Abstract
Objective
To investigate the distribution and alteration of lymphatic vessels and draining function in knee joints of normal and osteoarthritic mice.
Methods
For the mouse models of osteoarthritis (OA), we used mice with meniscal-ligamentous injury or mice with conditional knockout of the gene for cartilage transforming growth factor β (TGF β) type II receptor. The severity of cartilage loss and joint destruction was assessed histologically. Capillary and mature lymphatic vessels were identified and analyzed using double immunofluorescence staining and a whole-slide digital imaging system. Lymphatic drainage of knee joints was examined using near-infrared lymphatic imaging. Patient joint specimens obtained during total knee or hip arthroplasty were evaluated to verify the content validity of the mouse findings.
Results
Lymphatic vessels were distributed in soft tissues (mainly around the joint capsule, ligaments, fat pads, and muscles of normal knees). The number of lymphatic vessels, particularly the number of capillaries, was significantly increased in joints of mice with mild OA, while the number of mature lymphatic vessels was markedly decreased in joints of mice with severe OA. OA knees exhibited significantly decreased lymph clearance. The number of both capillary and mature lymphatic vessels was significantly decreased in the joints of patients with OA.
Conclusion
The whole-slide digital imaging system is a powerful tool, enabling the identification and assessment of lymphatic microvasculature in the entire mouse knee. Lymphatic capillaries and mature vessels are present in various soft tissues around articular spaces. Abnormalities of lymphatic vessels and draining function, including significantly reduced numbers of mature vessels and impaired clearance, are present in OA joints.
Lymphatic vasculature is widely distributed in body tissue and is uniquely tethered to the surrounding interstitial matrix through anchoring filaments (1,2). Plasma is continuously filtered from the arterial capillary bed into the interstitial space, where excess fluid drains through permeable lymphatic endothelial cell junctions in lymphatic capillaries along with immune cells, antigens, lipids, and macromolecules (which collectively comprise the lymph). Lymph moves from the lymphatic capillaries into mature lymphatic vessels and from there to draining lymph nodes. Lymphatic capillaries are blind-ended tubes composed of single-layer lymphatic endothelial cells without a basement membrane; as such, their morphology, structural composition, and function differ from that of mature lymphatic vessels. At the tissue level, lymphatic capillaries can be identified by immunostaining with antibodies to lymphatic vessel endothelial receptor 1 (LYVE-1) (3–6) or podoplanin (5,7,8), proteins specifically expressed by lymphatic endothelial cells. Mature lymphatic vessels are characterized by the presence of lymphatic endothelial cells, a smooth muscle cell (SMC) layer, a basement membrane, and valves for unidirectional lymph flow (9,10). The SMCs exhibit spontaneous and phasic contractions that enable mature lymphatic vessels to pump lymph (11) and are critical for the draining function of lymphatic vessels. Since SMCs express α-smooth muscle actin (α-SMA), double immunofluorescence staining with anti-podoplanin and α-SMA antibodies is used in most studies to identify lymphatic vessels (12,13).
The lymphatic system plays important roles in maintaining body fluid homeostasis, immune cell trafficking, and lipid transport under normal physiologic conditions. It also participates in many pathologic processes, including tumor cell dissemination, metabolic syndrome, and chronic inflammation (10). Clinical studies have revealed increased numbers of lymphatic vessels in joints of patients with rheumatoid arthritis (RA) (14–16). We have confirmed that numbers of lymphatic vessels were increased in joint sections from mice with RA and demonstrated that enhancement of lymph flow attenuates joint tissue damage, providing experimental evidence for the potential benefit of a lymphatic enhancement–based therapy in RA (4,17).
Osteoarthritis (OA) is a degenerative joint disease characterized by cartilage loss, relatively mild syno-vial inflammation, and subchondral bone destruction. Critical pathologic events in OA include elevated production of catabolic enzymes (such as matrix metalloproteinases and aggrecanases, which break down various joint tissue components) and the development of mild chronic joint effusion.
Currently, the only effective therapy for end-stage OA is total joint replacement. OA patients often receive physical therapy, such as massage to reduce joint symptoms (18–20), during the early stages of disease, but the mechanism behind the joint swelling is not clear. Massage can enhance lymphatic drainage and has been recommended in the treatment of RA to improve lymphatic flow (21–23). We speculate that the lymphatic system may also be involved in the pathogenesis of OA. Immunostaining for LYVE-1 or podoplanin expression in joint sections from OA patients showed increased (14) or decreased (24) numbers of lymphatic vessels. This discrepancy may be a reflection of the sampling location or of the different patient populations studied. These descriptive studies did not distinguish between lymphatic capillaries and mature lymphatic vessels, since it is difficult to perform double immunofluorescence staining on paraffin-embedded joint samples that have been decalcified for 3 weeks. It is also very difficult to perform histomorphometric analysis on immunofluorescence-stained sections, since identification of lymphatic vessels requires high-magnification views, and only a small area can be observed before the fluorescence is quenched. Because of these technical challenges, the distribution and alteration of lymphatic vessels have not been studied in animal models of OA.
In this study, we used a whole-slide digital imaging system to scan and digitize immunofluorescence-stained sections; a high-magnification view of the digitized images was used to visualize lymphatic vessels and thus differentiate capillaries from mature vessels. Paraffin-embedded joint sections from mice with OA of differing degrees of severity were double immunofluorescence–stained with anti-podoplanin and α-SMA antibodies. The whole-slide imaging system was used to address the following questions: 1) whether we can identify lymphatic capillaries and mature lymphatic vessels and perform reliable histomorphometric analyses of their densities and areas, 2) whether and how lymphatic vessels are distributed in mouse knee joints, 3) whether lymphatic vessels change in OA joints, and 4) whether there is any alteration of lymphatic vessels associated with the severity of arthritic damage. Whole-slide imaging allowed us to clearly distinguish lymphatic capillaries from mature lymphatic vessels and to quantify their numbers and area in knee joint sections. We found that mature lymphatic vessels decreased in number in OA joints and that this decrease became more marked as OA progressed, accompanied by significantly reduced lymph clearance. The decreased numbers of lymphatic vessels were confirmed in joint sections from OA patients. The present findings indicate that lymphatic vessel formation and draining function are impaired in OA joints and emphasize the need for further investigation of the lymphatic system in the pathogenesis and treatment of OA.
MATERIALS AND METHODS
Animal models of OA
All murine experiments were conducted using procedures that were approved by the University Committee on Animal Resources at the University of Rochester Medical Center. We used 2 mouse models of OA: meniscal ligamentous injury (MLI)–induced OA and cartilage-specific knockout of transforming growth factor β receptor type II (TGFβRII)–induced OA. Each group contained 5–13 mice.
Ten-week-old male C57BL/6J mice purchased from The Jackson Laboratory underwent MLI on the right knee and sham surgery on the left knee, as described previously (25,26). Briefly, a 5-mm–long incision was made on the medial aspect of the right knee joint and the medial collateral ligament was transected to open the joint space. The medial meniscus was detached from its anterior attachment to the tibia, and a portion of the detached meniscus was removed. Sham surgery involved a skin incision made at the same location on the left knee. Mice were assessed 12 weeks and 20 weeks after surgery. In a separate set of mice, sham surgery involving incision of the joint capsule and isolation of ligaments was performed as previously described (27,28).
To generate cartilage-specific depletion of Tgfbr2, we crossed Col2a1-CreERT2 mice (29) with Tgfbr2fx/fx mice (30) (kindly provided by Dr. H. L. Moses, Vanderbilt University School of Medicine, Nashville, TN) to generate Tgfbr2fx/fx/Col2a1-CreERT2 mice (Tgfbr2–conditional knockout [cKO]) mice. Tgfbr2-cKO mice and their Cre-negative littermates (Tgfbr2fx/fx) were administered tamoxifen (1 mg/10 gm of body weight, intraperitoneally for 5 days) at age 2 weeks, and mice were killed 6 or 12 weeks later. The OA phenotype of Tgfbr-cKO mice has been previously described (31).
Tissue harvest and immunofluorescence staining of lymphatic vessels
Isolated knee joints were fixed in 10% neutral buffered formalin and decalcified in 10% EDTA. All joints were embedded in the same orientation. Blocks were serially sectioned in the midsagittal plane through the medial compartment of the joint. The first section was collected at the beginning of the articular cavity in the midsagittal plane. A series of 4-μm–thick sections were cut, and a total of 15 sections were collected and divided into 3 levels. Each level was ~50 μm from the previous level. One section from each of the 3 levels was assessed for Alcian blue and orange G staining and Osteoarthritis Research Society International (OARSI) scoring and histomorphometry (32). One section was chosen from level 2 and one section was chosen from level 3 for immunostaining (17). Briefly, deparaffinized sections were subjected to antigen retrieval, blocked in 5% bovine serum albumin–phosphate buffered saline, and then incubated with hamster monoclonal anti-mouse podoplanin (1:1,000 dilution; Abcam) at 4°C overnight, followed by Alexa Fluor 546–conjugated goat anti-hamster podoplanin (1:400 dilution; Invitrogen/Molecular Probes) and fluorescein isothiocyanate (FITC)–conjugated anti-mouse α-SMA (1:400 dilution; Sigma). The sections were mounted with an aqueous mounting medium, Vector H-1000, as previously described (4,33).
Histomorphometric analysis
Semiquantitative grading of OA pathology was carried out on Alcian blue and orange G–stained sections using a murine scoring system established by the OARSI Histopathology Initiative (32). The tibial articular cartilage area was measured using OsteoMeasure software (OsteoMetrics), as previously described (34). To evaluate capillaries and mature lymphatic vessels, double immunofluorescence–stained slides were scanned using an Olympus VS-110 whole-slide imaging system. The entire knee joint, including the distal femur and proximal tibia, was scanned and multiple electronic images were saved. Images were analyzed using Olympus VS-110 software version 2.3. We outlined regions of interest (ROIs), which included synovial capsules, ligaments, meniscus, and adjacent soft tissues (but excluded synovial space, articular cartilage, and bones). The total tissue area of the ROI was determined automatically with Olympus VS-110 software; it ranged from 0.998 mm2 to 1.368 mm2. The area and numbers of lymphatic vessels within the entire ROI were measured at a final magnification of 100× using a grid-counting method as we have previously described (4,17,35), and calculated by dividing the lymphatic area or numbers by the total tissue area. Podopalin+/α-SMA– vessels were defined as lymphatic capillaries and podopalin+/α-SMA+ vessels were defined as mature lymphatic vessels, according to published literature (8,11,12,36).
Indocyanine green (ICG) near-infrared (NIR) lym phatic imaging
ICG NIR lymphatic imaging was performed by intraarticularly injecting 6 μl (0.1 μg/μl) of ICG into knee joints. The dynamics of ICG fluorescence over the entire leg were visualized using an NIR imaging system that we developed (17,33,37). Briefly, the target area was excited with NIR illumination provided by a tungsten halogen nondichroic MR16 light bulb in a conventional voltage-regulated microscope light source. The infrared blocking filter typically placed between the bulb and proximal end of a fiberoptic ring illuminator was replaced with an excitation filter suitable for ICG fluorescence excitation. The ring was placed immediately below the lens (Zoom 7000; Navitar). A fluorescence emission filter without a mounting ring was placed behind the lens and in front of a high-sensitivity 1.4-megapixel CCD camera sensor (Prosilica GC1380; Allied Vision Technologies). The camera, NIR excitation, and background illumination were controlled using software developed in the LabView programming environment (National Instruments).
Human sample collection
The study of human samples was approved by the Shanghai University Communications Research Ethics Service (AO20120012). Samples were obtained during total knee arthroplasty, from patients at Putuo Hospital, Guanghua Hospital, and the 6th People's Hospital in Shanghai, China who satisfied American College of Rheumatology criteria for knee OA (38). Synovium adjacent to the medial tibial plateau, as well as mid-coronal slices of the medial tibial plateau were used. These sites were selected based on the higher prevalence and severity of OA in the medial tibial plateau (24). Control samples (the whole layer of the anterior articular capsule) were collected from patients without OA (during total hip replacement arthroplasty or hemiarthroplasty) who had a fresh (within 24–48 hours) subcapital fracture of the femoral neck. Patients with acute inflammation, diabetes, and other diseases that affect the lymphatic vessels were excluded.
Statistical analysis
Mean ± SD values were calculated. Differences between 2 groups were analyzed using Student's unpaired t-test, while comparisons among >2 groups were performed using one-way analysis of variance, followed by Dunnett's test with Bonferroni correction. Statistical analyses were performed with SPSS 16.0 statistical software. P values less than 0.05 were considered significant. Dot plots were generated using GraphPad biostatistics software.
RESULTS
Distribution of lymphatic vessels around knee joints of wild-type (WT) mice
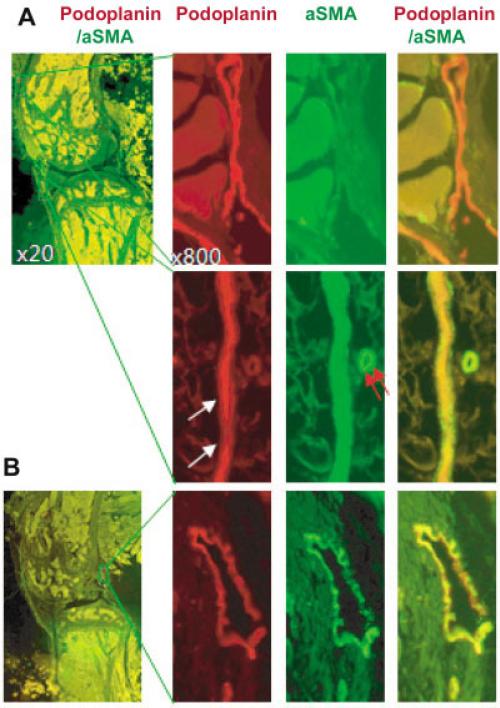
To study the distribution of lymphatic vessels in mouse knees, tissue sections from C57BL/6J WT mice were double stained with anti-podoplanin and anti–α-SMA antibodies. Podoplanin+ vessels had red fluorescence (Alexa Fluor 546), α-SMA+ vessels had green fluorescence (FITC), and podoplanin+/α-SMA+ vessels had yellow fluorescence. According to the literature (8,11,12,36), we defined podoplanin+/α-SMA– vessels as lymphatic capillaries, since they are not covered by α-SMA–expressing SMCs. We defined podoplanin+/α-SMA+ vessels as mature lymphatic vessels, since they are surrounded by a thin layer of SMCs. We observed 3 types of podoplanin+ vessels: podoplanin+/α-SMA– vessels with thin walls and open lumens (a characteristic of lymphatic capillaries) and podoplanin+ vessels covered by continuous α-SMA+ SMCs (Figure 1A) or sparse α-SMA+ SMCs (which are features of mature lymphatic vessels) (Figure 1B).
Figure 1.
Identification of lymphatic capillaries and mature lymphatic vessels. Paraffin-embedded knee sections from a 6-month-old wild-type mouse were stained with anti-podoplanin and anti–α-smooth muscle actin (anti–α-SMA) antibodies. Sections were scanned using an objective lens at 20× magnification in an Olympus VS-110 whole-slide imaging system and analyzed using the VS fluorescence software package. To help distinguish lymphatic capillaries and mature lymphatic vessels, images were observed at 100–1,000× magnification. A, Low-magnification photomicrograph showing the region of interest (ROI) (left). Boxed areas are shown at higher magnification in the top right and bottom right panels. A podoplanin+/α-SMA– lymphatic capillary is shown (top right). A podoplanin+/α-SMA+ mature lymphatic vessel (white arrows) and a podoplanin–/α-SMA+ blood vessel (red arrows) are also shown (bottom right). B, Low-magnification photomicrograph showing the ROI (left). Boxed area is shown at higher magnification in the right panels (original magnification × 800). A mature (precollecting) lymphatic vessel that is positive for podoplanin, but is covered by sparse α-SMA+ smooth muscle cells, is shown (right).
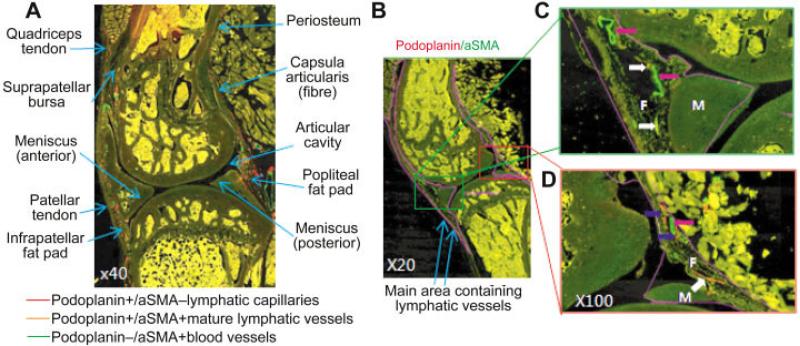
We observed numerous podoplanin+ lymphatic vessels in the soft tissues around the knee joint and deep in the synovial membrane, the fat pad, the fibrous layer of the articular capsule, the articular ligaments, and the patellar tendon region (Figure 2A). Lymphatic vessels were mainly localized in the fat pad near the meniscus, in fibrous tissues adjacent to the synovial membrane, and between ligament/tendon and bone (Figure 2A). No podoplanin staining was detected within the meniscus, articular cartilage, or subchondral bones. To evaluate alterations in lymphatic vessels, we outlined a large region that included all lymphatic vessels in the knee joint (Figure 2B), and found that almost all of the vessels could be observed clearly at 100× magnification (Figures 2C and D).
Figure 2.
Distribution of lymphatic vessels around the knee joint of a wild-type mouse. Knee sections were stained with anti-podoplanin and anti–α-smooth muscle actin (anti–α-SMA) antibodies and scanned as described in Figure 1. A, Photomicrograph showing the distribution of podoplanin+/α-SMA– lymphatic capillaries (red), podoplanin+/α-SMA+ mature lymphatic vessels (orange), and podoplanin–/α-SMA+ blood vessels (green) in the entire knee joint. Tissue areas that have lymphatic vessels are also indicated. B, Low-magnification photomicrograph of the whole joint, showing the area in which lymphatic vessels are distributed (indicated with a purple line). C and D, Higher-magnification views of the boxed areas in B, showing the anterior portion (C) and the posterior portion (D) of the joint. Lymphatic capillaries (podoplanin+/α-SMA–) (blue arrows), mature lymphatic vessels (podoplanin+/α-SMA+) (white arrows), and small blood vessels (podoplanin–/α-SMA+) (pink arrows) are shown. F = fat pad; M = meniscus.
Abnormal lymphatic vessels in knee joints of mice with osteoarthritis
To investigate if there are any changes in lymphatic vessels in osteoarthritic joints, we used 2 models of OA (26,31). We examined lymphatic vessels in joints from mice 12 weeks and 20 weeks after MLI or sham surgery.
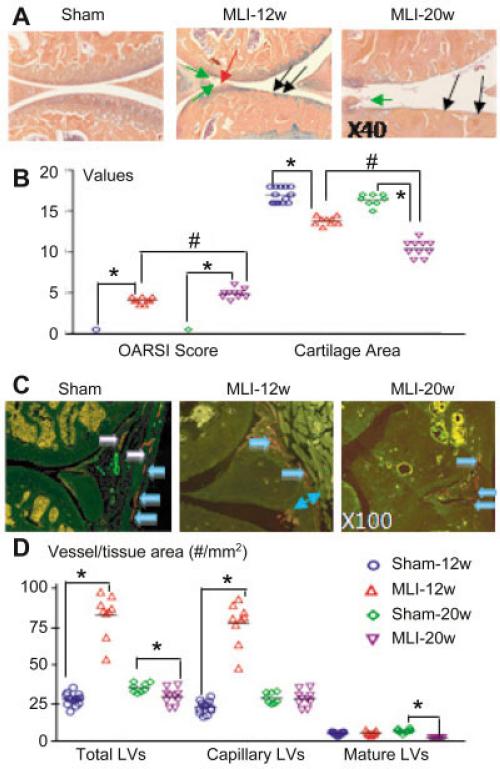
We confirmed that at 12 weeks post-MLI, mice developed mild OA-like histologic changes (Figure 3A), including clefts, floating cartilage, and articular cartilage erosion and loss. These histologic abnomalities became more severe in mice 20 weeks post-MLI (Figure 3B). In knee sections from mice 12 weeks post-MLI, we observed lymphatic vessels in all regions of the synovial membrane, including the superficial, middle, and deep zones of the subintima–the fibrous layer of the articular capsule and ligaments (Figure 3C). The synovial membrane was thicker in OA joints than in control knees, and inflammatory cell infiltration was found where lymphatic vessels were often abundant. The majority of lymphatic vessels were lymphatic capillaries, and <10% of them had a mature lymphatic phenotype (i.e., covered completely or partially by α-SMA+ cells).
Figure 3.
Decreased lymphatic vessel formation in joints of mice with meniscal ligamentous injury (MLI)–induced osteoarthritis (OA). Knees from mice 12 weeks after MLI (MLI-12w) and 20 weeks after MLI (MLI-20w) were examined (n = 9–11 mice per group). A, Orange G–Alcian blue–stained sections show features of OA, including clefts in cartilage (red arrow), floating cartilage (green arrows), and cartilage loss (black arrows). B, Osteoarthritis Research Society International (OARSI) scores and cartilage area measurements in sham-operated and MLI mice 12 and 20 weeks after surgery are shown. C, Sections were double stained with anti-podoplanin and α-smooth muscle actin antibodies and scanned as described in Figure 1. Photomicrographs show lymphatic capillaries (blue arrows) and mature lymphatic vessels (LVs) (white arrows) in the posterior portion of a sham-operated knee, abundant lymphatic capillaries in the thicker synovial membrane of a mouse 12 weeks post-MLI, and decreased numbers of lymphatic capillaries in a mouse 20 weeks post-MLI. Decreased numbers of mature lymphatic vessels are detected in mice at both 12 weeks post-MLI and 20 weeks post-MLI. D, The number of lymphatic vessels per vessel area was determined by histomorphometric analysis. In B and D, each symbol represents a single sample; bars show the mean. * and # = P < 0.05.
In knee sections 20 weeks post-MLI (Figure 3C), no obvious changes in lymphatic capillaries were detected, but the numbers of mature lymphatic vessels were markedly reduced. The numbers of total lymphatic vessels in joints from mice 12 weeks post-MLI were increased significantly and comprised mainly capillaries. No changes in the numbers of mature lymphatic vessels were detected. In contrast, the numbers of mature lymphatic vessels were decreased significantly in the joints of mice with severe OA. Similar results were obtained when the area of the lymphatic vessels was assessed (data not shown). In addition, the ratio of mature lymphatic vessels to total lymphatic vessels or lymphatic capillaries was significantly lower in joints with either mild or severe MLI-induced OA as compared to control joints (mature lymphatic vessel area/total lymphatic vessel area [mean ± SD percent] 20 ± 4 in mice 12 weeks after sham operation versus 12 ± 3 in mice 12 weeks post-MLI and 16 ± 3 in mice 20 weeks after sham operation versus 5.7 ± 1.2 in mice 20 weeks post-MLI; mature lymphatic vessel area/capillary lymphatic vessel area [mean ± SD percent] 25 ± 6 in mice 12 weeks after sham operation versus 14 ± 3 in mice 12 weeks post-MLI and 19 ± 4 in mice 20 weeks after sham operation versus 6.1 ± 1.3 in mice 20 weeks post-MLI).
While podoplanin was mainly expressed by lymphatic endothelium, expression of messenger RNA and protein for podoplanin has been reported in other cells or tissues including bone and cartilage (39,40). We found that anti-podoplanin antibody stains only lymphatic vessels in joint sections from WT mice, and it stains lymphatic vessels and other cells in joint sections from mice with severe joint inflammation. Under this condition, anti-podoplanin antibody also stains CD11b+ inflammatory cells (data not shown). To confirm that anti-podoplanin antibody stains only lymphatic vessels, we performed immunostaining on a set of sections with anti–LYVE-1 antibody, another antibody that has been extensively used to identify lymphatic vessels (3–6), and obtained a result similar to the result we obtained when we used anti-podoplanin antibody (data available upon request from the corresponding author).
In the current study, we performed sham surgery by creating a skin incision that, based on our previous findings (26), does not induce OA-like changes. However, it is possible that changes in lymphatic vessels in samples from mice with MLI-induced OA are due to surgery-related local soft tissue trauma rather than association with OA progression. To address this question, we performed sham surgery with arthrotomy/ capsulotomy without ligament transection. No significant differences in OARSI score and lymphatic vessels were observed between the 2 sham-operated groups, indicating that changes of lymphatic vasculature seen in OA joints are unlikely due to the surgery procedure itself (data available upon request from the corresponding author).
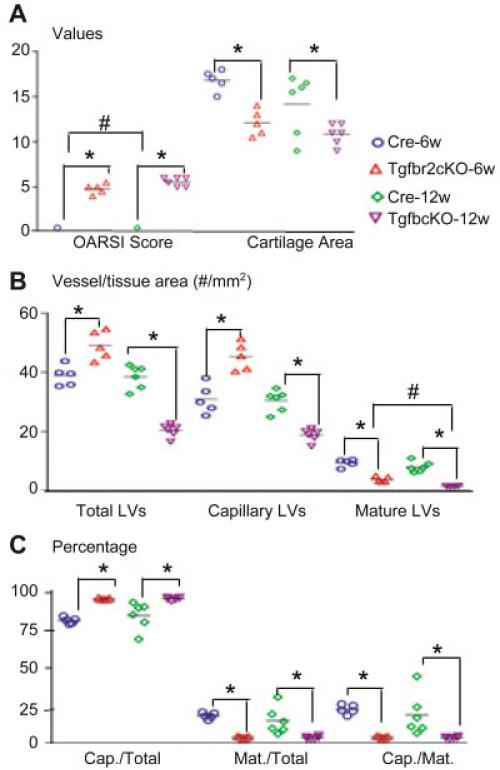
To determine if abnormal lymphatic vasculature is a common feature of OA, we examined lymphatic vessel formation in Tgfbr2-cKO mice. We first confirmed that Tgfbr-cKO mice had OA-like changes at 6 weeks and 12 weeks after tamoxifen induction. The OA-like changes in Tgfbr2-cKO mice 12 weeks after tamoxifen induction were more severe than those in mice 20 weeks post-MLI (Figure 4A), where OA-like changes included complete loss of articular cartilage, floating cartilage fragments, and exposure of subchondral bone (data not shown). Similar to mice 12 weeks post-MLI in Figure 3B, Tgfbr2-cKO mice 6 weeks after tamoxifen induction also had increased numbers of lymphatic capillaries and a slightly reduced number of mature lymphatic vessels. The decrease in number of lymphatic vessels was much more dramatic in Tgfbr2-cKO mice 12 weeks after tamoxifen induction than in mice 20 weeks post-TLI (Figures 4B and C). These findings suggest that changes in lymphatic structure, distribution, and function are associated with the severity of the OA tissue lesions.
Figure 4.
Decreased lymphatic vessel formation in joints from Tgfbr2– conditional knockout (cKO) mice. Knee joints from Tgfbr-cKO or Cre control mice at 6 weeks or 12 weeks after tamoxifen induction were examined (n = 5–11 mice per group). A, OARSI scores and cartilage area measurements of orange G–Alcian blue–stained sections are shown. B, Sections were double stained with anti-podoplanin and α-smooth muscle actin antibodies and scanned as described in Figure 1. Histomorphometric analyses of lymphatic vessel density were performed. C, The percentage of lymphatic capillary (Cap.) vessels or mature (Mat.) lymphatic vessels of the total lymphatic vessels was calculated. Each symbol represents a single sample; bars show the mean. * and # = P < 0.05. See Figure 3 for other definitions.
Decreased lymphatic draining function in joints with MLI-induced OA
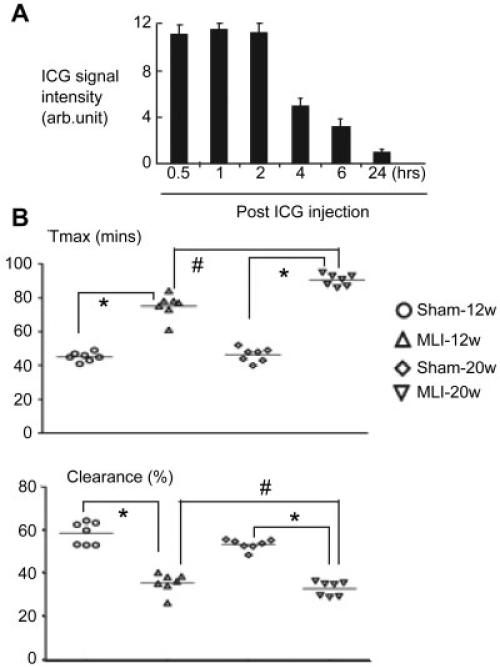
A critical function of the lymphatic system is removal of interstitial fluid. We have reported that lymphatic drainage protects joints against tissue damage in mice with RA (17). To examine if abnormal lymphatic vessels in OA joints are associated with changes in draining function, we performed ICG NIR imaging on the knees of mice with OA to assess lymphatic drainage. We first established outcome measures of lymphatic draining function in mouse knee joints. In contrast to the rapid appearance of connecting lymphatic vessels after ICG footpad injection in ankle lymphatic imaging (17), bright lymphatic vessels were not observed when ICG was injected into knee joints; instead, we observed that the ICG signal in knees reached a peak after ~30 minutes to 1 hour and disappeared within 6 hours (Figure 5A). When we opened the abdominal cavity, we observed a fluorescent lymphatic vessel extended to the iliac lymph nodes, as previously described (33,37).
Figure 5.
Impaired lymphatic function in joints with MLI-induced OA. A, Indocyanine green (ICG) was injected into the knee joints of 3-month-old wild-type (WT) mice, and ICG near-infrared lymphatic imaging was performed. The ICG fluorescence signal intensity was recorded at several time points after injection by designating a region of interest (ROI) over the knee and computing the median intensity in a fixed-size oval ROI for each animal and time point. The use of the median value permitted accurate estimation despite some pixel saturation in the ROI. Values are mean ± SD of 5 mice. Arb. = arbitrary. B, ICG was injected into the knee joints of WT mice (n = 7) 12 weeks and 20 weeks after MLI surgery on the right knee and sham surgery on the left knee, and ICG near-infrared lymphatic imaging was performed. Tmax and the percentage of ICG clearance were measured. Each symbol represents a single sample; bars show the mean. * and # = P < 0.05. See Figure 3 for other definitions.
Based on the nature of lymphatic drainage of knee joints, we developed 2 outcome measures: 1) Tmax, the time that it takes to reach the maximum signal intensity in the ROI, and 2) ICG clearance, the percentage of ICG that has been removed from the injection site 3–6 hours after the injection. We examined knee lymphatic draining function in mice 12 and 20 weeks post-MLI. Compared to the sham-operated knees, OA knees in the same mice had markedly longer Tmax and delayed clearance (Figure 5B). Alteration of Tmax and ICG clearance was more significant in knees of mice 20 weeks post-MLI as compared to knees of mice 12 weeks post-MLI. To confirm that lymphatic drainage is impaired in OA knees, we injected ICG into knee joints of mice 20 weeks post-MLI and examined ICG fluorescence intensity of iliac lymph nodes, upstream lymph nodes that drain lymph from knee areas. We observed prominently fluorescent lymphatic vessels extending to iliac lymph nodes in mice without MLI surgery, but after MLI surgery, fluorescence intensity in both lymphatic vessels and iliac lymph nodes was markedly decreased (with a mean ± SD ICG signal intensity in iliac lymph nodes of 4.2 ± 0.9 arbitrary units in OA knees versus 19.4 ± 2.9 in sham-operated knees [n = 4 per group]; P < 0.05).
Decreased lymphatic vessel formation in joint sections from OA patients
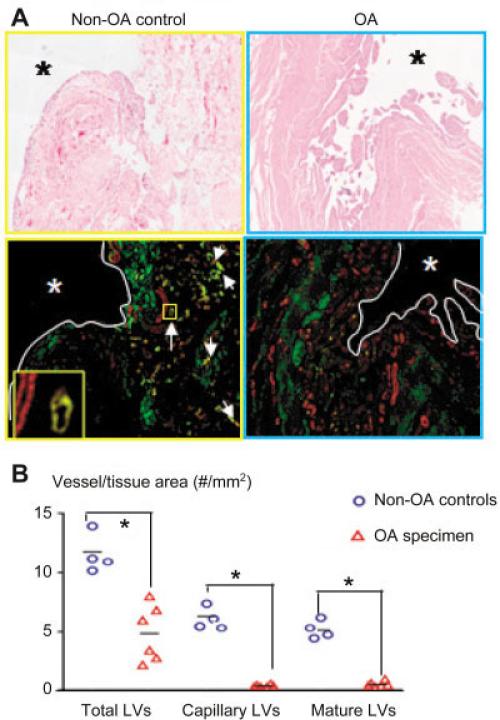
To determine if lymphatic vessel abnormalities occur in human OA, we stained lymphatic vessels in sections from joints of patients with severe OA who underwent total knee arthroplasty (n = 6) and in sections from normal joints (femoral heads) obtained from patients without an OA diagnosis but with a fresh subcapital fracture who were undergoing total hip arthroplasty or hemiarthroplasty (n = 4). The numbers and area of lymphatic vessels were significantly decreased in OA joints (Figure 6), indicating that reduced lymphatic function may also occur in human OA.
Figure 6.
Decreased lymphatic vessel formation in joints from patients with OA. Knee joint samples from patients with severe OA who underwent total knee arthroplasty (n = 6) or from patients without OA who had a fresh subcapital fracture of the femoral neck and had undergone total hip arthroplasty or hemiarthroplasty (n = 4) were used. A, Sections were stained with hematoxylin and eosin and double stained with anti-podoplanin antibodies (green) and α-smooth muscle actin (α-SMA) antibodies (red) and scanned as described in Figure 1. Photomicrographs show numerous lymphatic capillaries (green) and mature vessels (yellow) (white arrows) in the subsynovial area of a representative non-OA sample. A podoplainin+/α-SMA+ mature lymphatic vessel adjacent to a blood vessel (podoplanin–/α-SMA+) (boxed area) is shown at higher magnification in the inset. Asterisks indicate synovial space. Original magnification × 40. B, Histomorpho-metric analyses of lymphatic vessel density were performed. Each symbol represents a single sample; bars show the mean. * = P < 0.05. See Figure 3 for other definitions.
DISCUSSION
We used a whole-slide imaging system to analyze the distribution and alteration of lymphatic capillaries and mature lymphatic vessels in knee joint sections from normal mice and mice with OA. Both capillary and mature lymphatic vessels were present in various soft tissues around normal joints. Lymphatic vessels were localized mainly in loose connective tissues and in the fat pad between the synovial membrane and joint tendons (Figure 2). The lymphatic capillary vessel area and the number of lymphatic capillary vessels were increased in joints of mice with mild OA. However, the numbers of mature lymphatic vessels were markedly decreased in joints of mice with severe OA. Delayed lymphatic drainage occurred in OA knees. Decreased numbers of lymphatic vessels were also observed in tissue samples from human OA joints. These findings reveal abnormal lymphatic vasculature and function in joints with evidence of OA, and indicate that function worsens as OA progresses.
Lymphatic vessels are present in most tissues, but their distribution in the entire knee joint has not been examined previously. We found lymphatic vessels in soft tissues around articular (synovial) spaces, which are enriched in adipose tissues between the meniscus and ligaments (Figure 2). The precise role of the lymphatic system in joint fluid homeostasis is not clear. Synovial fluid is produced by synoviocytes and it helps to nourish joint cartilage and keep it lubricated. In OA, catabolic factors, such as collagenases, metalloproteinases, cytokines, and chemokines, are generated by various cell types in the joints and are released into the synovial fluid. The concentrations of these factors in synovial fluid or joint lavage specimens have been used as biomarkers for OA disease severity because of the detrimental effects these factors have on cartilage and other tissue components (41). However, it is not yet known if catabolic factors are cleared from joints via lymphatic vessels and if this clearance mechanism plays a role in the pathogenesis of OA.
Early clinical imaging studies indicated that the articular lymphatic system removes macromolecules from the articular cavity (synovial space) into draining lymph nodes (42,43) and that this action is delayed in joints of patients with OA (44). We confirmed that lymphatic drainage was impaired in mouse OA joints (Figure 5). The capacity of lymphatic drainage is determined by multiple factors, including capillary lymphatic vessels that collect fluid from the interstitial space, mature lymphatic vessels that transfer lymph within the lymphatic vessels, valves that control unidirectional flow of lymph, and draining lymph nodes that receive lymph and its contents (45–47). In this study, we found that mild OA is associated with increased numbers of lymphatic capillaries, while severe OA is accompanied by significantly decreased numbers of lymphatic vessels, particularly, decreased numbers of mature lymphatic vessels. Lymphatic capillaries are not responsible for transferring lymph, and the percentage of mature lymphatic vessels is actually reduced in mild OA, which may be why joints with mild OA also have reduced lymph clearance.
The mechanisms that mediate the decrease in the number of mature lymphatic vessels are not known because most studies have not distinguished between mature lymphatic vessels and capillaries. A unique feature of mature vessels is the presence of SMCs, the contraction of which moves lymph within the lymphatic system. Enhancement of smooth muscle function by platelet-derived growth factor BB promotes lymphatic vessel maturation and increases lymph flow (36,48). We have found decreased SMC coverage of lymphatic vessels in mice with RA (Xing L, et al: unpublished observations), suggesting that dysfunction of lymphatic SMCs affects lymphatic vessel maturation and function. Injection of vascular endothelial growth factor C into mouse RA joints attenuates tissue damage, which is associated with increased SMC coverage of lymphatic vessels and lymphatic drainage (17). Thus, administration of lymphatic growth factors might promote lymphatic vessel maturation. However, it is also possible that inflammation releases factors that inhibit lymphatic maturation. For example, vasoactive intestinal peptide (VIP), a neuro-immunomodulator with antiinflamma-tory properties, is released by nerve terminals and inflammatory cells; VIP inhibits lymphatic vessel pump activity by affecting lymphatic muscle cells (49). This process might become critical during inflammation, resulting in decreased lymph drainage, edema formation, and compromised immune cell trafficking. We do not know if similar mechanisms apply to impaired lymphatic maturation and drainage in OA. This will require further study.
RA and OA differ in many ways, with different etiology, pathogenesis, and therapy. Inflammation in OA joints is less severe than that in RA joints. Thus, it is important to identify lymphatic factors that are generated only in OA joints.
In summary, we demonstrated an increase in the number of capillary lymphatic vessels in mild OA, but a decrease in the number of mature lymphatic vessels in severe OA. Lymphatic draining function from the OA knee to the upstream iliac lymph nodes was remarkably reduced in both mild and severe OA. We confirmed that the number of both capillary and mature lymphatic vessels was significantly decreased in OA samples from humans. Currently, we do not know if lymphatic abnormality is associated with the pathogenesis of OA. We also do not know which molecular mechanisms inhibit lymphatic maturation in OA joints. To address the first question, we can investigate whether treating mice with lymphatic inhibitors and then inducing OA accelerates the development and severity of OA. To address the second question, we can study how lymphatic vessel maturation is affected by known factors that are generated and released by cells in OA joints. Answering these questions will help us determine if the lymphatic system plays an important role in OA pathogenesis and has therapeutic potential.
ACKNOWLEDGMENTS
We thank Ms Yanyun Li for technical assistance with whole-slide scanning, Mr. Robert Hoff for immunostaining, and Dr. Yinghui Ma (Department of Joint Surgery, Guanghua Hospital of Traditional and Western Medicine, Shanghai, China) for providing human samples.
Supported by the NIH (Public Health Service grants AR-43510 and 1S10-RR-027340-01 to Dr. Boyce and AR-48697 to Dr. Xing). Dr. Shi's work was supported by the Key Constructive Disciplines of State Administration of Traditional Chinese Medicine (grant T0303 and grant 2013GQ001I) and Putuo Hospital, Shanghai, China (grant 2013QK15). Dr. Xu's work was supported in part by the National Natural Science Foundation of China (through International Cooperation Programs grant 81220108027 to Dr. Wang).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Xing had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Shi, Liang, Zuscik, Shen, D. Chen, Xu, Wang, Y. Chen, Wood, Boyce, Xing.
Acquisition of data. Shi, Liang, Zuscik, Shen, Xu, Wood, Xing.
Analysis and interpretation of data. Shi, Liang, Zuscik, Shen, D. Chen, Xu, Wang, Y. Chen, Wood, Li, Boyce, Xing.
ClinicalTrials.gov identifier: NCT1430559.
REFERENCES
- 1.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–18. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–57. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–9. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 6.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-κB and Prox1. Blood. 2010;115:418–29. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noda Y, Amano I, Hata M, Kojima H, Sawa Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem Cytochem. 2010;43:61–8. doi: 10.1267/ahc.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 10.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–80. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 11.Von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–53. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–20. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–69. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis. 2003;62:1227–9. doi: 10.1136/ard.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takiwaki H, Adachi A, Kohno H, Ogawa Y. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases. J Am Acad Dermatol. 2004;50:585–90. doi: 10.1016/j.jaad.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Canete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmarti R, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor κ therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:751–6. doi: 10.1136/ard.2008.089284. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 2011;63:2318–28. doi: 10.1002/art.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurtkuran M, Kocagil T. TENS, electroacupuncture and ice massage: comparison of treatment for osteoarthritis of the knee. Am J Acupunct. 1999;27:133–40. [PubMed] [Google Scholar]
- 19.Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166:2533–8. doi: 10.1001/archinte.166.22.2533. [DOI] [PubMed] [Google Scholar]
- 20.Perlman AI, Ali A, Njike VY, Hom D, Davidi A, Gould-Fogerite S, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248. doi: 10.1371/journal.pone.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joos E, Bourgeois P, Famaey JP. Lymphatic disorders in rheumatoid arthritis. Semin Arthritis Rheum. 1993;22:392–8. doi: 10.1016/s0049-0172(05)80031-9. [DOI] [PubMed] [Google Scholar]
- 22.Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M, Gradalski T. Tissue fluid pressure and flow during pneumatic compression in lymphedema of lower limbs. Lymphat Res Biol. 2011;9:77–83. doi: 10.1089/lrb.2009.0025. [DOI] [PubMed] [Google Scholar]
- 23.Hurkmans EJ, Li L, Verhoef J, Vliet Vlieland TP. Physical therapists’ management of rheumatoid arthritis: results of a Dutch survey. Musculoskeletal Care. 2012;10:142–8. doi: 10.1002/msc.1011. [DOI] [PubMed] [Google Scholar]
- 24.Walsh DA, Verghese P, Cook GJ, McWilliams DF, Mapp PI, Ashraf S, et al. Lymphatic vessels in osteoarthritic human knees. Osteoarthritis Cartilage. 2012;20:405–12. doi: 10.1016/j.joca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Sampson ER, Beck CA, Ketz J, Canary KL, Hilton MJ, Awad H, et al. Establishment of an index with increased sensitivity for assessing murine arthritis. J Orthop Res. 2011;29:1145–51. doi: 10.1002/jor.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4–knockout mice. Arthritis Rheum. 2004;50:2547–58. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 28.Kozawa E, Nishida Y, Cheng XW, Urakawa H, Arai E, Futamura N, et al. Osteoarthritic change is delayed in a Ctsk-knockout mouse model of osteoarthritis. Arthritis Rheum. 2012;64:454–64. doi: 10.1002/art.33398. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Lichtler A, Sheu T, Xie C, Zhang X, O'Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-β type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, et al. Deletion of the transforming growth factor β receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;65:3107–19. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasson SS, Chambers MG, van den Berg WB, Little CB. The OARSI Histopathology Initiative–recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Wood R, Schwarz EM, Wang YJ, Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 2010;62:1881–9. doi: 10.1002/art.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Kim KO, Sampson ER, Chen D, Awad H, O'Brien T, et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008;58:3132–44. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyce BF. Bone biopsy and histomorphometry in metabolic bone disease. In: Stevenson JC, editor. New techniques in metabolic bone disease. Butterworths; London: 1990. pp. 110–31. [Google Scholar]
- 36.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–66. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Zhou Q, Wood RW, Kuzin I, Bottaro A, Ritchlin CT, et al. CD23+/CD21hi B-cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthritis Res Ther. 2011;13:R138. doi: 10.1186/ar3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Melrose J. Podoplanin is expressed by a sub-population of human foetal rib and knee joint rudiment chondrocytes. Tissue Cell. 2011;43:39–44. doi: 10.1016/j.tice.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ariizumi T, Ogose A, Kawashima H, Hotta T, Li G, Xu Y, et al. Expression of podoplanin in human bone and bone tumors: new marker of osteogenic and chondrogenic bone tumors. Pathol Int. 2010;60:193–202. doi: 10.1111/j.1440-1827.2009.02510.x. [DOI] [PubMed] [Google Scholar]
- 41.Seifer DR, Furman BD, Guilak F, Olson SA, Brooks SC, III, Kraus VB. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage. 2008;16:1532–8. doi: 10.1016/j.joca.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer W, Short CL, Bennett GA. The manner of removal of proteins from normal joints. J Exp Med. 1933;57:419–33. doi: 10.1084/jem.57.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levick JR. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980;306:445–61. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rayan V, Thonar EJ, Chen LM, Lenz ME, Williams JM. Regional differences in the rise in blood levels of antigenic keratan sulfate and hyaluronan after chymopapain induced knee joint injury. J Rheumatol. 1998;25:521–6. [PubMed] [Google Scholar]
- 45.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4:217–28. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 46.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–2. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 48.Maejima D, Kawai Y, Ajima K, Ohhashi T. Platelet-derived growth factor (PDGF)-BB produces NO-mediated relaxation and PDGF receptor β-dependent tonic contraction in murine iliac lymph vessels. Microcirculation. 2011;18:474–86. doi: 10.1111/j.1549-8719.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- 49.Von der Weid PY, Rehal S, Dyrda P, Lee S, Mathias R, Rahman M, et al. Mechanisms of VIP-induced inhibition of the lymphatic vessel pump. J Physiol. 2012;590:2677–91. doi: 10.1113/jphysiol.2012.230599. [DOI] [PMC free article] [PubMed] [Google Scholar]