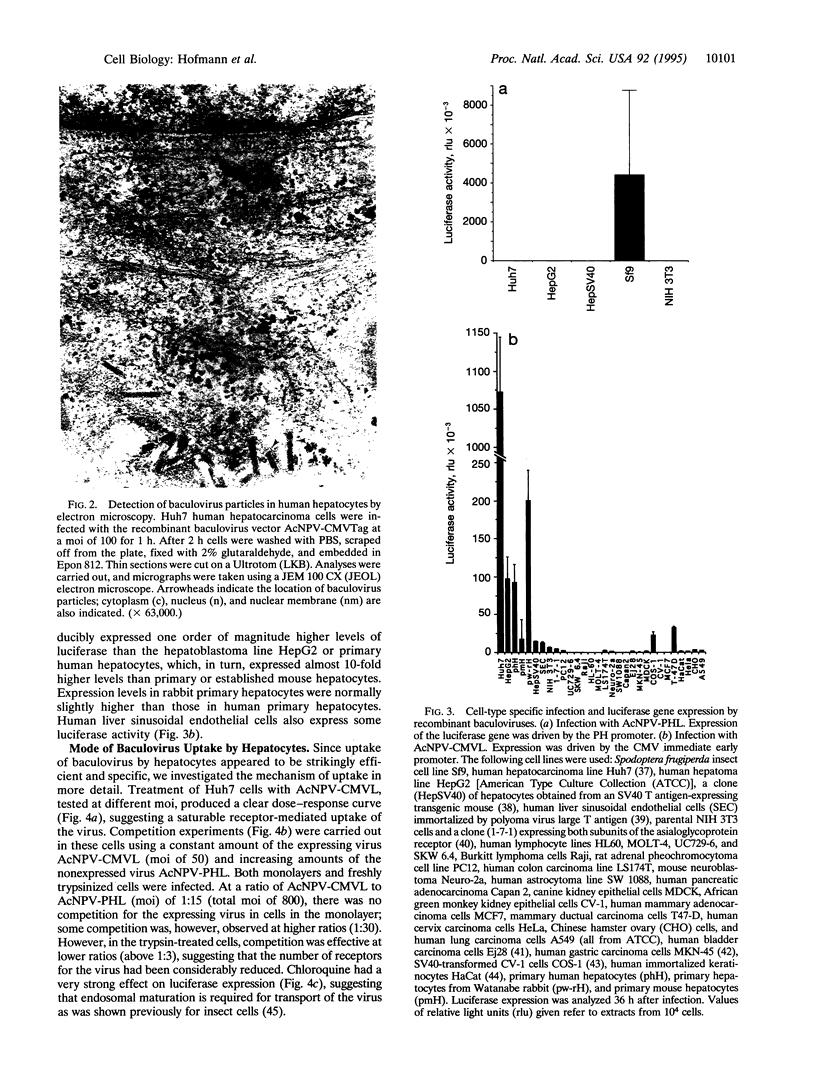
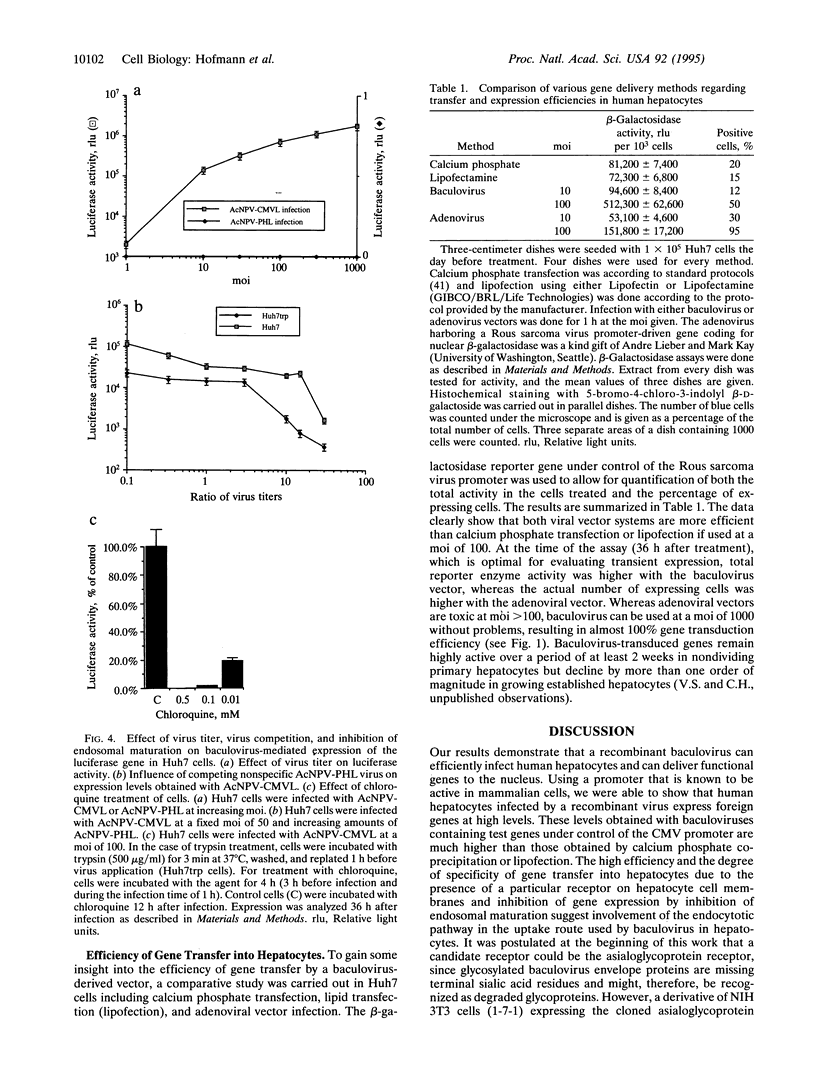
Abstract
Viral vectors are the most efficient tools for gene delivery, and the search for tissue-specific infecting viruses is important for the development of in vivo gene therapy strategies. The baculovirus Autographa californica nuclear polyhedrosis virus is widely used as a vector for expression of foreign genes in insect cells, and its host specificity is supposed to be restricted to arthropods. Here we demonstrate that recombinant A. californica nuclear polyhedrosis virus is efficiently taken up by human hepatocytes via an endosomal pathway. High-level reporter gene expression from heterologous promoters was observed in human and rabbit hepatocytes in vitro. Mouse hepatocytes and some other epithelial cell types are targeted at a considerably lower rate. The efficiency of gene transfer by baculovirus considerably exceeds that obtained by calcium phosphate or lipid transfection. These properties of baculovirus suggest a use for it as a vector for liver-directed gene transfer but highlight a potential risk in handling certain recombinant baculoviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. F., Klowden M. J., Miller L. K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985 Oct;56(1):153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. F., Miller L. K. Baculovirus interaction with nontarget organisms: a virus-borne reporter gene is not expressed in two mammalian cell lines. Appl Environ Microbiol. 1987 Jul;53(7):1412–1417. doi: 10.1128/aem.53.7.1412-1417.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton C. A., Volkman L. E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology. 1993 Nov;197(1):245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- Cristiano R. J., Smith L. C., Woo S. L. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2122–2126. doi: 10.1073/pnas.90.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Wagner E., Cotten M., Birnstiel M. L., Agarwal S., Li C. M., Loechel S., Hu P. C. High-efficiency gene transfer mediated by adenovirus coupled to DNA-polylysine complexes. Hum Gene Ther. 1992 Apr;3(2):147–154. doi: 10.1089/hum.1992.3.2-147. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Ye X., Doranz B., Wilson J. M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Grossman M., Raper S. E., Kozarsky K., Stein E. A., Engelhardt J. F., Muller D., Lupien P. J., Wilson J. M. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994 Apr;6(4):335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- Gröner A., Granados R. R., Burand J. P. Interaction of Autographa californica nuclear polyhedrosis virus with two nonpermissive cell lines. Intervirology. 1984;21(4):203–209. doi: 10.1159/000149522. [DOI] [PubMed] [Google Scholar]
- Hastings R. J., Franks L. M. Cellular heterogeneity in a tissue culture cell line derived from a human bladder carcinoma. Br J Cancer. 1983 Feb;47(2):233–244. doi: 10.1038/bjc.1983.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S., Griffin B. E., Strauss M. Immortalization of human fetal sinusoidal liver cells by polyoma virus large T antigen. Exp Cell Res. 1991 Jul;195(1):1–7. doi: 10.1016/0014-4827(91)90492-d. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Sandig V., Kirillova I., Jennings G., Rudolph M., Schlag P., Strauss M. Hepatocyte-specific binding of L/S-HBV particles expressed in insect cells. Biol Chem Hoppe Seyler. 1995 Mar;376(3):173–178. doi: 10.1515/bchm3.1995.376.3.173. [DOI] [PubMed] [Google Scholar]
- Horwich A. L. Inherited hepatic enzyme defects as candidates for liver-directed gene therapy. Curr Top Microbiol Immunol. 1991;168:185–200. doi: 10.1007/978-3-642-76015-0_9. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Li Q., Liu T. J., Leland F., Toman C., Finegold M., Woo S. L. Hepatic gene therapy: persistent expression of human alpha 1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992 Dec;3(6):641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Woo S. L. Gene therapy for metabolic disorders. Trends Genet. 1994 Jul;10(7):253–257. doi: 10.1016/0168-9525(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Li Q., Kay M. A., Finegold M., Stratford-Perricaudet L. D., Woo S. L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993 Aug;4(4):403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Paul D., Höhne M., Pinkert C., Piasecki A., Ummelmann E., Brinster R. L. Immortalized differentiated hepatocyte lines derived from transgenic mice harboring SV40 T-antigen genes. Exp Cell Res. 1988 Apr;175(2):354–362. doi: 10.1016/0014-4827(88)90199-1. [DOI] [PubMed] [Google Scholar]
- Perales J. C., Ferkol T., Beegen H., Ratnoff O. D., Hanson R. W. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandig V., Lieber A., Bähring S., Strauss M. A phage T7 class-III promoter functions as a polymerase II promoter in mammalian cells. Gene. 1993 Sep 15;131(2):255–259. doi: 10.1016/0378-1119(93)90302-j. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Lodish H. F. The two subunits of the human asialoglycoprotein receptor have different fates when expressed alone in fibroblasts. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1158–1162. doi: 10.1073/pnas.86.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Argani P., Mohr I. J., Gluzman Y. Studies on the origin-specific DNA-binding domain of simian virus 40 large T antigen. J Virol. 1987 Oct;61(10):3326–3330. doi: 10.1128/jvi.61.10.3326-3330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M. Liver-directed gene therapy: prospects and problems. Gene Ther. 1994 May;1(3):156–164. [PubMed] [Google Scholar]
- Tjia S. T., zu Altenschildesche G. M., Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983 Feb;125(1):107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- Versland M. R., Wu C. H., Wu G. Y. Strategies for gene therapy in the liver. Semin Liver Dis. 1992 Aug;12(3):332–339. doi: 10.1055/s-2008-1040402. [DOI] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Foisner R., Birnstiel M. L. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Wilson J. M., Shalaby F., Grossman M., Shafritz D. A., Wu C. H. Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem. 1991 Aug 5;266(22):14338–14342. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987 Apr 5;262(10):4429–4432. [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]





