Abstract
Honey bees can form distinct spatiotemporal memories that allow them to return repeatedly to different food sources at different times of day. Although it is becoming increasingly clear that different behavioral states are associated with different profiles of brain gene expression, it is not known whether this relationship extends to states that are as dynamic and specific as those associated with foraging-related spatiotemporal memories. We tested this hypothesis by training different groups of foragers from the same colony to collect sucrose solution from one of two artificial feeders; each feeder was in a different location and had sucrose available at a different time, either in the morning or afternoon. Bees from both training groups were collected at both the morning and afternoon training times to result in one set of bees that was undergoing stereotypical food anticipatory behavior and another that was inactive for each time of day. Between the two groups with the different spatiotemporal memories, microarray analysis revealed that 1329 genes were differentially expressed in the brains of honey bees. Many of these genes also varied with time of day, time of training or state of food anticipation. Some of these genes are known to be involved in a variety of biological processes, including metabolism and behavior. These results indicate that distinct spatiotemporal foraging memories in honey bees are associated with distinct neurogenomic signatures, and the decomposition of these signatures into sets of genes that are also influenced by time or activity state hints at the modular composition of this complex neurogenomic phenotype.
Keywords: circadian, appetitive behavior, honey bee, memory, microarray
INTRODUCTION
Honey bees (Apis mellifera) have the remarkable ability to form and use spatiotemporal memories in order to direct their foraging behavior. This is adaptive because different species of flowering plants bloom at different times of day (Linnaeus, 1751), and nectar and pollen are available for pollinators only during discrete, but consistent, windows of time (Gimenes et al., 1993; Doorn and Meeteren, 2003; Matile, 2006). Honey bees can learn not only where to fly to collect food, but also when to do so (Beling, 1929; Wahl, 1932). This ability is based, in part, on an endogenous time-keeping mechanism (Beling, 1929; Renner, 1957).
Although much is known about the molecular basis of basic clock function in animals, including honey bees (Ruben et al., 2006), less is known about the molecular mechanisms underlying food-related activity rhythms. Like honey bees, mice and other animals can be trained to forage at specific times of day, resulting in rhythmic anticipatory appetitive behavior (reviewed by Mistlberger, 2009). It now appears that clocks in various parts of the brain (Angeles-Castellanos et al., 2007) and the liver (Vollmers et al., 2009) are necessary for circadian food anticipatory activity in mammals. The molecular basis of food anticipatory states has yet to be elucidated in insects, perhaps because it has not been possible to train Drosophila to anticipate food availability in a circadian manner (Oishi et al., 2004). Honey bees are thus an attractive organism for such studies.
Although it is becoming increasingly clear that different behavioral states are associated with different profiles of brain gene expression (Robinson et al., 2008), it is not known whether this relationship extends to states that are as dynamic and specific as those associated with foraging-related spatiotemporal memories. Transcriptional profiles in honey bee brains have been shown to be strongly correlated with certain behaviors such as nursing (brood care) and foraging (Whitfield et al., 2003; Alaux et al., 2009a; Alaux et al., 2009b). The duration of the nursing and foraging states in honey bees is 1 week or longer, and comparisons between the brains of nurses and foragers revealed large gene expression differences [∼20 to 40% of transcripts tested in Alaux et al. (Alaux et al., 2009b) and Whitfield et al. (Whitfield et al., 2003), respectively]. Other behaviors that are more transient in duration, such as guarding the hive, comb building and undertaking (the removal of dead bees from the colony), each last for only a day or two and are associated with fewer differences in gene expression (Cash et al., 2005). These findings, plus the known influences of gene expression on rhythmic behavior (Allada and Chung, 2010) and various forms of learned behavior (Barrett and Wood, 2008) in other organisms, led us to hypothesize that transcriptional differences exist between bees trained to have distinct foraging-related spatiotemporal memories.
MATERIALS AND METHODS
We trained different bees from the same hive to forage at different times of day, at different locations and with different scents. Our experimental design enabled us to search separately for gene expression patterns associated with differences in activity state, time of day or specific spatiotemporal memories.
Bees, training and collection
Two unrelated typical colonies of European honey bees (Apis mellifera Linnaeus 1758, a mixture of European subspecies, largely ligustica) were each placed into a clear plastic-walled observation hive (97×96 cm) with eight frames of honeycombs. Each colony was headed by a naturally mated queen and was composed of ∼12,000 workers at the start of the study. The hives were kept at the former Marine Corps Armory site, warrant of East Tennessee State University, Johnson City, TN, USA. Each hive was kept in a small wooden shed to allow for observations without exposing the colony to direct sunlight, and the bees were allowed to adjust to the new environment for at least 1 week before training commenced. The sides of the hive were constructed of 16 clear plastic windows that could be opened to allow for the collection of bees with minimal disturbance to the colony. Two replicates of the experiment were performed, one for each colony, from 14 to 19 and 21 to 28 August 2007. Over the duration of the experiment, several different species of wildflowers were in bloom at the study site.
The field component of this study consisted of three phases: orientation, training and collection. The orientation phase lasted 1 or 2 days, during which the bees learned the location of the artificial feeder. Filter paper soaked with sucrose solution was used to lure bees out of the hive and to transfer them from the hive entrance to a feeder located 1 m away. The feeder consisted of a 96-well tissue culture plate that was filled with 2 mol l–1 sucrose solution and placed on a piece of scented filter paper disk situated on top of a short (40 cm) table. Once a steady number of bees (∼5 min–1) were observed flying to the feeder on their own, the filter paper transfer was stopped and the feeder was moved several meters away from the hive. When the foragers again found the feeder at a steady rate, the feeder was moved again several more meters away. This procedure was repeated until the feeder reached the desired training location, ∼120 m away from the hive. Training was performed at two different times of day, once in the morning from 09:00 to 10:15 h and again in the afternoon from 17:00 to 18:15 h. At the end of each training period, the feeder was emptied and washed, and the filter paper was replaced with a new unscented sheet. Different locations and different scents were used for each of the two training times, morning and afternoon. Essential oil extracts of lilac and lavender flowers were used for scents in the morning and afternoon, respectively.
The training phase consisted of adding scent and sucrose solution to the feeder during the training time and marking – with an individual identifying paint code (see von Frisch, 1967) – foragers that were recruited to the training location by the trained bees from the orientation phase. Their time of arrival was recorded for each foraging trip. Although most bees displayed fidelity to a single training time, some did not; any bee bearing the paint code indicating that she had obtained a food reward at the other training time was removed to prevent cross-recruitment based on residual smells. Training continued for 4–7 days until there were a sufficient number (N≈40) of trained bees at both training times.
On collection day, the feeder was left empty and unscented to ensure that any gene expression differences were a result of memory and not recent experience with the food source. All bees used for molecular analysis were collected from the hive, 15–45 min before the onset of the training time. The bees were collected directly off the combs to help prevent confounding effects associated with the energy expenditure from flying to the feeder. Once focal bees were located and identified by their paint code, the windows of the observation hive were flipped up and soft forceps were used to collect the bees directly into liquid nitrogen to flash-freeze the transcriptional profile. Both ‘anticipating bees’ (bees whose training time was soon approaching) and ‘inactive bees’ (bees trained to the other time) were collected simultaneously out of the hive at both times, resulting in four experimental groups (Fig. 1).
Fig. 1.
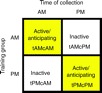
Experimental design and the four behavioral groups analyzed in this study. Two groups of honey bees (Apis mellifera) from the same colony were trained to forage at an artificial feeder either in the morning (tAM) or in the afternoon (tPM). Bees from each training group were collected just prior to the onset of each training time, in the morning (cAM) or afternoon (cPM). Bees collected just before their training time demonstrated stereotypical anticipatory behavior that precedes flying to a food source whereas bees collected outside of their training time were inactive.
Anticipating bees were collected near the entrance of the hive, as described by Körner (Körner, 1939) and Moore et al. (Moore et al., 1989). These bees monitor returning foragers for scents or dances that indicate that the food source to which they are trained has already become available. If left undisturbed, these bees would have eventually flown to the feeding location even without exposure to other cues (von Frisch, 1967). Inactive bees were collected farther away from the hive entrance in areas associated with honey storage. These bees were not observed performing other behaviors and appeared to be in a sleep-like state, as described by previous investigators (von Frisch, 1967; Kaiser and Steiner-Kaiser, 1983). The four behavioral groups analyzed in this study were: morning-trained, morning-collected (tAM-cAM); morning-trained, afternoon-collected (tAM-cPM); afternoon-trained, afternoon-collected (tPM-cPM); and afternoon-trained, morning-collected (tPM-cAM).
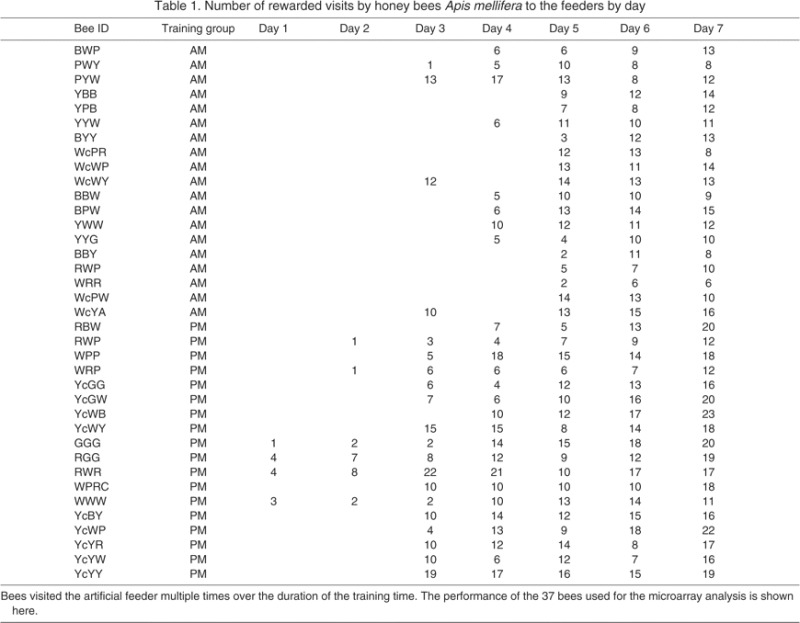
Bees representing these four groups were collected for microarray analysis if they received at least 3 days of training. Furthermore, each bee included in the microarray analysis (N=76) flew to the feeder and received a food reward at least two, six and six times on the last 3 days of training, respectively. This criterion identified the most highly trained and motivated bees. Experiments performed at the same study site in previous years (Moore and Doherty, 2009) (B.N.V.N., A. E. Wagner and D.M., unpublished) demonstrated that >90% of bees with this level of training will fly to the empty, unscented feeder at the appropriate time on the following day. Of the 76 bees identified according to this criterion, we selected the 12 bees from each of the four experimental groups (six from each replicate, two replicates) with the most visits to the feeder during the training phase (N=48) for microarray analysis. Table 1 shows the distribution of visits by bees during the training phase, for all bees that were selected for microarray analysis.
Table 1.
Number of rewarded visits by honey bees Apis mellifera to the feeders by day
Microarray analysis
The heads of the bees were freeze-dried (–80°C and 40 Pa) and the brains dissected out while submerged in dry ice/ethanol (see Schulz and Robinson, 1999). The brains were homogenized and nucleic acids were extracted using phenol/chloroform and RNeasy kits (Qiagen, Valencia, CA, USA). Then samples were treated with DNase (Qiagen). From the resulting RNA, 500 ng was amplified using MessageAmpII kits (Ambion/Applied Biosystems, Austin, TX, USA). Amplified RNA was labeled with Cy3 or Cy5 dyes using a Kreatech ULS labeling system (Amsterdam, The Netherlands). The 48 individuals were hybridized in pairs with a loop design that used 113 microarrays (printed by W. M. Keck Center for Comparative and Functional Genomics, University of Illinois Urbana-Champaign, Urbana, IL, USA). The microarrays contained 13,440 distinct 70-mer oligonucleotides based on information from the honey bee genome (Honeybee Genome Sequencing Consortium, 2006). The oligonucleotide sequences were chosen based on predicted gene models and expressed sequence tags, and each was spotted twice on the array. This second-generation honey bee microarray was characterized by Alaux et al. (Alaux et al., 2009b), and from this point on, each representative sequence will be referred to as a ‘gene’. Hybridized arrays were scanned and analyzed using Axon GenePix 6.0 (Molecular Devices, Sunnyvale, CA, USA) software. As in Sen Sarma et al. (Sen Sarma et al., 2010), spots flagged with ‘–100’ by Axon GenePix 6.0 were removed from the analysis and genes that are highly expressed in the hypophgaryngeal glands were also excluded because of the risk of contamination during brain dissection (Alaux et al., 2009a). The remaining data were filtered using the median of control elements for microarray and dye as the threshold. LOWESS normalization was carried out on the log2-transformed intensities, and the measurements were then adjusted for global dye and microarray effects. A total of 8209 genes remained after these exclusions, representing the number of genes in the brain expressed above threshold for all individuals.
Statistical analysis of microarray results
A linear mixed-effects model was used to analyze the normalized log2-transformed fluorescent intensities for each gene (Alaux et al., 2009a; Alaux et al., 2009b). The model accounted for the effects of dye, microarray, individual sample and source colony. Two-way ANOVA with post hoc contrasts was used to determine significant differences based on time of collection, training group and activity state.
Principal component and hierarchical clustering analyses of the gene expression values showed that 11 individuals, relatively evenly distributed across all four experimental groups, were clustered together. Investigation revealed that the RNA amplification of all 11 of these individuals took place on two sequential days, and the affected 11 yielded the lowest final quantity of RNA on those days. For this reason, these 11 individuals and the corresponding 37 arrays they were measured with were dropped and the ANOVA was rerun. Only results of this reanalysis are reported here. Genes showing differential expression at a false discovery rate (FDR)-corrected P-value of <0.01 were considered significant.
Genes identified as differentially expressed in this experiment were compared with those from a previously published study (Alaux et al., 2009b) to probe for similarities and differences across different behavioral states. Gene list overlap analysis was performed using an exact hypergeometric probability test (one-tailed) in Microsoft Excel (Redmond, WA, USA). As in Alaux et al. (Alaux et al., 2009a), the representation factor (rf) was calculated as the number of observed overlapping genes divided by the expected number; the expected number of overlapping genes is defined as the product of the number of genes differentially expressed in each list, divided by the total number of genes analyzed.
Functional insights into differentially expressed genes were obtained by conducting a gene ontology (GO) term enrichment analysis. This analysis was performed using the FlyBase identification number representing the best BLAST hit for each honey bee gene (Honeybee Genome Sequencing Consortium, 2006) and the DAVID Bioinformatics Resources Functional Annotation tool (Huang et al., 2009). GO terms returned by DAVID with a modified Fisher's exact P-value <0.05 were considered significantly enriched.
Quantitative reverse-transcriptase PCR analysis and array validation
Quantitative reverse-transcriptase PCR (qRT-PCR) was used to confirm some of the results obtained from microarray analysis. Twenty-four genes were selected for validation based on significance, fold change and functions of interest (supplementary material Fig. S1). Two hundred nanograms of the original unamplified RNA from all original 76 individuals in the study were used to synthesize cDNAs using ArrayScript (Ambion) reverse transcriptase. qRT-PCR was performed in triplicate 10 μl reactions in 384-well plates using PerfeCTa SYBR Green Fastmix (Quanta Biosystems, Gaithersburg, MD, USA). Reactions not within 0.5 Ct (cycle threshold) agreement with the others were discounted. The results were analyzed by standard weighted means two-way ANOVA with post hoc contrasts using SAS (SAS Institute Inc., Cary, NC, USA). Agreement of gene expression results between microarray and qRT-PCR analysis was tested using standardized data (‘standardize’ procedure in SAS, mean=0, s.d.=1), which were then analyzed using linear discriminant analysis (‘lda’ function) in the MASS package (Venables and Ripley, 2002) for R (R Development Core Team, 2006).
RESULTS
Behavioral evidence for spatiotemporal memories
Over the course of the two replicates, 184 trained foragers were marked individually and followed throughout the experiment. Of these bees, 28 were captured upon their arrival at the wrong feeding station, i.e. the one to which they were not initially trained; they were removed from the study. Thus, the majority (85%) of the time-trained bees specialized and foraged exclusively at one training time or the other, as expected (Kleber, 1935; Moore et al., 1989). Twenty-five bees (14%) that were marked and trained did not appear on the final training day and were not found during the collections from the hive. These disappearances represent natural mortality expected among forager honey bees (Winston, 1987).
Honey bees clearly exhibited food anticipatory behavior over the course of the experiment. Outside of the training time, trained bees were typically found scattered in areas of the hive away from the entrance; however, as the training time approached, the trained bees began to cluster near the entrance and dance floor. Furthermore, on training days, bees frequently flew to the feeder before food or scent had been applied for that day. The frequency of these reconnaissance flights increased as the training time approached (Fig. 2). As previously reported (Moore and Rankin, 1983), afternoon-trained bees exhibited anticipatory behavior for a longer period of time before the onset of the training time than morning-trained bees.
Fig. 2.
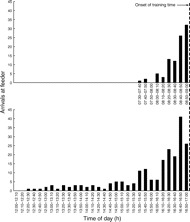
Anticipatory behavior in honey bees. Trained bees frequently flew to the feeder in anticipation of food availability, and the frequency of these flights increased as the training time approached. Data are from 18 Aug 2007, the day prior to the day of collection for the first replicate of the experiment.
Brain gene expression differences associated with time of collection
To determine whether there were genes showing brain expression differences associated with time of collection, independent of time of training, we used two-way ANOVA to contrast the gene expression values for tAM-cAM and tPM-cAM with tAM-cPM and tPM-cPM bees (Fig. 1). There were 798 genes showing differences in brain gene expression between morning- and afternoon-collected bees (FDR<0.01). This represents ∼10% of the genes on the microarray analyzed in this study.
GO analysis revealed 80 categories significantly enriched (modified Fisher's exact test, P<0.05) for genes with differences in brain gene expression associated with time of day (supplementary material Table S1). Among enriched terms for genes with higher expression in the morning-collected bees were ‘organelle ATP synthesis coupled electron transport’ and numerous mitochondria-associated terms (supplementary material Table S2). There were also multiple terms associated with ribosomes and translation as well as proteasomes and catabolic processes. Enriched terms for genes more highly expressed in afternoon-collected bees included ‘cell adhesion’, ‘lipid metabolic process’, ‘lipid transport’ and ‘protein localization’ (supplementary material Table S3). Terms associated with circadian behavior – such as ‘circadian rhythm’, ‘rhythmic behavior’, ‘locomotory behavior’ and ‘sleep’ – all appear exclusively for the list of genes with higher expression in the afternoon.
Brain gene expression differences associated with time of training
To determine whether there were genes showing brain expression differences associated with time of training, independent of time of collection, we contrasted tAM-cAM and tAM-cPM with tPM-cAM and tPM-cPM bees. Relative to other categories, fewer genes (229) were found to be differentially expressed between the two groups of bees trained to forage either in the morning or afternoon, regardless of whether they were collected in an anticipatory or inactive state (FDR<0.01). GO analysis showed an enrichment of upregulated genes involved with carbohydrate catabolic processes and kinase regulator activity in the morning-trained groups (supplementary material Tables S4, S5). Afternoon-trained groups showed enrichment for genes associated with ‘response to heat’ and ‘oxidoreductase activity, acting on the CH-CH group of donors’ (supplementary material Table S6). Among the genes showing time of training differences were foraging and clock, both of which had higher expression in morning-trained bees.
Brain gene expression differences associated with state of food anticipation
To determine whether there were genes showing brain expression differences associated with food anticipatory state, independent of time of training or collection, we contrasted tAM-cAM and tPM-cPM with tAM-cPM and tPM-cAM bees. Bees collected 15–45 min prior to their time of training were actively anticipating food availability and preparing for flight whereas bees trained to the other time were inactive. This difference in activity state was associated with a very large number of genes: 2028 differentially expressed brain transcripts or ∼25% of all genes on the microarray analyzed in this study.
Genes upregulated in anticipating bees showed significant GO enrichment for many categories associated with nervous system function, including ‘cation transport’, ‘transmission of nerve impulse’, ‘neurotransmitter secretion’ and ‘synaptic transmission’ (supplementary material Tables S7, S8). Behavioral categories were also present, including ‘rhythmic process’ and ‘circadian behavior’, as well as ‘response to chemical stimulus’, ‘response to light stimulus’ and ‘memory’, even though the bees had not recently encountered the environment outside the hive. ankyrin, GB16541, the heat shock proteins hsp8 and hsp90a, and the insulin receptor inR were all confirmed as correlated with the state of food anticipation via qRT-PCR (see Validation of microarray results with qRT-PCR).
Inactive bees showed a remarkably strong signature of ribosome-related genes in their transcriptional profile (supplementary material Table S9). In all, 99 genes annotated as being associated with the ribonucleoprotein complex were significantly upregulated in inactive bees, with the GO term ‘structural constituent of the ribosome’ having the strongest enrichment found in the entire experiment (modified Fisher's exact test, P=1.07E–46). Other significantly enriched terms include ‘mitochondrial part’, ‘mitochondrial ribosome’, ‘proteasome complex’, ‘DNA-directed RNA polymerase activity’ and ‘spliceosome’. Some of the genes upregulated in inactive bees and confirmed by qRT-PCR include the histone H1 and the canonical clock genes period and cryptochrome (Ruben et al., 2006).
The list of genes differentially expressed between anticipating and inactive groups was compared with a list of genes generated by Alaux et al. (Alaux et al., 2009b) for differences between (non-foraging) nurse and forager honey bees. There was significant overlap for these lists (rf=1.2, P<0.0001), with 66% of genes either up- or down-regulated in a concordant way for both anticipating bees in this study and foragers in Alaux et al. (Alaux et al., 2009b).
Brain gene expression differences associated with distinct spatiotemporal memories
To determine whether there were genes showing brain expression differences associated with distinct spatiotemporal memories, we contrasted tAM-cAM bees with tPM-cPM bees. These two groups were both anticipating food availability, but at a different time of day and for a different location. A total of 1329 genes were differentially expressed in the brains between these two groups (t-test, P<0.01). This large number of genes included many genes that were differentially expressed in the analyses described above. Of the 1329 differentially expressed genes, 624 also varied significantly with time of day, 180 also varied significantly with time of training and 618 also varied significantly with state of food anticipation (Fig. 3). The same pattern of decomposition is reflected in the GO enrichment analysis. For example, terms such as ‘cell adhesion’ and ‘cellular macromolecule catabolic process’ appeared in the analysis of tAM-cAM vs tPM-cPM bees as well as in the time of day analysis (Table 2). GO enrichment also revealed other differences between the tAM-cAM and tPM-cPM bees, including ‘lipid metabolic process’, ‘carbohydrate metabolic process’ and ‘chemosensory behavior’ (supplementary material Tables S10–S12).
Fig. 3.
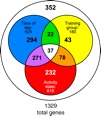
Decomposition of brain gene expression differences associated with distinct spatiotemporal memories in honey bees: effects of time of collection (Time of day), time of training (Training group) and activity state. A total of 1329 genes were differentially expressed between the two active groups with distinct spatiotemporal memories. Expression of a majority of these genes was also influenced by time of day, time of training and/or activity state. Three-hundred fifty-two of the 1329 genes did not have significant differences in expression in these other analyses and represent unique transcriptional signatures associated with a unique spatiotemporal memory.
Table 2.
Selected results of gene ontology (GO) enrichment analysis to identify molecular functions and biological processes that are associated with the lists of genes showing expression differences in association with time of collection, time of training, activity state or differences in spatiotemporal memories
Removing all the genes that varied with time of day, time of training and/or anticipatory state still left 352 genes that showed significant differences in brain expression between tAM-cAM and tPM-cPM bees. These expression differences are associated with other unknown aspects that either contribute to the formation of the two unique spatiotemporal memories or reflect their existence. GO enriched terms for these putative spatiotemporal memory-related genes include ‘extracellular structure organization and biogenesis’ and ‘synaptogenesis’ (supplementary material Tables S13–S15).
Effects of time of day, time of training and anticipatory state on brain expression of clock genes
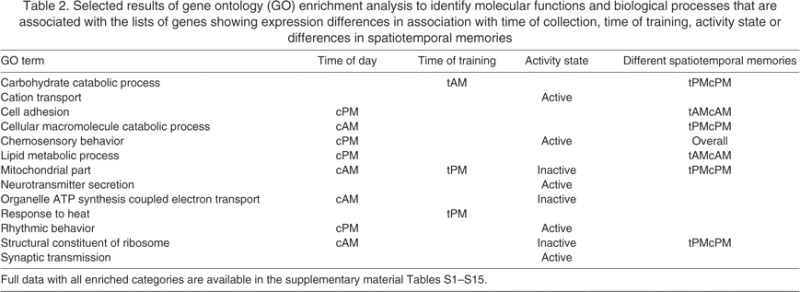
The molecular basis of circadian function involves a small set of interacting transcription factors, some with negative and others with positive feedback effects on each other's expression (Allada and Chung, 2010). cycle (cyc), a gene comprising part of the positive feedback mechanism in the bee's circadian clock, was robustly differentially expressed in terms of time of day of collection (ANOVA, P<0.0001) but was not affected by time of training or activity state (Fig. 5). Another canonical clock gene in the positive feedback mechanism, clock (clk), showed a significant difference based on time of training (P=0.0035) but no effect based on anticipatory state or time of collection; there was higher expression among morning-trained bees, both active and inactive. By contrast, period (per), a gene with negative feedback effects, was differentially expressed as a function of both time of day of collection (P=0.03) and activity state (P<0.0001), but not time of training. cryptochrome (cry), which is also involved in negative feedback regulation of the clock, was differentially expressed based on both time of day of collection (P<0.0001) and activity state (P<0.0001) but not time of training.
Fig. 5.
Expression of canonical clock genes as a function of time training in honey bees. Genes representing the set of transcription factors comprising the negative feedback element of the molecular clock (per, cry and tim2) have expression patterns that are more closely associated with the activity state of the bee rather than time of day. Genes representing the positive feedback element of the molecular clock (cyc and clk) do not show any significant differences based on activity state, although cyc shows clear differences based on the time of day. See supplementary material Table S16 for significance values.
Validation of microarray results with qRT-PCR
qRT-PCR validation of the microarray results was challenging in this study because each gene could show significant expression differences for each of the time of collection, time of training, activity state and/or unique memory analyses. We therefore used linear discriminant analysis (LDA) to examine the gestalt of similarities and differences between the four experimental groups across the two different expression measurement platforms. Expression values for each of the genes, generated by both microarray and qRT-PCR, are found in supplementary material Fig. S1. LDA revealed that overall expression patterns were similar between microarray and qRT-PCR analysis (Fig. 4). The qRT-PCR results showed strong clustering for individuals within the four experimental groups and nice separation of the four groups, with only the data from the 24 genes chosen for validation. This result further reflects the robustness of the differences between the four groups and supports the hypothesis that spatiotemporal foraging rhythms are associated with unique neurogenomic states.
Fig. 4.
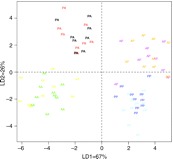
Linear discriminant analysis indicates strong similarities between results obtained with microarrays and qRT-PCR and supports the hypothesis that distinct neurogenomic states exist for each of the four honey bee groups in the experiment. Microarray and qRT-PCR data for the same 37 individuals are shown here. For clarity of presentation, abbreviations were condensed from what appears in Fig. 1: AA, AM (morning) trained, AM collected; AP, AM trained, PM (afternoon) collected; PA, PM trained, AM collected; PP, PM trained, PM collected. Black, orange, yellow and light blue represent the microarray data whereas red, pink, green and dark blue represent the qRT-PCR data.
DISCUSSION
The principal significance of these results is that they demonstrate that the ability of honey bees to form distinct spatiotemporal foraging memories is associated with the formation of distinct neurogenomic signatures of gene expression in the brain. Furthermore, these signatures can be partially decomposed into sets of genes that are influenced by time or activity state, and still others that are not influenced by these variables. This finding hints at the modular composition of complex neurogenomic phenotypes. It is not known which, if any, of these extensive gene expression changes are causal. However, differences in gene expression are known to have causal effects on various forms of both circadian (Allada and Chung, 2010) and learned behavior (Barrett and Wood, 2008) in the laboratory, suggesting that at least some of the changes we detected function to enable bees to form and utilize different spatiotemporal memories during natural foraging behavior.
This study identified a relatively large number of genes that showed differences in expression as a function of time of collection, i.e. genes whose expression varied in the morning vs the afternoon. Ueda et al. (Ueda et al., 2002) reported that a similar number of genes varied in expression as a function of light–dark conditions in Drosophila melanogaster heads. In our study, genes upregulated in the morning included those associated with ATP synthesis, mitochondria and NADH oxioreductase activity, as well as the canonical clock gene cycle (bmal1 in mammals). These findings are consistent with previous reports of a link between the positive feedback element of the molecular clock and the regulation of basic metabolic processes (Eckel-Mahan and Sassone-Corsi, 2009; Ramsey et al., 2009). Genes upregulated in the afternoon included cell adhesion molecules, which have been implicated in the synchronization of circadian oscillators (Shen et al., 1997; Miche and Colwell, 2001) and the regulation of learning and memory (Welzl and Stork, 2003). Perhaps this finding reflects the occurrence of memory consolidation in foragers during the night.
Some genes showed differences associated with the time at which the bee was trained, regardless of whether it was then collected in the morning or afternoon, or was anticipating or inactive. This might reflect either differences in the environment encountered as a result of the different training times or a natural proclivity to respond to training at one time versus another. Regarding the latter, Kraus et al. (Kraus et al., in press) have reported genotypic differences in the preferences of bees to forage either in the morning or afternoon. Among the genes differentially expressed based on the time of training were genes associated with metabolism as well as foraging and clock. Upregulation of foraging has been implicated in increased phototactic behavior in honey bees (Ben-Shahar et al., 2003), and increased phototaxis could make the foragers more likely to respond to the morning feeder (which coincides more closely with sunrise) than the afternoon feeder. Other genetic differences for similar phenomena include polymorphisms in human clock associated with differences in the times at which individuals prefer to either be active or sleeping (Katzenberg et al., 1998).
The largest differences in brain gene expression detected in this study were related to differences in the activity state of the bees, either anticipating flight to a specific food reward or inactive. Bees collected just before their time of training were observed displaying the stereotypical anticipatory behavior that precedes exiting the hive to fly to the feeder. Renner demonstrated that anticipatory behavior in bees arises as a function of an internal circadian clock that allows the bees to time the availability of food (Renner, 1957). Results of GO analyses suggest that mechanisms involved in stimulus perception and response are reactivated in a circadian manner during the anticipatory state, perhaps ‘priming’ the brain for subsequent activity, after over 22 h of inactivity. In addition, GO categories such as ‘cation transport,’ ‘transmission of nerve impulse’ and ‘neurotransmitter secretion’ point toward possible mechanisms that the brain would use to initiate behavior. Mammalian pacemaker neurons found in the suprachiasmatic nucleus generate autonomous circadian activity patterns when isolated (Green and Gillette, 1982), and cation channels play a role in this autoactivation (Meredith et al., 2006; Wang and Huang, 2006; Kononenko and Berezetskaya, 2010).
There was also a significant enrichment of genes encoding the heat shock proteins that are upregulated in anticipating bees. These genes could play a role in preparing foragers for the stresses of foraging (Williams et al., 2008). It is also possible that heat shock proteins play more of a direct role in behavior; knock-down of heat shock proteins affects behavioral rhythmicity in flies, even though the core molecular oscillators are unaffected (Hung et al., 2009).
A large number of the genes upregulated in inactive bees were associated with transcription, translation and proteasome function. Inactive foragers have long been thought to enter into sleep-like states (von Frisch, 1967; Kaiser and Steiner-Kaiser, 1983; Kaiser, 1988; Eban-Rothschild and Bloch, 2008; Klein et al., 2008); this speculation is strengthened by work in Drosophila rigorously demonstrating that insects sleep (Hendricks et al., 2000; Shaw et al., 2000) (reviewed by Harbison et al., 2009). Perhaps inactive bees are showing evidence for the kind of purging of old proteins and building of new proteins that has been associated with sleep in other organisms (reviewed by Mackiewicz et al., 2008).
Further insights into the possible significance of the molecular differences between active and inactive bees were gained by comparing their differences in brain gene expression with the differences between nurse and forager bees (Alaux et al., 2009b). Anticipating bees have a brain expression profile that resembles the forager profile whereas the profile of inactive bees more closely resembles that of the nurse. Among the genes differentially expressed in both contexts is the insulin receptor (inR), which shows higher expression in both foragers compared with nurses and in anticipating foragers compared with inactive foragers. Insulin and other nutritional signaling pathways play a part in the division of labor among honey bee workers (Ament et al., 2008), and RNAi knock-down of the insulin receptor alters honey bee foraging behavior (Wang et al., 2010). These results imply that some of the same genes involved in the regulation of foraging over the lifetime of the bee are also involved in the regulation of daily foraging rhythms.
Genes involved in synaptogenesis are significantly overrepresented in the group of genes that vary with spatiotemporal memory but not with time of training, time of collection or activity state. This is consistent with the idea that different spatiotemporal memories have unique representations in the brain. In addition, the overrepresentation of genes previously shown to be involved in chemosensory behavior perhaps reflects the possibility that some aspects of the olfactory system are differentially utilized in the learning of the unique scents used in the spatiotemporal training.
Some of the genes well known to be involved in the regulation of circadian rhythms in other organisms showed interesting patterns of expression in this study. Expression of per and cry in bees trained in the morning was consistent with patterns reported by Ruben et al. (Ruben et al., 2006) under ad libitum feeding conditions; however, an altered pattern was seen in the afternoon-trained bees. This is perhaps because the time-training procedures used in this experiment uncoupled activity state from time of day, and food acted as a zeitgeber to set elements of the internal clock, which then dictated foraging rhythms on subsequent days. Intriguingly, another clock gene, cyc, did not show this altered expression pattern. The differences observed in the time-trained bees suggest that, in the honey bee, the two sets of molecular regulatory feedback loops have become uncoupled, with the positive element synchronizing gene expression oscillations with day–night cycles whereas the negative element synchronizes expression oscillations with the time of food availability. This is supported by the recent finding that genes involved in insulin signaling are involved in setting the pace of the negative element of the clock in Drosophila (Zheng and Seghal, 2010).
It should be noted that this study was performed using whole brains. The altered pattern of gene expression due to spatiotemporal training that we report here could have occurred only in a subset of neurons, and different neural circuits could have different expression profiles. Previous studies in mice have found that restricted feeding schedules shifted expression of per, but only in specific brain regions (Angeles-Costellanos et al., 2007) (reviewed by Webb et al., 2009). Furthermore, peripheral oscillators in other tissues such as the insect fat body (a metabolic tissue analogous to vertebrate livers) could play a role in behavioral rhythmicity as it relates to feeding (Xu et al., 2008; Vollmers et al., 2009) (reviewed by Escobar et al., 2009). Additional studies that probe the specific neuroanatomical location of the differences in gene expression reported here might help clarify the neurobiological basis of these findings and will contribute to our understanding of learning and memory under natural conditions.
This study is the first to separate genes involved with activity rhythms from genes influenced by time of day in an insect without the confounding effects of sleep deprivation. We identified genes associated with motivation in the form of food anticipation, and we have demonstrated that spatiotemporal training creates neurogenomic profiles that are distinct for each training regime. These findings highlight the surprisingly close relationship between changes in brain gene expression and naturally occurring behavioral plasticity (Robinson et al., 2005; Robinson et al., 2008). A challenge for the future is to learn how time training exerts these effects on the genome and how, in turn, spatiotemporally encoded neurogenomic profiles impact foraging behavior.
Supplementary Material
Acknowledgments
We thank K. S. Pruiett for assistance with the bees, and T. Newman and T. Nguyen for assistance with various molecular techniques.
LIST OF ABBREVIATIONS
- tAM-cAM
a bee trained to the morning feeder and collected in the morning
- tAM-cPM
a bee trained to the morning feeder and collected in the afternoon
- tPM-cAM
a bee trained to the afternoon feeder and collected in the morning
- tPM-cPM
a bee trained to the afternoon feeder and collected in the afternoon
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.053421/-/DC1
We acknowledge funding support from National Science Foundation Frontiers in Biological Research grant BeeSpace (EF 0425852, principal investigator B. Schatz), the Illinois Sociogenomics Initiative and USDA grant 2006-35302-7278 (principal investigator D.M.). Microarray data generated in this study meet Minimum Information About Microarray Experiment (MIAME) standards and are available at ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/) under accession number E-MTAB-489.
REFERENCES
- Alaux C., Sinha S., Hasadsri L., Hunt G. J., Guzman-Novoa E., DeGrandi-Hoffman G., Uribe-Rubio J. L., Southey B. R., Rodriguez-Zas S., Robinson G. E. (2009a). Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl. Acad. Sci. USA 106, 15400-15405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux C., Le Conte Y., Adams H. A., Rodriguez-Zas S., Grozinger C. M., Sinha S., Robinson G. E. (2009b). Regulation of brain gene expression in honey bees by brood pheromone. Genes Brain Behav. 8, 309-319 [DOI] [PubMed] [Google Scholar]
- Allada R., Chung B. Y. (2010). Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 72, 605-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament S. A., Corona M., Pollock H. S., Robinson G. E. (2008). Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 105, 4226-4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Castellanos M., Mendoza J., Escobar C. (2007). Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 144, 344-355 [DOI] [PubMed] [Google Scholar]
- Barrett R. M., Wood M. A. (2008). Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 15, 460-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beling I. (1929). Uber das Zeitgedachtnis der Bienen. Z. Vgl. Physiol. 9, 259-338 [Google Scholar]
- Ben-Shahar Y., Leung H. T., Pak W. L., Sokolowski M. B., Robinson G. E. (2003). cGMP-dependent changes in phototaxis: a possible role for the foraging gene in honey bee division of labor. J. Exp. Biol. 206, 2507-2515 [DOI] [PubMed] [Google Scholar]
- Cash A. C., Whitfield C. W., Ismail N., Robinson G. E. (2005). Behavior and the limits of genomic plasticity: power and replicability in microarray analysis of honey bee brains. Genes Brain Behav. 4, 267-271 [DOI] [PubMed] [Google Scholar]
- Doorn W. G. v., Meeteren U. v. (2003). Flower opening and closure: a review. J. Exp. Bot. 54, 1801-1812 [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A. D., Bloch G. (2008). Differences in the sleep architecture of forager and young honey bees (Apis mellifera). J. Exp. Biol. 211, 2408-2416 [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K., Sassone-Corsi P. (2009). Metabolism control by the circadian clock and vice versa. Nat. Struct. Mol. Biol. 16, 462-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C., Cailotto C., Angeles-Castellanos M., Delgado R. S., Bujis R. M. (2009). Peripheral oscillators: the driving force for food-anticipatory activity. Eur. J. Neurosci. 30, 1665-1675 [DOI] [PubMed] [Google Scholar]
- Gimenes M., Benedito-Silva A. A., Marques M. D. (1993). Chronobiologic aspects of a coadaptive prodess: the interaction of Ludwigia elegans flowers and their more frequent bee visitors. Chronobiol. Int. 10, 20-30 [DOI] [PubMed] [Google Scholar]
- Green D. J., Gillette R. (1982). Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 245, 198-200 [DOI] [PubMed] [Google Scholar]
- Harbison S. T., Mackay T. F., Anholt R. R. (2009). Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 25, 262-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., Sehgal A., Pack A. I. (2000). Rest in Drosophila is a sleep-like state. Neuron 26, 295-298 [DOI] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium (2006). Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443, 931-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44-57 [DOI] [PubMed] [Google Scholar]
- Hung H. C., Kay S. A., Weber F. (2009). HSP90, a capacitor of behavioral variation. J. Biol. Rhythms 24, 183-192 [DOI] [PubMed] [Google Scholar]
- Kaiser W. (1988). Busy bees need rest, too: behavioural and electromyographical sleep signs in honeybees. J. Comp. Physiol. A. 163, 565-584 [Google Scholar]
- Kaiser W., Steiner-Kaiser J. (1983). Neuronal correlates of sleep, wakefulness, and arousal in a diurnal insect. Nature 301, 707-709 [DOI] [PubMed] [Google Scholar]
- Katzenberg D., Young T., Finn L., Lin L., King D. P., Takahashi J. S., Mignot E. (1998). A CLOCK polymorphism associated with human diurnal preference. Sleep 21, 569-576 [DOI] [PubMed] [Google Scholar]
- Kleber E. (1935). Hat das zeitgedachtnis der bienen biologische bedeutung? Z. Vgl. Physiol 22, 221-262 [Google Scholar]
- Klein B. A., Olzsowy K. M., Klein A., Saunders K. M., Seeley T. D. (2008). Caste-dependent sleep of worker honey bees. J. Exp. Biol. 211, 3028-3040 [DOI] [PubMed] [Google Scholar]
- Kononenko N. I., Berezetskaya N. M. (2010). Modeling the spontaneous activity in suprachiasmatic nucleus neurons: role of cation single channels. J. Theor. Biol. 265, 115-125 [DOI] [PubMed] [Google Scholar]
- Körner I. (1939). Zeitgedächtnis und alarmierung bei den Bienen. Z. Vgl. Physiol. 27, 445-459 [Google Scholar]
- Kraus F. B., Gerecke E., Moritz R. F. A. (in press). Shift work has a genetic basis in honeybee pollen foragers. Behav. Genet. (DOI: 10.1007/s10519-010-9382-9). [DOI] [PubMed] [Google Scholar]
- Linnaeus C. (1751). Philosophia Botanica. New York, NY: Oxford University Press; [Google Scholar]
- Mackiewicz M., Naidoo N., Zimmerman J. E., Pack A. I. (2008). Molecular mechanisms of sleep and wakefullness. Ann. N. Y. Acad. Sci. 1129, 335-349 [DOI] [PubMed] [Google Scholar]
- Matile P. (2006). Circadian rhythmicity of nectar secretion in Hoya carnosa. Bot. Helv. 116, 1-7 [Google Scholar]
- Meredith A. L., Wiler S. W., Miller B. H., Takahashi J. S., Fodor A. A., Ruby N. F., Aldrich R. W. (2006). BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat. Neurosci. 9, 1041-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miche S., Colwell C. S. (2001). Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol. Int. 18, 579-600 [DOI] [PubMed] [Google Scholar]
- Mistlberger R. E. (2009). Food-anticipatory circadian rhythms: concepts and methods. Eur. J. Neurosci. 30, 1718-1729 [DOI] [PubMed] [Google Scholar]
- Moore D., Doherty P. (2009). Acquisition of a time-memory in forager honey bees. J. Comp. Physiol. A 195, 741-751 [DOI] [PubMed] [Google Scholar]
- Moore D., Rankin M. A. (1983). Diurnal changes in the accuracy of the honeybee foraging rhythm. Biol. Bull. 164, 471-482 [Google Scholar]
- Moore D., Siegfried D., Wilson R., Rankin M. A. (1989). The influence of time of day on the foraging behavior of the honeybee, Apis mellifera. J. Biol. Rhythms 4, 305-325 [DOI] [PubMed] [Google Scholar]
- Oishi K., Shiota M., Sakamoto K., Kasamatsu M., Ishida N. (2004). Feeding is not a more potent Zeitgeber than the light–dark cycle in Drosophila. NeuroReport 15, 739-743 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2006). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; [Google Scholar]
- Ramsey K. M., Yoshino J., Brace C. S., Abrassart D., Kobayashi Y., Marcheva B., Hong H. K., Chong J. L., Buhr E. D., Lee C., et al. (2009). Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M. (1957). Neue versuche uber den zeitsinn der honigbiene. Z. Vgl. Physiol. 40, 85-118 [Google Scholar]
- Robinson G. E., Grozinger C. M., Whitfield C. W. (2005). Sociogenomics: social life in molecular terms. Nat. Rev. Genet. 6, 257-270 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Fernald R. D., Clayton D. F. (2008). Genes and social behavior. Science 322, 896-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben E. B., Shemesh Y., Cohen M., Elgavish S., Robertson H. M., Bloch G. (2006). Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 16, 1352-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D. J., Robinson G. E. (1999). Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 185, 481-488 [DOI] [PubMed] [Google Scholar]
- Sen Sarma M., Rodriguez-Zas S. L., Gernat T., Nguyen T., Newman T., Robinson G. E. (2010). Distance-responsive genes found in dancing honey bees. Genes Brain Behav. 9, 825-830 [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Cirelli C., Greenspan R. J., Tononi G. (2000). Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834-1837 [DOI] [PubMed] [Google Scholar]
- Shen H., Watanabe M., Tomasiewicz H., Rutishauser U., Magnuson T., Glass J. D. (1997). Role of neural cell adhesion molecule and polysialic acid in mouse circadian clock function. J. Neurosci. 17, 5221-5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H. R., Matsumoto A., Kawamura M., Iino M., Tanimura T., Hashimoto S. (2002). Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J. Biol. Chem. 277, 14048-14052 [DOI] [PubMed] [Google Scholar]
- Venables W. N., Ripley B. D. (2002). Modern Applied Statistics Using S, 4th edition New York, NY: Springer; [Google Scholar]
- Vollmers C., Gill S., DiTacchio L., Pulivarthy S. R., Le H. D., Panda S. (2009). Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 106, 21453-21458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K. (1967). The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press; [Google Scholar]
- Wahl O. (1932). Neue untersuchungen uber das zeitgedachtnis der bienen. Z. Vgl Physiol. 16, 529-589 [Google Scholar]
- Wang Y., Mutti N. S., Ihle K. E., Siegel A., Dolezal A. G., Kaftanoglu O., Amdam G. V. (2010). Down-regulation of honey bee IRS gene biases behavior towards food rich in protein. PLoS Genet. 6, e1000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. C., Huang R. C. (2006). Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J. Neurophysiol. 96, 109-118 [DOI] [PubMed] [Google Scholar]
- Williams J. B., Roberts S. P., Elekonich M. M. (2008). Age and natural metabolically intensive behavior affect oxidative stress and antioxidant mechanisms. Exp. Gerentol. 43, 538-549 [DOI] [PubMed] [Google Scholar]
- Winston M. L. (1987). The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; [Google Scholar]
- Webb I. C., Baltazar R. M., Lehman M. N., Coolen L. M. (2009). Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? Eur. J. Neurosci. 30, 1739-1748 [DOI] [PubMed] [Google Scholar]
- Welzl H., Stork O. (2003). Cell adhesion molecules: key players in memory consolidation? News Physiol. Sci. 18, 147-150 [DOI] [PubMed] [Google Scholar]
- Whitfield C. W., Cziko A. M., Robinson G. E. (2003). Gene expression profiles in the brain predict behavior in individual honey bees. Science 302, 296-299 [DOI] [PubMed] [Google Scholar]
- Xu K., Zheng X., Seghal A. (2008). Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 8, 289-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Sehgal A. (2010). AKT and TOR signaling set the pace of the circadian pacemaker. Curr. Biol. 20, 1203-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


