Abstract
Establishment of the functional pulmonary vasculature requires intimate interaction between the epithelium and mesenchyme. Previous genetic studies have led to inconsistent conclusions about the contribution of epithelial Wnts to pulmonary vasculature development. This discrepancy is possibly due to the functional redundancy among different Wnts. Here, we use Shh-Cre to conditionally delete Gpr177 (the mouse ortholog of Drosophila Wntless, Wls), a chaperon protein important for the sorting and secretion of Wnt proteins. Deletion of epithelial Gpr177 reduces Wnt signaling activity in both the epithelium and mesenchyme, resulting in severe hemorrhage and abnormal vasculature, accompanied by branching defects and abnormal epithelial differentiation. We then used multiple mouse models to demonstrate that Wnt/β-catenin signaling is not only required for the proliferation and differentiation of mesenchyme, but also is important for the maintenance of smooth muscle cells through the regulation of the transcription factor Kruppel-like factor 2 (Klf2). Together, our studies define a novel mechanism by which epithelial Wnts regulate the normal development and maintenance of pulmonary vasculature. These findings provide insight into the pathobiology of congenital lung diseases, such as alveolar capillary dysplasia (ACD), that have abnormal alveolar development and dysmorphic pulmonary vasculature.
Keywords: Wntless, Wnt, Klf2, Lung morphogenesis, Hemorrhage, Mouse, Wls
INTRODUCTION
Generation of a functional pulmonary vasculature requires close interplay of epithelium and mesenchyme. Previous studies have shown that epithelial signaling molecules, including sonic hedgehog and vascular endothelial growth factors (VEGFs), regulate the proliferation and differentiation of mesenchymal cells (Healy et al., 2000; Miller et al., 2004; Del Moral et al., 2006; White et al., 2006; Chen et al., 2010). However, recent studies regarding the role of epithelial Wnts in vasculature development generate inconsistent conclusions (Shu et al., 2002; Rajagopal et al., 2008). In one study, deletion of Wnt7b leads to hemorrhage and abnormal vasculature (Shu et al., 2002). In another study, where multiple Wnt7b mutant alleles have been used, Wnt7b disruption has minimal effects on the formation of the pulmonary vasculature (Rajagopal et al., 2008). We reason that compensation among multiple Wnt proteins present in the epithelium in different genetic backgrounds may underlie this discrepancy. To circumvent this issue, we used a mouse line harboring floxed Gpr177 (Wls - Mouse Genome Informatics) the mouse ortholog of Drosophila Wntless that regulates the sorting and secretion of Wnt proteins (Bänziger et al., 2006; Bartscherer et al., 2006; Fu et al., 2009). Conditional deletion of Gpr177 in the epithelium results in poorly developed vasculature with reduced proliferation and differentiation of mesenchyme. Further analysis revealed that Wnt/β-catenin is required for the maintenance of pulmonary vascular smooth muscle cells through the transcription factor Kruppel-like factor 2 (Klf2).
MATERIALS AND METHODS
Mice
The Shh-Cre, Dermo1-Cre, smooth muscle myosin heavy chain (SMMHC)-CreER (MHC-CreER), Gpr177loxp/loxp, β-Cateninloxp/loxp and Axin2-lacZ mouse strains, and genotyping methods have been reported previously (Brault et al., 2001; Yu et al., 2003; Harfe et al., 2004; Yu et al., 2005; Wirth et al., 2008; Fu et al., 2011). All mouse experiments were approved by the IACUC at the University of Rochester.
Tissue preparation, histology, immunostaining and X-gal staining
The immunostaining and histochemistry were performed as previously described (Que et al., 2009; Rodriguez et al., 2010; Liu et al., 2013). The antibodies used for immunohistochemistry include: rabbit anti-Sox2 (Seven Hills), rabbit anti-Sox9 (Millipore), rabbit anti-cleaved caspase 3 (Cell Signaling), rat anti-Scgb1a1 (R&D), mouse anti-α-tubulin (Sigma), rabbit anti-SpC (Millipore), rat anti-T1a (Hamster), rat anti-phosphorylated Histone H3 (Sigma), rat anti-Pecam1 (BD Biosciences), rabbit anti-laminin (Sigma), rat anti-tenascin C (Abcam), rabbit anti-ABCA3 (Seven Hills), goat anti-Klf2 (Santa Cruz) and mouse anti-smooth muscle actin (Sigma). The secondary antibodies were either fluorescence- or DAB-conjugated, and images were acquired with a Leica SP1 confocal microscope. Whole-mount X-gal staining was performed as previously described (Rodriguez et al., 2010; Chen et al., 2012).
In situ hybridization
In situ hybridization analysis was performed as described previously (Que et al., 2006; Fu et al., 2009).
Cell culture, gene knockdown and luciferase assay
Rat lung vascular smooth muscle cell line PAC1 was maintained in 10% FBS DMEM medium. Klf2 shRNA were purchased from Sigma-Aldrich. shRNA-mediated knockdown was performed as previously described (Liu et al., 2013). To test whether the Klf2 promoter is regulated by Wnt/β-catenin signaling, a 3 kb promoter region of the Klf2 gene was cloned into the pGL3 luciferase report vector, and then co-transfected into PAC1 cells with CatcLEF or Wnt3a plasmids. Point mutation of the potential binding sites was generated with QuickChange II XL Site-Directed Mutagenesis kit (Aligent). Luciferase activity was determined 24-48 hours after transfection using a dual reporter luciferase kit (Promega).
Reverse transcription PCR
RNA extraction and cDNA synthesis were performed as previously described (Rodriguez et al., 2010). The primer sequences are provided in supplementary material Table S1.
Western blot analysis
Primary antibodies were Klf2 (Chemicon) and β-actin (Abcam); the blots were processed as previously described (Que et al., 2007).
Statistical analysis
For the measurement of the proliferation index, five different high-magnification views for each tissue section and total 20 sections were examined for both mutant and wild-type lungs at E12.5 and E14.5. Data are expressed as mean±s.e.m. Differences between two samples were analyzed by Student’s t-test. P-values of 0.05 or less were considered statistically significant.
RESULTS AND DISCUSSION
Deletion of epithelial Gpr177 in Shh-Cre;Gpr177loxp/loxp mutants leads to neonatal lethality and severe pulmonary hemorrhage
Wnt signaling is crucial for the initiation of lung morphogenesis (Goss et al., 2009; Harris-Johnson et al., 2009; Chen et al., 2010). Previous characterization studies have shown that lung epithelial cells express Wnt7b and Wnt11 (Lako et al., 1998; Cohen et al., 2009). To obtain a comprehensive picture of Wnts expressed in the early lung, we first performed RT-PCR and found that Wnt2, Wnt2b, Wnt3a, Wnt5a, Wnt5b, Wnt6, Wnt7b, Wnt8a, Wnt9a, Wnt11 and Wnt16 are present at E11.5, whereas Wnt1, Wnt3, Wnt4, Wnt7a, Wnt8b, Wnt9b, Wnt10a and Wnt10b fall below detectable levels at this stage (supplementary material Fig. S1A). We further observed that Wnt3a, Wnt5a, Wnt6, Wnt7b, Wnt11 and Wnt16 are expressed in the epithelium at this stage (supplementary material Fig. S1A).
Gpr177 is ubiquitously expressed in the E9.5 lung (supplementary material Fig. S1B), and we used Shh-Cre to delete Gpr177 in the epithelium (Gpr177Δ/Δ). The separation of the trachea and esophagus is completed, and the lung budding is also normal. These findings are different from the Shh-Cre;β-cateninloxp/loxp mutants, which lack the trachea and lung (Harris-Johnson et al., 2009), supporting the hypothesis that mesenchymal Wnts (Wnt2/2b) are important for the specification of respiratory cells in the early foregut (Goss et al., 2009). Consistent with the role of Gpr177 in the regulation of Wnt secretion, but not production, the transcript levels of epithelial Wnts, including Wnt6, Wnt7b and Wnt11 are not altered significantly after Gpr177 deletion at E11.5 (P>0.05, supplementary material Fig. S1C). By contrast, canonical Wnt signaling is decreased in both the epithelium and mesenchyme of the E12.5 Gpr177Δ/Δ;Axin2-lacZ lung (Fig. 1A,B). Immunostaining of activated β-catenin confirms the decrease of signals in the epithelium and mesenchyme (supplementary material Fig. S1D,E). These findings were further supported by the decrease in the transcript levels of Wnt/β-catenin downstream targets Axin2 and Lef1 (supplementary material Fig. S1F).
Fig. 1.
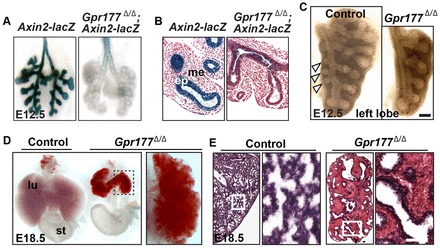
Loss of epithelial Gpr177 in the Shh-Cre;Gpr177loxp/loxp (Gpr177Δ/Δ) mutants leads to reduced Wnt signaling and severe pulmonary hemorrhage. (A,B) Deletion of epithelial Gpr177 reduces canonical Wnt signaling in both the epithelium and mesenchyme of the E12.5 lungs, as shown by the Axin2-lacZ allele. (C) Whole-mount microscopy shows reduced branching in the left lobe of E12.5 mutant lung. Arrowheads indicate lateral secondary branches in the normal lung. (D) Gpr177 deletion leads to hypoplastic lung with severe hemorrhage. (E) Hematoxylin and Eosin staining shows interstitial space in the mutant lung is filled with blood cells. ep, epithelium; me, mesenchyme; lu, lung; st, stomach. Scale bars: 50 μm.
All of the Gpr177Δ/Δ mutants (n=23) die at neonatal stage with reduced lung sizes [7.1±3.6 mg versus 25.4±2.9 mg (P<0.01)], and the lung branching seems delayed in the mutants (Fig. 1C; supplementary material Fig. S1G). More importantly, severe hemorrhaging is present throughout the interstitial space, apparently more severe than previously reported in the Wnt7b-null mutants (Shu et al., 2002) (Fig. 1D,E). Gpr177 was also deleted in the mesenchyme with Dermo1-Cre (Derm1-Cre), which is activated at ∼E10.5 (De Langhe et al., 2008), and the mutants die between E15.5 and 17.5 without hemorrhage or visible vasculature defects (supplementary material Fig. S1I,J). In both Gpr177Δ/Δ and Derm1-Cre;Gpr177loxp/loxp mutants, the airways are enlarged, which is more pronounced in the former ones (supplementary material Fig. S1H,J). Previous studies have shown that the only function for Gpr177 is to regulate the sorting and secretion of Wnt proteins (Bänziger et al., 2006; Port and Basler, 2010; Fu and Hsu, 2013). Therefore, these findings suggest that the Gpr177-mediated production of epithelial Wnts is important for pulmonary vasculature development, and multiple Wnts in the epithelium possibly compensate for the loss of Wnt7b (Shu et al., 2002; Rajagopal et al., 2008).
Limited alveolar development in the lungs of Shh-Cre;Gpr177loxp/loxp mutants
We next asked whether there are defects in the differentiation of the epithelium. The proximal-distal patterning of the epithelial progenitor cells seems unaffected, as shown by normal distribution of Sox2+ and Sox9+ cells in the E14.5 mutant lungs (Fig. 2A) (Que et al., 2009; Rawlins, 2011). Similarly, Sox2 expression is maintained in the proximal airways, while Sox9 is lost in the distal lung in both mutants and controls at E18.5 (Fig. 2B). All five epithelial types (Clara, ciliated, neuroendocrine, type I and II cells) are present in the mutants (Fig. 2C-G). However, the numbers of type I and type II alveolar cells are reduced in the mutants. Although Clara [Cc10 (Scgb1a1)+], neuroendocrine (CGRP+) and ciliated cells (α-acetylated tubulin+) are lined along the bronchial and bronchiolar airways in the proximity of type I (T1α) and type II cells (SpC+ and ABCA3+) in the control lungs (Fig. 2C-G and data not shown), the enlarged airways in the central region of the mutant lungs are packed with Clara and ciliated cells (Fig. 2C,D), and only a small number of type I and type II cells are present at the peripheral region (Fig. 2E-G). A previous study showed that deletion of the β-catenin gene in the Spc-rtTA;(tetO)7CMV-Cre;β-cateninloxp/loxp mutants also affects the differentiation of the epithelium (Mucenski et al., 2003). Our findings therefore support the observation that epithelial Wnts control epithelial morphogenesis in an autocrine fashion. However, it is also possible that the mesenchymal defects seen in the Gpr177Δ/Δ mutants impact epithelial development and contribute to the abnormal phenotypes. Previous studies have shown that signaling molecules (e.g. Fgf10) from the mesenchyme regulate lung morphogenesis (Weaver et al., 2000; Volckaert et al., 2011). Moreover, heterozygous loss of Foxf1, a transcription factor enriched in the mesenchyme, also leads to abnormal vasculature development and lung branching defects (Lim et al., 2002; Stankiewicz et al., 2009). Intriguingly, the transcript levels of Foxf1 are reduced in the Gpr177Δ/Δ mutants (supplementary material Fig. S1K). In future studies it will be interesting to determine whether the reduced levels of Foxf1 also contribute to the abnormal lung morphogenesis in the Gpr177Δ/Δ mutants. Notably, heterozygous loss-of-function FOXF1 mutations have been associated with the pathobiology of alveolar capillary dysplasia (ACD), which is characterized by the dramatically reduced numbers of capillary vessels and poor alveolar development (Bishop et al., 2011; Mestan and Steinhorn, 2011).
Fig. 2.
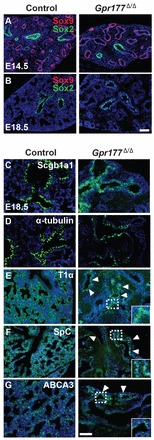
Loss of epithelial Gpr177 leads to limited alveolar development in the E18.5 lung. (A) Proximal-distal specification of epithelial progenitor cells is unaffected in the E14.5 mutant lung. Proximal and distal epithelial progenitor cells are labeled with Sox2 and Sox9, respectively. (B) Sox2 is maintained in the proximal airways of the normal and mutant lungs at E18.5. (C,D) Clara cells (Scgb1a1+) and ciliated cells (α-acetylated tubulin+) are present in the proximal airways of the normal and mutant lungs. (E-G) The numbers of type I cells (T1α+, arrowheads) and type II cells (SpC+ and ABCA3+, arrowheads) are reduced in the mutant lungs. The insets are the high magnification view of the boxed region. Scale bars: 50 μm.
Deletion of epithelial Gpr177 affects vasculature development and reduces the proliferation of mesenchymal cells
The severe hemorrhaging in the Gpr177 mutant lung prompted us to examine pulmonary vasculature development. The numbers of blood vessels (Pecam1+) in the E11.5 mutant lung are decreased when compared with the control (supplementary material Fig. S2A), and the difference becomes more prominent at E14.5 (Fig. 3A). Similarly, the numbers of smooth muscle cells (SMA+) surrounding blood vessels and airways are reduced at E12.5 and E14.5 (Fig. 3B; supplementary material Fig. S2B), and the transcript levels of Pecam1 and SMA are also reduced in the E12.5 mutant lung (supplementary material Fig. S2C). In addition, deletion of Gpr177 results in significant decrease in the proliferation of mesenchymal cells at E12.5 and E14.5 (P<0.05, Fig. 3C,D; supplementary material Fig. S2D,E), whereas the proliferation of epithelial cells is slightly increased (Fig. 3C,D; supplementary material Fig. S2D,E). Moreover, the proliferation of smooth muscle cells and endothelium is also significantly reduced at E14.5 (P<0.05, supplementary material Fig. S2F,G). We have also examined the apoptosis of epithelium and mesenchyme at E14.5 and E18.5, and no difference between mutants and controls was observed (data not shown).
Fig. 3.
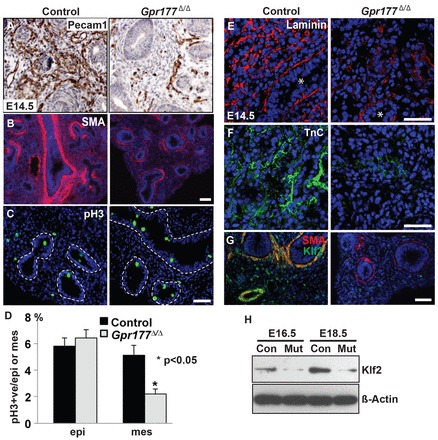
Deletion of epithelial Gpr177 disrupts mesenchymal morphogenesis. (A) The density of blood vessels (Pecam1+) is reduced in the E14.5 mutant lung. (B) Gpr177 deletion leads to reduced numbers of vascular and airway smooth muscle cells. (C) Representative section to show decreased proliferating cells (pH3+) in the mesenchyme of the mutant lung. Dotted lines indicate the basement membrane. (D) Quantification of proliferating cells in the epithelium and mesenchyme of the control and mutant lung. (E) Loss of Gpr177 results in reduced expression of the matrix protein laminin. Asterisks indicate bronchiolar airways. (F) Gpr177 deletion leads to the reduced expression of tenascin-C (TnC). (G) The expression of Klf2 is reduced in the mesenchyme of the Gpr177 mutant lung. (H) Deletion of Gpr177 leads to decreased protein levels of Klf2 in the E16.5 and E18.5 lung, as shown by western blot analysis. Scale bars: 50 μm.
Loss of Gpr177 reduces the levels of basement membrane proteins laminin and tenascin C (TnC) in the mutant lung at E14.5 (Fig. 3E,F). Electronic microscopy (EM) further shows thinning of the basement membrane and disjointed endothelial junctions at E16.5 (supplementary material Fig. S3A). TnC has previously been identified as a downstream player in the Wnt/β-catenin pathway (Cohen et al., 2009), suggesting that Gpr177 in the epithelium modulates mesenchymal differentiation in a canonical Wnt signaling-dependent manner. Consistently, deletion of β-catenin in the mesenchyme with Derm1-Cre leads to reduced numbers of blood vessels (supplementary material Fig. S3B) (De Langhe et al., 2008). Through examining the expression of genes that are known to regulate vasculature development, we determined that VEGF-c is reduced in the E16.5 Gpr177Δ/Δ mutant lung (supplementary material Fig. S3C). Intriguingly, both the transcript and protein levels of Kruppel-like factor 2 (Klf2) are also consistently reduced in the mutant lungs at E12.5, E14.5, E16.5 and E18.5 (Fig. 3G,H; supplementary material Fig. S3D). Klf2 is a zinc-finger transcription factor that has been shown to regulate the proliferation and migration of vascular smooth muscle cells (Wu et al., 2008). Klf2 deletion leads to destabilized blood vessels and severe intra-embryonic and intra-amniotic hemorrhage (Kuo et al., 1997). Taken together, these findings indicate epithelial Gpr177 regulates pulmonary vasculature development through modulating canonical Wnt signaling in the mesenchyme, and they also suggest that Klf2 is a Wnt signaling mediator.
Wnt/β-catenin regulates the proliferation of vascular smooth muscles through Klf2
A stabilized and mature vessel wall is required for maintaining the integrity of blood vessels. We asked whether canonical Wnt signaling is required for the maintenance of the vascular smooth muscle cells lining the vessel walls. To address this issue, we deleted β-catenin in the smooth muscle cells using the SMMHC-CreER mouse line (Wirth et al., 2008; Gomez et al., 2013). Tamoxifen was injected into the mothers that harbor E11.5 SMMHC-CreER;β-cateninloxp/loxp and control SMMHC-CreER;β-cateninloxp/+ embryos, and the lungs were examined at E14.5 (Fig. 4A). Removal of β-catenin leads to the reduced number of smooth muscle cells surrounding both the bronchioles and blood vessels (Fig. 4B-C′), accompanied by reduced expression of Klf2 proteins (Fig. 4D,E).
Fig. 4.
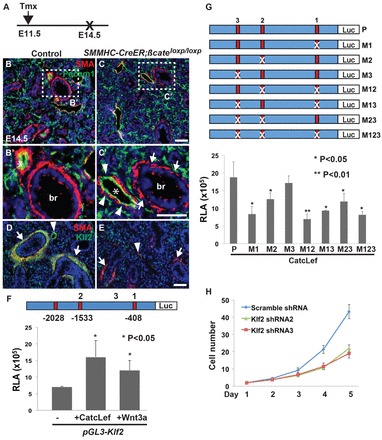
Wnt/β-catenin signaling maintains vascular smooth muscle cells through Klf2. (A) Tamoxifen was injected into mothers bearing E11.5 SMMHC-CreER;β-cateninloxp/loxp mutants and their littermates, and the embryos were harvested at E14.5. (B-C′) Loss of β-catenin leads to decreased number of smooth muscle cells surrounding the blood vessels and airways in the SMMHC-CreER;β-cateninloxp/loxp mutants. There is a discontinuous smooth muscle ring surrounding the blood vessels (arrowheads) and airways (arrows). Asterisks indicate blood vessels. (D,E) β-Catenin deletion leads to reduced levels of Klf2 in the smooth muscle cells of the blood vessels (arrowheads) and airways (arrows). Klf2 expression is maintained in a subpopulation of mesenchymal cells (SMA negative). (F) Activation of the Wnt/β-catenin pathway by co-transfection with plasmids encoding active β-catenin-Lef fusion protein (CatcLef) or Wnt3a leads to increased promoter activity of the Klf2 gene in rat pulmonary vascular smooth muscle PAC1 cells. Three potential β-catenin-binding sites are present in the 3 kb promoter region of the Klf2 gene. A dual luciferase assay was used to quantify the promoter-driven luciferase activities. (G) A point mutation was generated for each of the three β-catenin-binding sites (M1, M2 and M3). Disruption of M1 and M2, but not of M3, significantly diminishes the luciferase activity driven by the Klf2 promoter. Combinatorial mutations (M12, M13, M23 and M123) also cause significant reduction in luciferase activities (*P<0.05). (H) Lentiviral shRNA-mediated knockdown of Klf2 results in decreased proliferation of PAC1 cells. Two different shRNA targeting distinct regions of Klf2 mRNA were used. Data are presented as mean±s.e.m. br, bronchus. Scale bars: 50 μm.
We then asked whether canonical Wnt signaling directly regulates the transcription of Klf2. Three potential β-catenin-binding sites were identified in the 3 kb promoter region of the Klf2 gene, and this 3 kb DNA was cloned into the pGL3 luciferase reporter construct (Fig. 4F). Upon co-transfection with a dominant Wnt signaling activator, CatcLEF (Fu et al., 2009), or of Wnt3a plasmids into PAC1 cells, the Klf2-driven luciferase reporter signal is increased by 2.4-fold and 1.6-fold, respectively (Fig. 4F). Furthermore, point mutation analysis revealed that the two distal binding sites, 408 bp and 1533 bp, upstream of the transcription start site are crucial for the functional regulation of Klf2 by β-catenin (Fig. 4G), supporting the observation that Klf2 is a direct Wnt/β-catenin downstream target. We next tested whether Klf2 is required for the proliferation of PAC1 cells by using virus-mediated shRNA to knockdown Klf2. The knockdown efficiency is ∼70% for both shRNA constructs that target two individual regions of the Klf2 mRNA sequence (supplementary material Fig. S3E). Significantly, knockdown of Klf2 leads to a 57% decrease in the proliferation of PAC1 cells (Fig. 4H). Together, these findings suggest that the canonical Wnt/β-catenin signaling is crucial for the expansion and maintenance of vascular smooth muscle cells, and this function is in part mediated through Klf2.
In summary, we use multiple mouse models to demonstrate that Gpr177 regulation of Wnt secretion from the lung epithelium is important for both epithelial and mesenchymal development. These findings are consistent with the crucial roles of Wnt signaling in lung development and maintenance (Morrisey and Hogan, 2010; Giangreco et al., 2012). Conditional deletion of epithelial Gpr177 affects lung branching, epithelial differentiation and pulmonary vasculature morphogenesis. Our further analysis revealed that Wnt/β-catenin is required for the maintenance of vascular smooth muscle cells through Klf2. Together, these findings not only provide important information about the normal mechanism by which epithelial Wnts control vasculature development, but provide important insights into birth defects, including alveolar capillary dysplasia, that present with abnormal alveologenesis and dysmorphic vasculature (Bishop et al., 2011; Mestan and Steinhorn, 2011).
Supplementary Material
Acknowledgments
We are thankful for the stimulating discussions with Drs Brigid Hogan and Barry Stripp (Duke University, Durham NC, USA), and Drs Michael O’Reilly and Thomas Mariani (University of Rochester, NY, USA). We are grateful that Dr Gary K. Owens and Laura Shankman (Cardiovascular Research Center of the University of Virginia, Charlottesville, USA) shared with us the SMMHC-CreER;R26R embryos.
Footnotes
Funding
This research is supported by the March of Dimes Basil O’Connor Starter Scholar Research Award (to J.Q.).
Competing interests statement
The authors declare no competing financial interests.
Author contributions
M.J. and J.Q. designed the experiments. M.J., W.K. and J.F. performed the experiments. S.O. provided the SMMHC-CreER mice. W.H. provided the Gpr177loxp mice. M.J. and J.Q. wrote the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.095471/-/DC1
References
- Bänziger C., Soldini D., Schütt C., Zipperlen P., Hausmann G., Basler K. (2006). Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D., Boutros M. (2006). Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523–533 [DOI] [PubMed] [Google Scholar]
- Bishop N. B., Stankiewicz P., Steinhorn R. H. (2011). Alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med. 184, 172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 [DOI] [PubMed] [Google Scholar]
- Chen F., Cao Y., Qian J., Shao F., Niederreither K., Cardoso W. V. (2010). A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 120, 2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li J., Li H., Hu Y., Tevebaugh W., Yamamoto M., Que J., Chen X. (2012). Transcript profiling identifies dynamic gene expression patterns and an important role for Nrf2/Keap1 pathway in the developing mouse esophagus. PLoS ONE 7, e36504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. D., Ihida-Stansbury K., Lu M. M., Panettieri R. A., Jones P. L., Morrisey E. E. (2009). Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J. Clin. Invest. 119, 2538–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe S. P., Carraro G., Tefft D., Li C., Xu X., Chai Y., Minoo P., Hajihosseini M. K., Drouin J., Kaartinen V., et al. (2008). Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One 3, e1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Moral P. M., Sala F. G., Tefft D., Shi W., Keshet E., Bellusci S., Warburton D. (2006). VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev. Biol. 290, 177–188 [DOI] [PubMed] [Google Scholar]
- Fu J., Hsu W. (2013). Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J. Invest. Dermatol. 133, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Jiang M., Mirando A. J., Yu H. M., Hsu W. (2009). Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. USA 106, 18598–18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Ivy Yu H. M., Maruyama T., Mirando A. J., Hsu W. (2011). Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev. Dyn. 240, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Lu L., Vickers C., Teixeira V. H., Groot K. R., Butler C. R., Ilieva E. V., George P. J., Nicholson A. G., Sage E. K., et al. (2012). β-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J. Pathol. 226, 575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D., Shankman L. S., Nguyen A. T., Owens G. K. (2013). Detection of histone modifications at specific gene loci in single cells in histological sections. Nat. Methods 10, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P., Morrisey E. E. (2009). Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P., Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528 [DOI] [PubMed] [Google Scholar]
- Harris-Johnson K. S., Domyan E. T., Vezina C. M., Sun X. (2009). beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy A. M., Morgenthau L., Zhu X., Farber H. W., Cardoso W. V. (2000). VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev. Dyn. 219, 341–352 [DOI] [PubMed] [Google Scholar]
- Kuo C. T., Veselits M. L., Barton K. P., Lu M. M., Clendenin C., Leiden J. M. (1997). The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako M., Strachan T., Bullen P., Wilson D. I., Robson S. C., Lindsay S. (1998). Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene 219, 101–110 [DOI] [PubMed] [Google Scholar]
- Lim L., Kalinichenko V. V., Whitsett J. A., Costa R. H. (2002). Fusion of lung lobes and vessels in mouse embryos heterozygous for the forkhead box f1 targeted allele. Am. J. Physiol. 282, L1012–L1022 [DOI] [PubMed] [Google Scholar]
- Liu K., Jiang M., Lu Y., Chen H., Sun J., Wu S., Ku W. Y., Nakagawa H., Kita Y., Natsugoe S., et al. (2013). Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell 12, 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan K. K., Steinhorn R. H. (2011). Fetal origins of neonatal lung disease: understanding the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. 301, L858–L859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. A., Wert S. E., Clark J. C., Xu Y., Perl A. K., Whitsett J. A. (2004). Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev. Dyn. 231, 57–71 [DOI] [PubMed] [Google Scholar]
- Morrisey E. E., Hogan B. L. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Wert S. E., Nation J. M., Loudy D. E., Huelsken J., Birchmeier W., Morrisey E. E., Whitsett J. A. (2003). beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 278, 40231–40238 [DOI] [PubMed] [Google Scholar]
- Port F., Basler K. (2010). Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11, 1265–1271 [DOI] [PubMed] [Google Scholar]
- Que J., Choi M., Ziel J. W., Klingensmith J., Hogan B. L. (2006). Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74, 422–437 [DOI] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K. T., Kurotani R., Morrisey E. E., Taranova O., Pevny L. H., Hogan B. L. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Luo X., Schwartz R. J., Hogan B. L. (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136, 1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal J., Carroll T. J., Guseh J. S., Bores S. A., Blank L. J., Anderson W. J., Yu J., Zhou Q., McMahon A. P., Melton D. A. (2008). Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development 135, 1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E. L. (2011). The building blocks of mammalian lung development. Dev. Dyn. 240, 463–476 [DOI] [PubMed] [Google Scholar]
- Rodriguez P., Da Silva S., Oxburgh L., Wang F., Hogan B. L., Que J. (2010). BMP signaling in the development of the mouse esophagus and forestomach. Development 137, 4171–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Jiang Y. Q., Lu M. M., Morrisey E. E. (2002). Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129, 4831–4842 [DOI] [PubMed] [Google Scholar]
- Stankiewicz P., Sen P., Bhatt S. S., Storer M., Xia Z., Bejjani B. A., Ou Z., Wiszniewska J., Driscoll D. J., Maisenbacher M. K., et al. (2009). Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am. J. Hum. Genet. 84, 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Dill E., Campbell A., Tiozzo C., Majka S., Bellusci S., De Langhe S. P. (2011). Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 121, 4409–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M., Dunn N. R., Hogan B. L. (2000). Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, 2695–2704 [DOI] [PubMed] [Google Scholar]
- White A. C., Xu J., Yin Y., Smith C., Schmid G., Ornitz D. M. (2006). FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 133, 1507–1517 [DOI] [PubMed] [Google Scholar]
- Wirth A., Benyó Z., Lukasova M., Leutgeb B., Wettschureck N., Gorbey S., Orsy P., Horváth B., Maser-Gluth C., Greiner E., et al. (2008). G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 [DOI] [PubMed] [Google Scholar]
- Wu J., Bohanan C. S., Neumann J. C., Lingrel J. B. (2008). KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J. Biol. Chem. 283, 3942–3950 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063–3074 [DOI] [PubMed] [Google Scholar]
- Yu H. M., Jerchow B., Sheu T. J., Liu B., Costantini F., Puzas J. E., Birchmeier W., Hsu W. (2005). The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132, 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


