Summary
Blood vessels deliver oxygen, nutrients, hormones and immunity factors throughout the body. To perform these vital functions, vascular cords branch, lumenize and interconnect. Yet, little is known about the cellular, molecular and physiological mechanisms that control how circulatory networks form and interconnect. Specifically, how circulatory networks merge by interconnecting ‘in parallel’ along their boundaries remains unexplored. To examine this process we studied the formation and functional maturation of the plexus that forms between the dorsal longitudinal anastomotic vessels (DLAVs) in the zebrafish. We find that the migration and proliferation of endothelial cells within the DLAVs and their segmental (Se) vessel precursors drives DLAV plexus formation. Remarkably, the presence of Se vessels containing only endothelial cells of the arterial lineage is sufficient for DLAV plexus morphogenesis, suggesting that endothelial cells from the venous lineage make a dispensable or null contribution to this process. The discovery of a circuit that integrates the inputs of circulatory flow and vascular endothelial growth factor (VEGF) signaling to modulate aortic arch angiogenesis, together with the expression of components of this circuit in the trunk vasculature, prompted us to investigate the role of these inputs and their relationship during DLAV plexus formation. We find that circulatory flow and VEGF signaling make additive contributions to DLAV plexus morphogenesis, rather than acting as essential inputs with equivalent contributions as they do during aortic arch angiogenesis. Our observations underscore the existence of context-dependent differences in the integration of physiological stimuli and signaling cascades during vascular development.
Key words: Angiogenesis, Circulatory flow, Endothelium, VEGF, Vascular development, Zebrafish
Introduction
Blood and lymphatic circulatory networks enable survival and homeostasis (Adams and Alitalo, 2007; Xu and Cleaver, 2011). Accordingly, formation of too many, too few, and/or ectopic vascular interconnections, like those between arteries and veins or blood and lymphatic vessels, is deleterious (Brouillard and Vikkula, 2007; Schulte-Merker et al., 2011). Blood vessel networks that are insufficiently interconnected provide poor circulatory flow, leading to ischemia, cognitive and/or motor deficits, and death (Vikkula, 2006; Covassin et al., 2009; Corti et al., 2011). Conversely, the reconnection of vascular networks disrupted by trauma is essential for wound healing, the incorporation of tissue grafts and transplanted organs, and recovery from both coronary artery disease and limb ischemia (Laschke et al., 2009).
Vascular networks arise by diverse mechanisms. For example, the vasculogenic coalescence of endothelial cell precursors forms the primary vascular plexi of the yolk sac and embryo (Patan, 2000), radial waves of sprouting angiogenesis drive the expansion of the primary retinal vascular plexus (Fruttiger, 2007) and bilateral segmental (Se) vessels interconnect ipsilaterally ‘in series’ to form the paired dorsal longitudinal anastomotic vessels (DLAVs) of the trunk that flank the roof of the neural tube (Isogai et al., 2003; Walls et al., 2008). However, little is known about how circulatory networks merge by interconnecting ‘in parallel’ along their boundaries to yield larger circuits.
To learn about this process we exploited the advantages of the zebrafish embryo as a model system for the study of vascular development (McKinney and Weinstein, 2008) to determine how the paired DLAVs interconnect with each other across the midline (see Isogai et al., 2001). The zebrafish DLAVs derive from chevron-shaped primary segmental vessel sprouts [primary Se; nomenclature according to Isogai et al. (Isogai et al., 2003)] that arise bilaterally at 21 hours post-fertilization (hpf) from the trunk axial artery (aorta) near the somite boundaries. As the primary Se bifurcate along the anteroposterior axis, near the dorsal side of the neural tube, they anastomose with their ipsilateral neighbors forming two continuous and independent DLAVs that diverge from each other anterogradely (Isogai et al., 2001; Childs et al., 2002; Isogai et al., 2003; Blum et al., 2008; Herwig et al., 2011). Shortly after (∼36 hpf), secondary segmental vessel sprouts [secondary Se; nomenclature according to Isogai et al. (Isogai et al., 2003)] start arising bilaterally from the axial vein. Some of these sprouts will contribute to the lymphatic vasculature. Others connect to an adjacent ipsilateral primary Se, thus transforming half of the segmental vasculature into veins (SeVs) after regression of the aortic roots of the primary Se. Others connect to an adjacent ipsilateral primary Se, thus transforming half of the segmental vasculature into veins (SeVs) after regression of the latter's aortic root. Henceforth, primary Se connected to the aorta are called Se arteries (SeAs). These two kinds of Se vessels generally occupy non-stereotypical, alternating positions along the trunk and can be distinguished according to the directionality of the flow they carry and via transgenic reporter expression (Isogai et al., 2003; Hogan et al., 2009a; Bussmann et al., 2010).
The formation of the simple and evolutionarily conserved anatomy of the DLAVs (Isogai et al., 2003; Levine et al., 2003; Doherty et al., 2007; Walls et al., 2008) and the eventual establishment of the plexus that interconnects them are vital. The DLAVs communicate in series with cephalic vessels (Isogai et al., 2001; Walls et al., 2008), are thought to be precursors for the perineural vascular plexus that later surrounds and invades the spinal cord (Walls et al., 2008; Bautch and James, 2009), and are likely to guide the migration of the neighboring dorsal lymphatic vasculature (see Hogan et al., 2009a; Hogan et al., 2009b; Bussmann et al., 2010). Thus, studying how the DLAV plexus arises and remodels should also illuminate the etiology of spinal cord vascular malformations, a major cause of debilitating myelopathies (Caragine et al., 2002).
Results
Development and anatomy of the DLAV plexus
To visualize the process of DLAV plexus morphogenesis we imaged the mid-trunk region (which approximately spans somites 8–13) of zebrafish embryos carrying the endothelial-specific reporter Tg(fli:EGFP)y1 (Lawson and Weinstein, 2002). In this trunk region DLAV assembly is complete by ∼32 hpf (Fig. 1A–D). Shortly after, at around 36–38 hpf, interconnections between the two DLAVs begin to form as angiogenic sprouts launch from the dorsal surface of the DLAVs and project in a dorsomedial fashion (Fig. 1E,F). These DLAV-derived sprouts connect either with their closest contra-lateral neighbors or to the opposite DLAV to form a plexus. For simplicity, from this stage onwards we collectively refer to the paired DLAVs and their interconnections as the DLAV plexus. The DLAV plexus progressively displays a ladder-like appearance in dorsal views, such that by 48 hpf this structure has many ‘rungs’ along its length (Fig. 1G–I). Almost simultaneously, these rungs branch at the midline in an anteroposterior fashion, anastomosing in series (supplementary material Movie 1), to yield by 56 hpf a third longitudinal vessel that runs along the midline at a more dorsal position than the DLAVs (Fig. 1J–L; supplementary material Movie 2). At this stage the DLAV plexus displays a complex and non-stereotypical braid-like organization in which the longitudinal vessels display different diameters along their length. By 60 hpf two or three longitudinal vessels are found along the DLAV plexus (Fig. 1M–M′′′).
Fig. 1.
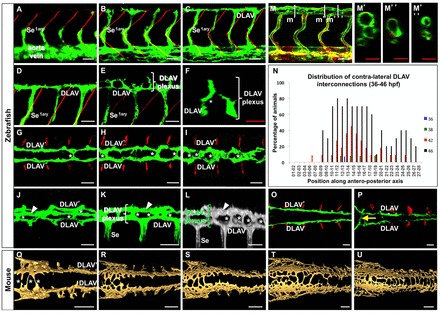
DLAV plexus morphogenesis in zebrafish (A–P) and mouse (Q–U) embryos. (A–D) Left lateral views (anterior, left; dorsal, up) of primary Se and DLAV development at 24 (A), 28 (B) and 32 hpf (C–D; D is a magnified detail of C). (E–M′″,O–P) Lateral (E,K,L–M; L is a 3D rendering), dorsal (G–I, O–P) and cross-sectional (F, from E; M′–M′″ correspond to levels m′–m′″ in M) views of DLAV plexus morphogenesis in the mid (E–M′″) and anterior (O–P) trunk regions at 38 (E–G), 46 (H), 48 (I,O), 56 (J–L), 60 (M–M′″) and 72 hpf (P). Anterior, left (lateral and dorsal views); dorsal, up (lateral and cross-sectional views). White asterisks (F–L,Q), examples of contra-lateral DLAV interconnections. White arrowhead (J–L), midline dorsal vessel). (N) Development of contra-lateral DLAV interconnections at 36–46 hpf). The x-axis shows their anteroposterior location with regards to consecutive Se vessels pairs (anterior-most Se vessel usually found at the third somite boundary level); n = 9–11 animals per stage. (A–P) Data from Tg(fli:EGFP)y1 embryos [M–M′″ includes Tg(flk1:ras-mCherry)s896]. Endothelium, green (A–K, M–M′″,O–P) and red (M–M′″) or gray (L). Somite boundaries, red (A–E,G–I,O–P). Scale bars: (A–M′″,O–P): 30 µm (white); 20 µm (red). (Q–U) 3D-rendered dorsal views of DLAV plexus morphogenesis in mouse embryos at the 22, 26, 27, 32, and 34-somite stage, respectively. Endothelium, gold. Anterior, left. Scale bars: 250 µm. See the Materials and Methods for details.
To determine the spatiotemporal dynamics of initial DLAV plexus formation we scored the position of DLAV–DLAV interconnections along the trunk and tail between 36 and 48 hpf (Fig. 1N). We found that the DLAV plexus forms first at the mid and posterior trunk regions, spreading both rostrally and caudally while becoming denser. Notably, the plexus is absent from the region corresponding approximately to the first eight somites (Fig. 1N,O–P), thus indicating that the anterior limit for DLAV plexus morphogenesis falls within the transition zone between anterior and posterior trunk somitogenesis (Holley, 2006). However, at 72 hpf a single vessel interconnects the DLAVs at the level of the putative hindbrain-spinal cord interface (Ma et al., 2010), namely between the third and fourth somites (Fig. 1P).
Next, we used endothelial PECAM-1 immunostaining to visualize the vasculature in mouse embryos to determine if and how DLAV plexus formation occurs. Our observations reveal that, at a minimum, the initial steps of this process are evolutionarily conserved between zebrafish and mammals. However, in the mouse DLAV plexus formation progresses in a rostrocaudal manner and occurs both along the entire length of the trunk (Fig. 1Q–U; supplementary material Fig. S1).
Endothelial cell behaviors associated with DLAV plexus assembly
To explore the cellular basis of DLAV plexus formation and remodeling we first quantified endothelial cell abundance within the two separate DLAVs or the DLAV plexus during development. To do this we co-visualized endothelial cell nuclei and blood vessel anatomy in Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 embryos (see Blum et al., 2008; Chi et al., 2008). Specifically, we scored endothelial cell abundance at 34, 48 and 72 hpf in two areas: the anterior region where the DLAVs remain separate due to lack of plexus formation (somites 3–5) and the DLAV plexus-forming mid-trunk region (somites 8–12). This analysis revealed that endothelial cell abundance increases significantly over time in both regions and that endothelial cell abundance is similar between both regions at each stage analyzed (Fig. 2A–C). These observations indicate that the lack of DLAV plexus formation in the anterior trunk is due to a key difference in the way endothelial cells distribute, rather than to an anterior deficit in endothelial cell abundance.
Fig. 2.
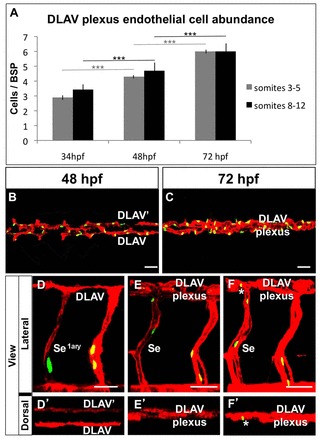
Endothelial cell abundance and behaviors during DLAV plexus morphogenesis. (A) Quantification of endothelial cell abundance within the developing DLAVs and DLAV plexus at 34, 48 and 72 hpf in the anterior and mid trunk (regions spanning somites 3–5 and 8–11, respectively); n = 10–11 animals per stage. Error bars show the s.e.m. ***P<0.001. (B,C) Dorsal views of the mid trunk (region spanning somites 8–12) DLAVs and DLAV plexus at 48 (B) and 72 (C) hpf; anterior, left. (A–C) Data from Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 embryos. (D–F′) Trunk vasculature (details) of a chimeric embryo from a Tg(flk1:EGFP-NLS) donor and a Tg(flk1:ras-mCherry)s896 host at 32 (D,D′), 56 (E,E′), and 72 (F,F′) hpf. Anterior, left. White asterisks, endothelial cell nuclei of donor origin found within DLAV plexus. (B–F′) Endothelium, red; endothelial cell nuclei, green. Scale bars: 30 µm.
We next asked whether endothelial cell migration and/or proliferation contribute to the observed increase in DLAV plexus endothelial cell abundance in the mid-trunk. To do this we performed cell transplantation experiments between Tg(flk1:EGFP-NLS) donors and Tg(flk1:ras-mCherry)s896 hosts and periodically imaged the chimeras to track changes in both the position and number of donor endothelial cells (Zygmunt et al., 2011). Consistent with prior reports describing complex, non-stereotypical cell rearrangements during DLAV formation (Blum et al., 2008) we found that donor endothelial cells found within the mid-trunk primary Se of the host at 32 hpf could later be found within the DLAV plexus at 72 hpf (Fig. 2D–F′). Hence, endothelial cells found within the primary Se after the DLAVs have already formed contribute to DLAV plexus morphogenesis by migrating dorsally. We also found that donor endothelial cells located within the DLAVs of the host at 32 hpf had migrated and proliferated by 72 hpf (supplementary material Fig. S2). Accordingly, time-lapse imaging of Tg(flk1:NLS-mCherry) embryos (32–44 hpf) confirms the occurrence of both endothelial cell proliferation and migration during DLAV plexus morphogenesis (supplementary material Movie 3).
The DLAV plexus communicates with SeAs and SeVs arranged in a generally alternating and non-stereotypical pattern (see Isogai et al., 2003; Hogan et al., 2009a; Bussmann et al., 2010). While SeAs are made exclusively of angiogenic cells of an arterial lineage (the aorta), SeVs contain angiogenic cells from both the axial artery and vein (Isogai et al., 2003; Hogan et al., 2009b; Bussmann et al., 2010). Thus, to investigate if endothelial cells from the venous lineage found within Se vessels are required for DLAV plexus formation, we analyzed wild-type (WT) embryos injected with a morpholino (MO) that induces a functional knockdown of ccbe1 (collagen and calcium-binding EGF domain-1). ccbe1 morphants lack venous secondary Se sprouts and thus cannot establish SeVs (Hogan et al., 2009a). As expected, comparison of WT embryos and ccbe1 morphants carrying the endothelial-specific reporter Tg(fli:EGFP)y1 revealed a lack of Se venous flow in ccbe1 morphants at both 48 and 72 hpf (supplementary material Fig. S3). However, we observed venous flow in the first, third and fourth Se vessels of ccbe1 morphants, consistent with the notion that these vessels are genetically hardwired to become veins (data not shown) (see Isogai et al., 2003; Hogan et al., 2009a). ccbe1 morphants do not exhibit obvious abnormalities in DLAV plexus morphogenesis despite abnormal DLAV plexus hemodynamics, including unidirectional anterograde circulatory flow and reduced venous outflow (data not shown). Collectively, these observations indicate that endothelial cells from the venous lineage make null or dispensable contributions to DLAV plexus morphogenesis in zebrafish (see Fig. 4H for a graphical summary of our findings) and that this process is not significantly impaired by a partial but significant reduction in its venous outflow.
Fig. 4.
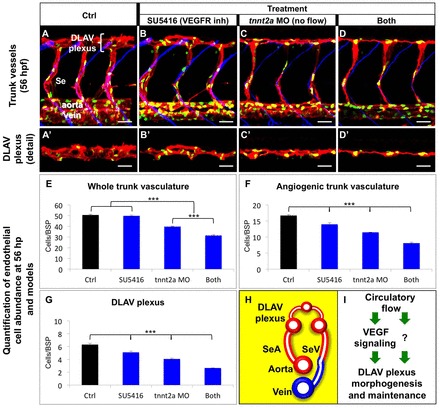
Circulatory flow and VEGF signaling make additive contributions to DLAV plexus morphogenesis. (A–D′) Lateral views of the mid trunk vasculature of 56 hpf Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 embryos under the indicated four conditions. Anterior, left; dorsal, up. Endothelium, red; endothelial cell nuclei, green; somite boundaries, blue (A,B,C,D). Scale bars: 30 µm. (E–G) Quantifications of the effect of treatments (see Materials and Methods) on the vascular; n = 6–10 animals per treatment and stage. Error bars, s.e.m. ***P<0.001. The normalized endothelial cell abundance within the whole trunk vasculature (E) or specific subsets of it (F–G) was determined. (H) Diagram showing a cross-sectional view of the trunk blood vasculature (∼56 hpf). Endothelium color-coded according to its arterial (red) or venous (blue) lineage; vessel lumens, white. Dorsal, up; left side, left. (I) Favored model of how DLAV plexus morphogenesis and maintenance are positively regulated by circulatory flow and VEGF signaling. It is likely that additional flow-dependent signals (‘?’) are involved in this process.
Characterization of circulatory flow during DLAV plexus morphogenesis
During embryogenesis, conserved genetic pathways shape the stereotypical anatomy of the early vessels in a circulation-independent manner. Yet, later, mechanical inputs and chemical factors provided by circulatory flow modulate vascular development (reviewed by Jones, 2011). Hence, as a first step to define the potential role of circulatory flow in DLAV morphogenesis we used confocal microangiography (Weinstein et al., 1995) to visualize lumens with active circulation in 32–72 hpf Tg(fli:EGFP)y1 embryos in which the blood plasma was fluorescently labeled with quantum dots (Qdots) (Rieger et al., 2005) (Fig. 3).
Fig. 3.
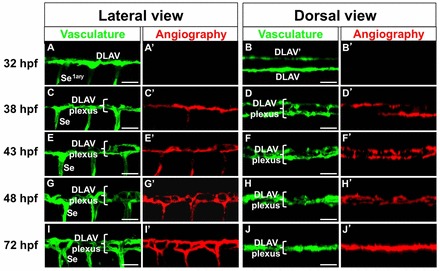
Maturation of DLAV and DLAV plexus circulatory function during development. (A–J′) Mid trunk DLAV and DLAV plexus vasculature (details). Endothelium is shown in green [Tg(fli:EGFP)y1]. Microangiograms of circulatory plasma flow is shown in red (Qdot-605). Anterior, left; dorsal, up (lateral views). Scale bars: 30 µm.
We found that at 32 hpf dorsal aorta of the trunk and axial vein display bright angiographic signals and carry robust erythrocyte circulation (data not shown) (Bussmann et al., 2010). In contrast, the DLAVs and the primary Se are angiographically negative (Fig. 3A,B′). This indicates that at this stage these structures have yet to complete tubulogenesis, i.e. form a continuous lumen (see Xu and Cleaver, 2011), consistent with prior observations based on the visualization of the luminal marker Podocalyxin-2 (Herwig et al., 2011). In contrast, at the start of DLAV plexus morphogenesis (38 hpf) the DLAVs/developing DLAV plexus and some of the primary Se carry circulatory flow (Fig. 3C,D′). Later, at 43 hpf most of the Se vasculature supports plasma flow (Fig. 3C,D′) and thus by 48 and 72 hpf the entire DLAV plexus and Se vasculature display strongly angiographically positive signals, indicating that tubulogenesis is complete across the entire network (Fig. 3G–J′). Hence, our microangiographic findings reveal that DLAV perfusion precedes plexus formation.
Next, to determine the directionality of circulatory flow through the DLAV plexus and Se vasculature we visualized erythrocyte movement. To do this we used Tg(fli:EGFP)y1; Tg(gata-1:DsRed)sd2 embryos, in which the endothelium and erythrocytes are labeled with fluorescent proteins of different colors (Lawson and Weinstein, 2002; Traver et al., 2003). We found that before 43 hpf erythrocytes do not circulate through the DLAV plexus and flow only through a small fraction of the Se vasculature, likely because they have yet to complete tubulogenesis and/or their luminal diameter is yet too small (data not shown). However, erythrocytes travel through both the DLAV plexus and the majority of SeAs and SeVs at 48 and 72 hpf, consistent with our angiographic findings. Notably, the DLAV plexus enables non-stereotypical patterns of erythrocyte flow in both anterior and posterior directions and from SeAs to both ipsilateral and contralateral SeVs, even those located at a different anteroposterior level (supplementary material Movies 4, 5, and data not shown). Hence, the DLAV plexus effectively dorsally merges the two Se networks.
Circulatory flow and VEGF signaling make additive contributions to DLAV plexus morphogenesis
Circulatory flow is a physiological factor that modulates signaling cascade outputs to control diverse aspects of cardiovascular development, including angiogenesis, hematopoiesis and cardiac maturation (Jones, 2011). For example, crosstalk between circulatory flow and VEGF signaling shapes aortic arch remodeling (Yashiro et al., 2007; Nicoli et al., 2010).
Our microangiographic findings suggest that DLAV plexus morphogenesis might require circulatory flow. In addition, VEGF ligands are expressed near (vegfaa and vegfab) or in (vegfc) the trunk vasculature (Lawson et al., 2002; Covassin et al., 2006; Hogan et al., 2009b; Gore et al., 2011). Moreover, components of the circuit that integrates hemodynamic flow and VEGF signaling during aortic arch angiogenesis, such as klf2a, mir-126a and the VEGF receptors kdrl and flt4 are expressed in the trunk vasculature. Finally, klf2a expression in the trunk vasculature is circulation dependent. Taken together, these observations suggest that circulatory flow and VEGF signaling might be functionally related in the context of DLAV plexus morphogenesis, as they are during aortic arch angiogenesis (Lawson et al., 2001; Wienholds et al., 2005; Covassin et al., 2006; Parmar et al., 2006; Choi et al., 2007; Siekmann and Lawson, 2007; Hogan et al., 2009b; North et al., 2009; He et al., 2011; Wang et al., 2011).
Hence, to evaluate if circulatory flow is required for DLAV plexus morphogenesis we first analyzed silent heart mutants (sihb109). These embryos lack heart contractility due to the inactivation of the tnnt2a/cardiac troponin-t2a gene, yet form continuous DLAVs on schedule (Sehnert et al., 2002; Isogai et al., 2003). We found that DLAV plexus formation is impaired, but not absent, in both 48 hpf sihb109 mutants and tnnt2a morphants (supplementary material Fig. S4, and data not shown), indicating that circulation promotes DLAV plexus morphogenesis. We next abrogated circulatory flow on demand by stopping the heartbeat with the action potential blocker tricaine (Muntean et al., 2010) or the myosin ATPase inhibitor BDM/2,3-butanedione 2-monoxime (Forster et al., 2004), as in Nicoli et al. (Nicoli et al., 2010). Surprisingly, we found that post-DLAV formation (32–48 hpf) circulatory flow abrogation with either tricaine or BDM prevented, rather than impaired, DLAV plexus morphogenesis in WT embryos. Moreover, DLAV plexus morphogenesis was absent in 48 hpf sihb109 mutants treated with tricaine during the same period. In addition, while both 48 hpf sihb109 mutants and tnnt2a morphants display prominent cerebral Central Arteries, these brain vessels fail to form in both WT and sihb109 mutants treated with either tricaine or BDM (data not shown) (see Bussmann et al., 2011; Corti et al., 2011). Hence, tricaine and BDM treatments induce circulation-independent effects that impair vascular development.
We sought to define whether VEGF signaling also modulates DLAV plexus morphogenesis. We began by analyzing VEGF receptor-2 ortholog kinase insert domain receptor-like mutants (kdrly17), which have impaired primary Se sprouting/growth and circulatory deficits but can form DLAVs (Covassin et al., 2006; Bussmann et al., 2008; Covassin et al., 2009; Bussmann et al., 2010). We found that 48 and 72 kdrly17 fail to form contra-lateral interconnections between the DLAVs (supplementary material Fig. S5A,B). To abrogate VEGF receptor (VEGFR) activation on demand we employed a dose of the highly specific tyrosine kinase inhibitor SU5416 that completely blocks primary Se angiogenesis even when VEGF signaling is enhanced (Mendel et al., 2000; Zygmunt et al., 2011). SU5416 abrogates the signaling activity of VEGFR1/Flt1 (Itokawa et al., 2002), VEGFR2/KDR (Mendel et al., 2000) and VEGFR3/Flt4 (Manley et al., 2004). All three VEGF receptors are implicated in the formation of the trunk vasculature (see Habeck et al., 2002; Covassin et al., 2006; Siekmann and Lawson, 2007; Hogan et al., 2009b; Krueger et al., 2011; Zygmunt et al., 2011). We found that chemical VEGFR inactivation post-DLAV formation (32–48 hpf) phenocopies the lack of DLAV plexus formation and circulatory deficits of 48 hpf kdrly17 (supplementary material Fig. S5C,D′; data not shown) and reduces 48 hpf DLAV/DLAV plexus endothelial cell abundance (supplementary material Fig. S5E).
Overall, the negative impact of reducing/abrogating VEGFR signaling on circulatory flow is consistent with the dependency of heart contractility on VEGF signaling and the susceptibility of both cardiac morphogenesis and functional maturation to circulatory deficits before 48 hpf (Rottbauer et al., 2005; de Pater et al., 2009; Vermot et al., 2009; Liu et al., 2010; Lin et al., 2011; Peshkovsky et al., 2011). However, due to the presence of circulatory deficits, these results fail to clarify the role of VEGFR signaling during DLAV plexus formation. Therefore to selectively analyze the role of VEGF signaling in DLAV plexus morphogenesis we sought to abrogate VEGFR activation while maintaining circulatory flow. We found that 43–56 hpf VEGFR inhibition achieved this (data not shown).
Hence, to define the relative contributions of VEGF signaling and circulatory flow to DLAV plexus morphogenesis, we compared 56 hpf DLAV plexus morphogenesis in animals subjected to four different conditions: treated starting at 43 hpf with the vehicle DMSO (control; ctrol), treated starting at 43 hpf with SU5416 (to abrogate VEGFR activation), injected with tnnt2a MO to prevent circulation [sih phenocopy (Sehnert et al., 2002)] and the combination of the latter two treatments (both). To do this we measured endothelial cell abundance within the whole trunk vasculature (axial vasculogenic and angiogenic vessels), the angiogenic vasculature (Se vessels and DLAV plexus) and the DLAV plexus (idem).
Overall, we found that lack of circulatory flow induces greater defects on DLAV plexus morphogenesis than VEGFR inactivation. In addition, the combination of these two conditions exacerbates the morphological defects induced by either treatment (Fig. 4A–G). In particular, the lack of both circulatory flow and VEGFR signaling has a synergistic effect on endothelial cell abundance within the whole trunk vasculature (Fig. 4E), but additive effects within both the angiogenic vasculature (Fig. 4F) and the DLAV plexus (Fig. 4G).
Next, we aimed to determine whether lack of circulatory flow and/or VEGFR inactivation affects DLAV plexus morphogenesis by impairing its development or inducing its regression (supplementary material Fig. S6). To do this we measured endothelial cell abundance in both control and tnnt2a morphant embryos at 43 hpf (supplementary material Fig. S6A,D,G) and compared these values with those exhibited by 56 hpf animals under the four conditions mentioned before (supplementary material Fig. S6B,C,E,F,H,I). We found that endothelial cell abundance in 56 hpf animals lacking both circulatory flow and VEGFR signaling is significantly smaller than that of both 43 hpf control and tnnt2a morphant embryos. Thus, our results support the notion that the DLAV plexus regresses when both circulatory flow and VEGFR signaling are absent.
Discussion
Our analysis of DLAV plexus formation in zebrafish and mouse embryos provides the first description of the cellular, molecular and physiological mechanisms by which circulatory networks merge by interconnecting in parallel along their boundaries.
While the initial stages of DLAV plexus morphogenesis are evolutionarily conserved, this process advances in a different way in these two species. This is surprising for two reasons. First, the sprouting of Se vessels (the DLAV precursors) progresses in a rostrocaudal direction in zebrafish, Xenopus and mouse, likely reflecting the evolutionarily conserved spatiotemporal dynamics of somitogenesis and somite maturation. Second, differences in somitic specification, formation and differentiation between the anterior trunk, posterior trunk and tail are thought to be evolutionarily conserved (Isogai et al., 2001; Levine et al., 2003; Bedell et al., 2005; Holley, 2007; Walls et al., 2008). Regardless, given that somitic signals modulate the organization of the spinal cord along its anteroposterior axis (see Lewis and Eisen, 2004; Holley, 2006) it is likely that DLAV plexus formation is modulated by somitic and/or spinal cord cues. Indeed, the results of our experiments and gene expression analyses (not shown) suggest that the cues that modulate DLAV plexus morphogenesis include VEGF ligands expressed in both tissues.
Zebrafish DLAV plexus formation involves classical angiogenic behaviors, such as endothelial cell proliferation and migration. In particular, our findings indicate that endothelial cells from the arterial lineage make up the bulk, if not the entirety, of the zebrafish DLAV plexus. However, whether this is the case also in mammals remains unexplored. We note that zebrafish DLAV plexus formation appears to rely on two uncommon morphogenetic mechanisms of vascular assembly. The first is the formation of a midline vessel from two lateral precursors. This resembles the way in which the midline basilar artery of the hindbrain forms from the two laterally positioned primordial hindbrain channels in zebrafish (Bussmann et al., 2011; Corti et al., 2011; Fujita et al., 2011; Ulrich et al., 2011). The second is ‘vessel fusion’, a unique process in which vessels coalesce by behaving like highly viscous liquid drops. This is the morphogenetic mechanism employed by amniotes to transform their bilateral aortae into a single axial artery (Drake and Little, 1999; Reese et al., 2004; Fleming et al., 2010; Garriock et al., 2010). Both the existence of regions within the DLAV plexus with two, rather than three longitudinal vessels (after the establishment of the third midline vessel) and our time-lapse imaging data (not shown) suggest that DLAV plexus remodeling involves vessel fusion events.
The interplay between circulatory flow and VEGF signaling is important for aortic arch angiogenesis and remodeling (Yashiro et al., 2007; Nicoli et al., 2010). For example, in the zebrafish, blood flow activates the expression of the mechano-sensitive transcription factor klf2a, which induces the expression of mir-126a. This microRNA inactivates downstream VEGF signaling inhibitors to enable angiogenesis of the fifth aortic arch. In this context, abrogation of either circulatory flow or VEGF signaling blocks angiogenesis (Nicoli et al., 2010). In contrast, we found that during DLAV plexus morphogenesis the effects of circulatory flow and VEGFR signaling are generally additive, consistent with two models. The first and most likely possibility (see Lawson et al., 2001; Wienholds et al., 2005; Covassin et al., 2006; Parmar et al., 2006; Choi et al., 2007; Siekmann and Lawson, 2007; Hogan et al., 2009b; North et al., 2009; He et al., 2011; Wang et al., 2011) is that circulatory flow promotes, but is not absolutely required, for VEGF signaling during DLAV plexus morphogenesis (Fig. 4I). This would explain why the DLAV plexus angiogenesis deficit of animals lacking flow is enhanced upon chemical inhibition of VEGFR signaling. The second alternative is that circulatory flow and VEGF signaling act in parallel pathways during DLAV morphogenesis (see Martienssen and Irish, 1999). Overall, our findings indicate that the angiogenic contributions of circulatory flow and VEGF signaling to DLAV plexus and aortic arch morphogenesis are quantitatively and qualitatively distinct (see Nicoli et al., 2010), highlighting the context-dependent nature of the circuits used by endothelial cells to integrate the inputs of physiological stimuli and signaling cascades.
Materials and Methods
Zebrafish husbandry and embryo collection
Animals were kept and handled using standard laboratory conditions (Kimmel et al., 1995) under New York University IACUC guidelines. Embryo collection, incubation, staging and blockage of melanin synthesis to preserve transparency performed as described previously (Zygmunt et al., 2011).
Zebrafish lines
We used the endothelial-specific fluorescent reporter lines Tg(fli:EGFP)y1 (cytosolic EGFP) (Lawson and Weinstein, 2002), Tg(flk1:ras-mCherry)s896 (membrane-targeted mCherry) (Chi et al., 2008), Tg(flk1:EGFP-NLS) (nuclear-targeted EGFP) (Blum et al., 2008), Tg(flk1:nlsmCherry) (nuclear-targeted mCherry) (Wang et al., 2010) and the erythrocyte-specific fluorescent reporter Tg(gata-1:DsRed)sd2 (Traver et al., 2003). Mutant alleles: silent heart (sihb109) mutants lack heartbeat due to a lesion in the cardiac troponin-t2a gene (Sehnert et al., 2002). The kinase dead hypomorphic allele of the duplicate canonical VEGFR2 kinase insert domain receptor-like (kdrly17) displays reduced angiogenesis (Covassin et al., 2006; Covassin et al., 2009). Lines were used alone or in combination.
Fixation and immunofluorescent staining of zebrafish embryos
Fixation and immunofluorescent staining of zebrafish embryos was carried out as described previously (Zygmunt et al., 2011).
Drug treatments of zebrafish embryos
Pools of 20 dechorionated embryos were treated in 4 ml aqueous solutions (standard embryo medium or SE medium) inside capped glass vials (Fisherbrand) with gentle rocking in a 28.5°C incubator. Treatment periods as indicated. Control: 0.025% DMSO/DMSO (CAS number 67-68-5; Sigma). VEGFR inhibition: 0.5 µM SU5416 (CAS number: 204005-46-9; Sigma), 0.025% DMSO. Flow inhibition: 0.66 mg/ml tricaine methanesulphonate/MS-222 (CAS number 886-86-2; Sigma-Aldrich) or BDM/2,3-butanedione 2-monoxime (CAS number 57-71-6; Sigma-Aldrich). Treatments were performed as described previously (Nicoli et al., 2010; Zygmunt et al., 2011).
Confocal imaging and image processing of zebrafish embryos
Live and fixed, immunostained embryos were mounted as described by Zygmunt et al. (Zygmunt et al., 2011). Confocal imaging performed with a Leica TCS SP5 microscope (Leipzig, Germany) using the water dipping 40× lens (NA = 0.8) and the 488, 561, 647 nm laser lines. Time-lapse imaging performed by scanning every 6 minutes; movies play at 10 fps. High-speed imaging performed by collecting 1 or 2–4 Z-stacks at a rate of 0.2 sec/stack for 2 minutes; movies play at 10 fps. ImageJ 1.42q (National Institutes of Health), Imaris (Bitplane Inc.) and Adobe Photoshop7 (Adobe) softwares were used for image processing.
Zebrafish confocal microangiography with quantum dots (Qdots) as contrast reagent
Tg(fli:EGFP)y1 embryos were briefly treated with a sub-effective Tricaine dose to slow the heartbeat. Immediately 9 nl of a 1 µM Qdot-605 streptavidin conjugate solution (Q10101MP, Invitrogen) were delivered in 3 nl steps into the duct of Cuvier at 32, 38, 43, 48 or 72 hpf by injection with a glass needle; as described previously (Rieger et al., 2005). Embryos were transferred to SE medium for 5 minutes to enable full circulatory recovery, fixed, immunostained (to highlight the vasculature) and confocal imaged.
MO injection into zebrafish embryos
MOs (Genetools, LLC) were delivered into single-cell stage embryos as follows. 7.5 ng of the ccbe1 translation blocker MO (5′-CGGGTAGATCATTTCAGACACTCTG-3′) were delivered into Tg(fli:EGFP)y1 embryos, as described by Hogan et al. (Hogan et al., 2009a). 4 ng of the tnnt2a translation blocker MO (5′-CATGTTTGCTCTGATCTGACACGCA-3′) were delivered into Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 embryos, as described previously (Sehnert et al., 2002).
Quantification of arterial and venous circulatory blood flow through zebrafish Se vessels
Erythrocyte flow directionality through Se vessels scored under transmitted light using an M165 FC stereomicroscope (Leica Microsystems) at 48 and 72 hpf in Tg(fli:EGFP)y1 embryos (uninjected and ccbe1 morphants). 10 consecutive left Se vessels scored per embryo at the approximate level of Se vessels 5–15. 20 embryos scored per condition and stage. Flow scoring: arterial (dorsal-bound); venous (ventral-bound); unscorable (lacking erythrocyte flow).
Endothelial cell abundance quantification in zebrafish embryos
Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 embryos were immunofluorescently stained to co-visualize the vascular anatomy, endothelial cell nuclei and somite boundaries using confocal imaging. Images collected in the anterior (somites 2–6) and mid trunk (somites 8–12) regions. 3D projections were built and EGFP-positive nuclei marked and counted across three consecutive somite pairs (delimited by the anterior boundary of the first somite and the posterior boundary of the third somite scored). Averages reported as cells/bilateral somite pair. Student's t-test (homocedastic, two-tail distribution) was used to analyze the differences between the means of data sets.
Mouse embryo collection and immunostaining
Mouse embryos collected and stained as described by Walls et al. (Walls et al., 2008). Wild-type Swiss-Webster embryos were collected between embryonic day (E) 9.5 (∼20–30 somites) and E10.0 (∼30–35 somites). Noon of the plug day was considered to be E0.5. Embryos were dissected from their deciduas and Reichert's membranes, then, to maintain natural shape, were fixed for 1 h in 4% paraformaldehyde before the remaining extra-embryonic tissues were removed. Endogenous peroxidase activity was quenched with 3% H2O2. Non-specific antibody binding was blocked by pre-incubating embryos in 1% heat inactivated FBS (Gibco, Grand Island NY) and 1% normal donkey serum (EMD Millipore, Billerica, MA). Embryos were then incubated overnight with 5 µg/ml of anti-PECAM-1 antibody (Mec13.3) (BD Pharmingen). Next, embryos were incubated overnight with a donkey anti-rat HRP secondary antibody (Jackson ImmunoResearch, WestGrove PA), followed by a 6-hour incubation in a 1∶50 dilution of tyramide-Cy3 reagent (PerkinElmer, Boston MA). Experiments approved by the Institutional Animal Care and Use Committee of Regeneron Pharmaceuticals (Tarrytown, New York).
Extended focal depth imaging of mouse embryos
Images of PECAM-1 immunofluorescently stained mouse embryos were collected at various positions of the focal plane using a Leica M205FA fluorescence stereomicroscope, an Olympus XM10 camera and Olympus MicroSuite software. Images were registered and aligned using the StackReg plugin for ImageJ (Thévenaz et al., 1998) and an extended depth of field image created using the Extended Depth of Field plugin for ImageJ (Forster et al., 2004).
Optical projection tomography imaging of mouse embryos and image processing
Samples were embedded in 1% low melting point agarose, dehydrated in methanol, and cleared in 1:2 benzyl alcohol:benzyl benzoate (BABB). Optical projection tomography imaging was performed as described previously (Walls et al., 2008) using a Qioptiq T100 telecentric lens system and a Regita Exi CCD camera. Data was 3D rendered using Amira software (Visage Imaging). Adobe Photoshop CS5.1 was used for image processing.
Supplementary Material
Acknowledgments
We thank M. Affolter, N. C. Chi, J. J. Essner, N. D. Lawson, B. Roman, D. Y. Stainier, B. M. Weinstein and D. Yelon for zebrafish lines, and C. M. Gay, H. Knaut, F. L. Marlow, S. Small and J. E. Treisman for comments.
Footnotes
Funding
This work was supported by grants from the American Heart Association [grant number 12GRNT8690000 to J.T.-V.]; the National Heart, Lung and Blood Institute [grant number 1R01HL092263-01A1 to J.T.-V.]; and a New York University McCracken Fellowship to T.Z. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.108555/-/DC1
References
- Adams R. H., Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 10.1038/nrm2183 [DOI] [PubMed] [Google Scholar]
- Bautch V. L., James J. M. (2009). Neurovascular development: The beginning of a beautiful friendship. Cell Adh. Migr. 3, 199–204 10.4161/cam.3.2.8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell V. M., Yeo S. Y., Park K. W., Chung J., Seth P., Shivalingappa V., Zhao J., Obara T., Sukhatme V. P., Drummond I. A.et al. (2005). roundabout4 is essential for angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 102, 6373–6378 10.1073/pnas.0408318102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum Y., Belting H. G., Ellertsdottir E., Herwig L., Lüders F., Affolter M. (2008). Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312–322 10.1016/j.ydbio.2008.01.038 [DOI] [PubMed] [Google Scholar]
- Brouillard P., Vikkula M. (2007). Genetic causes of vascular malformations. Hum. Mol. Genet. 16, R140–R149 [DOI] [PubMed] [Google Scholar]
- Bussmann J., Lawson N., Zon L., Schulte–Merker S. Zebrafish Nomenclature Committee (2008). Zebrafish VEGF receptors: a guideline to nomenclature. PLoS Genet. 4, e1000064 10.1371/journal.pgen.1000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J., Bos F. L., Urasaki A., Kawakami K., Duckers H. J., Schulte–Merker S. (2010). Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653–2657 10.1242/dev.048207 [DOI] [PubMed] [Google Scholar]
- Bussmann J., Wolfe S. A., Siekmann A. F. (2011). Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development 138, 1717–1726 10.1242/dev.059881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragine L. P., Jr, Halbach V. V., Ng P. P., Dowd C. F. (2002). Vascular myelopathies-vascular malformations of the spinal cord: presentation and endovascular surgical management. Semin. Neurol. 22, 123–132 10.1055/s-2002-36535 [DOI] [PubMed] [Google Scholar]
- Chi N. C., Shaw R. M., De Val S., Kang G., Jan L. Y., Black B. L., Stainier D. Y. (2008). Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 10.1101/gad.1629408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S., Chen J. N., Garrity D. M., Fishman M. C. (2002). Patterning of angiogenesis in the zebrafish embryo. Development 129, 973–982 [DOI] [PubMed] [Google Scholar]
- Choi J., Dong L., Ahn J., Dao D., Hammerschmidt M., Chen J. N. (2007). FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 304, 735–744 10.1016/j.ydbio.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti P., Young S., Chen C. Y., Patrick M. J., Rochon E. R., Pekkan K., Roman B. L. (2011). Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138, 1573–1582 10.1242/dev.060467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L. D., Villefranc J. A., Kacergis M. C., Weinstein B. M., Lawson N. D. (2006). Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA 103, 6554–6559 10.1073/pnas.0506886103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L. D., Siekmann A. F., Kacergis M. C., Laver E., Moore J. C., Villefranc J. A., Weinstein B. M., Lawson N. D. (2009). A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Dev. Biol. 329, 212–226 10.1016/j.ydbio.2009.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater E., Clijsters L., Marques S. R., Lin Y. F., Garavito–Aguilar Z. V., Yelon D., Bakkers J. (2009). Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136, 1633–1641 10.1242/dev.030924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J. R., Johnson Hamlet M. R., Kuliyev E., Mead P. E. (2007). A flk-1 promoter/enhancer reporter transgenic Xenopus laevis generated using the Sleeping Beauty transposon system: an in vivo model for vascular studies. Dev. Dyn. 236, 2808–2817 [DOI] [PubMed] [Google Scholar]
- Drake C. J., Little C. D. (1999). VEGF and vascular fusion: implications for normal and pathological vessels. J. Histochem. Cytochem. 47, 1351–1356 [DOI] [PubMed] [Google Scholar]
- Fleming P. A., Argraves W. S., Gentile C., Neagu A., Forgacs G., Drake C. J. (2010). Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev. Dyn. 239, 398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B., Van De Ville D., Berent J., Sage D., Unser M. (2004). Complex wavelets for extended depth-of-field: a new method for the fusion of multichannel microscopy images. Microsc. Res. Tech. 65, 33–42 10.1002/jemt.20092 [DOI] [PubMed] [Google Scholar]
- Fruttiger M. (2007). Development of the retinal vasculature. Angiogenesis 10, 77–88 10.1007/s10456-007-9065-1 [DOI] [PubMed] [Google Scholar]
- Fujita M., Cha Y. R., Pham V. N., Sakurai A., Roman B. L., Gutkind J. S., Weinstein B. M. (2011). Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 138, 1705–1715 10.1242/dev.058776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock R. J., Czeisler C., Ishii Y., Navetta A. M., Mikawa T. (2010). An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development 137, 3697–3706 10.1242/dev.051664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A. V., Swift M. R., Cha Y. R., Lo B., McKinney M. C., Li W., Castranova D., Davis A., Mukouyama Y. S., Weinstein B. M. (2011). Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138, 4875–4886 10.1242/dev.068460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck H., Odenthal J., Walderich B., Maischein H., Schulte–Merker S. Tübingen 2000screen consortium </emph>(2002). Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr. Biol. 12, 1405–1412 10.1016/S0960-9822(02)01044-8 [DOI] [PubMed] [Google Scholar]
- He X., Yan Y. L., DeLaurier A., Postlethwait J. H. (2011). Observation of miRNA gene expression in zebrafish embryos by in situ hybridization to microRNA primary transcripts. Zebrafish 8, 1–8 10.1089/zeb.2010.0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig L., Blum Y., Krudewig A., Ellertsdottir E., Lenard A., Belting H. G., Affolter M. (2011). Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr. Biol. 21, 1942–1948 10.1016/j.cub.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Hogan B. M., Bos F. L., Bussmann J., Witte M., Chi N. C., Duckers H. J., Schulte–Merker S. (2009a). Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 10.1038/ng.321 [DOI] [PubMed] [Google Scholar]
- Hogan B. M., Herpers R., Witte M., Heloterä H., Alitalo K., Duckers H. J., Schulte–Merker S. (2009b). Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136, 4001–4009 10.1242/dev.039990 [DOI] [PubMed] [Google Scholar]
- Holley S. A. (2006). Anterior-posterior differences in vertebrate segments: specification of trunk and tail somites in the zebrafish blastula. Genes Dev. 20, 1831–1837 10.1101/gad.1453706 [DOI] [PubMed] [Google Scholar]
- Holley S. A. (2007). The genetics and embryology of zebrafish metamerism. Dev. Dyn. 236, 1422–1449 [DOI] [PubMed] [Google Scholar]
- Isogai S., Horiguchi M., Weinstein B. M. (2001). The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 230, 278–301 10.1006/dbio.2000.9995 [DOI] [PubMed] [Google Scholar]
- Isogai S., Lawson N. D., Torrealday S., Horiguchi M., Weinstein B. M. (2003). Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281–5290 10.1242/dev.00733 [DOI] [PubMed] [Google Scholar]
- Itokawa T., Nokihara H., Nishioka Y., Sone S., Iwamoto Y., Yamada Y., Cherrington J., McMahon G., Shibuya M., Kuwano M.et al. (2002). Antiangiogenic effect by SU5416 is partly attributable to inhibition of Flt-1 receptor signaling. Mol. Cancer Ther. 1, 295–302 [PubMed] [Google Scholar]
- Jones E. A. (2011). The initiation of blood flow and flow induced events in early vascular development. Semin. Cell Dev. Biol. 22, 1028–1035 10.1016/j.semcdb.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Krueger J., Liu D., Scholz K., Zimmer A., Shi Y., Klein C., Siekmann A., Schulte–Merker S., Cudmore M., Ahmed A.et al. (2011). Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138, 2111–2120 10.1242/dev.063933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschke M. W., Vollmar B., Menger M. D. (2009). Inosculation: connecting the life-sustaining pipelines. Tissue Eng. Part B Rev. 15, 455–465 10.1089/ten.teb.2009.0252 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 10.1006/dbio.2002.0711 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Scheer N., Pham V. N., Kim C. H., Chitnis A. B., Campos–Ortega J. A., Weinstein B. M. (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675–3683 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Vogel A. M., Weinstein B. M. (2002). sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell 3, 127–136 10.1016/S1534-5807(02)00198-3 [DOI] [PubMed] [Google Scholar]
- Levine A. J., Munoz–Sanjuan I., Bell E., North A. J., Brivanlou A. H. (2003). Fluorescent labeling of endothelial cells allows in vivo, continuous characterization of the vascular development of Xenopus laevis. Dev. Biol. 254, 50–67 10.1016/S0012-1606(02)00029-5 [DOI] [PubMed] [Google Scholar]
- Lewis K. E., Eisen J. S. (2004). Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity. Development 131, 891–902 10.1242/dev.00981 [DOI] [PubMed] [Google Scholar]
- Lin Y. F., Swinburne I., Yelon D. (2012). Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Dev. Biol. 362, 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Bressan M., Hassel D., Huisken J., Staudt D., Kikuchi K., Poss K. D., Mikawa T., Stainier D. Y. (2010). A dual role for ErbB2 signaling in cardiac trabeculation. Development 137, 3867–3875 10.1242/dev.053736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. H., Gilland E., Bass A. H., Baker R. (2010). Ancestry of motor innervation to pectoral fin and forelimb. Nat. Commun. 1, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley P. W., Bold G., Brüggen J., Fendrich G., Furet P., Mestan J., Schnell C., Stolz B., Meyer T., Meyhack B.et al. (2004). Advances in the structural biology, design and clinical development of VEGF-R kinase inhibitors for the treatment of angiogenesis. Biochim. Biophys. Acta 1697, 17–27 10.1016/j.bbapap.2003.11.010 [DOI] [PubMed] [Google Scholar]
- Martienssen R., Irish V. (1999). Copying out our ABCs: the role of gene redundancy in interpreting genetic hierarchies. Trends Genet. 15, 435–437 10.1016/S0168-9525(99)01833-8 [DOI] [PubMed] [Google Scholar]
- McKinney M. C., Weinstein B. M. (2008). Chapter 4. Using the zebrafish to study vessel formation. Methods Enzymol. 444, 65–97 10.1016/S0076-6879(08)02804-8 [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Schreck R. E., West D. C., Li G., Strawn L. M., Tanciongco S. S., Vasile S., Shawver L. K., Cherrington J. M. (2000). The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin. Cancer Res. 6, 4848–4858 [PubMed] [Google Scholar]
- Muntean B. S., Horvat C. M., Behler J. H., Aboualaiwi W. A., Nauli A. M., Williams F. E., Nauli S. M. (2010). A comparative study of embedded and anesthetized zebrafish in vivo on myocardiac calcium oscillation and heart muscle contraction. Front. Pharmacol. 1, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S., Standley C., Walker P., Hurlstone A., Fogarty K. E., Lawson N. D. (2010). MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 464, 1196–1200 10.1038/nature08889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. E., Goessling W., Peeters M., Li P., Ceol C., Lord A. M., Weber G. J., Harris J., Cutting C. C., Huang P.et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748 10.1016/j.cell.2009.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K. M., Larman H. B., Dai G., Zhang Y., Wang E. T., Moorthy S. N., Kratz J. R., Lin Z., Jain M. K., Gimbrone M. A.Jr et al (2006). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58 10.1172/JCI24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. (2000). Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50, 1–15 10.1023/A:1006493130855 [DOI] [PubMed] [Google Scholar]
- Peshkovsky C., Totong R., Yelon D. (2011). Dependence of cardiac trabeculation on neuregulin signaling and blood flow in zebrafish, Dev. Dyn. 240, 446–456 [DOI] [PubMed] [Google Scholar]
- Reese D. E., Hall C. E., Mikawa T. (2004). Negative regulation of midline vascular development by the notochord. Dev. Cell 6, 699–708 10.1016/S1534-5807(04)00127-3 [DOI] [PubMed] [Google Scholar]
- Rieger S., Kulkarni R. P., Darcy D., Fraser S. E., Koster R. W. (2005). Quantum dots are powerful multipurpose vital labeling agents in zebrafish embryos. Dev. Dyn. 234, 670–681 [DOI] [PubMed] [Google Scholar]
- Rottbauer W., Just S., Wessels G., Trano N., Most P., Katus H. A., Fishman M. C. (2005). VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 19, 1624–1634 10.1101/gad.1319405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte–Merker S., Sabine A., Petrova T. V. (2011). Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 10.1083/jcb.201012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert A. J., Huq A., Weinstein B. M., Walker C., Fishman M., Stainier D. Y. (2002). Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 31, 106–110 10.1038/ng875 [DOI] [PubMed] [Google Scholar]
- Siekmann A. F., Lawson N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 10.1038/nature05577 [DOI] [PubMed] [Google Scholar]
- Thévenaz P., Ruttimann U. E., Unser M. (1998). A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I. (2003). Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 10.1038/ni1007 [DOI] [PubMed] [Google Scholar]
- Ulrich F., Ma L. H., Baker R. G., Torres–Vázquez J. (2011). Neurovascular development in the embryonic zebrafish hindbrain. Dev. Biol. 357, 134–151 10.1016/j.ydbio.2011.06.037 [DOI] [PubMed] [Google Scholar]
- Vermot J., Forouhar A. S., Liebling M., Wu D., Plummer D., Gharib M., Fraser S. E. (2009). Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 7, e1000246 10.1371/journal.pbio.1000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikkula M. (2006). Pathogénie et génétique des anomalies vasculaires. Ann. Chir. Plast. Esthet. 51, 282–286 10.1016/j.anplas.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Walls J. R., Coultas L., Rossant J., Henkelman R. M. (2008). Three-dimensional analysis of vascular development in the mouse embryo. PLoS ONE 3, e2853 10.1371/journal.pone.0002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kaiser M. S., Larson J. D., Nasevicius A., Clark K. J., Wadman S. A., Roberg–Perez S. E., Ekker S. C., Hackett P. B., McGrail M.et al. (2010). Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 137, 3119–3128 10.1242/dev.048785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang P., Wei Y., Gao Y., Patient R., Liu F. (2011). A blood flow-dependent klf2a-NO signaling cascade is required for stabilization of hematopoietic stem cell programming in zebrafish embryos. Blood 118, 4102–4110 10.1182/blood-2011-05-353235 [DOI] [PubMed] [Google Scholar]
- Weinstein B. M., Stemple D. L., Driever W., Fishman M. C. (1995). Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1, 1143–1147 10.1038/nm1195-1143 [DOI] [PubMed] [Google Scholar]
- Wienholds E., Kloosterman W. P., Miska E., Alvarez–Saavedra E., Berezikov E., de Bruijn E., Horvitz H. R., Kauppinen S., Plasterk R. H. (2005). MicroRNA expression in zebrafish embryonic development. Science 309, 310–311 10.1126/science.1114519 [DOI] [PubMed] [Google Scholar]
- Xu K., Cleaver O. (2011). Tubulogenesis during blood vessel formation. Semin. Cell Dev. Biol. 22, 993–1004 10.1016/j.semcdb.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K., Shiratori H., Hamada H. (2007). Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450, 285–288 10.1038/nature06254 [DOI] [PubMed] [Google Scholar]
- Zygmunt T., Gay C. M., Blondelle J., Singh M. K., Flaherty K. M., Means P. C., Herwig L., Krudewig A., Belting H. G., Affolter M.et al. (2011). Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev. Cell 21, 301–314 10.1016/j.devcel.2011.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


