Abstract
The neural crest is a transient structure unique to vertebrate embryos that gives rise to multiple lineages along the rostrocaudal axis. In cranial regions, neural crest cells are thought to differentiate into chondrocytes, osteocytes, pericytes and stromal cells, which are collectively termed ectomesenchyme derivatives, as well as pigment and neuronal derivatives. There is still no consensus as to whether the neural crest can be classified as a homogenous multipotent population of cells. This unresolved controversy has important implications for the formation of ectomesenchyme and for confirmation of whether the neural fold is compartmentalized into distinct domains, each with a different repertoire of derivatives. Here we report in mouse and chicken that cells in the neural fold delaminate over an extended period from different regions of the cranial neural fold to give rise to cells with distinct fates. Importantly, cells that give rise to ectomesenchyme undergo epithelial-mesenchymal transition from a lateral neural fold domain that does not express definitive neural markers, such as Sox1 and N-cadherin. Additionally, the inference that cells originating from the cranial neural ectoderm have a common origin and cell fate with trunk neural crest cells prompted us to revisit the issue of what defines the neural crest and the origin of the ectomesenchyme.
Keywords: Neural crest, Ectomesenchyme, Neural fold
INTRODUCTION
The neural crest of the vertebrate embryo has been defined as the dorsal portion of the neural tube epithelium, which appears when the neural folds elevate and fuse in the midline to form the neural tube (Hörstadius, 1950; Marshall, 1879). During neurulation, cells residing within the neural crest leave the dorsal neural epithelium through an epithelial-mesenchymal transition (EMT) and disperse into the adjacent embryonic interstitial spaces, where they are generally termed migrating neural crest cells. Experiments involving dorsal neural fold epithelia (Hörstadius, 1950) suggested that the neural crest is the source of multipotent stem cells that give rise to a remarkable diversity of cell types, including pigment cells, neurons and glia of the peripheral and enteric nervous systems. In addition, the neural crest was widely considered to be the source of mesenchymal connective tissues that entered the branchial arches to form components of the craniofacial skeleton and connective tissue of the dorsal fin at the trunk axial levels of fishes and amphibia (Hörstadius, 1950; Le Douarin and Kalcheim, 1999; Raven, 1936).
Grafting studies in avian embryos (Le Douarin and Teillet, 1974; Nakamura and Ayer-le Lievre, 1982) suggested that developmental potential differed among crest-derived cells from different axial levels. Such inferences were based on three assumptions: (1) the entire neural fold epithelial domain was presumed to be neural crest (Dupin et al., 2007; Hörstadius, 1950); (2) these cell populations were initially developmentally homogeneous; and (3) the putative neural crest epithelia involved in these marking and grafting studies at trunk and cranial axial levels were qualitatively the same. Recent publications now question these assumptions (Breau et al., 2008; Weston et al., 2004).
Developmental heterogeneity exists within neural crest-derived populations, at least when they first emerge from the neural epithelium in vitro (Henion and Weston, 1997) and in vivo (Krispin et al., 2010; McKinney et al., 2013; Nitzan et al., 2013; Shoval and Kalcheim, 2012). Moreover, a population of mesenchyme cells precociously emerges from lateral cranial neural fold epithelium and enters the branchial arches before other cells emerge from the neural tube (Hill and Watson, 1958; Nichols, 1981). This implied early developmental heterogeneity in the cranial neural fold epithelium compared with the trunk, which led to the suggestion that skeletogenic ectomesenchyme might arise from a distinct epithelial domain of the neural fold, designated as ‘metablast’, which, in contrast to trunk neural crest cells, expressed a unique combination of ectodermal and mesodermal markers, such as platelet-derived growth factor receptor alpha (PDGFRα) (Weston et al., 2004). This idea is supported by the finding that these cells were found in established mouse strains that label the ectomesenchyme (Breau et al., 2008).
Studies have yet to directly demonstrate that craniofacial skeletal tissues are formed from the lateral non-neural epithelium of the cranial neural folds (Breau et al., 2008). To test this, we provide a detailed immunohistological and cell fate analysis of the neural fold in the midbrain of both mouse and chicken embryos and show that there are two distinct regions from which cells delaminate. In the midbrain, cells originating from the neural ectoderm labeled through the use of Sox1-Cre give rise predominantly to neuronal derivatives. Direct DiI labeling of corresponding regions within the neural fold in chicken embryos shows that the neural ectoderm gives rise to neuronal derivatives, whereas non-neural ectoderm gives rise to ectomesenchyme. We conclude that, in both species, the cranial neural fold can be broadly divided into two developmentally distinct domains - the neural and the non-neural ectoderm - that undergo temporally distinct episodes of delamination and give rise to neuronal and ectomesenchymal derivatives, respectively.
RESULTS
Cranial neural fold contains two phenotypically distinct epithelial domains and premigratory cells are initially only found in the non-neural ectoderm
During early development, neural induction results in two epithelial domains that can be distinguished within the neural fold: the neural and the non-neural ectoderm. The neural ectoderm in embryos of both mouse and chicken is characterized by the expression of Sox1 and N-cadherin (cadherin 2), whereas the non-neural ectoderm is characterized by the expression of E-cadherin (cadherin 1) (Dady et al., 2012; Edelman et al., 1983; Hatta and Takeichi, 1986; Nose and Takeichi, 1986; Pevny et al., 1998; Wood and Episkopou, 1999). To characterize the neural fold in mouse embryos, we used E-cadherin antibodies to delineate the non-neural ectoderm and Sox9 as a specific marker for cells that are destined to delaminate. At the onset of neurulation at 2 somites, Sox1 was already expressed in the neural ectoderm (Fig. 1Aa,e) and E-cadherin in the non-neural ectoderm (Fig. 1Ac,g). Some residual E-cadherin is found in the Sox1-expressing neural ectoderm, probably owing to the stability of E-cadherin in the entire ectoderm at earlier stages (Carver et al., 2001). However, at this stage, Sox9 (Fig. 1Ab,f) was co-expressed with E-cadherin in the non-neural ectoderm in a restricted region adjacent to, but not overlapping, the Sox1-positive neural epithelium (Fig. 1Ad,h; supplementary material Fig. S1A).
Fig. 1.
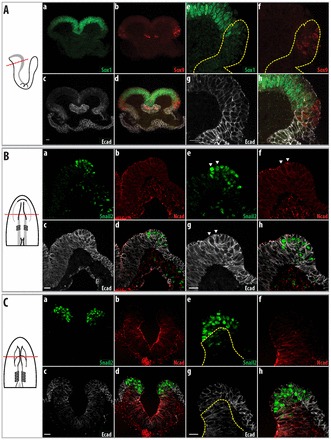
The cranial neural fold in mouse and chicken embryos contains neural and non-neural ectoderm. At early stages, cells destined to delaminate are only found in the non-neural ectoderm. To the left are schematics of the embryos shown in the images, with the plane of section illustrated. Parts a-d show an overview, whereas e-h show a higher magnification of the neural fold. Neural ectoderm is indicated by the expression of Sox1 (Aa,e) in mouse embryos and N-cadherin in chicken embryos (Bb,f, Cb,f), whereas non-neural ectoderm is indicated by expression of E-cadherin (Ac,g, Bc,g, Cc,g). (A) Two-somite mouse embryo. Sox9 is only expressed in the non-neural ectoderm, which is marked by E-cadherin (Ecad); regions with higher E-cadherin levels are outlined (yellow dotted lines). (B) Two-somite chicken embryo. Snail2 is expressed in the non-neural ectoderm, as marked by E-cadherin. N-cadherin (Ncad) is localized apically in the neural ectoderm. Snail2-expressing cells showing apical staining of N-cadherin (arrowheads). (C) Four-somite chicken embryo. Snail2 is expressed only in the non-neural ectoderm; the region expressing N-cadherin is demarcated by the yellow dotted line. At this stage, N-cadherin is localized apically and laterally in the neural ectoderm, as compared with earlier stages when it was only found apically (B). Scale bars: 20 μm.
We used E-cadherin (L-CAM) and N-cadherin for the non-neural and neural ectoderm, respectively, and Snail2 to identify cells that were fated to delaminate. N-cadherin was co-expressed with Sox1 in the neural ectoderm in chicken embryos (supplementary material Fig. S2). Thus, the expression pattern of N-cadherin in chicken embryos is comparable to that of Sox1 in mouse embryos, and both factors define the neural ectoderm. Snail2 has been shown to be the earliest indicator of delaminating cells in the epithelium (Nieto et al., 1994). At 2 somites (Fig. 1B), E-cadherin was found in both the neural and non-neural ectoderm (Fig. 1Bc,g); cells found medially in the neural ectoderm showed reduced expression of E-cadherin as compared with cells located more laterally in the non-neural ectoderm (Fig. 1Bc; supplementary material Fig. S1Ba). The neural ectoderm also began to express N-cadherin apically (Fig. 1Bb,f). During this time, the majority of Snail2-expressing cells were found in the non-neural ectoderm lying adjacent to the N-cadherin-expressing neural ectoderm (Fig. 1Ba,e), with a few Snail2-expressing cells found in the neural ectoderm. At 4 somites, the neural ectoderm expressed N-cadherin apically and laterally, whereas the non-neural ectoderm did not express N-cadherin (Fig. 1Cb,f). E-cadherin expression in the neural ectoderm was also diminished compared with the non-neural ectoderm; its expression was found mainly apically (Fig. 1Cg; supplementary material Fig. S1Ba,b). Snail2 was predominantly expressed in a region that does not express N-cadherin but showed reduced E-cadherin expression compared with lateral areas (Fig. 1Cd,h; supplementary material Fig. S1Ba,b). The in situ hybridization pattern of E-cadherin staining in the embryos at 4 somites is similar to that seen by immunostaining (supplementary material Fig. S3A) - that is, E-cadherin is found at higher levels in the non-neural ectoderm as well as in the Snail2 expression region.
In summary, prior to the delamination of cells, the neural fold consists of neural and non-neural ectoderm in both mouse and chicken embryos. The majority of cells that are going to delaminate are found in the lateral non-neural ectoderm.
Precocious EMT of cells within the non-neural ectoderm of mouse embryos
At 4 somites in the midbrain region, most delaminating cells expressing Sox9 (Fig. 2Ab,f) continued to co-express E-cadherin in the lateral non-neural ectoderm (Fig. 2Ad,h; supplementary material Fig. S1). The dorsal neural ectoderm expressed lower levels of Sox1 than more medial regions (Fig. 2Aa,e; supplementary material Fig. S1) and a few cells appeared with low levels of Sox1 and Sox9 expression (blue asterisk, Fig. 2Ad,h). However, E-cadherin expression in this domain was further reduced and Sox9-positive mesenchymal cells beneath the epithelium showed low levels of E-cadherin within the cytoplasm (blue dotted line, Fig. 2Ac,g); this suggests that these cells have recently delaminated from the E-cadherin-expressing epithelium (see also Breau et al., 2008). To further determine the origin of early delaminating cells, we examined the integrity of the epithelium via the basement membrane component laminin α1. Prior to the delamination of any cells (3-4 somites) there were gaps in laminin α1 immunostaining (yellow dotted line, Fig. 2Bc,g) in the region that was negative for Sox1 (Fig. 2Ba,e). At premigratory stages, Sox9 was expressed only in the non-neural ectoderm (Fig. 1A); this suggests that cells are directly delaminating from the non-neural ectoderm.
Fig. 2.
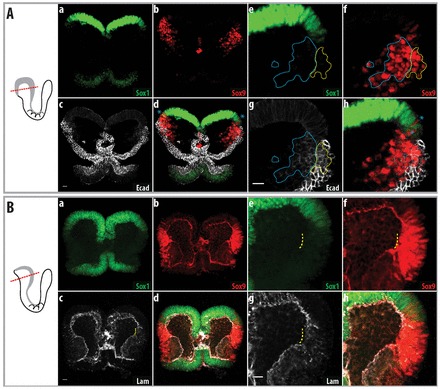
Cells delaminate from the non-neural ectoderm at ∼4 somites in mouse embryos. Delamination of cells in the non-neural ectoderm in mouse embryos. To the left are schematics of the embryos shown in the images, with the plane of section illustrated. Parts a-d of each figure show an overview, whereas e-h show a higher magnification of the neural fold. (A) Four-somite mouse embryo. Sox9 is still expressed in the non-neural ectoderm, as indicated by co-expression with E-cadherin. Sox9-positive cells that have delaminated retain their expression of E-cadherin (at lower levels, blue dotted line). Sox9-expressing cells that co-express low levels of Sox1 are indicated by blue asterisks. Sox9-expressing cells in the non-neural ectoderm are indicated by the yellow dotted line. (B) Three-somite mouse embryo, before cell delamination. Breakage of the basement membrane below the non-neural ectoderm is indicated by the yellow dotted line. Sox9-expressing cells in this region do not express Sox1. Lam, laminin α1. Scale bars: 20 μm.
Cells in the non-neural ectoderm delaminate first in chicken embryos
In mouse, we have shown that cells are delaminating from the non-neural ectoderm. However, because of the topology of the chicken neural fold, it is not known whether the Snail2-expressing cells in the non-neural ectoderm also delaminate in chicken embryos or how this would occur. In a previous study, it was shown that there is a general lateral-to-medial movement during neural tube closure in the midbrain region (Brouns et al., 2005). To test this idea, we labeled the non-neural ectoderm with DiI and recorded the movement of these cells during neural tube closure. Supplementary material Movie 1 shows the general lateral-to-medial movement of cells during neural tube closure, which is consistent with a previous study (Brouns et al., 2005), but more importantly, also shows that cells in the non-neural ectoderm undergo EMT and disperse into the embryo.
Epithelial cells are polarized, as revealed by the apical and basal localizations of zona occludens 1 (ZO1; also known as Tjp1) and β-dystroglycan (dystroglycan 1), respectively. During EMT, this apical/basal polarization of cells is lost. At 5 somites, when the two neural folds become juxtaposed, we found that Snail2- and E-cadherin-expressing cells (Fig. 3Aa) within the neural fold (Fig. 3Ad) exhibit reduced apical expression of ZO1 compared with cells found more ventrally or laterally (yellow dotted lines, Fig. 3Ae). Similarly, β-dystroglycan (Fig. 3Bb) was found throughout the cortex (yellow dotted area, Fig. 3Be) in these cells. By contrast, Snail2-expressing cells found more ventrally in the neural ectoderm retained their polarized expression of ZO1 and β-dystroglycan (Fig. 3Ae,Be).
Fig. 3.
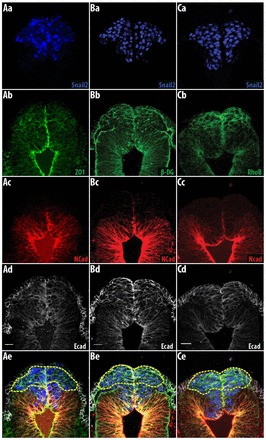
Snail2-expressing cells in the non-neural ectoderm are depolarized in chicken embryos. Cells in the lateral non-neural ectoderm are depolarized and express RhoB. (A,B) Five-somite chicken embryo. (A) ZO1 is localized apically in Snail2-positive cells within the neural ectoderm, whereas cells in the lateral non-neural ectoderm exhibit reduced apical localization of ZO1. (B) β-dystroglycan (β-DG) is found throughout the cell cortex in the Snail2-expressing cells in the lateral non-neural ectoderm (yellow dotted line), whereas β-dystroglycan in Snail2-expressing cells in the neural ectoderm is basally localized. (C) Six-somite chicken embryo. RhoB is only co-expressed with Snail2 and E-cadherin in the non-neural ectoderm (yellow dotted line). Snail2-expressing cells are found more ventrally and do not express RhoB. Scale bars: 20 μm.
Small GTPases control the organization of the cytoskeleton in association with changes in cell morphology and migratory behavior. It was previously shown that RhoB is specifically expressed by neural crest cells but not during the early stages of neural crest formation (Del Barrio and Nieto, 2004; Liu and Jessell, 1998). At 5 somites, RhoB (yellow dotted lines, Fig. 3Cb,e) was only co-expressed in the non-neural ectoderm with Snail2 (Fig. 3Ca) and E-cadherin (Fig. 3Cd), and not in the Snail2-expressing cells situated more medioventrally. The loss of apical/basal polarity and the differential expression of RhoB in Snail2-expressing cells in the non-neural ectoderm indicate that these cells delaminate first. These data, together with the behavioral data of the DiI-labeled cells in the corresponding neural fold epithelium, suggest that RhoB is expressed in the epithelial cells that are the first to delaminate from the neural fold.
A second population of delaminating cells originates from the neural ectoderm in cranial regions
Delamination occurs over a prolonged timeframe in cranial regions compared with the trunk region. To determine whether spatiotemporal changes occur during cell delamination, the pattern of expression of Sox9 and Snail2 was established in the neural and non-neural ectoderm, respectively, of mouse and chicken embryos.
The boundary between the neural and non-neural ectoderm was distinct in mouse embryos at 8 somites (Fig. 4A). The dorsal region of the neural ectoderm expressed lower levels of Sox1 than more ventral regions (Fig. 4Aa,e) and Sox9 was expressed in this region of the ectoderm (blue asterisk, Fig. 4Ad,h; supplementary material Fig. S1A) but not in the non-neural ectoderm (Fig. 4Ac,g).
Fig. 4.
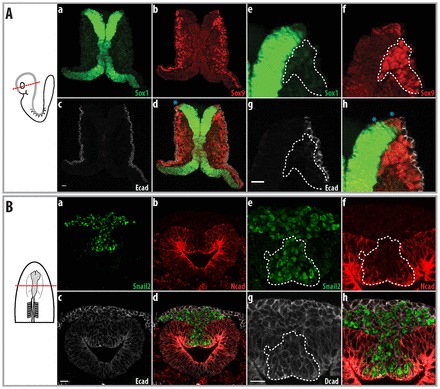
Cells subsequently delaminate from the neural ectoderm in both mouse and chicken embryos. A second population of cells delaminates from the neural ectoderm. To the left are schematics of the embryos shown in the images, with the plane of section illustrated. Parts a-d show an overview, whereas e-h show a higher magnification of the neural fold. (A) Eight-somite mouse embryo. Sox9 is expressed in the dorsal neural ectoderm that is expressing Sox1; delaminated cells are indicated (white dotted line). (B) Seven-somite chicken embryo. N-cadherin is more strongly expressed in the neural ectoderm, whereas E-cadherin expression in the neural ectoderm is reduced. Snail2 is expressed in the N-cadherin-positive neural epithelia (white dotted line) and in migrating cells juxtaposed between the neural and non-neural ectoderm. Scale bars: 20 μm.
The neural tube in the midbrain of chicken embryos is closed at 7 somites. N-cadherin was strongly expressed in the neural ectoderm (Fig. 4Bb,f), and lower levels of E-cadherin were found in Snail2-expressing cells and the neural ectoderm (Fig. 4Bc,g; supplementary material Fig. S1Ba,b). At this stage, Snail2-expressing cells were observed between the neural and non-neural ectoderm (Fig. 4Bd,h) and Snail2-expressing cells were found in the neural ectoderm (white dotted lines, Fig. 4Bd,h; supplementary material Fig. S1Ba,b). E-cadherin mRNA was only found in the non-neural ectoderm, whereas E-cadherin immunostaining showed that neural ectoderm and delaminated cells are also positive, indicating that E-cadherin protein is stable (supplementary material Fig. S3b). At this stage, Snail2-expressing cells in the neural ectoderm were no longer polarized, as shown by the uniform distribution of ZO1 and β-dystroglycan (yellow dotted line, supplementary material Fig. S4A,B). Furthermore, these cells also began to express RhoB (yellow dotted line, supplementary material Fig. S4C). Similar to the mouse, we noted a second population of delaminating cells in the chicken neural ectoderm.
Orderly delamination of cells in the chicken neural fold
To more definitively show that there are two populations of cells delaminating in the neural fold of chicken embryos and to assess whether these cells delaminate in an orderly fashion, we labeled different regions of the neural fold and followed their fates shortly after delamination. The non-neural ectoderm at the level of the mesencephalon was labeled by depositing a small drop of DiI onto the non-neural ectoderm using a micromanipulator; care was taken not to pierce the epithelium (supplementary material Fig. S5). When the non-neural ectoderm was labeled (Fig. 5A), these labeled cells were found at the leading edge of migrating cells. By contrast, when the neural ectoderm was labeled, the labeled cells were found at the trailing edge of the migrating cells (Fig. 5B). Fig. 5C shows an embryo in which the cells in the non-neural ectoderm and the neural ectoderm were labeled with DiO and DiI, respectively. These two groups constitute opposite ends of the migrating cells, with little or no cell intercalation. This further shows that cells in the non-neural ectoderm delaminate first, whereas cells in the neural ectoderm delaminate later (Fig. 5D,E).
Fig. 5.
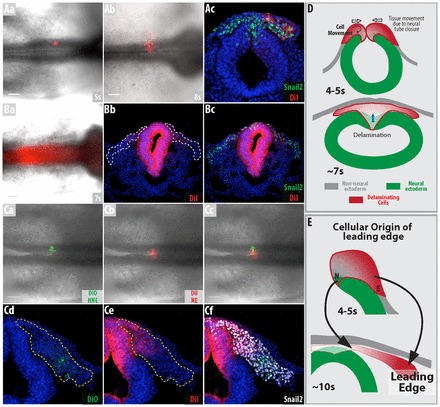
Cells delaminate from the non-neural ectoderm before the neural ectoderm in chicken embryos. (A-C) DiI and DiO were used to label different regions of the neural fold. (A) Non-neural ectoderm was DiI labeled at 5 somites. The embryos were grown until 8 somites (Ab,c). Labeled cells can be seen at the leading edge of the migrating cells. (B) Neural ectoderm was labeled with DiI at 7 somites. The embryos were grown until 11 somites (Bb,c). Labeled cells are found in the trailing edge of the migrating cells; the white dotted line represents migrating Snail2-positive cells. (C) Labeling was performed at 4 somites and embryos were grown until 10 somites (Cd-f). These two populations of labeled cells do not intermingle. (Cd) DiO-labeled cells form the leading edge of the migrating cells, whereas (Ce) DiI-labeled cells form the trailing edge; yellow dotted line represents migrating Snail2-positive cells. (D,E) Schematics of (D) cell delamination and movement away from the neural fold at 4 and 7 somites based on the results from general tissue movements, loss of basal/apical polarity and the short-term tracing of cells and (E) how they contribute to the migrating mass of cells. The red gradient in the figures represents the two waves of delaminating cells, with red and white representing cells originating from the non-neural and neural ectoderm, respectively. s, somites. Scale bars: 100 μm.
Cells originating from the neural ectoderm give rise to neurons and glia in mouse embryos
Takashima et al. reported that branchial arch (BA) 1 in Sox1-Cre/YFP embryos was not well labeled when compared with P0-Cre/YFP embryos (Takashima et al., 2007), a transgenic mouse line used to label neural crest derivatives. We showed that Sox1 is expressed in the neural ectoderm (Fig. 1Aa,e) and that the neural ectoderm is YFP-labeled in Sox1-Cre/YFP embryos (supplementary material Fig. S6) prior to cell delamination, suggesting that the neuroectoderm does not give rise to ectomesenchyme.
Cells originating from the midbrain neural folds give rise to cells within BA1 as well as to cells within the trigeminal ganglion (Osumi-Yamashita et al., 1994). In embryonic day (E) 11.5 Sox1-Cre/YFP embryos, there were few YFP-positive cells in BA1 that were also expressing Sox9 (Fig. 6A,C,D), a marker of bone/cartilage precursors (Akiyama et al., 2005; Mori-Akiyama et al., 2003). By contrast, the trigeminal ganglia was relatively well labeled, with the majority of glial cells in the trigeminal ganglia co-expressing YFP and Sox10 (Fig. 6B,E), a glial cell marker (Kuhlbrodt et al., 1998). Furthermore, some neurons within the trigeminal ganglia, which originated from the neural crest, were also labeled and showed co-expression of YFP and HuC/D (Elavl3/4), a neuronal marker (Fig. 6E).
Fig. 6.
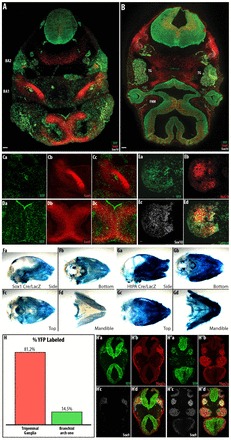
Neuronal and glial derivatives in the trigeminal ganglia are preferentially labeled in Sox1-Cre. In cranial regions, Sox1-Cre preferentially labels neuronal and glial derivatives compared with the ectomesenchyme. (A-E) E11.5 Sox1-Cre/YFP mouse embryo. Overview of YFP-labeled cells in cranial regions of Sox1-Cre/YFP embryos at the level of BA1 (A) and the trigeminal ganglia (B). (C,D) Cartilage/bone precursors in BA1 (C) and the nasal septum (D) are not well labeled in these regions. (E) More cells in the trigeminal ganglia are labeled with YFP. Sox1-Cre/lacZ (F) and HtPA-Cre/lacZ (G) P0 mouse skull preparations. (H) The percentage of YFP-labeled cells in the anlage of the trigeminal ganglia and BA1 was determined in E9.5 Sox1-Cre/YFP embryos. (H’,H′) Representative sections in which the numbers of YFP-labeled and Sox9/Tfap2a-expressing cells were counted in the anlage of the trigeminal ganglia (H′) and BA1 (H′). Region counted indicated by blue and green dotted lines for the trigeminal ganglia and branchial arch, respectively. n=2. TG, trigeminal ganglia; FNM, frontal nasal process. Scale bars: 40 μm.
HtPA-Cre/lacZ will label all cells that originate from the neural fold (Pietri et al., 2003). We compared bone/cartilage derivatives in the skulls of postnatal day 0 mice that had been labeled with HtPA-Cre/lacZ or Sox1-Cre/lacZ. Our results showed that the overall intensity of β-galactosidase was much weaker in Sox1-Cre/lacZ skull preparations than in those labeled with HtPA-Cre/lacZ (Fig. 6F,G). This was especially the case for the mandible, which derives from BA1; the bones and cartilages within this region were not well labeled (Fig. 6Fd,Gd).
We further quantified the proportion of bone/cartilage and neuronal precursors in E9.0 Sox1-Cre/YFP embryos (15 somites) after both the trigeminal ganglia (blue dotted lines, Fig. 6H′d) and BA1 (green dotted lines, Fig. 6H′d) had formed. We found far greater numbers of YFP-positive cells in the trigeminal ganglia than in BA1, indicating that Sox1-Cre preferentially labels glial and neuronal derivatives (81.2%, Fig. 6H) compared with ectomesenchyme (14.5%, Fig. 6H). These neural fold-derived cells also expressed Tfap2a (Fig. 6H′b,H′b) as well as Sox9 (Fig. 6H′c,H′c); both of these markers are expressed by migratory cells (Mitchell et al., 1991; Wright et al., 1995). Sox1 was not expressed in the trigeminal ganglion at E9.0 (data not shown), which rules out the possibility of labeling because of de novo expression of Sox1 in the trigeminal ganglion. Overall, we have shown that the ectomesenchyme in BA1 is not well labeled, whereas the neuronal derivatives are well labeled in Sox1-Cre/YFP embryos.
The incomplete labeling of the trigeminal ganglion prompted us to examine the activation of the YFP reporter at the onset of cell delamination. We found that at 5 somites some YFP-expressing cells are present within the E-cadherin-expressing non-neural ectoderm (blue asterisk, Fig. 7Ad,Bd). Although these cells expressed YFP, they did not express Sox1 protein. As mentioned above, the dorsal region of the neural ectoderm expressed lower levels of Sox1 than the more ventral regions (yellow dotted line, Fig. 7Bd). It is important to note that in Sox1-Cre/YFP embryos the border region between the neural and non-neural ectoderm had a mosaic appearance, i.e. cells were sporadically labeled with YFP. These YFP-labeled cells in the non-neural ectoderm could still be observed at 7 somites.
Fig. 7.
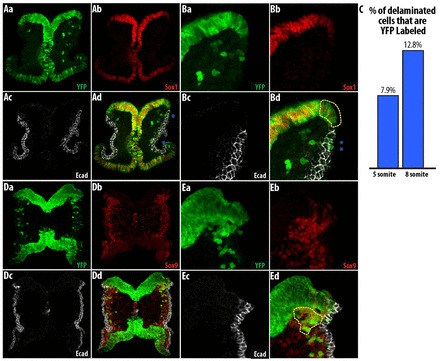
Sox1-Cre labels some cells in the lateral non-neural ectoderm. The majority of cells labeled in Sox1-Cre originate from the neural ectoderm but there are some ectopically labeled non-neural ectoderm cells. (A,B) YFP-labeled cells in the non-neural ectoderm do not express Sox1 (blue asterisks). YFP-labeled dorsal neural ectoderm expressing lower levels of Sox1 is indicated by the yellow dotted line. (C) There are few YFP-labeled cells in the Sox1-Cre/YFP embryo at the early stages of cell delamination. n=2. (D,E) Cells delaminating from the neural ectoderm are better labeled at 10 somites, prior to the formation of the trigeminal ganglia.
The formation of BA1 at 8 somites and anlage of the trigeminal ganglia after 10 somites (Chan and Tam, 1988; Theiler, 1989) coincides with the switch in expression of Sox9 from the non-neural ectoderm to the neural ectoderm. We determined the percentage of YFP-labeled migratory cells in Sox1-Cre/YFP embryos as 7.9% at 6 somites and 12.8% at 8 somites (Fig. 7C). At ∼10 somites, the cells that had recently delaminated from the neural ectoderm were YFP labeled (Fig. 7D,E), consistent with the percentage of YFP-labeled cells found in older embryos in this study. The early delaminating cells are most probably derived from the non-neural ectoderm and are ectopically labeled by Sox1-Cre. Thus, although we cannot fully exclude the possibility that some ectomesenchymal derivatives originate from the neural epithelial domain of the cranial neural fold in Sox1-Cre/YFP embryos, these labeled cells most likely originated from cells that ectopically expressed Sox1-Cre in the non-neural epithelial domain.
Non-neural ectoderm-derived and neural epithelial-derived cells give rise to ectomesenchymal cells and neuronal derivatives, respectively, in chicken embryos
We performed DiI labeling of the non-neural and neural ectoderm in chicken embryos to determine the fate of labeled cells 2 days later. In whole-mounts of these embryos, the majority of labeled cells were found in BA1 (Fig. 8A) or in the periocular mesenchyme. Particular cells within the trigeminal ganglia were also labeled with DiI in 2 out of 10 embryos (Fig. 8A,C). When these embryos were sectioned, the DiI-labeled mesenchymal cells within BA1 showed Tfap2a expression (Fig. 8B) (Minarcik and Golden, 2003). It is possible that the DiI-labeled cells within the trigeminal ganglion might be neurons derived from the trigeminal placode, since these two regions are adjacent to each other. However, because of the intermingling of the neurons and glia within the ganglion and the punctate DiI signal, we cannot distinguish exactly whether these labeled cells are glia or neurons. To determine whether these cells are derived from the trigeminal placode, we looked at earlier stages when the DiI signal was stronger (n=14). Pax3 expression in neural crest cells is lost after 16 somites but Pax3 is expressed by the trigeminal placode and its derivatives (Xu et al., 2008); this provides a means to identify the trigeminal placode as well as its derivatives. In two embryos in which we could observe DiI-labeled cells in the trigeminal ganglia at 20 somites, DiI-labeled cells in the trigeminal ganglia expressed Pax3 (supplementary material Fig. S7A). Furthermore, analysis of non-neural ectoderm-labeled embryos at early stages revealed three embryos with DiI-labeled cells in the Pax3-positive trigeminal placode (supplementary material Fig. S7B,C). Therefore, since we did not consistently observe derivatives in the trigeminal ganglia in all labeled embryos, and as we also demonstrated that the trigeminal placode and its derivatives are labeled in some cases, it is likely that the DiI-positive cells that we observed in the trigeminal ganglion at Hamburger-Hamilton stage (HH) 18 were derived from the trigeminal placode.
Fig. 8.
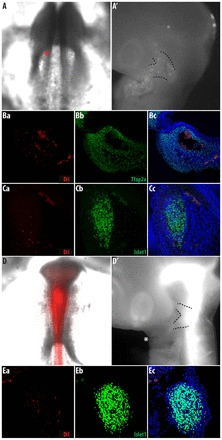
Two populations of delaminating cells in the neural fold give rise to distinct cell fates. Non-neural ectoderm and neural ectoderm give rise to derivatives in the branchial arches and trigeminal ganglia, respectively. (A-C) Non-neural ectoderm was Dil labeled at 4 somites. All labeled chicken embryos have labeled ectomesenchyme in the periocular or branchial arch 1 and only two embryos have cells labeled in the trigeminal ganglia; n=10. Chicken embryos showing initial DiI labeling of the non-neural ectoderm (A) and labeled derivatives after 2 days of culture (A′). Note that the majority of labeled cells are in the branchial arch (one of two embryos with labeled cells in trigeminal ganglia). Transverse section of (B) branchial arch one (BA1) immunostained for Tfap2a, which is expressed in ectomesenchymal cells and (C) the trigeminal ganglia immunostained for Islet1/2, which is expressed by neuronal cells. (D,E) Neural ectoderm was labeled by DiI at 7 somites; n=8. Chicken embryos showing initial DiI labeling of the neural ectoderm (D) and labeled derivatives after 2 days of culture (D′). Note the absence of labeled cells in the branchial arches. (E) Transverse section of the trigeminal ganglia immunostained for Islet1/2. Dotted lines indicated the trigeminal ganglia.
Injecting DiI into the lumen of the neural tube at 7 somites only labels cells that originate from the neural ectoderm, as the neural tube is already closed. In whole-mounts of these neural ectoderm-labeled embryos, there were no DiI-labeled cells within BA1 (Fig. 8D). The strong fluorescent signal from the underlying neural tube meant that it was difficult to determine, in whole-mounts, whether labeled cells were present in the trigeminal ganglion. Upon sectioning these embryos, we found numerous DiI-labeled cells within the trigeminal ganglia (Fig. 8E). In this case, because only the neural ectoderm was labeled, these DiI-labeled cells could not have originated from the trigeminal placode.
From these labeling experiments, we conclude that cells within distinct domains of the neural fold give rise to cells with different cell fates. Cells originating from the non-neural ectoderm give rise to mesectodermal derivatives, whereas cells originating from the neural ectoderm give rise to neuronal derivatives.
DISCUSSION
Ectomesenchyme is present in all vertebrates; however, its origin was often debated in early studies because of the considerable variation in the morphogenesis of the head across different classes of vertebrates. In addition, the terms ‘dorsal neural tube in the trunk’ and ‘cranial neural fold’ are considered to describe equivalent tissues. Unlike the single episode of delamination in the trunk region, there are two episodes of cell delamination from the neural fold in the midbrain region in both chicken and mouse embryos (summarized in Fig. 9). In cranial regions only the second wave of delaminating cells shares a common origin and cell fate with cells that delaminate in the trunk. Our study provides compelling evidence that these two regions are not equivalent, and that the unique formation of the cranial neural folds may permit the formation of ectomesenchyme.
Fig. 9.
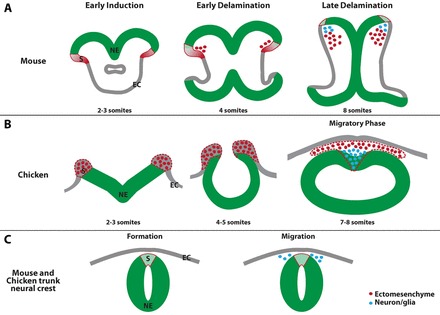
Model of cell delamination in the midbrain and trunk. There are two waves of cell delamination and these cells adopt distinct cell fates in the midbrain of both mouse (A) and chicken (B) embryos. In the trunk, cells only delaminate when the neural and non-neural ectoderm are already separated (C). EC, non-neural ectoderm; NE, neural ectoderm; S, region where cells delaminate.
In the lineage analysis of chicken embryos, we do not address the issue of cell fate potential, as we did not challenge these cells. Furthermore, the small number of Snail2-expressing cells in the neural ectoderm at very early stages could be precursors of cells that delaminate from the neural ectoderm. Below we address a number of issues regarding the definition and pluripotency of these cells at different axial levels.
Origin of ectomesenchyme and topology of the neural fold
The initial study on the origin of the ectomesenchyme showed that it was derived from the lateral non-neural ectoderm (Platt, 1893), a region distinct from the neural crest that originated from the dorsal neural ectoderm and gave rise to cranial ganglia. However, subsequent studies dismissed the notion that there were two distinct waves of delaminating cells by conflating the definition of neural crest and neural fold (Hörstadius, 1950; Landacre, 1921). This was probably also complicated by the lateral-to-medial movement of cells during cranial neural tube closure (Brouns et al., 2005). Uncertainties remained as to whether cells were delaminating only from the neural epithelium or also from the overlying non-neural ectoderm, and this was true even in the trunk (Beard, 1888; Hörstadius, 1950). The origin of trunk neural crest from the neural ectoderm was unequivocally shown through grafting of neural tube in avian embryos (Weston, 1963). However, the continuity of the neural and non-neural ectoderm in cranial regions (Johnston, 1966) made it impossible for grafting experiments to determine the origin of cells with sufficient spatial and temporal resolution.
In mammalian embryos, cranial neural tube closure occurs after cell delamination and the neural fold adopts an extended structure with an underlying interstitial space. This creates a scenario in which the origin of delaminating cells can be accurately determined. A number of studies have shown that the lateral non-neural ectoderm gives rise to ectomesenchyme found in the branchial arches in mammals (Breau et al., 2008; Chan and Tam, 1988; Hill and Watson, 1958; Nichols, 1981; Vaglia and Smith, 2003; Weston et al., 2004). We have also observed expression of so-called ‘neural crest specifiers’ such as Tfap2, Pax3 and Pax7, in mouse and chicken embryos in both the non-neural and neural ectoderm (data not shown). This would explain why a number of transgenic mouse lines, including Wnt1-Cre, HtPA-Cre, Pax3-Cre and Pax7-Cre, label both the neuronal as well as ectomesenchymal derivatives (Danielian et al., 1998; Engleka et al., 2005; Murdoch et al., 2012; Pietri et al., 2003). These observations support the idea that there are two distinct episodes of delamination from cranial neural fold epithelium.
Our results disagree with the findings from a recent report concluding that there is no reduced expression of E-cadherin in the non-neural ectoderm (Dady et al., 2012). On the contrary, we observed increased expression of E-cadherin in the non-neural ectoderm as compared with the neural ectoderm. Indeed, the E-cadherin in situ hybridization results corroborate the results from our immunostaining data and also show that E-cadherin protein is relatively stable. Based on this observation, we define the non-neural ectoderm as the region of ectoderm that expresses higher E-cadherin levels and has an absence of N-cadherin. The pattern of cell delamination in chicken embryos is comparable to that of mouse embryos, as Snail2 is initially found in a region devoid of Sox1 or N-cadherin expression, whereas at later stages Snail2 expression is found in the neural ectoderm (compare Fig. 9A with 9B).
The ventral-to-dorsal formation of derivatives - early fate restriction
Numerous studies have shown that neural crest derivatives are formed in a ventral-to-dorsal sequence (Baker et al., 1997; Krispin et al., 2010; Lumsden et al., 1991; McKinney et al., 2013; Nitzan et al., 2013; Osumi-Yamashita et al., 1994; Shoval and Kalcheim, 2012). It is interesting to note that, in the study by Lumsden et al. (Lumsden et al., 1991), although no attempt was made to label distinct regions of the neural fold, the authors found that, when the neural fold in the midbrain was labeled at early stages, only labeling of the ectomesenchyme was observed in the majority of cases. This is also consistent with our current findings. By contrast, the study by Baker et al. (Baker et al., 1997) shows that early migrating cells can give rise to all of the different derivatives. The discrepancy could be because of the different methodology employed, as the grafts used in the study by Baker et al. would comprise significantly more cells than the number of cells that we labeled with DiI.
Recent studies disagree as to whether trunk neural crest cells are fate restricted prior to delamination (Krispin et al., 2010; McKinney et al., 2013; Nitzan et al., 2013). Krispin et al. and McKinney et al. looked at whether neural crest cells delaminate from the neural tube in an orderly fashion and whether fate restriction occurs prior to delamination. The only consensus from these studies was that precursors of the sympathetic ganglia were the first cells to delaminate and they were fate restricted. However, Nitzan et al. showed that Foxd3 expression in the dorsal neural tube is heterogeneous and that melanoblast precursors downregulate Foxd3 before delamination. Perturbing the expression of Foxd3 in the neural tube caused these cells to adopt neuronal or melanocytic cell fates. This is corroborated by the expression of melanoblast markers prior to delamination (Erickson and Goins, 1995; Wehrle-Haller and Weston, 1995; Wilson et al., 2004). In addition to melanoblasts, in the dorsal neural tube of mice there is a population of neurogenin 2-expressing trunk neural crest cells that preferentially give rise to cells within the dorsal root ganglia (Zirlinger et al., 2002). Similarly, in zebrafish, it has been shown that both the cranial and trunk neural crest cells are already fate restricted prior to delamination (Raible and Eisen, 1994; Schilling and Kimmel, 1994). This shows that it is highly likely that fate restriction occurs prior to delamination.
Previously, it was shown that downregulation of Sox2 was required for the induction of neural crest cells (Remboutsika et al., 2011; Wakamatsu et al., 2004). Sox1, like Sox2, is expressed at lower levels in the dorsal region of the neural epithelia, indicating that these factors might be involved in the positioning of the neural plate border (Wakamatsu et al., 2004). The expression level of Sox1 might be important for inducing cells to adopt a neuronal fate and for generating heterogeneity within the neural fold, since Sox1 is differentially expressed by the two populations of delaminating cells. In mice, the removal of Sox1 causes only microphthalmia (Nishiguchi et al., 1998); however, this could result from compensation by other members of SoxB1 gene family.
Is ectomesenchyme a unique derivative of the cranial neural fold?
When transplanted into the trunk, the cranial neural fold readily forms ectopic cartilage and bone (Le Douarin and Teillet, 1974); however, the trunk neural crest does not seem to give rise to cartilage or bone when transplanted into cranial regions (Nakamura and Ayer-le Lievre, 1982). This has led to questioning as to whether ectomesenchyme is a unique derivative of the cranial neural fold. It has been suggested that both cranial and trunk neural crest cells have the same developmental potential and that formation of the ectomesenchyme is due to inductive environmental factors within the branchial arches (Abzhanov et al., 2003; Baker et al., 1997; Blentic et al., 2008; Lumsden, 1988; McGonnell and Graham, 2002). However, a study has shown that trunk and cranial ‘neural crest’ respond differently to the same signal, and that the formation of ectomesenchyme by trunk neural crest cells involves their conversion to a more cranial-like state (Abzhanov et al., 2003). This might indicate that trunk neural crest only forms ectomesenchyme via extensive cellular reprograming. Although Sox1-Cre was used to show that, in the trunk, mesenchymal stem cells (MSCs) were derived from the neural epithelium, the significance of this is unclear, as MSCs were found only in embryos, and trunk neural crest does not normally give rise to mesenchymal lineages. In some vertebrates, such as the turtle, the trunk neural crest appears to give rise to ectomesenchymal derivatives in the plastron and fins (Clark et al., 2001; Raven, 1936; Smith and Hall, 1990). However, at least in zebrafish, fin rays and scales are not derived from the neural crest (Lee et al., 2013; Mongera et al., 2013). By contrast, the cranial neural fold, when transplanted into the trunk, readily forms ectopic cartilage and bone, even in a foreign environment (Le Douarin and Teillet, 1974). This implies that there could be intrinsic differences between the cranial and trunk ectodermal regions.
In 1950, Hörstadius stated that it was unclear whether the neural crest should be considered an outgrowth from the central nervous system or a separate rudiment (Hörstadius, 1950). Other than the ability of neural crest cells to undergo EMT, it has remained challenging to define these cells in terms of their origin or differentiation potential. Based on the presence of two separate epithelial domains in the cranial neural fold - one giving rise to ectomesenchyme and the other sharing an origin and cell fate with cells that delaminate in the trunk - it might be time to reconsider the nature of the neural crest and the origin of ectomesenchyme. The term ‘metablast’ was proposed by Weston et al. to explain the unique potential of the cranial neural fold to give rise to ectomesenchyme (Weston et al., 2004). This term was coined to take into account the unique population of cells that delaminates in the cranial region, which has a different origin and fate from the neural crest. Alternatively, we can adopt a broader definition of the neural crest: neural crest cells originate from the border of the neural and non-neural ectoderm and subpopulations of such broadly defined neural crest cells can be further refined.
Given the close proximity of the two epithelial domains within the cranial neural fold, we are faced with the dilemma of how to define the cells delaminating from the non-neural ectoderm. Further work is needed to adequately assess how these two regions segregate and to determine their relatedness. This might also serve to deepen our understanding of the emergence of ectomesenchyme in vertebrates and of how different derivatives are formed.
MATERIALS AND METHODS
Mouse strains
All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Agency for Science, Technology and Research (A*STAR) of Biomedical Science Institutes. Sox1-Cre (Takashima et al., 2007) and HtPA-Cre (Pietri et al., 2003) were bred with reporter strains Rosa26-YFP (Srinivas et al., 2001) and Rosa26-lacZ (Soriano, 1999) for lineage analysis.
Chicken embryo manipulation
Chicken embryos (Chew’s Agriculture, Singapore; Shiroyama Farm, Kanagawa, Japan) used for lineage analysis were cultured by a modification of the cornish pasty method (Nagai et al., 2011). Two embryos were joined together, forming a flat disc, but only one embryo was DiI or DiO labeled. The DiI-labeled embryo was immobilized by the addition of 0.1% Bacto Agar and cultured at the air/medium interface to increase the survival of the labeled embryos. After 2 days of culture, the embryos reached HH16-18, similar to in ovo development. DiI labeling was performed using a PLI-100 microinjector (Harvard Apparatus). DiI was used at 1 mg/ml in dimethylformamide for lineage tracing.
Chicken embryos were cultured dorsal side upward on agarose dishes using the paper culture method (Chapman et al., 2001). A small hole was made in the vitelline membrane and the non-neural ectoderm of the neural fold was labeled with DiI. Time-lapse video microscopy was carried out using an Olympus BX51W1 microscope fitted with an incubator box.
Histology and immunohistochemistry
Embryos were fixed in 4% paraformaldehyde, washed with PBS, equilibrated in 30% sucrose and embedded in Tissue-Tek OCT compound (Sakura Finetek). Sectioning was performed on a Leica CM1900 cryostat. Immunohistochemistry was performed as described (Breau et al., 2008). Images were acquired using a Zeiss LSM700 or an Olympus FV1000 confocal microscope.
Immunohistochemistry was performed using: goat anti-Sox1 (1:100, AF3369, R&D Systems) and rat anti-N-cadherin (1:100, 205605, NCD2, Calbiochem); rabbit anti-Sox9 (1:1000, AB5535, Millipore); rat anti-E-cadherin (1:100, U3254, Decma, Sigma); goat anti-L-CAM [1:400, chicken E-cadherin (Thiery et al., 1984)]; rabbit anti-Snail2 (1:100, #9585, Cell Signaling Technology); mouse anti-RhoB (56.4H7), mouse anti-Islet1/2 (39.4D5), mouse anti-Pax3 (Pax3) and mouse anti-Tfap2a (3B5) (1:100, DSHB); chicken anti-GFP (1:500, ab13970, Abcam); mouse anti-β-dystroglycan (1:100, B-DG-CE, Novocastro Laboratories); mouse anti-HuC/D (A21271) and mouse anti-ZO-1 (339100) (1:100, Invitrogen); and rat anti-laminin α1 (1:200, MAB1905, Chemicon). RhoB immunostaining was performed as described (Liu and Jessell, 1998). Quantification of images was carried out using Fiji (Schindelin et al., 2012) (supplementary material Fig. S1).
For lacZ staining of skull preparations, X-gal staining was performed as described (Pietri et al., 2004).
Fluorescent in situ hybridization (FISH)
E-cadherin FISH in chicken embryos was performed using the Fluorescein Tyramide Signal Amplification Kit (Perkin Elmer). Chicken E-cadherin probe (provided by J. L. Duband, University Pierre and Marie Curie-CNRS, Paris, France) was used at 75 ng/ml, with hybridization performed at 70°C. After FISH, the chicken embryos were sectioned and immunostained for Snail2.
Supplementary Material
Acknowledgments
The antibodies developed by T. M. Jessell and S. Brenner-Morton (RhoB and Islet1/2), C. P. Ordal (Pax3) and T. William (Tfap2a) were obtained from the Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242, USA. We thank Warren Gallin for providing chicken E-cadherin antibody, Shin-Ichi Nishikawa for the Sox1-Cre mouse, Jean-Loup Duband for providing chicken E-cadherin in situ probe, and Thomas J. Carney and Rebecca Jackson for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
R.T.H.L., H.N. and Y.N. performed the experiments. R.T.H.L., G.S. and P.A.T. analyzed the data. R.T.H.L., J.A.W. and J.P.T. conceived the study and wrote the manuscript.
Funding
This work was supported by the Biomedical Research Council of A*STAR (Agency for Science, Technology and Research), Singapore. Research in the P.A.T. laboratory was supported by the Stowers Institute for Medical Research and the National Institute for Dental and Craniofacial Research [DE 016082]. Work in the Laboratory for Early Embryogenesis was supported by Riken. Deposited in PMC for immediate release.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.094680/-/DC1
References
- Abzhanov A., Tzahor E., Lassar A. B., Tabin C. J. (2003). Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 130, 4567–4579 [DOI] [PubMed] [Google Scholar]
- Akiyama H., Kim J.-E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T., et al. (2005). Osteochondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. V., Bronner-Fraser M., Le Douarin N. M., Teillet M. A. (1997). Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development 124, 3077–3087 [DOI] [PubMed] [Google Scholar]
- Beard J. (1888). Memoirs: morphological studies. II. The Development of the Peripheral Nervous System of Vertebrates (part I. Elassmobranchii and Aves). Q. J. Microsc. Sci. s2-s29, 153–228 [Google Scholar]
- Blentic A., Tandon P., Payton S., Walshe J., Carney T. J., Kelsh R. N., Mason I., Graham A. (2008). The emergence of ectomesenchyme. Dev. Dyn. 237, 592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau M. A., Pietri T., Stemmler M. P., Thiery J. P., Weston J. A. (2008). A nonneural epithelial domain of embryonic cranial neural folds gives rise to ectomesenchyme. Proc. Natl. Acad. Sci. USA 105, 7750–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns M. R., Afman L. A., Vanhauten B. A. M., Hekking J. W. M., Köhler E. S., van Straaten H. W. M. (2005). Morphogenetic movements during cranial neural tube closure in the chick embryo and the effect of homocysteine. Anat. Embryol. (Berl.) 210, 81–90 [DOI] [PubMed] [Google Scholar]
- Carver E. A., Jiang R., Lan Y., Oram K. F., Gridley T. (2001). The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol. 21, 8184–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. Y., Tam P. P. L. (1988). A morphological and experimental study of the mesencephalic neural crest cells in the mouse embryo using wheat germ agglutinin-gold conjugate as the cell marker. Development 102, 427–442 [DOI] [PubMed] [Google Scholar]
- Chapman S. C., Collignon J., Schoenwolf G. C., Lumsden A. (2001). Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 220, 284–289 [DOI] [PubMed] [Google Scholar]
- Clark K., Bender G., Murray B. P., Panfilio K., Cook S., Davis R., Murnen K., Tuan R. S., Gilbert S. F. (2001). Evidence for the neural crest origin of turtle plastron bones. Genesis 31, 111–117 [DOI] [PubMed] [Google Scholar]
- Dady A., Blavet C., Duband J.-L. (2012). Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev. Dyn. 241, 1333–1349 [DOI] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- Del Barrio M. G., Nieto M. A. (2004). Relative expression of Slug, RhoB, and HNK-1 in the cranial neural crest of the early chicken embryo. Dev. Dyn. 229, 136–139 [DOI] [PubMed] [Google Scholar]
- Dupin E., Calloni G. W., Real C., Gonçalves-Trentin A., Le Douarin N. M. (2007). Neural crest progenitors and stem cells. C. R. Biol. 330, 521–529 [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gallin W. J., Delouvée A., Cunningham B. A., Thiery J. P. (1983). Early epochal maps of two different cell adhesion molecules. Proc. Natl. Acad. Sci. USA 80, 4384–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka K. A., Gitler A. D., Zhang M., Zhou D. D., High F. A., Epstein J. A. (2005). Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev. Biol. 280, 396–406 [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Goins T. L. (1995). Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development 121, 915–924 [DOI] [PubMed] [Google Scholar]
- Hatta K., Takeichi M. (1986). Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320, 447–449 [DOI] [PubMed] [Google Scholar]
- Henion P. D., Weston J. A. (1997). Timing and pattern of cell fate restrictions in the neural crest lineage. Development 124, 4351–4359 [DOI] [PubMed] [Google Scholar]
- Hill J. P., Watson K. M. (1958). The early development of the brain in marsupials; preliminary communication. J. Anat. 92, 493–497 [PMC free article] [PubMed] [Google Scholar]
- Hörstadius S. (1950). The Neural Crest; its Properties and Derivatives in the Light of Experimental Research. London, UK: Oxford University Press; [Google Scholar]
- Johnston M. C. (1966). A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat. Rec. 156, 143–155 [DOI] [PubMed] [Google Scholar]
- Krispin S., Nitzan E., Kassem Y., Kalcheim C. (2010). Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 137, 585–595 [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., Hermans-Borgmeyer I., Wegner M. (1998). Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 18, 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landacre F. L. (1921). The fate of the neural crest in the head of the urodeles. J. Comp. Neurol. 33, 1–43 [Google Scholar]
- Le Douarin N. M., Kalcheim C. (1999). The Neural Crest. Cambridge, UK: Cambridge University Press; [Google Scholar]
- Le Douarin N. M., Teillet M. A. (1974). Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 41, 162–184 [DOI] [PubMed] [Google Scholar]
- Lee R. T., Thiery J. P., Carney T. J. (2013). Dermal fin rays and scales derive from mesoderm, not neural crest. Curr. Biol. 23, R336–R337 [DOI] [PubMed] [Google Scholar]
- Liu J. P., Jessell T. M. (1998). A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125, 5055–5067 [DOI] [PubMed] [Google Scholar]
- Lumsden A. G. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103 Suppl, 155–169 [DOI] [PubMed] [Google Scholar]
- Lumsden A., Sprawson N., Graham A. (1991). Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113, 1281–1291 [DOI] [PubMed] [Google Scholar]
- Marshall A. (1879). The morphology of the vertebrate olfactory organ. Q. J. Microsc. Sci. 19, 300–340 [Google Scholar]
- McGonnell I. M., Graham A. (2002). Trunk neural crest has skeletogenic potential. Curr. Biol. 12, 767–771 [DOI] [PubMed] [Google Scholar]
- McKinney M. C., Fukatsu K., Morrison J., McLennan R., Bronner M. E., Kulesa P. M. (2013). Evidence for dynamic rearrangements but lack of fate or position restrictions in premigratory avian trunk neural crest. Development 140, 820–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarcik J. C., Golden J. A. (2003). AP-2 and HNK-1 define distinct populations of cranial neural crest cells. Orthod. Craniofac. Res. 6, 210–219 [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Timmons P. M., Hébert J. M., Rigby P. W., Tjian R. (1991). Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 5, 105–119 [DOI] [PubMed] [Google Scholar]
- Mongera A., Singh A. P., Levesque M. P., Chen Y.-Y., Konstantinidis P., Nüsslein-Volhard C. (2013). Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development 140, 916–925 [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y., Akiyama H., Rowitch D. H., de Crombrugghe B. (2003). Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA 100, 9360–9365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B., DelConte C., García-Castro M. I. (2012). Pax7 lineage contributions to the mammalian neural crest. PLoS ONE 7, e41089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Lin M.-C., Sheng G. (2011). A modified cornish pasty method for ex ovo culture of the chick embryo. Genesis 49, 46–52 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Ayer-le Lievre C. S. (1982). Mesectodermal capabilities of the trunk neural crest of birds. J. Embryol. Exp. Morphol. 70, 1–18 [PubMed] [Google Scholar]
- Nichols D. H. (1981). Neural crest formation in the head of the mouse embryo as observed using a new histological technique. J. Embryol. Exp. Morphol. 64, 105–120 [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835–839 [DOI] [PubMed] [Google Scholar]
- Nishiguchi S., Wood H., Kondoh H., Lovell-Badge R., Episkopou V. (1998). Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12, 776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan E., Krispin S., Pfaltzgraff E. R., Klar A., Labosky P. A., Kalcheim C. (2013). A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development 140, 2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Takeichi M. (1986). A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J. Cell Biol. 103, 2649–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi-Yamashita N., Ninomiya Y., Doi H., Eto K. (1994). The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev. Biol. 164, 409–419 [DOI] [PubMed] [Google Scholar]
- Pevny L. H., Sockanathan S., Placzek M., Lovell-Badge R. (1998). A role for SOX1 in neural determination. Development 125, 1967–1978 [DOI] [PubMed] [Google Scholar]
- Pietri T., Eder O., Blanche M., Thiery J. P., Dufour S. (2003). The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev. Biol. 259, 176–187 [DOI] [PubMed] [Google Scholar]
- Pietri T., Eder O., Breau M. A., Topilko P., Blanche M., Brakebusch C., Fässler R., Thiery J. P., Dufour S. (2004). Conditional beta1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development 131, 3871–3883 [DOI] [PubMed] [Google Scholar]
- Platt J. B. (1893). Ectodermic origin of the cartilages of the head. Anat. Anz. 8, 506–509 [Google Scholar]
- Raible D. W., Eisen J. S. (1994). Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development 120, 495–503 [DOI] [PubMed] [Google Scholar]
- Raven C. P. (1936). Zur Entwicklung der Ganglienleiste. V. Über die Differenzierung des Rumpfganglienleistenmaterials. Wilhelm Roux Arch. Entwickl. Mech. Org. 134, 122–146 [DOI] [PubMed] [Google Scholar]
- Remboutsika E., Elkouris M., Iulianella A., Andoniadou C. L., Poulou M., Mitsiadis T. A., Trainor P. A., Lovell-Badge R. (2011). Flexibility of neural stem cells. Front. Physiol. 2, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T. F., Kimmel C. B. (1994). Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120, 483–494 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoval I., Kalcheim C. (2012). Antagonistic activities of Rho and Rac GTPases underlie the transition from neural crest delamination to migration. Dev. Dyn. 241, 1155–1168 [DOI] [PubMed] [Google Scholar]
- Smith M. M., Hall B. K. (1990). Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol. Rev. Camb. Philos. Soc. 65, 277–373 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y., Era T., Nakao K., Kondo S., Kasuga M., Smith A. G., Nishikawa S.-I. (2007). Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129, 1377–1388 [DOI] [PubMed] [Google Scholar]
- Theiler K. (1989). The House Mouse: Atlas of Embryonic Development. New York, NY, USA: Springer Verlag; [Google Scholar]
- Thiery J. P., Delouvée A., Gallin W. J., Cunningham B. A., Edelman G. M. (1984). Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev. Biol. 102, 61–78 [DOI] [PubMed] [Google Scholar]
- Vaglia J. L., Smith K. K. (2003). Early differentiation and migration of cranial neural crest in the opossum, Monodelphis domestica. Evol. Dev. 5, 121–135 [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y., Endo Y., Osumi N., Weston J. A. (2004). Multiple roles of Sox2, an HMG-box transcription factor in avian neural crest development. Dev. Dyn. 229, 74–86 [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B., Weston J. A. (1995). Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development 121, 731–742 [DOI] [PubMed] [Google Scholar]
- Weston J. A. (1963). A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 6, 279–310 [DOI] [PubMed] [Google Scholar]
- Weston J. A., Yoshida H., Robinson V., Nishikawa S., Fraser S. T., Nishikawa S. (2004). Neural crest and the origin of ectomesenchyme: neural fold heterogeneity suggests an alternative hypothesis. Dev. Dyn. 229, 118–130 [DOI] [PubMed] [Google Scholar]
- Wilson Y. M., Richards K. L., Ford-Perriss M. L., Panthier J.-J., Murphy M. (2004). Neural crest cell lineage segregation in the mouse neural tube. Development 131, 6153–6162 [DOI] [PubMed] [Google Scholar]
- Wood H. B., Episkopou V. (1999). Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86, 197–201 [DOI] [PubMed] [Google Scholar]
- Wright E., Hargrave M. R., Christiansen J., Cooper L., Kun J., Evans T., Gangadharan U., Greenfield A., Koopman P. (1995). The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 9, 15–20 [DOI] [PubMed] [Google Scholar]
- Xu H., Dude C. M., Baker C. V. H. (2008). Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev. Biol. 317, 174–186 [DOI] [PubMed] [Google Scholar]
- Zirlinger M., Lo L., McMahon J., McMahon A. P., Anderson D. J. (2002). Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc. Natl. Acad. Sci. USA 99, 8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


