Abstract
Neural crest cells generate a range of cells and tissues in the vertebrate head and trunk, including peripheral neurons, pigment cells, and cartilage. Neural crest cells arise from the edges of the nascent central nervous system, a domain called the neural plate border (NPB). NPB induction is known to involve the BMP, Wnt and FGF signaling pathways. However, little is known about how these signals are integrated to achieve temporally and spatially specific expression of genes in NPB cells. Furthermore, the timing and relative importance of these signals in NPB formation appears to differ between vertebrate species. Here, we use heat-shock overexpression and chemical inhibitors to determine whether, and when, BMP, Wnt and FGF signaling are needed for expression of the NPB specifiers pax3a and zic3 in zebrafish. We then identify four evolutionarily conserved enhancers from the pax3a and zic3 loci and test their response to BMP, Wnt and FGF perturbations. We find that all three signaling pathways are required during gastrulation for the proper expression of pax3a and zic3 in the zebrafish NPB. We also find that, although the expression patterns driven by the pax3a and zic3 enhancers largely overlap, they respond to different combinations of BMP, Wnt and FGF signals. Finally, we show that the combination of the two pax3a enhancers is less susceptible to signaling perturbations than either enhancer alone. Taken together, our results reveal how BMPs, FGFs and Wnts act cooperatively and redundantly through partially redundant enhancers to achieve robust, specific gene expression in the zebrafish NPB.
Keywords: FGF, Neural crest, Wnt, Enhancer, Pax3, Zic3
INTRODUCTION
A major theme in developmental biology is the need for cells to integrate multiple signals to make decisions about their fate. To achieve this, regulatory DNA must be structured so developmental control genes are expressed only when a cell is in the correct signaling environment. Furthermore, this regulation must be robust so development can proceed normally even when signaling levels fluctuate with environmental variability.
Neural crest development is a classic example of how multiple signals are integrated to make cell fate decisions. Neural crest precursors are initially specified at the border between the neural plate and the epidermal ectoderm, a region called the neural plate border (NPB). This specification involves signals from the Wnt, FGF and BMP pathways. Wnt and FGF ligands are secreted by the neural plate and underlying mesoderm (Bang et al., 1999; Monsoro-Burq et al., 2003; García-Castro et al., 2002), while the epidermal ectoderm produces BMP ligands and the neural plate secretes BMP antagonists, resulting in a BMP signaling gradient along the dorsoventral axis (Marchant et al., 1998; Smith and Harland, 1992; Sasai et al., 1995; Endo et al., 2002). The NPB is specified in the region of the ectoderm that receives an intermediate dose of BMP signaling, as well as Wnts and/or FGFs (LaBonne and Bronner-Fraser, 1998; Streit and Stern, 1999; Villanueva et al., 2002). The combination of these signals leads to the expression of NPB specifier genes, including members the Pax3/7 and Zic families (Sato et al., 2005; Monsoro-Burq et al., 2003; Bang et al., 1999). Experiments in Xenopus indicate that the combination of Pax3/7 and Zic genes is sufficient to induce neural crest (Sato et al., 2005).
How Wnt, FGF and BMP signaling specify the NPB is an area of active research. Much of what we know about this process comes from experiments in Xenopus. It is generally agreed that an intermediate level of BMP signaling is crucial for establishing the NPB and recent studies have shed light on the time dependence of the BMP and Wnt requirement (Steventon et al., 2009; Steventon and Mayor, 2012; Patthey et al., 2009). The relative importance of Wnts and FGFs in this process has been controversial. Studies in the past decade have fuelled a debate about whether FGF and Wnt signaling act in parallel to induce NPB genes (Monsoro-Burq et al., 2005) or whether FGF acts indirectly by activating the expression of Wnt ligands (Hong et al., 2008). The interactions of these pathways in NPB specification have been less well studied in zebrafish. The ease of transgenic zebrafish generation (Kawakami, 2004), coupled with excellent methods for temporally controlled gene overexpression make zebrafish an attractive model for studying NPB gene regulation.
To better understand how and when BMP, Wnt and FGF signals specify the NPB in zebrafish, we knocked down Wnt and FGF signaling, and overexpressed BMP ligand at various developmental time points and monitored the expression of the NPB specifiers pax3a and zic3. We find that full pax3a NPB expression requires all three signals during mid-to-late gastrulation, whereas zic3 NPB expression only requires Wnt signaling and attenuation of BMP signaling. We also show that modulating BMP signaling affects both the position and intensity of pax3a and zic3 expression within the ectoderm, while perturbing Wnt and/or FGF expression mainly affects expression intensity. We then investigated how BMP, FGF and Wnt signals are integrated by the cis-regulatory machinery of zic3 and pax3a. To do this, we isolated four enhancers from the Takifugu rubripes zic3 and pax3a loci that drive reporter expression in the forming zebrafish NPB and then tested their responses to Wnt, FGF and BMP perturbations. We used the Fugu genome for these enhancer studies because it is more compact than the zebrafish genome, allowing enhancers to be located more easily. We found that Wnts, FGFs and BMPs act in different combinations through these enhancers to achieve NPB expression. Based on these findings, we propose that the parallel influences of FGFs and Wnts on partially redundant enhancers drive sharp, intense NPB specifier expression and buffer the regulation of NPB genes against variability in signaling levels. Furthermore, we propose that lineage-specific differences in the relative influence of these evolutionarily conserved enhancers could account for observed differences in the importance of FGF versus Wnt signaling during NPB induction in frog and chick.
MATERIALS AND METHODS
Zebrafish stocks and husbandry
Adult fish were kept at 28.5°C on a 14 hour light/10 hour dark cycle. Embryos were obtained by natural crosses and staged as previously described (Kimmel et al., 1995).
Generation of transgenic zebrafish
Putative enhancer fragments were amplified from Takifugu rubripes genomic DNA and cloned upstream of the mouse Fos promoter and GFP using a Gateway-based technique similar to a previously described method (Fisher et al., 2006). A dual fluorescence vector was created by cloning mCherry under control of the ubiquitously active Xenopus EF1α promoter downstream of GFP. Supplementary material Table S1 shows primer sequences.
dkk1 and bmp2b were amplified using RT-PCR and cloned into a Gateway vector. hsp70l-dkk1-mCherry and hsp70l-bmp2b tol2 plasmids were constructed using vectors from the Tol2 Gateway kits (Villefranc et al., 2007; Kwan et al., 2007).
Transgenesis constructs were injected as previously described (Kawakami, 2004; Kwan et al., 2007). Transgenic founders were identified using pair-wise crosses to wild-type fish. F1 fish were raised and their offspring used in experiments.
Site-specific mutagenesis was performed using PCR-based methods. Wild-type and mutant enhancers were cloned into the dual fluorescence vector. Mutant and wild-type constructs were injected on the same morning so GFP intensity could be directly compared. At the appropriate stage, GFP levels were scored in embryos expressing mCherry in the NPB or dorsal neural tube.
Signaling pathway manipulations
F1 fish containing reporter constructs were crossed to fish heterozygous for the hsp70l-dkk1-mCherry or hsp70l-bmp2b transgene. Similar transgenic lines have been described previously (Stoick-Cooper et al., 2007; Chocron et al., 2007). hsp70:bmp2b fish were incubated at 37°C for 1 hour then 28.5°C until fixed. hsp70:dkk1-mCherry fish were incubated at 39°C for 30 minutes then 33°C until fixed. Double in situ hybridizations were performed with probes for the gene of interest and bmp2b or mCherry. Coloration was carried out using NBT-BCIP for the gene of interest and Fast Red for bmp2b or mCherry. Embryos were sorted into mock heat shock and bmp2b- or dkk1-overexpressing groups based on Fast Red staining.
The wnt8a morpholino was a kind gift from Kristin Artinger (University of Colorado, Aurora, USA). The MO sequence is the same as wnt8a MO1 (Lekven et al., 2001). We injected the MO as described by Lewis et al. (Lewis et al., 2004).
SU5402 treatments were performed by incubating dechorionated embryos in 90 μM SU5402 in 1% DMSO/system water. Control embryos were incubated in 1% DMSO/system water alone. At the appropriate stage, embryos were removed from the treatment solution and immediately fixed.
A GR-Lef1-βcat construct was kindly provided by Yevgenya Grinblat (University of Wisconsin, Madison, USA). GR-Lef1-βcat mRNA was synthesized and injected as previously described (Ramel and Lekven, 2004). Dechorionated embryos were treated with dexamethasone and cycloheximide as previously described (Martin and Kimelman, 2008).
In situ hybridization, photography and mounting
Whole-mount in situ hybridization was performed as described previously (Thisse et al., 1993) with modifications (Melby et al., 1997). Digoxygenin- or fluorescien-labeled RNA probes were synthesized from cDNA templates. Embryos were cleared and mounted as described previously (Griffin et al., 1998).
RESULTS
Proper BMP, FGF and Wnt signaling during late gastrulation are crucial for pax3a and zic3 expression at the NPB
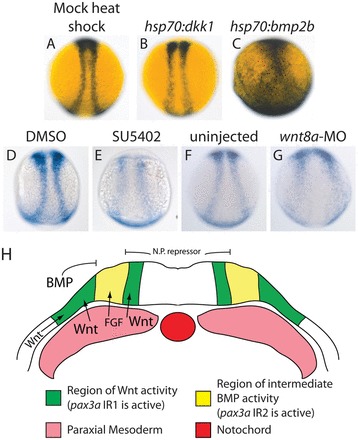
Previous studies have shown that Wnt and FGF signals from the mesoderm and epidermis along with an intermediate level of BMP signaling are important for NPB induction (LaBonne and Bronner-Fraser, 1998; García-Castro et al., 2002). To determine the temporal requirement for these signals in zebrafish we used a heat-shock strategy to overexpress the Wnt antagonist dickkopf1 (dkk1) or the BMP ligand bmp2b. Previous studies indicate that events during mid- to late gastrulation are important in NPB specification. For this reason we heatshocked embryos containing a hsp70l:dkk1-mcherry or hsp70l:bmp2b transgene beginning between 30% epiboly (4.7 hpf) and tailbud stage (10 hpf) and monitored NPB gene expression at 6 somites (12 hpf) and phenotype at 24 hpf. We found that shield stage (6 hpf) heatshock had a similar effect to earlier heat shocks and that heat shocks later than 75% epiboly had minor effects on gene expression (data not shown). For this reason, we concentrated on embryos that were heat shocked at shield stage (6 hpf) or 75% epiboly (8 hpf) and fixed at 6 somites (12 hpf). We used the FGF signaling inhibitor SU5402 to test the requirement for FGF signaling in regulating these genes during late gastrulation. SU5402 treatments have been shown to knockdown expression of direct FGF signaling targets in zebrafish (Roehl and Nüsslein-Volhard, 2001). We started SU5402 treatments at stages between 30% epiboly and tailbud stages and found that treatments beginning at 65% epiboly (7 hpf) disrupted NPB gene expression to a degree similar to earlier treatments, without confounding effects on gastrulation or axis formation. To confirm the efficiency of these manipulations, we monitored gene expression of the hindbrain marker krox20 and the neural plate markers sox2 and sox3, and observed the phenotypes of treated embryos at 24 hpf agreed with previously described phenotypes (supplementary material Fig. S1). The observed changes in gene expression patterns and phenotypes agreed with previously described results. We monitored pax3a and zic3 expression in these embryos by in situ hybridization. pax3a is expressed in the anterior paraxial mesoderm near 12 hpf, so we used optical sections to confirm that any changes observed in pax3a expression occurred in the NPB (Fig. 1S-T).
Fig. 1.
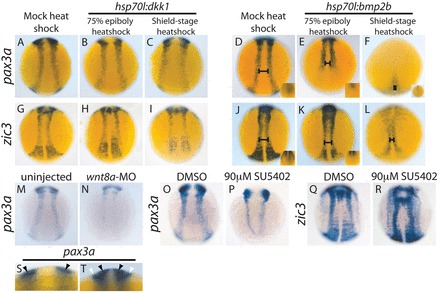
BMP and Wnt signaling regulate pax3a and zic3, and FGF signaling regulates pax3a during late gastrulation. (A-C,G-I) pax3a and zic3 expression decreases upon heat shock-mediated dkk1 overexpression. pax3a and zic3 expression decreases slightly in hsp70l:dkk1 embryos heat shocked at 75% epiboly [pax3a, B versus A (81%, n=27); zic3, H versus G (93%, n=28)] and a shield stage heat shock leads to a greater decrease in expression [pax3a, C versus A (92%, n=49); zic3, I versus G (100%, n=33)]. (D-F,J-L) pax3a and zic3 expression in the NPB decreases and shifts medially upon heat shock-mediated bmp2b overexpression. A 75% epiboly heat shock leads to a loss of posterior NPB expression (E versus D and K versus J, insets) and a slight medial shift of the anterior NPB domain (D,E,J,K, bars) (pax3a, 100%, n=22; zic3, 94%, n=18). A shield stage heat shock leads to a drastic decrease in pax3a and zic3 expression throughout the anterior-posterior axis, and NPB expression shifts medially into a teardrop shape [pax3a, F versus D (100%, n=28); zic3, L versus J (100%, n=39)]. (M,N) Morpholino-mediated knockdown of wnt8a leads to a greater decrease in pax3a expression than does dkk1 overexpression [N versus M (90%, n=20)]. (O-R) Inhibition of FGF signaling by SU5402 treatment beginning at 7 hpf leads to a decrease in pax3a expression by 12 hpf (84%, n=44) (P versus O). The same SU5402 treatment does not decrease NPB zic3 expression (100%, n=27) (R versus Q). (S,T) Optical cross-sections allow the distinction between NPB and mesodermal pax3a expression. An optical cross-section of the trunk at ~12 hpf reveals pax3a expression in the ectoderm only (black arrowheads, S), whereas an optical cross-section slightly later reveals pax3a mRNA in the ectoderm (black arrowheads, T) and paraxial mesoderm (white arrowheads, T). The insets in D-F,J,K are posterior dorsal views, all other pictures are dorsal trunk views with anterior upwards.
zic3 NPB expression decreases dramatically with dkk1-mcherry overexpression (Fig. 1H,I versus 1G) and pax3a mRNA exhibits a more modest decrease (Fig. 1B,C versus 1A). The effect is stronger with a shield stage heat shock, indicating that Wnt signaling is crucial at late gastrulation for proper levels of NPB pax3a and zic3 expression. Previous work suggests Wnt8a is the Wnt ligand involved in neural border/neural crest specification (Lewis et al., 2004). Interestingly, the effect of dkk1 overexpression on pax3a expression was milder than the effect of wnt8a knockdown by morpholino (Lewis et al., 2004) (Fig. 1N versus 1M) (supplementary material Fig. S1). This difference suggests that the wnt8a MO may be disrupting pax3a NPB expression both directly and indirectly, potentially through its disruption of DV axis formation (Ramel and Lekven, 2004). Embryos treated with SU5402 beginning at 65% epiboly (7 hpf) exhibited decreased pax3a NPB expression (Fig. 1P versus 1O). The same SU5402 treatment did not decrease the level of zic3 NPB expression, but led to a less organized zic3 expression pattern (Fig. 1R versus 1Q).
hsp70l:bmp2b embryos heat shocked at 75% epiboly did not express pax3a or zic3 in the posterior NPB (Fig. 1E versus 1D and 1K versus 1J, insets) and exhibited a slight medial shift in trunk NPB expression (Fig. 1E versus 1D and 1K versus 1J, bars). pax3a and zic3 expression decreased dramatically when hsp70l:bmp2b embryos were heat shocked at shield stage and both expression patterns shifted to the midline (Fig. 1F versus 1D and 1L versus 1J). pax3a expression and the trunk and tail aspects of zic3 expression are limited to a small tear-drop shape (Fig. 1F,L). This medial shift of pax3a and zic3 expression is consistent with the idea that NPB gene expression requires a moderate level of BMP signaling. The decrease in expression accompanying the medial shift is consistent with the presence of a repressor of these genes in the neural plate. Our results indicate that BMP signaling during late gastrulation is important for the placement of the NPB. The temporal requirement for this signaling proceeds from anterior to posterior, as posterior gene expression is more severely affected by the 75% epiboly heat shock.
These results suggest BMP signaling positions pax3a and zic3 expression in the ectoderm whereas Wnt signaling is required for the genes to be expressed at the proper level. FGF signaling is required for the proper level of pax3a expression, but not that of zic3.
Wnt and FGF signaling act redundantly to amplify NPB gene expression
The data described above demonstrate that a knockdown of Wnt or FGF signaling can decrease the expression level of pax3a, whereas FGF knockdown does not decrease the level of NPB zic3 expression. We hypothesized that Wnt signaling may be sufficient to mask any effects of FGF knockdown on zic3. To test this, we treated hsp70l:dkk1-mCherry embryos with SU5402 while heat shocking them (Fig. 2). The combination of Wnt and FGF knockdown decreases zic3 expression more than dkk1 overexpression alone (Fig. 2H versus 2G), demonstrating that these two pathways function redundantly to amplify zic3 gene expression at the NPB. Similarly, pax3a expression decreases with Wnt or FGF knockdown (Fig. 2B,C versus 2A), and decreases further when both are inhibited (Fig. 2D).
Fig. 2.
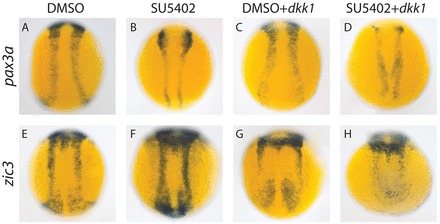
Wnt and FGF signaling play partially redundant roles in regulating NPB specifiers. (A-H) Embryos treated with SU5402 starting at 7 hpf exhibited decreased pax3a expression (100%, n=28) (B versus A), but no decrease in zic3 expression (100%, n=16) (F versus E). dkk1 overexpression induced by 7 hpf heat shock decreases expression of pax3a (100%, n=24) (C versus A) and zic3 (100%, n=16) (G versus E). When the Wnt and FGF signaling pathways are both attenuated with heat shock-induced dkk1 overexpression and SU5402 treatment starting at 7 hpf, pax3a and zic3 mRNA levels decrease more than when either pathway is knocked down alone [pax3a, D versus B,C (100%, n=24); zic3, H versus F,G (100%, n=17)]. All pictures are dorsal trunk views with anterior upwards.
Two evolutionarily conserved enhancers drive pax3a expression in the NPB
We wanted to elucidate the relationship between the BMP, Wnt and FGF pathways in pax3a regulation by determining how they act on individual pax3a enhancers. To identify these enhancers, we used comparative genomics to detect conserved non-coding sequences (Fig. 3A; supplementary material Fig. S2A). We identified two regions from the fourth intron of Fugu pax3a that drive gene expression in the NPB during early segmentation and later in the dorsal neural tube. We call these enhancers IR1 and IR2. pax3a IR1 contains two regions of high sequence identity between Fugu and human, a 5′ 286 bp region with 76% sequence identity and a 3′ 334 bp region with 65% sequence identity. The 3′ region is a likely homolog of a previously described mouse enhancer (Degenhardt et al., 2010). pax3a IR1 drives gene expression along the entire length of the NPB during early segmentation (Fig. 3B-G). This band of enhancer activity is wider than endogenous pax3a NPB expression (Fig. 3C,E versus Fig. 1A,S), suggesting other elements are needed to refine pax3a expression. A double in situ hybridization for GFP and pax3a confirms that pax3a expression and IR1 activity overlap (Fig. 3F,G). pax3a IR2 contains a 142 bp region with 65% sequence identity between Fugu and human. IR2 drives gene expression along the NPB during early segmentation (Fig. 3I-N) in a pattern more similar to endogenous pax3a expression than IR1 (Fig. 3J,L versus Fig. 1A,S). A double in situ hybridization for GFP and pax3a confirms that pax3a expression and IR2 activity overlap (Fig. 3M). Both pax3a enhancers drive gene expression in the dorsal neural tube at 24 hpf (Fig. 3H,O). We generated five independent zebrafish lines containing the pax3a IR1:GFP transgene and three independent lines containing the pax3a IR2:GFP transgene. The GFP expression patterns observed were consistent between lines.
Fig. 3.
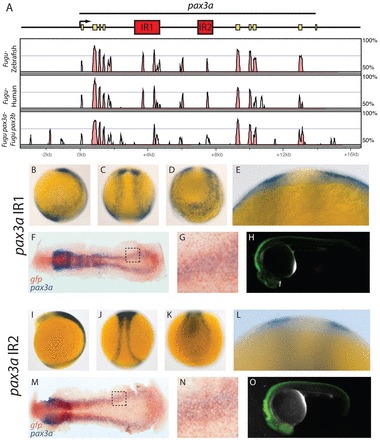
Two enhancers in pax3a intron 4 drive gene expression in the NPB and dorsal neural tube. (A) The Fugu pax3a locus is shown along with a Vista plot (Frazer et al., 2004; Mayor et al., 2000) of sequence identity between Fugu and zebrafish, Fugu and human, and Fugu pax3a and its paralog pax3b. Enhancer regions are shown in red and pax3a exons are shown in yellow. (B-O) GFP expression driven by IR1 (B-H) and IR2 (I-O) is shown as detected by in situ hybridization for embryos at 12 hpf (B-G,I-N) and by fluorescence at 24 hpf (H,O). Optical cross-sections through the trunk region confirm that IR1 and IR2 drive gene expression in the ectoderm (E,L) and double in situ hybridization for GFP (brown) and pax3a (purple) demonstrate that pax3a expression overlaps with the activity of IR1 and IR2 (F,G,M,N). G and N are higher magnification views of the areas outlined in F,M, respectively. B,H,I,O are lateral views; C,J are dorsal trunk views; D,K are posterior dorsal views.
The pax3a IR1 and IR2 enhancers have distinct responses to Wnt, BMP and FGF perturbations
To determine how the Wnt-, BMP- and FGF-regulated aspects of pax3a transcription are divided between the two enhancers, we crossed pax3a IR1 and IR2 transgenic fish into the hsp70l:dkk1-mcherry and hsp70l:bmp2b lines, and treated fish containing the transgenes with SU5402. pax3a IR1 activity dramatically decreases with dkk1 overexpression (Fig. 4B,C versus 4A), whereas pax3a IR2 is unaffected by dkk1 overexpression (Fig. 4E,F versus 4D). The decrease in pax3a IR1 activity is much greater than that of the endogenous gene (Fig. 4C versus Fig. 1C), indicating that additional enhancers buffer pax3a expression against variations in Wnt signaling levels.
Fig. 4.
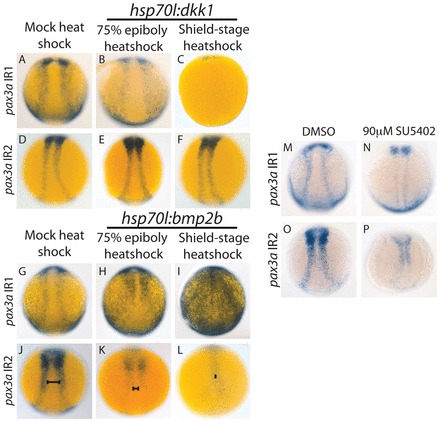
pax3a IR1 requires Wnt and FGF signaling, and pax3a IR2 requires BMP and FGF signaling for full activity. (A-F) dkk1 was overexpressed by heat shock in embryos with pax3a IR1:GFP or pax3a IR2:GFP. pax3a IR1 activity decreased with dkk1 overexpression induced with a 75% epiboly heatshock (100%, n=25) (B versus A) and was almost completely lost with a shield stage heat shock (100%, n=12) (C versus A). pax3a IR2:GFP activity did not decrease when dkk1-mCherry overexpression was induced by heat shock at 75% epiboly (92%, n=12) (E versus D) or shield stage (89%, n=18) (F versus D). (G-L) bmp2b was overexpressed by heat shock. pax3a IR1activity intensifies and expands into the neural plate and epidermis when bmp2b overexpression is induced with a 75% epiboly heat shock (93%, n=41) (H versus G) and heat shock at shield stage leads to an even greater activity increase and causes expansion into the entire neural plate (84%, n=19) (I versus G). pax3a IR2 activity decreases and shifts medially (J-L, bars) upon bmp2b induction by 75% epiboly heat shock (100%, n=33) (K versus J) and further decreases and shifts medially into a tear-drop shape with a shield-stage heat shock (100%, n=14) (L versus J). (M-P) SU5402 treatment (90 μM) beginning at 7 hpf causes a decrease in the activity of pax3a IR1 (94%, n=17) (N versus M) and pax3a IR2 (100%, n=25) (P versus O). All pictures are dorsal trunk views with anterior upwards.
pax3a IR1 and IR2 activities decrease dramatically upon SU5402 treatment (Fig. 4N versus 4M and 4P versus 4O), indicating FGF signaling is crucial for their function. Thus, pax3a IR2 is FGF dependent, but Wnt independent. Assuming that IR2 regulates pax3a, this demonstrates that at least some pax3a regulation by FGF signaling is not mediated by Wnts. This is in contrast to work in Xenopus suggesting FGFs regulate NPB genes mainly by inducing Wnt ligand expression (Hong et al., 2008).
pax3a IR2 activity decreases upon bmp2b overexpression and the activity shifts medially into a teardrop shape (Fig. 4K,L versus 4J). This is similar to the bmp2b response observed for endogenous pax3a. pax3a IR1 activity also shifts medially in the trunk upon bmp2b overexpression, but its activity intensifies and expands into the posterior epidermis (Fig. 4H,I versus 4G). The fact that the gene expression driven by IR1 intensifies and expands medially indicates that it is not repressed by factors in the neural plate, whereas pax3a IR2 is. The expression of neural markers also shifts medially upon bmp2b overexpression (supplementary material Fig. S1), but IR1 is able to drive expression in the most medial parts of the embryo, indicating that it is not highly repressed in these regions. The increase in pax3a IR1 activity upon bmp2b overexpression could result from wnt8a upregulation with increased BMP levels (supplementary material Fig. S3).
The combined activity of two pax3a enhancers is less susceptible to perturbation than either enhancer alone
Our results demonstrate that endogenous pax3a expression is less susceptible to Wnt and BMP perturbations than the activity of pax3a IR1 or IR2. We hypothesized that if we linked IR1 and IR2, the resulting transgene would be less sensitive to signaling perturbations. To test this, we made two independent stable transgenic lines in which pax3a IR2 and IR1 were placed upstream of the mouse Fos promoter driving GFP. Both lines expressed GFP in the NPB in a narrow band, similar to that driven by pax3a IR2 alone and endogenous pax3a (Fig. 5A). When dkk1 was overexpressed by a shield stage heat shock we observed a slight decrease in GFP expression (Fig. 5B versus 5A). This decrease was not as dramatic as when the same manipulation was performed for pax3a IR1 alone (Fig. 5B versus Fig. 4C), indicating the sensitivity of pax3a IR1 to changes in Wnt signaling levels can be masked by pax3a IR2. However, the activity of the compound enhancer is more significantly reduced upon wnt8a MO injection (Fig. 5G versus 5F), similar to the response observed for endogenous pax3a (Fig. 1N versus 1M). When bmp2b overexpression was induced in these fish, NPB GFP expression shifted medially in the trunk and expanded into the neural plate and epidermis in the posterior, while increasing in intensity (Fig. 5C versus 5A). This indicates that neural plate factors that repress pax3a IR2 cannot repress IR1 when the two enhancers are linked in this way. pax3a probably contains additional regulatory information that prevents IR1 from activating high levels of pax3a expression in the neural plate upon bmp2b overexpression. One likely candidate for this function is the pax3a promoter region, which has regulatory activity in mice (Milewski et al., 2004). SU5402 treatment beginning at 65% epiboly decreased IR2:IR1 activity similar to either enhancer alone (Fig. 5E versus 5D). Thus, the presence of these two enhancers cannot buffer against fluctuations in FGF levels. The drastic downregulation of endogenous pax3a upon SU5402 treatment demonstrates that endogenous pax3a is also not well buffered against decreases in FGF signaling (Fig. 1P versus 1O).
Fig. 5.
The combination of pax3a IR1 and IR2 is less susceptible to signaling perturbations than either enhancer alone. (A-G) GFP expression driven by a pax3a IR2+IR1 composite enhancer is shown under various treatments. dkk1-mCherry overexpression beginning with a shield stage heat shock mildly decreases enhancer activity (60%, n=42) (B versus A). bmp2b overexpression beginning with a shield stage heat shock causes increased enhancer activity, a shift medially in the trunk region and expansion in the posterior (87%, n=31) (C versus A). SU5402 treatment starting at 7 hpf drastically decreases the activity of the composite enhancer (100%, n=14) (E versus D). wnt8a-MO injection causes a more severe decrease in activity than dkk1 overexpression (80%, n=10) (G versus F). (H) A model for pax3a IR1 and IR2 activity. IR1 is activated by Wnt and FGF signaling in a wide band surrounding the NPB (green). IR2 activity is precisely positioned at the NPB by BMP signaling and a repressive neural plate factor and is amplified by FGF signaling (yellow). All pictures are dorsal trunk views with anterior upwards.
Two evolutionarily conserved enhancers drive zic3 expression in overlapping regions of the NPB
We aligned the genomic regions containing zic3 and zic6 from Fugu and zebrafish (zic3+6) and the region containing ZIC3 from human [zic6 has been lost from the tetrapod lineage (Keller and Chitnis, 2007)]. Several non-coding sequences in this region are conserved between fish and human (Fig. 6A), and we placed some of these upstream of a minimal promoter driving GFP to test for enhancer activity. For a more detailed description of our enhancer search strategy see supplementary material Fig. S2B.
Fig. 6.
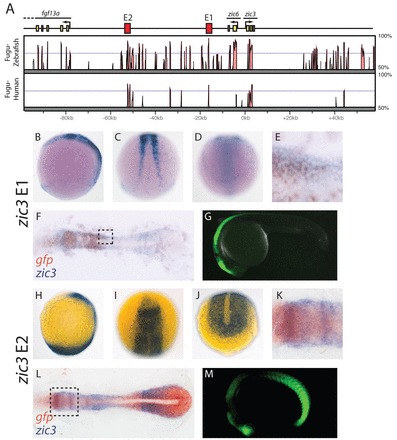
Two zic3 enhancers drive gene expression in the NPB and dorsal neural tube. (A) The Fugu zic3/zic6 locus is shown along with a Vista plot of the level of sequence identity between Fugu, zebrafish and human zic3 loci. Enhancers are shown in red and exons are shown in yellow. (B-M) GFP expression driven by E1 (B-G) and E2 (H-M) is shown as detected by in situ hybridization for embryos at 12 hpf (B-F,H-L) and by GFP fluorescence at 24 hpf (G,M). Double in situ hybridization for GFP (brown) and zic3 (purple) demonstrate that zic3 expression overlaps with the activity of E1 and E2 (E,F,K,L). E,K are higher magnification views of the areas outlined in F,L, respectively. B,H,G,M are lateral views; D,J are posterior dorsal views; C is a trunk dorsal view; I is an anterior dorsal view.
We found two regions that drive gene expression in the NPB and refer to these regions as E1 and E2. E1 lies 16 kb upstream of zic3 and 11 kb downstream of zic6 and contains a conserved region of 283 bp with 73% sequence identity between Fugu and human (Fig. 6A). This region drives gene expression along the NPB during early segmentation (Fig. 6B-F) and later in the dorsal neural tube (Fig. 6G). A double in situ hybridization for GFP and zic3 indicates that E1 activity overlaps with zic3 expression (Fig. 6E,F). Interestingly, the sequence of E1 is similar to a previously identified zic2a/zic5 NPB enhancer (Nyholm et al., 2007) and both of these are in roughly the same location relative to the genes they regulate. This suggests that these enhancers are paralogous and conservation of this enhancer sequence is partly responsible for the expression of vertebrate Zic genes in the developing neural crest.
zic3+6 E2 lies 53 kb upstream of zic3 and 46.5 kb downstream of zic6. It contains a conserved 352 bp region with 70% sequence identity between Fugu and human (Fig. 6A). During early segmentation, zic3+6 enhancer region E2 drives GFP expression in the posterior paraxial mesoderm and in the anterior neural plate and NPB (Fig. 6H-L). Later, this sequence is active in the anterior dorsal neural tube and the posterior paraxial mesoderm (Fig. 6M). A double in situ hybridization of GFP and zic3 demonstrates that E2 activity and zic3 expression overlap (Fig. 6K,L). The mesodermal expression is similar to endogenous zic3 expression. The zic3+6 E2-driven anterior dorsal neural tube expression is similar to that of zic6 at 24 hpf (supplementary material Fig. S4). The ectodermal and mesodermal activities of this E2 are separable (supplementary material Fig. S5), but we performed analyses using full-length E2 to avoid losing important sequences not present in the trimmed-down NPB enhancer. zic3 E2 is closer to fgf13a than is zic3 or zic6 (Fig. 6A), but fgf13a is not expressed before the 14-somite stage (Thisse et al., 2004), suggesting that it is not regulated by E2. Although these enhancers probably regulate both zic3 and zic6, we refer to them as zic3 enhancers as both have homologs in animals without a zic6 gene. We generated two independent zebrafish lines with zic3 E1:GFP and three independent zebrafish lines with zic3 E2:GFP. The GFP expression patterns observed were consistent among lines.
zic3 E1 and E2 respond similarly to Wnt and BMP perturbations, but differently to FGF inhibition
We overexpressed dkk1 in zic3 E1 and E2 transgenic lines by heat shock at shield stage and 75% epiboly. We fixed zic3 E1 embryos at six somites and zic3 E2 embryos at four somites (11 hpf). The zic3 E2 embryos were fixed earlier to observe GFP expression in the anterior NPB before neural closure in this region. zic3 E1 activity decreased with dkk1 overexpression induced by 75% epiboly heat shock (Fig. 7B versus 7A), and decreased more severely with shield stage heat shock (Fig. 7C versus 7A). zic3 E2 activity decreased slightly upon 75% epiboly heat shock (Fig. 7E versus 7D), and its activity in the NPB was almost eliminated upon shield stage heat shock (Fig. 7F versus 7D).
Fig. 7.
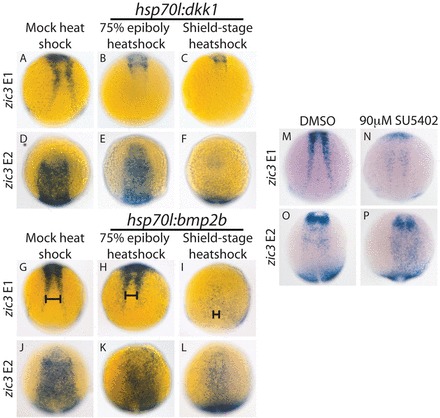
zic3 E1 is regulated by Wnt, BMP and FGF signaling, and zic3 E2 is regulated by Wnt and BMP signaling. (A-F) dkk1 was overexpressed by heat shock in embryos with zic3 E1:GFP or zic3 E2:GFP. dkk1 overexpression beginning with a 75% epiboly heat shock decreases zic3 E1 activity (100%, n=23) (B versus A) and slightly decreases zic3 E2 activity (88%, n=50) (E versus D). The NPB activity of both enhancers is almost completely lost, with dkk1 overexpression induced with a shield stage heat shock [E1, C versus A (100%, n=21); E2, F versus D (100%, n=55)]. (G-L) bmp2b was overexpressed by heat shock. zic3 E1 loses activity in the posterior NPB and shifts medially in the anterior (H, bar) upon bmp2b induction with 75% epiboly heat shock (93%, n=29) (H versus G). Shield-stage heat shock causes a dramatic reduction of E1 activity throughout and causes a drastic medial shift (I, bar) (96%, n=23) (I versus G). zic3 E2 activity does not change when bmp2b overexpression is induced with a 75% epiboly heat shock (79% with wild-type GFP level, n=38) (K versus J), but decreases with a shield-stage heat shock (100%, n=24) (L versus J). (M-P) SU5402 treatment beginning at 65% epiboly dramatically decreases zic3 E1 activity (100%, n=27) (N versus M), but not zic3 E2 activity (81% with wild-type GFP level, n=22) (P versus O). All E1 pictures are dorsal views of the trunk with anterior upwards; all E2 pictures are anterior dorsal views with anterior upwards.
We overexpressed bmp2b in zic3 E1 and E2 transgenic lines by heat shock at shield stage and 75% epiboly. zic3 E1 activity was eliminated from the posterior NPB with the later heat shock (Fig. 7H versus 7G) and drastically decreased throughout the NPB upon shield stage heatshock (Fig. 7I versus 7G). E1-driven NPB GFP expression shifted medially with bmp2b overexpression similar to endogenous zic3 (Fig. 7G-I, bars). zic3 E2 activity did not noticeably decrease in hsp70l:bmp2b embryos upon 75% epiboly heat shock (Fig. 7K versus 7J), but decreased dramatically with shield stage treatment (Fig. 7L versus 7J). The fact that both enhancers drive BMP-responsive NPB gene expression demonstrates that they are partially functionally redundant, possibly facilitating more robust control of zic3 expression in the anterior ectoderm.
To determine whether zic3 E1 and E2 require FGF signaling, we treated transgenic embryos with SU5402 beginning at 65% epiboly. SU5402 treatment decreases zic3 E1 activity (Fig. 7N versus 7M), while zic3 E2 drives GFP expression at a similar level with SU5402 treatment (Fig. 7P versus 7O). The sensitivity of zic3 E1 to SU5402 is surprising given the insensitivity of endogenous zic3 expression to FGF inhibition. This suggests that other zic3 enhancers can compensate for decreased E1 activity upon FGF signaling fluctuations, building additional robustness into zic3 NPB expression. The Wnt/FGF knockdown experiments presented in Fig. 2 demonstrate Wnt signaling can mask the effect of FGF knockdown on zic3 expression.
pax3a IR1, zic3 E1 and zic3 E2 are probably direct targets of canonical Wnt signaling
pax3a IR1 contains six putative high-affinity binding sites for Tcf/Lef transcription factors within its two regions of high sequence conservation (Fig. 8A; supplementary material Fig. S6A). Mutating these sites drastically decreased the activity of the enhancer (Fig. 8D,E versus 8B,C).
Fig. 8.
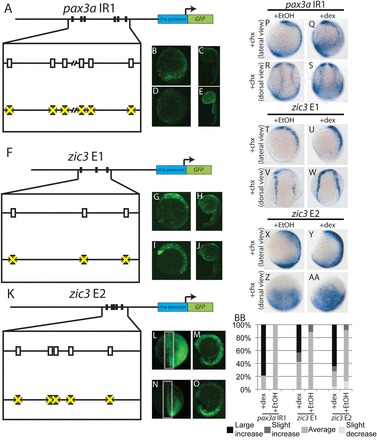
pax3a IR1 and zic3 E1 and E2 are probably direct targets of canonical Wnt signaling. (A-E) Mutating six putative Tcf/Lef-binding sites from pax3a IR1 (rectangles in A) decreases enhancer activity at 12 hpf (92%, n=36) (D versus B) and 24 hpf (100%, n=32) (E versus C). (F-J) Mutating three putative Tcf/Lef-binding sites (rectangles, F) does not affect zic3 E1 activity at 12 hpf (89%, 16 out of 18 with wild-type GFP levels) (I versus G) and 24 hpf (61%, 11 of 18 with wild-type GFP levels) (J versus H). (K-O) Mutating five putative Tcf/Lef-binding sites (rectangles, K) reduces zic3 E2 activity in the NPB at 12 hpf (87%, n=20) (N versus L) and the dorsal neural tube at 24 hpf (100%, n=40) (O versus M). (P-AA) Embryos containing pax3a IR1:GFP, zic3 E1:GFP or zic3 E2:GFP were injected with GR-Lef1-βcat mRNA. The activity of all three enhancers significantly increases with dexamethasone and cycloheximide treatment relative to ethanol and cycloheximide treatment [pax3a IR1, Q,S versus P,R (69%, P=7x10−7, n=16); zic3 E1, U,W versus T,V (57%, P=0.007, n=14); zic3 E2, Y,AA versus X,Z (73%, P=5×10−9, n=37)]. (BB) The distribution of GFP staining levels in embryos injected with GR-Lef1-βcat mRNA and treated with cycloheximide and dexamethasone or ethanol is shown for each enhancer. B-E,G-J,M,O,P,Q,T,U,X,Y are lateral views with dorsal towards the right; L,N,Z,AA are anterior-dorsal views with anterior upwards; R,S,V,W are dorsal trunk views with anterior upwards.
zic3 E1 contains three putative Tcf/Lef-binding sites in the most conserved region of the enhancer (Fig. 8F; supplementary material Fig. S6B). No decrease in enhancer activity was observed when we mutated these sites (Fig. 8I,J versus 8G,H).
zic3 E2 contains five putative Tcf/Lef-binding sites in the best conserved region of the enhancer (Fig. 8K; supplementary material Fig. S6C). Mutating these sites greatly decreases enhancer activity in the NPB and dorsal neural tube (Fig. 8N,O versus 8L,M).
To determine whether Tcf/Lef factors can induce pax3a IR1, zic3 E1 or zic3 E2 activity in the absence of translation, we injected embryos containing pax3a IR1:GFP, zic3 E1:GFP or zic3 E2:GFP transgenes with mRNA encoding the human Lef1 DNA-binding domain fused to the glucocorticoid receptor and β-catenin. This protein is a constitutively active Lef1 and is inducible by treatment with dexamethasone (Ramel and Lekven, 2004). Treatment of injected embryos with dexamethasone alone results in an increase in the activity of all three enhancers (supplementary material Fig. S7). We treated with cycloheximide beginning at 65% epiboly and added dexamethasone or ethanol 30 minutes later. We fixed the embryos at the two-somite stage and stained for GFP mRNA. In all three cases, GR-Lef1-βcat induction increases GFP expression in the absence of translation (Fig. 8P-BB), strongly suggesting the enhancers are direct targets of canonical Wnt signaling. This signaling is probably mediated through the putative Tcf/Lef-binding sites shown in Fig. 8A,K in the cases of pax3a IR1 and zic3 E2.
DISCUSSION
zic3 and pax3a NPB expression is established by intermediate levels of BMP signal and amplified by Wnt and FGF signals during mid to late gastrulation
In frog and chick, neural crest induction requires intermediate levels of BMP signaling coupled with Wnt signals (LaBonne and Bronner-Fraser, 1998; García-Castro et al., 2002; Liem et al., 1995). In frog, an FGF requirement has also been shown (Mayor et al., 1997; Monsoro-Burq et al., 2003), though FGFs may act largely through Wnts to exert their influence (Hong et al., 2008). In both species these signals induce neural crest cells in part by activating NPB specifiers from the Zic and Pax3/7 families (Monsoro-Burq et al., 2003; Bang et al., 1999), which are necessary and sufficient for the expression of neural crest specifiers such as foxd3 and soxE (Sato et al., 2005; Basch et al., 2006; Meulemans and Bronner-Fraser, 2004). BMPs and Wnts have also been shown to be important for neural crest development in zebrafish (Lewis et al., 2004; Dorsky et al., 1998; Nguyen et al., 1998). However, the earlier roles of these signals in zebrafish have not been intensively investigated. We find that an intermediate level of BMP signaling is required, starting at mid-gastrulation to position and activate zic3 and pax3a in the NPB (Fig. 1). We further find that Wnt and FGF act additively during this period to achieve maximum pax3a expression (Fig. 2). Wnts are necessary for maximum zic3 NPB expression, with the redundant role of FGFs becoming apparent only when Wnt signaling is attenuated. Thus, in zebrafish, intermediate levels of BMP during gastrulation establish the NPB, with Wnts and FGFs acting in parallel to amplify NPB specifier expression. Interestingly, Wnt inhibition at tailbud stage causes a dramatic reduction in the expression of the neural crest specifiers foxd3 and sox10 (Lewis et al., 2004). This probably reflects a later role for Wnts in inducing neural crest specifier expression distinct from its earlier role in establishing the NPB during gastrulation (Monsoro-Burq et al., 2005; Sato et al., 2005). Consistent with this, Wnts directly regulate the neural crest specifier snail through Tcf/Lef-binding sites in its promoter (Vallin et al., 2001).
zic3 and pax3a use similar cis-regulatory strategies to integrate Wnt, FGF and BMP signals
Our data suggest that zic3 and pax3a use the same signals during the same time window to achieve similar NPB expression. We asked whether these signals are integrated by similar cis-regulatory systems. We isolated enhancers driving zic3 and pax3a expression at the NPB, and tested their individual responsiveness to BMP, Wnt and FGF perturbations (Figs 3, 4, 5 6 and 7). We found that zic3 has two NPB enhancers: one responding to Wnt, FGF and BMP signals, and one responding to Wnt and BMP only (Fig. 9C). pax3a also has two NPB enhancers, one that requires both Wnt and FGF signaling and is upregulated by BMPs, and one that requires FGF signaling and is downregulated by BMPs (Fig. 9A). Thus, both zic3 and pax3a have BMP responsiveness encoded in both enhancers and each enhancer is responsive to FGF and/or Wnt signaling.
Fig. 9.
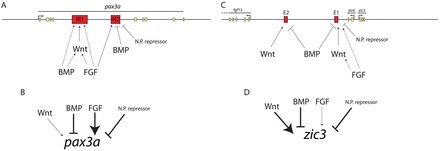
pax3a and zic3 are regulated by integrating inputs from multiple enhancers and signaling pathways. (A) pax3a IR1 requires Wnt and FGF signaling for its full activity and is activated by BMP signaling. The effect of Wnt signaling is probably direct. FGF and BMP signaling could be acting directly on IR1 or indirectly through Wnts (broken lines) as Wnt ligands are upregulated upon bmp2b overexpression (supplementary material Fig. S3) and Wnts are upregulated upon FGF overexpression in Xenopus (Hong et al., 2008). pax3a IR2 is repressed by BMP overexpression, requires FGF signaling for full activity and is probably repressed by a neural plate factor (N.P. repressor). (B) NPB pax3a expression has a strong requirement for FGF signaling and a weaker requirement for Wnt signaling. pax3a expression is repressed by BMP overexpression and probably by a repressor in the neural plate. (C) zic3 E1 requires FGF and Wnt signaling for full activity and is repressed by BMPs. FGF could be acting indirectly by inducing expression of Wnt ligands. zic3 E2 requires Wnt signaling for NPB activity and is repressed by BMP overexpression. (D) zic3 NPB expression has a strong requirement for Wnt signaling and a cryptic requirement for FGF signals that only becomes apparent when Wnt signaling is attenuated. zic3 in the NPB is repressed by BMP overexpression and probably also repressed by a factor in the neural plate.
Although each pax3a and zic3 enhancer recapitulates most aspects of endogenous NPB gene expression (except zic3 E2, which is not active in the posterior), no single enhancer fully mimics the response of its cognate gene to signaling perturbations. In both cases, endogenous gene expression is less susceptible to perturbations than are the individual enhancers, indicating that gene expression driven by a combination of multiple enhancers imparts robustness. However, our experiments demonstrate that pax3a expression is poorly buffered against FGF fluctuations. These results are consistent with recent Drosophila studies indicating that multiple ‘shadow’ enhancers can buffer gene expression against environmental fluctuations (Perry et al., 2010). Our experiments with the pax3a IR1-IR2 combination indicate that this compound enhancer increases robustness and specificity over either single enhancer alone even in the absence of additional regulatory DNA (Fig. 5).
zic3 expression is buffered against variability by cryptic responsiveness to FGF hardwired into zic3 E1
zic3 uses FGF and Wnt signals in a partially redundant manner to further buffer its expression (Fig. 2E-H). zic3 E1 is very sensitive to FGF signaling disruption, whereas SU5402 treatment does not decrease endogenous zic3 NPB expression levels (Fig. 7). This suggests additional enhancers buffer zic3 expression against FGF fluctuations. We find that zic3 expression in the NPB decreases upon SU5402 treatment when Wnt signaling is also attenuated. So if the Wnt pathway is intact, it can compensate for decreased FGF signaling in zic3 regulation. Thus, zic3 expression is buffered both by the presence of redundant enhancers and by partially redundant regulation by Wnts and FGFs. NPB induction is a process that is probably susceptible to noise in the form of random signal variation, so it follows that zic3 expression is well buffered against it.
pax3a achieves sharp, intense neural border expression by synergistic interactions between two enhancers with differential responsiveness to BMP and Wnts
Separate enhancers mediate the Wnt and BMP components of pax3a regulation at the NPB (Fig. 4), providing an opportunity to study these inputs independently. pax3a IR1, the Wnt-regulated enhancer, is active in a wider band at the NPB than endogenous pax3a, suggesting there is a broad zone in the ectoderm where Wnt signaling levels are permissive for pax3a expression (Fig. 5H, green region). This zone may correspond to the region of the ectoderm close enough to receive Wnt signals from the paraxial mesoderm. pax3a IR2 is active in a narrower band at the NPB than IR1 in a pattern closer to that of the endogenous gene, suggesting IR2 contains precise spatial information for pax3a expression (Fig. 5H, yellow zone). bmp2b overexpression shifts IR2 activity medially and weakens it, consistent with IR2 being active at intermediate BMP signaling levels. We propose that the decreased IR2 activity is the result of a repressor being present in the neural plate. Interestingly, pax3a IR1 activity increases when it expands into the neural plate, suggesting that it is not repressed by neural factors. The increase in IR1 activity may result from increased Wnt ligand expression upon bmp2b overexpression (supplementary material Fig. S3).
Our pax3a enhancer results indicate that BMP signaling positions NPB gene expression into a precise band within a larger area of the ectoderm that is receiving the proper Wnt signaling level (Fig. 5H). The combination of the pax3a IR1 and IR2 enhancers is able to read out this combination of signaling molecules and drive a strong, narrow band of pax3a expression in the NPB, although this enhancer combination does not work perfectly outside of its natural genomic context (Fig. 5). This type of enhancer synergy was recently described in Drosophila gap gene regulation and those experiments also indicate that genomic context is important for enhancer synergy to work properly (Perry et al., 2011). Gap gene and NPB gene regulation have much in common. Both gene types need to be activated in sharply defined regions within areas of broadly distributed regulatory molecules. Enhancer synergy may be a common strategy used to interpret such broad signal gradients.
Differences in the dominance of evolutionarily conserved enhancers could result in species-specific responses to experimental perturbations
Experiments in Xenopus and chick provide conflicting results as to whether FGF signaling specifies the NPB indirectly by acting through Wnt signaling (Liem et al., 1995; Hong et al., 2008; LaBonne and Bronner-Fraser, 1998) or through a Wnt-independent mechanism (Monsoro-Burq et al., 2003; Monsoro-Burq et al., 2005). The fact that pax3a IR2 is downstream of FGF signaling, but is insensitive to dkk1 overexpression, clearly demonstrates that FGF signaling has a Wnt-independent role in zebrafish NPB induction (Fig. 4D-F,O,P).
Although our findings reveal a Wnt-independent function for FGFs in zebrafish neural border induction, they also suggest how NPB induction could evolve to rely on different combinations BMP, Wnt and FGF signals in divergent vertebrates. Our results reveal that zic3 and pax3a are regulated by evolutionarily conserved enhancers responsive to different combinations of BMP, FGF and Wnt signals. Changes in the relative influence of these enhancers could thus cause zic3 and pax3/7 expression to respond differently to Wnt and FGF perturbations in different species. For example, if pax3a IR1 was lost, or pax3a IR2 somehow became dominant, pax3a would appear to be Wnt independent, whereas if pax3a IR2 was lost, or pax3a IR1 evolved to be dominant, pax3a regulation would be strongly Wnt dependent. Aside from the simple loss of partially redundant enhancers, changes in enhancer dominance could be driven by changes in the strength of transcription factor-mediated enhancer/promoter interactions. For example, one enhancer could potentially ‘out-compete’ the other by evolving to bind the basal transcriptional apparatus more stably. This mechanism would be similar to how long-range repressor elements ‘squelch’ transcription by blocking enhancer/promoter associations (Barolo and Levine, 1997; Gray et al., 1994).
Supplementary Material
Acknowledgements
We thank the members of the Medeiros lab for useful discussions, Michael Brent Hawkins for excellent fish care, David Stock for generosity with ideas and equipment, and Kristin Artinger for the wnt8a morpholino.
Footnotes
Funding
This work was supported by a University of Colorado Innovative Seed Grant to D.M.M., by a University of Colorado Undergraduate Research Opportunities Program Research Assistantship to T.A.S. and by a National Science Foundation grant awarded in 2009 to A.T.G. [DBI-0905991].
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.081497/-/DC1
References
- Bang A. G., Papalopulu N., Goulding M. D., Kintner C. (1999). Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev. Biol. 212, 366-380 [DOI] [PubMed] [Google Scholar]
- Barolo S., Levine M. (1997). hairy mediates dominant repression in the Drosophila embryo. EMBO J. 16, 2883-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch M. L., Bronner-Fraser M., García-Castro M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218-222 [DOI] [PubMed] [Google Scholar]
- Brand M., Heisenberg C. P., Jiang Y. J., Beuchle D., Lun K., Furutani-Seiki M., Granato M., Haffter P., Hammerschmidt M., Kane D. A., Kelsh R. N., et al. (1996). Mutations in zebrafish genes affecting the formation of the boundary between the midbrain and hindbrain. Development 123, 179-190 [DOI] [PubMed] [Google Scholar]
- Chocron S., Verhoeven M. C., Rentzsch F., Hammerschmidt M., Bakkers J. (2007). Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev. Biol. 305, 577-588 [DOI] [PubMed] [Google Scholar]
- Degenhardt K. R., Milewski R. C., Padmanabhan A., Miller M., Singh M. K., Lang D., Engleka K. A., Wu M., Li J., Zhou D., et al. (2010). Distinct enhancers at the Pax3 locus can function redundantly to regulate neural tube and neural crest expressions. Dev. Biol. 339, 519-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky R. I., Moon R. T., Raible D. W. (1998). Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370-373 [DOI] [PubMed] [Google Scholar]
- Endo Y., Osumi N., Wakamatsu Y. (2002). Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development 129, 863-873 [DOI] [PubMed] [Google Scholar]
- Fisher S., Grice E. A., Vinton R. M., Bessling S. L., Urasaki A., Kawakami K., McCallion A. S. (2006). Evaluating the biological relevance of putative enhancers using Tol2 transposon-mediated transgenesis in zebrafish. Nat. Protoc. 1, 1297-1305 [DOI] [PubMed] [Google Scholar]
- Frazer K. A., Pachter L., Poliakov A., Rubin E. M., Dubchak I. (2004). VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32, W273-W279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Castro M. I., Marcelle C., Bronner-Fraser M. (2002). Ectodermal Wnt function as a neural crest inducer. Science 297, 848-851 [DOI] [PubMed] [Google Scholar]
- Gray S., Szymanski P., Levine M. (1994). Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 8, 1829-1838 [DOI] [PubMed] [Google Scholar]
- Griffin K. J., Amacher S. L., Kimmel C. B., Kimelman D. (1998). Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development 125, 3379-3388 [DOI] [PubMed] [Google Scholar]
- Hong C. S., Park B. Y., Saint-Jeannet J. P. (2008). Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 135, 3903-3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K. (2004). Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201-222 [DOI] [PubMed] [Google Scholar]
- Keller M. J., Chitnis A. B. (2007). Insights into the evolutionary history of the vertebrate zic3 locus from a teleost-specific zic6 gene in the zebrafish, Danio rerio. Dev. Genes Evol. 217, 541-547 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099 [DOI] [PubMed] [Google Scholar]
- LaBonne C., Bronner-Fraser M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403-2414 [DOI] [PubMed] [Google Scholar]
- Lekven A. C., Thorpe C. J., Waxman J. S., Moon R. T. (2001). Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell 1, 103-114 [DOI] [PubMed] [Google Scholar]
- Lewis J. L., Bonner J., Modrell M., Ragland J. W., Moon R. T., Dorsky R. I., Raible D. W. (2004). Reiterated Wnt signaling during zebrafish neural crest development. Development 131, 1299-1308 [DOI] [PubMed] [Google Scholar]
- Liem K. F., Jr, Tremml G., Roelink H., Jessell T. M. (1995). Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82, 969-979 [DOI] [PubMed] [Google Scholar]
- Marchant L., Linker C., Ruiz P., Guerrero N., Mayor R. (1998). The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 198, 319-329 [PubMed] [Google Scholar]
- Martin B. L., Kimelman D. (2008). Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell 15, 121-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor C., Brudno M., Schwartz J. R., Poliakov A., Rubin E. M., Frazer K. A., Pachter L. S., Dubchak I. (2000). VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 16, 1046-1047 [DOI] [PubMed] [Google Scholar]
- Mayor R., Guerrero N., Martínez C. (1997). Role of FGF and noggin in neural crest induction. Dev. Biol. 189, 1-12 [DOI] [PubMed] [Google Scholar]
- Melby A. E., Kimelman D., Kimmel C. B. (1997). Spatial regulation of floating head expression in the developing notochord. Dev. Dyn. 209, 156-165 [DOI] [PubMed] [Google Scholar]
- Meulemans D., Bronner-Fraser M. (2004). Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291-299 [DOI] [PubMed] [Google Scholar]
- Milewski R. C., Chi N. C., Li J., Brown C., Lu M. M., Epstein J. A. (2004). Identification of minimal enhancer elements sufficient for Pax3 expression in neural crest and implication of Tead2 as a regulator of Pax3. Development 131, 829-837 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Fletcher R. B., Harland R. M. (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 130, 3111-3124 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Wang E., Harland R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167-178 [DOI] [PubMed] [Google Scholar]
- Nguyen V. H., Schmid B., Trout J., Connors S. A., Ekker M., Mullins M. C. (1998). Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 199, 93-110 [DOI] [PubMed] [Google Scholar]
- Nyholm M. K., Wu S. F., Dorsky R. I., Grinblat Y. (2007). The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development 134, 735-746 [DOI] [PubMed] [Google Scholar]
- Patthey C., Edlund T., Gunhaga L. (2009). Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 136, 73-83 [DOI] [PubMed] [Google Scholar]
- Perry M. W., Boettiger A. N., Bothma J. P., Levine M. (2010). Shadow enhancers foster robustness of Drosophila gastrulation. Curr. Biol. 20, 1562-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. W., Boettiger A. N., Levine M. (2011). Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 108, 13570-13575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel M. C., Lekven A. C. (2004). Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131, 3991-4000 [DOI] [PubMed] [Google Scholar]
- Roehl H., Nüsslein-Volhard C. (2001). Zebrafish pea3 and erm are general targets of FGF8 signaling. Curr. Biol. 11, 503-507 [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336 [DOI] [PubMed] [Google Scholar]
- Sato T., Sasai N., Sasai Y. (2005). Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 132, 2355-2363 [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829-840 [DOI] [PubMed] [Google Scholar]
- Steventon B., Mayor R. (2012). Early neural crest induction requires an initial inhibition of Wnt signals. Dev. Biol. 365, 196-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B., Araya C., Linker C., Kuriyama S., Mayor R. (2009). Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 136, 771-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper C. L., Weidinger G., Riehle K. J., Hubbert C., Major M. B., Fausto N., Moon R. T. (2007). Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134, 479-489 [DOI] [PubMed] [Google Scholar]
- Streit A., Stern C. D. (1999). Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech. Dev. 82, 51-66 [DOI] [PubMed] [Google Scholar]
- Thisse B., Thisse C. (2004). Fast release clones: A high throughput expression anaylsis. ZFIN Direct Submission. [Google Scholar]
- Thisse C., Thisse B., Schilling T. F., Postlethwait J. H. (1993). Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119, 1203-1215 [DOI] [PubMed] [Google Scholar]
- Vallin J., Thuret R., Giacomello E., Faraldo M. M., Thiery J. P., Broders F. (2001). Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J. Biol. Chem. 276, 30350-30358 [DOI] [PubMed] [Google Scholar]
- Villanueva S., Glavic A., Ruiz P., Mayor R. (2002). Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 241, 289-301 [DOI] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077-3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


