Abstract
Patients with amyotrophic lateral sclerosis (ALS) have a motor disorder and cognitive difficulties, including difficulty with action verbs. However, the basis for the action verb impairment is unknown. Thirty-six participants with ALS and 22 with Parkinson’s disease (PD) were assessed on a simple, two-alternative forced-choice associativity judgment task, where performance was untimed and did not depend on motor functioning. We probed 120 frequency-matched action verbs, cognition verbs, concrete nouns and abstract nouns. Performance was related to T1 MRI imaging of gray matter atrophy. Patients with ALS were significantly impaired relative to healthy senior control participants only for action verbs. Patients with PD did not differ from controls for all word categories. Regression analyses related action verb performance in ALS to motor-associated cortices, but action verb judgments in PD were not related to cortical atrophy. These findings are consistent with the hypothesis that action verb difficulty in ALS is related in part to the degradation of action-related conceptual knowledge represented in motor-associated cortex.
Keywords: Comprehension, Amyotrophic lateral sclerosis, Parkinson’s disease
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative condition of the motor system that has increasingly been associated with cognitive deficits [1]. This includes difficulty with action words [2, 3], although the basis for this is unclear. One possible explanation is that action words are a type of verb, with verbs serving as the grammatical anchor of sentences. Recent work has demonstrated impaired language, including grammatical deficits, in ALS [4]. Another possibility for this difficulty is that verbs depend on executive resources because of the multiple semantic and grammatical components that require integration for verb processing. Considerable evidence underlines limited executive resources in ALS [5, 6]. Finally, ALS compromises neocortical motor-associated regions. Sensory-motor theories of semantic memory hypothesize that word meaning depends in part on knowledge represented in brain regions important for processing the corresponding attribute. Consistent with other disorders associated with declines in language processing, pyramidal/neuromuscular disorders such as ALS may be associated with degraded motor-action knowledge [3, 7, 8].
In this study, we examined the cognitive and neuro-anatomic basis for the action word deficit in ALS with three converging approaches. First, we compared ALS action verb performance with other word categories, including cognition verbs with grammatical and executive demands but minimal associated action. We also assessed executive functioning (letter fluency and category fluency), grammatical comprehension, naming (Boston Naming Test) and semantic memory (Pyramids and Palm Trees). Second, we administered the same protocol to patients with Parkinson’s spectrum disorders (PD) who have a motor disorder evidencing minimal disease in motor cortex. Difficulty with action verbs in PD has been related to limited executive resources [9–11], although some have related this deficit to degraded motor knowledge [12–14]. Third, we examined gray matter (GM) atrophy, and related action word performance directly to the anatomic distribution of GM atrophy in ALS.
Methods
Subjects
We studied 36 right-handed, high school-educated, non-aphasic native English speakers with ALS. Demographic features are summarized in Table 1. Five of these patients differed by 1.5 S.D. from controls in their performance on the two executive measures reported in Table 2, and these patients were considered to have ALS-MCI [15]. We also included four ALS patients with mild, co-occurring frontotemporal degeneration (FTD) [16] so that this series was representative of the ALS clinical spectrum. We also studied 22 right-handed, high school-educated, non-aphasic native English speakers with PD spectrum disorder, including clinically definite PD (n = 8), PD with mild cognitive impairment (PD-MCI, n = 3) [17], Dementia with Lewy Bodies (PD-DLB, n = 3) [18], and PD with dementia (PDD, n = 8) [19]. Patients who were receiving dopaminergic supplementation (16 taking carbidopalevodopa, two taking ropinerole, four unmedicated) were maintained on a stable dose. These groups were matched for demographic features, except that ALS patients were significantly younger than PD patients, in keeping with the known demographic distribution of disease in these patient groups. Moreover, there was no correlation between age and performance on action verbs in the associativity task (r = 0.046; p = 0.701). Patients were mildly impaired cognitively, and average MMSE scores were in the non-demented range. Table 2 summarizes executive, semantic, grammatical functioning and naming in participants. Participants were excluded based on the presence of other neurological, psychiatric or medical conditions that could contribute to a cognitive deficit, and were not taking any sedating medications. We also studied 13 right-handed, high school-educated healthy seniors. All individuals (and their responsible caregivers, as appropriate) participated in an informed consent procedure approved by the University of Pennsylvania that is consistent with the Declaration of Helsinki.
Table 1.
Mean (±SD) demographic and clinical characteristics of patients with amyotrophic lateral sclerosis spectrum and Parkinson’s disease spectrum disorders
| Amyotrophic lateral sclerosis | Parkinson’s disease | Healthy seniors | |
|---|---|---|---|
| Age (years)a | 59.28 ± 11.25 | 70.82 ± 7.26 | 68.85 ± 9.56 |
| Education (years) | 14.46 ± 2.81 | 15.68 ± 2.03 | 16 ± 3.48 |
| Disease duration (years) | 3.53 ± 2.65 | 9.20 ± 4.97 | N/A |
| Motor symptom severity, ALS (ALS functional rating scale, max = 48) | 29.2 ± 10.09 | N/A | N/A |
| Motor symptom severity, PD (UPDRS part 3) | N/A | 29.24 ± 9.04 | N/A |
| MMSE (max = 30) | 27.47 ± 3.08* | 26.59 ± 4.32* | 29.25 ± .27 |
UPDRS Unified Parkinson’s Disease Rating Scale
According to paired t tests,
differs from healthy seniors p <0.01
ALS patients were significantly younger than healthy seniors (t = 2.95; p <0.008) and patients with PD spectrum disorder (t = 4.29; p <0.001)
Table 2.
Mean (±SD) cognitive findings
| Amyotrophic lateral sclerosisa | Parkinson’s diseasea | Healthy seniors | |
|---|---|---|---|
| Language | |||
| Boston naming test (max = 30) | 27.16 ± 2.34*,+,@ | 26.77 ± 3.48* | 29.80 ± 0.44 |
| Executive functioning | |||
| Letter fluency (# words/min) | 36.71 ± 14.10*,+,@ | 38.62 ± 19.48+,@ | 52.40 ± 11.54 |
| Category fluency (# words/min) | 18.45 ± 6.40+,@ | 15.90 ± 7.20*,+,@ | 24.80 ± 4.60 |
| Grammatical comprehension (max = 72) | 70.5 ± 1.81+,@ | 67.76 ± 5.44^ | 71.33 ± 0.57 |
| Pyramids and palm trees test (max = 104) | 99.71 ± 3.70+,@ | 97.68 ± 7.25+,@ | 104 |
| Associativity judgment task | |||
| Verb judgments—action | 91.7 ± 8.0^ | 93.0 ± 7.4 | 95.6 ± 4.2 |
| Verb judgments—cognition | 90.3 ± 9.8 | 89.4 ± 9.2 | 90.6 ± 7.6 |
| Noun judgments—object | 92.6 ± 5.2 | 90.7 ± 7.5 | 92.6 ± 6.6 |
| Noun judgments—abstract | 90.8 ± 8.0 | 88.9 ± 8.5 | 93.1 ± 6.8 |
According to paired t-tests,
differs from healthy seniors p < 0.01;
differs from healthy seniors p < 0.05. ALS and PD did not differ in their performance on neuropsychological measures Following Bonferroni correction for multiple comparisons,
correlates with action word associativity judgments p <0.05;
correlates with associativity judgments for other word classes p < 0.05
Neuropsychological measures obtained within 1 month of the associativity judgment task include: Letter fluency: the number of unique words beginning with a target letter listed in 1 min, administered for three different letters (F, A and S); category fluency: the number of different animals named in 1 min; Boston Naming Test: assessment of confrontation naming evaluating the number of correctly named line drawings of objects using a 30-item version; Pyramids and Palm Trees test: assessment of semantic memory evaluating the number of correct items in a two-alternative forced-choice associativity judgment task for identical word or picture representations of objects; grammatical comprehension: a two-alternative forced-choice sentence-picture matching task, where subjects matched a sentence featuring cleft and center-embedded phrase structures in subject-relative or object-relative voice to one of two pictures
Associativity judgment task
We developed a two-alternative forced-choice associativity judgment task to examine knowledge of verb and noun meaning. Participants are given as much time as needed to indicate which of two choices printed in the lower half of a computer screen is best associated with the target word printed in the center of the upper half of the screen. We chose this untimed technique because the task has minimal resource demands, and performance does not depend on motor response in a particular modality. Probed categories included 60 verb triads (half motion, half cognition) and 60 noun triads (half concrete and half abstract). The correct choice for each category was equally distributed on the left and the right. We frequency-matched stimulus materials across all word categories (p > 0.2), and assessed each triad for concreteness and imageability to ensure that abstract nouns and cognitive verbs were less concrete and less imageable than the concrete nouns and action verbs, respectively.
Imaging methods and procedures
Subsets of ALS patients (n = 19, including two ALS-FTD patients) and PD patients (n = 8, including one PD-MCI, three PDD, and two DLB patients) agreed to participation in the imaging component of this study. Patients were not included because of concurrent medical illness, difficulty breathing in a supine position, difficulty with transportation to our facility, or unwillingness to consent to magnetic resonance imaging (MRI). Participants underwent T1 MRI to examine GM atrophy. Imaged patients matched the respective larger cohorts from which they were drawn in action verb judgment accuracy (ALS: t = 0.39; p = 0.70; PD: t = 0.23; p = 0.82) and on demographic, neuropsychological, and language measures (Appendix e-1). A T1-weighted three-dimensional spoiled gradient-echo sequence was acquired on one of two Siemens 3.0T Trio scanners using an eight-channel head coil with TR = 1620 ms, TE = 3 ms, flip angle = 15°, matrix = 192 × 256, slice thickness = 1 mm, and in-plane resolution = 0.9 × 0.9 mm. A covariate was included in the imaging analysis to remove any potential scanner effect on the analysis. Imaging was acquired within an average of 5.38 (±4.00) months of the associativity judgment task. Imaging was also collected on an independent group of 31 healthy seniors.
As described previously [20, 21], the ANTS toolkit implements a validated, reliable diffeomorphic and symmetric registration and normalization method. Images were normalized to a standard space and segmented using the PipeDream interface (http://sourceforge.net/projects/neuropipedream/) to the ANTS toolkit (http://www.picsl.upenn.edu/ANTS/). The ANTS toolkit implements the most reliable diffeomorphic and symmetric registration and normalization method available [22]. A local T1 template of 1 mm resolution was built using ANTS from 25 healthy seniors and 25 neurodegenerative disease patients. Subject images were registered to the local template, the Atropos tool in ANTS [23] used template-based priors to guide three-tissue segmentation (GM, WM, and cerebrospinal fluid), and GM probability (GMP) images were calculated. GMP images were transformed into Montreal Neurological Institute (MNI) space and down-sampled to 2 mm3 resolution. Images were smoothed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) using a 4-mm full-width half-maximum (FWHM) Gaussian kernel to minimize individual gyral variations. GM atrophy was determined relative to controls in SPM8 using a whole-brain voxel-wise analysis with a height threshold of p <0.05 (uncorrected), a minimum cluster size of 100 voxels, and a peak voxel threshold of p <0.001. The multiple regressions module in SPM8 related GM atrophy to action verb accuracy, constrained to regions of GM atrophy. Regression analyses were accepted as significant using a height threshold of p <0.05 (uncorrected) with a minimum cluster size of 100 voxels and a peak voxel threshold of p <0.001.
Results
Cognitive studies
Figure 1 shows that individuals with ALS performed significantly worse than seniors only on action verbs (t = 2.25, p = 0.03). We found no significant differences in performance between ALS and controls on other word categories (Table 2). Within-group assessments showed that controls find action verbs easier than cognition verbs (t = 2.44; p = 0.03), but ALS patients performed at the same, reduced level on action verbs as on cognition verbs (t = 1.17; p > 0.2). ALS patients were more impaired than controls on measures of executive functioning (letter fluency) and naming (Boston Naming Test). Action word associativity judgments correlated with performance on neuropsychological measures following Bonferroni correction for multiple comparisons (Table 2), but these correlations were not selective for action verbs, and performance on other word categories also correlated with these measures.
Fig. 1.
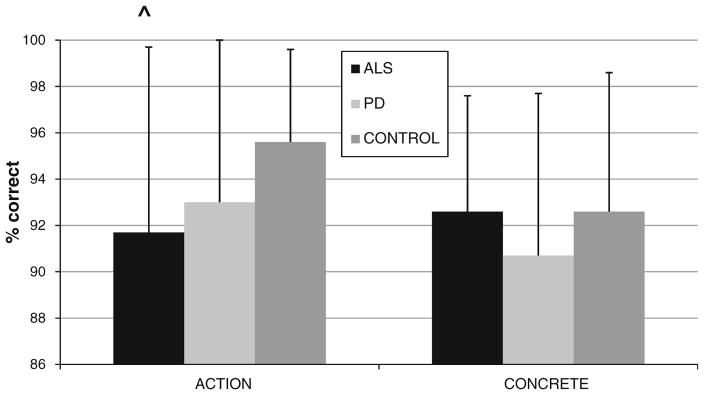
Mean (+SD) accuracy on action verb and concrete noun word categories in amyotrophic lateral sclerosis, Parkinson’s disease, and controls
We found no significant differences between PD and seniors for all categories of words, including action verbs. PD patients, like controls, performed significantly better on action verbs than on cognitive verbs (t = 2.345; p = 0.029). While PD patients were impaired on measures of executive functioning and naming relative to controls, these did not correlate selectively with action verb performance (Table 2).
Imaging studies
Figure 2 illustrates that significant GM atrophy was found in ALS relative to controls in several regions of the frontal lobe bilaterally, as summarized in Table 3, including motor cortex. Regression analyses related action verb judgments to GM atrophy in bilateral frontal regions, including motor association cortex and pre-frontal regions. While there was fairly extensive GM atrophy in the PD cohort, there were no significant regressions relating action verb judgments in PD to GM atrophy.
Fig. 2.
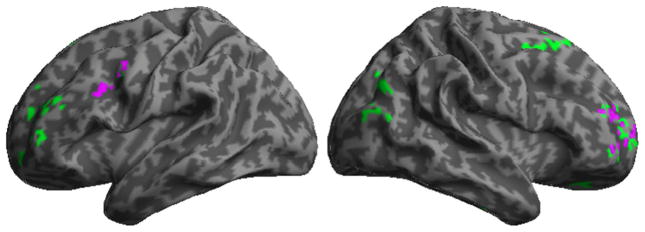
Gray matter atrophy in amyotrophic lateral sclerosis, and regression relating action verb performance to gray matter atrophy. Green areas indicate significant atrophy; regression analysis relating action verb performance to atrophy is in purple
Table 3.
Regressions of language performance with gray matter atrophy in amyotrophic lateral sclerosis and Parkinson’s disease
| Anatomic locus (Brodmann area) | MNI coordinates
|
Z score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Gray matter atrophy | |||||
| ALS < control | |||||
| L middle frontal gyrus (9) | −32 | 12 | 34 | 3.19 | 145 |
| L precentral gyrus (6)* | −46 | 0 | 44 | N/A | N/A |
| L cingulate gyrus (32) | −10 | 46 | 10 | 4.46 | 226 |
| L middle frontal gyrus (10) | −26 | 52 | 4 | 3.30 | 307 |
| L fusiform gyrus (20) | −42 | −28 | −24 | 3.23 | 135 |
| L fusiform gyrus (37) | −26 | −42 | −18 | 3.98 | 160 |
| R cingulate gyrus (32) | 20 | 4 | 48 | 3.45 | 164 |
| R medial frontal gyrus (10) | 2 | 42 | −8 | 3.18 | 558 |
| R middle frontal gyrus (10) | 24 | 56 | 4 | 4.62 | 376 |
| R middle temporal gyrus (39) | 50 | −74 | 20 | 3.30 | 241 |
| PD < control | |||||
| L fusiform gyrus (20) | −42 | −28 | −22 | 5.09 | 22,854 |
| L cingulate gyrus (23) | −6 | −42 | 22 | 3.48 | 521 |
| L superior frontal gyrus (6) | −24 | −8 | 72 | 3.13 | 109 |
| R precuneus (31) | 12 | −60 | 12 | 3.93 | 384 |
| R superior frontal gyrus (6) | 18 | 2 | 68 | 3.90 | 1,421 |
| Regression of action verb accuracy with gray matter atrophy | |||||
| ALS | |||||
| L precentral gyrus (6) | −44 | 2 | 46 | 4.20 | 131 |
| L cingulate gyrus (24) | −10 | 40 | 8 | 3.79 | 100 |
| R middle frontal gyrus (10) | 30 | 50 | 14 | 3.95 | 198 |
cluster subpeak in this location
Discussion
Non-aphasic individuals with ALS have language impairments, including difficulty with verbs. In this study, we examined the basis for this deficit. Using a simple, untimed task with minimal executive and motor demands, we found significant difficulty with action verb associativity judgments in ALS. This deficit appeared to be relatively selective, since difficulty was not evident for cognition verbs or concrete nouns. Performance was related to GM atrophy in motor association and prefrontal regions bilaterally. Patients with PD also have a motor disorder, but in PD this is due to a disturbance of the extrapyramidal system. We are unaware of previous comparative studies assessing action verbs in ALS and PD, and here we found that action verb associativity judgments are not significantly disturbed in PD. These findings are consistent with the hypothesis that the action verb deficit in ALS is related in part to the degraded representation of action feature knowledge in motor-associated cortical regions.
Non-aphasic patients with ALS appear to have some language deficits [1, 4]. However, the basis for these deficits has been difficult to establish because of confounding motor and executive components of many measures assessing language. For example, letter fluency is a timed task that depends on production in a specific (oral or written) modality. These confounds are important to recognize because ALS results in both a motor deficit and executive limitations, and these may interfere with task performance [1, 5, 6]. Limited executive resources may interfere with verb processing in a more insidious manner as well because this class of words is relatively complex and involves the coordinated processing of multiple grammatical and semantic features. While executive dysfunction may explain in part some difficulty with verbs, there are also examples of significant language deficits in ALS patients who appear to have normal executive functioning [4]. In the present study, we found a selective deficit for action verbs. While few studies have assessed cognition verbs in these patients, we did not find a deficit for cognition verbs. Thus, it is less likely that limited executive functioning can fully explain the action verb deficit in ALS. Although we found a correlation between action verb associativity judgments and measures of executive functioning, this effect was not selective for action verbs and was found for all word categories. Cognition verbs and abstract nouns were significantly more difficult than action verbs for PD patients and controls, consistent with a wellknown “concreteness effect” [24]. However, performance with action verbs was as difficult as that for cognition verbs and abstract nouns in ALS, consistent with the ALS-specific selective deficit for action verbs.
Another hypothesis attributes verb difficulty in ALS to a grammatical deficit. Verbs are grammatically complex and grammatical deficits may be evident in some ALS patients [4], although there has been scant work assessing grammatical processing in ALS [7]. In the present study, the observation of selective difficulty with action verbs but not cognition verbs is less consistent with the possibility that verb deficits can be easily explained by an impairment associated with the major grammatical category of verbs. Although action verb associativity judgments correlated with performance on a measure of grammatical comprehension, this correlation was not selective for action verbs and applied to other word classes as well.
Other studies have proposed that motor system disease degrades the representation of action knowledge associated with verb meaning. This hypothesis is based on the sensory-motor or embodied model of semantic memory that relates word meaning in part to the representation of pertinent feature knowledge in sensory-motor association cortices [25]. Previous work has thus attributed relative difficulty with action verbs compared to concrete nouns to a deficit with action features of verbs [3, 7]. Observations in the present study now emphasize selective difficulty with action verbs relative to preserved performance with verbs of cognition.
Additional evidence associating action verb deficits at least in part with degraded representations of action features comes from imaging studies relating action verb associativity judgments to frontal GM atrophy. This includes motor-associated cortices. Converging evidence relating action verbs to motor-associated cortices comes from functional MRI studies of healthy adults that relate passive viewing of action verbs involving face, arm and leg actions to the topographic representation of these components of the motor system in motor association cortices [26]. While we previously related difficulty with action verbs in ALS to motor-associated cortices [3], here we demonstrate the specificity of our finding for motor-associated cortical disease in ALS but no such deficit for PD.
We contrasted performance in ALS with performance in PD spectrum disorders. While previous work observed a deficit for verbs in PD-related disorders, the basis for this deficit has not been clear. Some reports described a deficit with action verbs compared to concrete nouns [12–14, 27]. However, this work did not evaluate verbs of cognition to determine whether the action verb deficit is related to the executive resource demands or grammatical demands associated with all classes of verbs. While patients receiving dopaminergic supplementation had more difficulty with action verbs compared to the patients “off” their medication regimen [27], dopaminergic supplementation did not appear to compromise action verb performance in the present study because these PD patients were relatively unimpaired, despite being “on” their medication regimen. Using a variety of techniques, others have pointed out that difficulty with verbs in PD may be related in part to the extensive executive resource demands associated with verbs [9–11]. PD-related disorders are not associated with significant disease burden in the pyramidal system that includes motor-associated cortices. Regression analyses did not relate action verb performance to cortical atrophy. Instead, PD typically implicates the striatum and other components of the extrapyramidal system that modulate motor performance.
Several caveats should be kept in mind when interpreting our findings. We studied a small number of patients. These patients had a limited range of motor impairments, and they were examined with a small number of action verbs. Additional work is needed to relate a wider range of action verbs to motor difficulty involving a particular anatomic distribution of disease. We did not confirm the pathologic basis for disease in these patients at autopsy. Although we minimized the executive component of task performance by presenting a simple, untimed choice between two alternatives, and although the same measure was used for all word categories, an on-line task without any decision component would have completely eliminated the possibility that an executive component contributed to task performance.
Supplementary Material
Acknowledgments
This work was supported in part by NIH (AG032953, AG017586, NS044266, AG038490, NS053488, AG043503), the ALS Association, and the Wyncote Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-014-7314-y) contains supplementary material, which is available to authorized users.
Conflicts of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Collin York, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Christopher Olm, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Ashley Boller, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Leo McCluskey, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Lauren Elman, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Jenna Haley, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Emily Seltzer, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Lama Chahine, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
John Woo, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Katya Rascovsky, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Corey McMillan, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA.
Murray Grossman, Email: mgrossma@upenn.edu, Department of Neurology and Penn Frontotemporal Degeneration Center, University of Pennsylvania, Philadelphia, PA, USA. Department of Neurology, 3 Gates, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104-4283, USA.
References
- 1.Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender NP, Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2012;83(1):102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- 2.Bak T, O’Donovan DG, Xuereb J, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain. 2001;124:103–120. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LJ, Brown RG, Tsermentseli S, Al-Chalabi A, Shaw CE, Ellis CM, Leigh PN, Goldstein LH. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2013;84(5):494–498. doi: 10.1136/jnnp-2012-303526. [DOI] [PubMed] [Google Scholar]
- 5.Libon DJ, McMillan C, Avants B, Boller A, Morgan B, Burkholder L, Chandrasekaran K, Elman L, McCluskey L, Grossman M. Deficit in concept formation in amyotrophic lateral sclerosis. Neuropsychology. 2012;26:422–429. doi: 10.1037/a0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12(4):368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- 7.Bak TH, Chandran S. What wires together dies together: verbs, actions and neurodegeneration in motor neuron disease. Cortex. 2012;48(7):936–944. doi: 10.1016/j.cortex.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain Lang. 2004;89:354–361. doi: 10.1016/S0093-934X(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 9.Auriacombe S, Grossman M, Carvell S, Gollomp S, Stern MB, Hurtig HI. Verbal fluency deficits in Parkinson’s disease. Neuropsychology. 1993;7:182–192. [Google Scholar]
- 10.Colman KSF, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R. The impact of executive functions on verb production in patients with Parkinson’s disease. Cortex. 2009;45(8):930–942. doi: 10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Grossman M, Stern MB, Gollomp S, Vernon G, Hurtig HI. Verb learning in Parkinson’s disease. Neuropsychology. 1994;8:413–423. [Google Scholar]
- 12.Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Herrera E, Rodriguez-Ferreiro J, Cuetos F. The effect of motion content in action naming by Parkinson’s disease patients. Cortex. 2010;48:900–904. doi: 10.1016/j.cortex.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Ferreiro J, Menendez M, Ribacoba R, Cuetos F. Action naming is impaired in Parkinson disease patients. Neuropsychologia. 2009;47:3271–3274. doi: 10.1016/j.neuropsychologia.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Later Scler. 2009;10(3):131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini M-L, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg YAL, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D. MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M for the Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 19.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn AD, Lees AJ, Levy R, Litvan I, McKeith IG, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 20.Grossman M, Peelle JE, Smith EE, McMillan CT, Cook PA, Powers JM, Dreyfuss M, Bonner MF, Richmond L, Boller A, Camp E, Burkholder L. Category-specific semantic memory: converging evidence from bold fMRI and Alzheimer’s disease. Neuroimage. 2013;68(0):263–274. doi: 10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein A, Andersson JLR, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee JC, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9(4):381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paivio A. Dual coding theory: retrospect and current status. Can J Psychol. 1991;45:255–287. [Google Scholar]
- 25.Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 26.Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- 27.Herrera E, Cuetos F. Action naming in Parkinson’s disease patients on/off dopamine. Neurosci Lett. 2012;513:219–222. doi: 10.1016/j.neulet.2012.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


