, cumulative distance the cursor traveled in each trial, normalized by the distance between center and outer targets).
We defined the level of co-activation between muscles as the Pearson correlation coefficient (R) between the filtered EMGs (control signals) during the period from outer target appearance to the end of the outer target hold time on consecutive trials that were concatenated. Statistical significance was assessed using paired t-tests.
To investigate the contribution of each muscle to movement to each target in more detail, we computed muscle tuning curves.12 These were computed from the average control signal during the outer target hold period for each trial during the post-training task and aligned to the mapping direction of each muscle.
Finally, we evaluated FMA-UEs before the first session and at the end of the last session. We surveyed subjects after the study using the following questions:
Did you notice any improvement in your arm function during the study? If so, what improved?
Was the amount of training too little, too much, or just right?
Did you enjoy participating in the study?
How would you recommend changing the MCI training paradigm to make it more enjoyable?
Results
Healthy subjects: task performance and reduction of co-activation
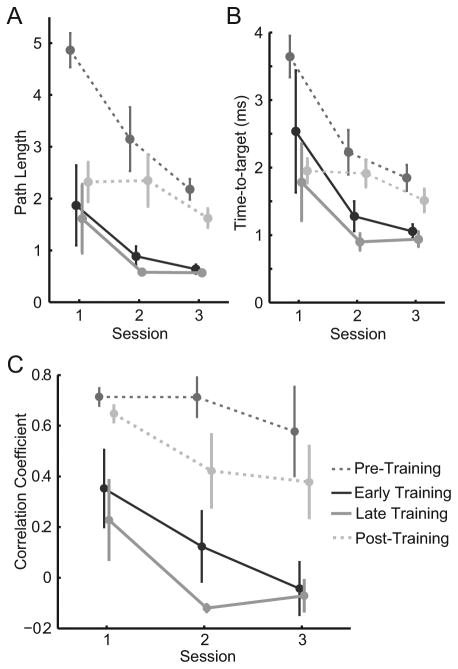
We first examined the healthy subjects' ability to learn to control the MCI in both tasks (Figure 2). Subjects learned the training task quickly, as evinced by the rapid improvements in path length (Figure 2A, solid lines) and time-to-target (Figure 2B, solid lines) from session 1 to 2. Path length improved by 69% (ΔPL=-1.3 ± 0.8, p=0.17, paired t-test) and time-to-target improved by 62% (ΔTT=-1.59 ± 0.9 s, p=0.16) from early training in session one to late training in session three. They learned the 8-target task quickly as well (Figure 2, dashed lines). Performance improvement persisted for one week.
Figure 2.
Healthy subjects' task performance and co-activation changes. (A) Time-to-target, (B) path length measures and (C) mean R between biceps and brachioradialis activity averaged across all subjects for pre- and post-training (dashed lines) and early and late training (solid lines). Error bars represent standard error (SE).
Healthy subjects readily learned the task of decoupling biceps and brachioradialis muscles within 2 sessions on consecutive days (Figure 2C). During the training task, correlation between these muscles decreased by an average (±SE) of 0.42 ± 0.18 from early training in session one to late training in session three, though not quite significantly (p = 0.055). Correlation decreased significantly from the pre-training phase of session one to the post-training phase of session three (ΔR = -0.33 ± 0.12, p = 0.046).
Stroke subjects: task performance
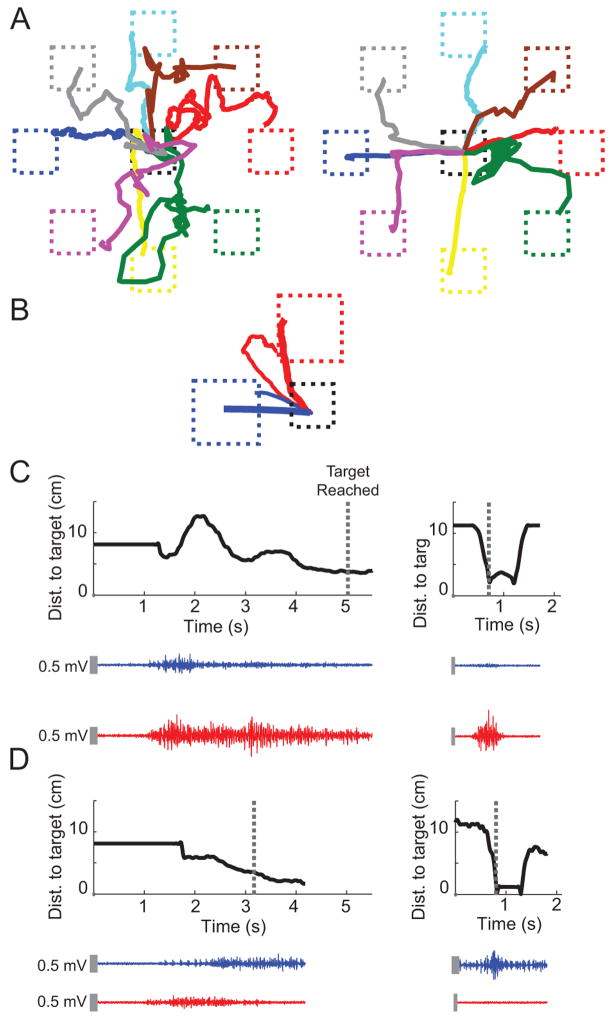
Despite being told the mapping direction of each muscle prior to starting the 8-target task, stroke subjects initially displayed poor control of the cursor (Figure 3A, left). By the final session, the movement trajectories more closely approached straight lines (Figure 3A, right) to each target. In the training task, trajectories tended to move along the diagonal between the two targets (Figure 3B, thin lines) in the first session, but over time, the trajectories became straighter (Figure 3B, thick lines). Representative EMG traces during movement in the anterior deltoid (Figure 3C) and biceps (Figure 3D) directions reveal a decrease in muscle coupling from the first session (left) to the last session (right); consistent with the cursor's path to the intended target.
Figure 3.
Mean movement trajectories to each target and representative EMG traces from a stroke subject during (A) pre-training in the first session (left) and post-training in the last session (right) and (B) early training in the first session (thin lines) and late training in the last session (thick lines). Blue and red trajectories represent cursor movement in the biceps direction and anterior deltoid direction, respectively. Representative EMG traces of biceps (blue) and anterior deltoid (red) for single trials during movement in the (C) anterior deltoid target direction and (D) biceps target direction show reduction of co-activation from early training in the first session (left) to late training in the last session (right). Black curves represent the cursor's distance to the intended target (dashed boxes in B) over time. Dashed lines in (C) and (D) represent the time at which target was reached. Vertical gray bars represent EMG scale (0.5 mV).
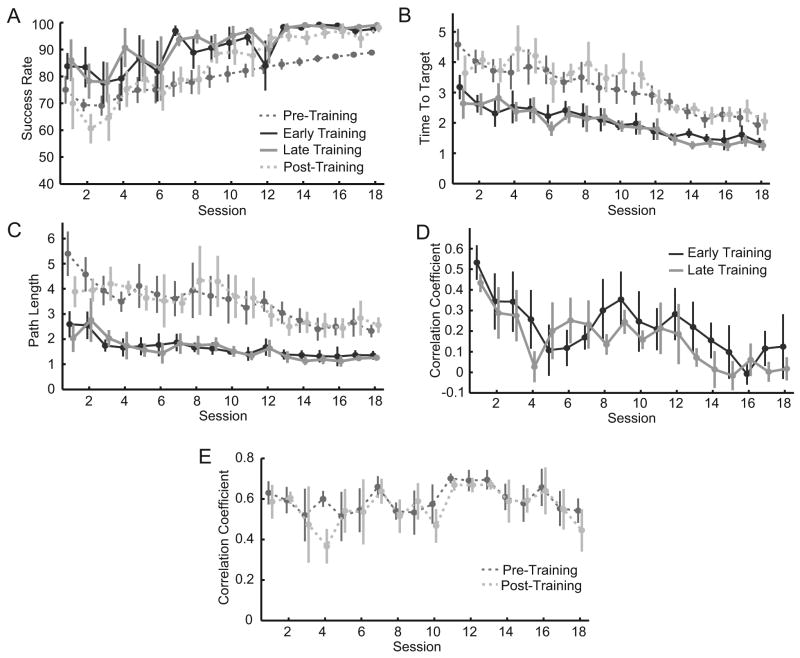
Subjects' task performance gradually improved in the 8-target task over time, as evinced by the changes in success rate (23 ± 5%, p = 0.008, Figure 4A), time to target (-2.5 ± 0.5 s, p = 0.008, Figure 4B) and path length (ΔPL = -2.8 ± 1.0, p = 0.05, Figure 4C) compared to the first session. Substantial improvement was also seen between early training in the first session and late training in the final session (Figure 4, ΔSR = 15 ± 5%, p = 0.03; ΔTT = -1.9 ± 0.5 s, p = 0.01; ΔPL = -1.3 ± 0.5, p = 0.056).
Figure 4.
Stroke subjects' task performance and co-activation changes. Performance measures, including (A) time-to-target, (B) path length, and (C) success rate averaged across all subjects show steady improvement during both training (solid lines) and pre- and post-training (dashed lines) tasks. Mean R between biceps and anterior deltoid during the (D) training and (E) pre- and post-training tasks. Correlations steadily and significantly decreased during the training task.
Stroke subjects: reduction of co-activation
Stroke subjects steadily learned to decouple biceps and anterior deltoid activity during the training task (Figure 4D). Correlations between the two muscles steadily decreased, with an average of 99% (ΔR = -0.52 ± 0.08, p = 0.005) reduction between early training of the first session and late training of the last session (Table 1). Correlations during pre- and post-training decreased by 23% (ΔR = -0.19 ± 0.15, p = 0.30, Figure 4E).
Table 1.
Details of performance changes.a
| Participant | ΔR
|
ΔR
|
Initial
|
Participants' Observations of
|
||
|---|---|---|---|---|---|---|
| 2-Target | 8-Target | FMA | ΔFMA | Improved FMA Items | Improved Movement | |
| 1 | −0.52 | −0.21 | 19 | +3 | Shoulder abduction/adduction, elbow flexion/extension | Improved arm function when washing dishes |
| 2 | −0.44 | 0.33 | 18 | +3 | Shoulder external rotation and adduction, elbow extension | Improved arm range of motion and increased ability to move |
| 3 | −0.83 | −0.61 | 23 | +3 | Elbow and shoulder flexion, mass flexion (power grasp) | Improved hand function |
| 4 | −0.42 | −0.26 | 20 | 0 | — | — |
| 5 | −0.36 | −0.17 | 14 | 0 | — | Increased arm use, improved arm function when bathing |
Abbreviation: FMA-UE, upper-extremity portion of the Fugl-Meyer Motor Assessment.
Shown are changes in R (ΔR) from the first to the last session during training (2-target), before to after training (8-target), initial and change in FMA-UE scores, the items in the FMA that improved for each participant, and the participants' reports of functional improvement in the survey.
Stroke subjects: muscle tuning curves
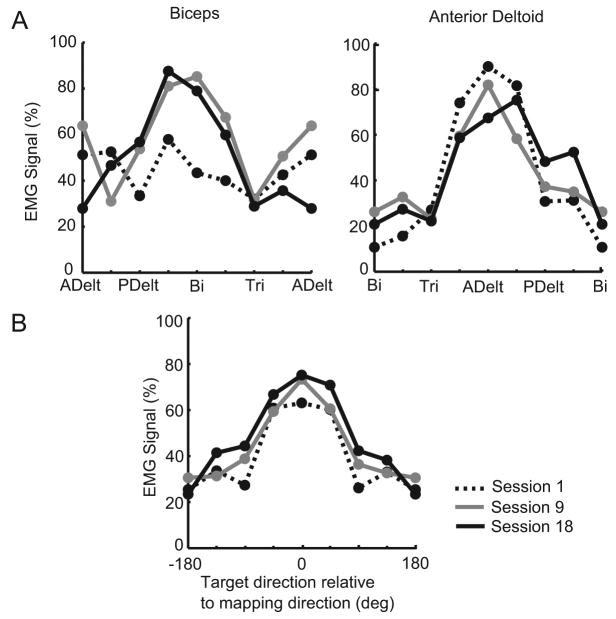
The average tuning curves across all stroke subjects (Figure 5A) demonstrate that biceps showed increased tuning toward the appropriate mapping direction and adjacent targets over time. Anterior deltoid was tuned predominantly in the appropriate direction from the start and therefore did not change greatly. When averaged over all muscles and subjects, a gradual increase in tuning depth (peak EMG amplitude) in the appropriate mapping direction was seen over time (Figure 5B). This also suggests better control of the muscles over time.
Figure 5.
Evolution of muscle tuning curves in stroke subjects. (A) Tuning curves of biceps and anterior deltoid averaged across subjects from post-training tasks of the first (dashed line), ninth (gray line), and last (black line) sessions. Control signals are normalized to the maximum level in both sessions and averaged across trials. (B) Tuning curves averaged across all 4 muscles and subjects over sessions. Tuning depth in all muscles gradually increased over time.
Effects of training on arm function
Four stroke subjects reported subjective improvement of arm or hand function in activities of daily living, including bathing, dressing, and washing dishes, during the study period (Table 1). All stroke subjects enjoyed performing the MCI training, but said it could be made even more enjoyable by integrating with a video game. Four of five asked for a larger dose of training and the remaining subject said the dose was just about right. Three subjects displayed improvement in FMA-UE scores by 3 points each; the other two subjects did not improve their scores. Reduced impairment correlated with reduced co-activation during training: ΔR= -0.52 ± 0.11 vs. -0.34 ± 0.09 for subjects whose FMA-UE scores did and did not improve, respectively.
Discussion
This study demonstrated that MCI training effectively enabled (1) healthy subjects to decouple two normally co-activating muscles and (2) stroke subjects to decouple two abnormally co-activating muscles. Moreover, the majority of stroke subjects displayed both subjective and objective evidence of reduced arm impairment after the training. Although changes in FMA scores were modest, this was not unexpected as they were achieved with a small amount of training and only aimed to decouple one pair of muscles. These results suggest that MCI training could be developed as a therapy to directly retrain muscle activation patterns and improve overall arm function in chronic stroke subjects.
The pathophysiology of abnormal co-activation is poorly understood, and there exists evidence, mostly indirect, to support origins both in increased bulbospinal outflow18 and in cortex.19-22 It is also unclear whether abnormal synergies are fixed and simply augmented by therapy23 or can be changed. The results in our study suggest that they may be malleable. However, this cannot be firmly established without examining the responses of the other arm muscles to the training of these two muscles. That is, it is not clear whether the subjects' central nervous system (CNS) learned to simply suppress a single muscle, or changed the entire synergy. It is also not clear at what level of the CNS (cortex, brainstem, or spinal cord) these changes occurred. Future studies may address these questions.
The MCI provides specific and intuitive feedback to the subject about their muscle activity. While a prior study has shown the ability to decouple joint torques in stroke survivors,11 to our knowledge, this is the first study to demonstrate the ability to directly decouple muscle activations in this patient population. Most studies using EMG biofeedback have attempted to increase strength in the target muscles,24 while a few have sought to decrease muscle activity.25-27 One study did show this ability in children with generalized dystonia within a single day.28 Another study did use EMG biofeedback of antagonist muscle to reduce co-contraction during agonist movement.29 However that study did not provide feedback in nearly an intuitive fashion as in the MCI, nor about the relative activation of both muscles, and required the user to produce a high level of elbow flexion force with the contractions. In contrast, the MCI provides intuitive feedback about the co-activation that only allows success when the muscles are decoupled. This could allow users to concentrate solely on succeeding in the task without explicitly thinking about activating individual muscles, which could take advantage of implicit motor learning mechanisms. Implicit motor learning is necessary for behavioral improvement, more durable over time, and less attentionally demanding than explicit learning.30-32 Further, since the MCI only requires EMG activity, not production of substantial forces, it could potentially be used in severely-impaired stroke survivors. This is critical, since these patients are often excluded from rehabilitative trials33 and are less likely to benefit from conventional therapy due to their extremely limited movement.34
Stroke subjects learned to reduce co-activation more slowly than did healthy subjects. This comparison is far from ideal, since healthy subjects were younger and were learning to decouple muscles that often, but not always, co-activate, while stroke subjects were learning to decouple abnormally co-activating muscles. Nevertheless, this result suggests that hemiparetic stroke survivors may have slower rates of motor learning in the paretic arm. This issue has not been well-studied to date,35 with one study showing possible impairment of learning in the paretic arm which was attributed to weakness.36 Although this is a difficult question to study,35 further study seems warranted, and the MCI could provide a means of circumventing the confounding effects of impaired movement since only EMG activity, not movement, is required to control the MCI.
While the reduction in coupling during the 8-target task did not reach significance, this could have been due a small sample size. In addition, the 8-target task was substantially more complex than the 2-target task, since it sometimes required the subject to co-activate pairs of muscles. Moreover, since each control signal was opposed directly by another, subjects could succeed in the task without decreasing co-activation simply by increasing activity in one muscle much more than in the opposing muscle; this would not be reflected in R-values. Due to these complexities, in retrospect we believe the 8-target task was not optimal for testing generalization of the learned decoupling. Indeed, reduced coupling during the training task correlated with FMA-UE scores, while reduced coupling during the 8-target task did not. Therefore, we place more emphasis on the subjective reports and improved FMA-UE scores, which suggest that the training did transfer to activities of daily living in stroke subjects.
Several issues remain to be addressed before the MCI can be tested as a clinical treatment. These include determining 1) appropriate dosage and duration of training, 2) the number of muscle pairs to decouple, 3) the use of isometric vs. non-isometric activations, and 4) the duration for which beneficial effects will persist. The optimal amount of training needed to gain significant improvement in functional outcome is an outstanding question in many rehabilitation paradigms. All subjects expressed interest in increasing the training intensity, which is strongly correlated with motor learning, cortical reorganization and recovery after stroke.37-39 Since the only requirements are surface EMG recording and relatively simple software, the system could easily be implemented in the community setting, and potentially in patients' homes, which would allow more frequent training. While subjects uniformly enjoyed the task, the MCI could be integrated into gaming environments that would further enhance motivation and training intensity. Many activities of daily living require non-isometric activations, and therefore it is possible that MCI training using unrestrained movements may translate to greater functional gains than the isometric training used here. We plan to investigate some of these issues in future studies.
Acknowledgments
We thank Robert Davisson Flint and Eric Lindberg for technical assistance and Mark Shapiro for the Delsys system. This work was supported by the Northwestern Memorial Foundation Dixon Translational Research Grants Initiative (part of NUCATS Institute NIH grant UL1RR025741).
References
- 1.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 2.Dewald J, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 3.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 4.Dewald J, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 6.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 7.Brunnström S. Movement therapy in hemiplegia: a neurophysiological approach. Facts and Comparisons. 1970 [Google Scholar]
- 8.Busse M, Wiles C, Van Deursen R. Muscle co-activation in neurological conditions. Phys Ther Rev. 2005;10:247–253. [Google Scholar]
- 9.Gowland C, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Phys Ther. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- 10.Chae J, Yang G, Park BK, Labatia I. Muscle weakness and cocontraction in upper limb hemiparesis: relationship to motor impairment and physical disability. Neurorehabil Neural Repair. 2002;16:241–248. doi: 10.1177/154596830201600303. [DOI] [PubMed] [Google Scholar]
- 11.Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JPA. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32:170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhakrishnan SM, Baker SN, Jackson A. Learning a novel myoelectric-controlled interface task. J Neurophysiol. 2008;100:2397–2408. doi: 10.1152/jn.90614.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1.a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 14.Georgopoulos A, Kalaska J, Caminiti R, Massey J. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 16.Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol. 2009;102:2026–2038. doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Origins of Spontaneous Firing of Motor Units in the Spastic–Paretic Biceps Brachii Muscle of Stroke Survivors. J Neurophysiol. 2010;104:3168–3179. doi: 10.1152/jn.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerachshenko T, Rymer WZ, Stinear JW. Abnormal corticomotor excitability assessed in biceps brachii preceding pronator contraction post-stroke. Clin Neurophysiol. 2008;119:683–692. doi: 10.1016/j.clinph.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan C, Dhaher Y. Corticospinal responses of quadriceps are abnormally coupled with hip adductors in chronic stroke survivors. Exp Neurol. 2012;233:400–407. doi: 10.1016/j.expneurol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res. 2008;185:509–519. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Chen A, Carmona C, Dewald JP. Cortical overlap of joint representations contributes to the loss of independent joint control following stroke. Neuroimage. 2009;45:490–499. doi: 10.1016/j.neuroimage.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dipietro L, Krebs HI, Fasoli SE, et al. Changing motor synergies in chronic stroke. J Neurophysiol. 2007;98:757. doi: 10.1152/jn.01295.2006. [DOI] [PubMed] [Google Scholar]
- 24.Woodford H, Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.CD004585.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basmajian J, Gowland C, Brandstater M, Swanson L, Trotter J. EMG feedback treatment of upper limb in hemiplegic stroke patients: a pilot study. Arch Phys Med Rehabil. 1982;63:613–616. [PubMed] [Google Scholar]
- 26.Moreland J, Thomson MA. Efficacy of electromyographic biofeedback compared with conventional physical therapy for upper-extremity function in patients following stroke: a research overview and meta-analysis. Phys Ther. 1994;74:534–543. doi: 10.1093/ptj/74.6.534. [DOI] [PubMed] [Google Scholar]
- 27.Wolf SL. Electromyographic biofeedback applications to stroke patients. A critical review. Phys Ther. 1983;63:1448–1459. doi: 10.1093/ptj/63.9.1448. [DOI] [PubMed] [Google Scholar]
- 28.Young SJ, van Doornik J, Sanger TD. Visual feedback reduces co-contraction in children with dystonia. J Child Neurol. 2011;26:37–43. doi: 10.1177/0883073810371828. [DOI] [PubMed] [Google Scholar]
- 29.Prevo A, Visser S, Vogelaar T. Effect of EMG feedback on paretic muscles and abnormal co-contraction in the hemiplegic arm, compared with conventional physical therapy. Scand J Rehabil Med. 1982;14:121–131. [PubMed] [Google Scholar]
- 30.Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Berry DC. Implicit learning: Theoretical and empirical issues. Psychology Press; 1993. [Google Scholar]
- 32.Jiménez L, Méndez C. Which attention is needed for implicit sequence learning? J Exp Psychol: Learning Memory Cogn. 1999;25:236. [Google Scholar]
- 33.Grotta JC, Noser EA, Ro T, et al. Constraint-induced movement therapy. Stroke. 2004;35:2699–2701. doi: 10.1161/01.STR.0000143320.64953.c4. [DOI] [PubMed] [Google Scholar]
- 34.Buch ER, Modir Shanechi A, Fourkas AD, Weber C, Birbaumer N, Cohen LG. Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain. 2012 doi: 10.1093/brain/awr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi CD, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. 2003;149:131–140. doi: 10.1007/s00221-002-1340-1. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt RA, Lee TD. Motor control and learning: A behavioral emphasis. 1999 [Google Scholar]
- 38.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 39.MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehab Neural Repair. 2011;25:740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]