Abstract
Some studies suggest that there are urban–rural variations in cancer incidence but whether these simply reflect urban–rural socioeconomic variation is unclear. We investigated whether there were urban–rural variations in the incidence of 18 cancers, after adjusting for socioeconomic status. Cancers diagnosed between 1995 and 2007 were extracted from the population-based National Cancer Registry Ireland and Northern Ireland Cancer Registry and categorised by urban–rural status, based on population density of area of residence at diagnosis (rural <1 person per hectare, intermediate 1–15 people per hectare, urban >15 people per hectare). Relative risks (RR) were calculated by negative binomial regression, adjusting for age, country and three area-based markers of socioeconomic status. Risks were significantly higher in both sexes in urban than rural residents with head and neck (males RR urban vs. rural = 1.53, 95 % CI 1.42–1.64; females RR = 1.29, 95 % CI 1.15–1.45), esophageal (males 1.21, 1.11–1.31; females 1.21, 1.08–1.35), stomach (males 1.36, 1.27–1.46; females 1.19, 1.08–1.30), colorectal (males 1.14, 1.09–1.18; females 1.04, 1.00–1.09), lung (males 1.54, 1.47–1.61; females 1.74, 1.65–1.84), non-melanoma skin (males 1.13, 1.10–1.17; females 1.23, 1.19–1.27) and bladder (males 1.30, 1.21–1.39; females 1.31, 1.17–1.46) cancers. Risks of breast, cervical, kidney and brain cancer were significantly higher in females in urban areas. Prostate cancer risk was higher in rural areas (0.94, 0.90–0.97). Other cancers showed no significant urban–rural differences. After adjusting for socioeconomic variation, urban–rural differences were evident for 12 of 18 cancers. Variations in healthcare utilization and known risk factors likely explain some of the observed associations. Explanations for others are unclear and, in the interests of equity, warrant further investigation.
Keywords: Urbanisation, Cancer, Incidence, Inequalities, Socioeconomic status
Introduction
The investigation of geographical variations in cancer has a long and distinguished history. For example, the International Agency for Research on Cancer, part of the World Health Organisation, has published ten volumes of cancer incidence in five continents1 documenting, in detail, cancers recorded by population-based cancer registries worldwide since the 1950s. This series was predicated on the belief that comparison of cancer rates between countries has the potential to suggest clues to aetiology (thereby advancing understanding of cancer) and identify populations which could potentially benefit from cancer control activities. Over the past two to three decades, there has been interest in investigating geographical variations in cancer within individual countries (see, for example2–6). These types of analyses can reveal more local variations in risk and highlight local differences in service provision or uptake (e.g. screening) or healthcare needs. Since inequalities need to be identified before they can be addressed, such analyses also inform the health equity agenda promoted in many countries7 and, increasingly, in individual cities.8
One approach in investigating the geographical distribution of cancer within countries has been to describe variations in rates between those resident in urban and rural areas. Many studies suggest that cancer rates are higher in urban than rural areas (see for example9–12). However, this is not a universal finding and reported patterns of association differ by country, time period, cancer site, gender and the measure of cancer burden considered.10,13–16 A particular difficulty relates to studies which focus on cancer mortality (of which there are many). Any urban–rural variations in mortality could be due to geographical differences in overall incidence, extent of disease at diagnosis or survival, and these effects cannot be distinguished if only deaths are considered. Moreover, significant proportions of cancer deaths occur in nursing homes17,18 and other locations which do not necessarily reflect where people lived during their lifetime, and this has the potential to induce spatial artifacts in mortality rates.
It is well recognized that exposure to many cancer risk factors varies across socioeconomic groups.19 In many countries, the socioeconomic composition of urban and rural areas differs, meaning that observed variations in cancer rates between urban and rural areas may simply reflect socioeconomic differences. However, many of the previous studies of this topic have not adjusted their analyses for measures of socioeconomic status. A recent study, from England and Wales, reported that differences in a series of health outcomes (including all cancers and lung cancer) between urban and rural areas were largely accounted for by adjusting for deprivation.20 This analysis was based on mortality data, and whether adjustment for socioeconomic status eliminates urban–rural variations in cancer incidence is less clear.
In a population-based study in two countries, we investigated urban–rural variations in the incidence of 18 common cancers, after adjusting for measures of socioeconomic status.
Methods
Setting
The study was conducted on the island of Ireland, which had, in 1995–2007, an average population of approximately 5.6 million. Almost 70 % (3.9 million) lived in the Republic of Ireland (RoI); the remainder lived in Northern Ireland (NI), which is part of the United Kingdom. The healthcare systems—and cancer services—of the two countries are separate. The RoI system involves a mixture of public and private provision while the NI system is predominantly public. In NI, population-based screening programs for cervical and breast cancers were in place before the mid-1990s. In RoI, population-based screening for breast cancer commenced in the Dublin area in 2000, with national roll-out achieved in 2007, while organized cervical screening was only available to women in a single area of the mid-west during the study period.
Data Sources
Data on cancers was derived from the two cancer registries in Ireland which, between them, record all cancers newly diagnosed in the populations usually resident in RoI (National Cancer Registry Ireland; www.ncri.ie) and NI (N. Ireland Cancer Registry; www.qub.ac.uk/research-centres/nicr/). Both registries operate according to internationally accepted registration and coding conventions. They identify incident cancers through a variety of hospital and community sources and collect patient (e.g. date of birth, address at diagnosis) and tumour details (e.g. cancer site, date of diagnosis) for each case. Completeness of case ascertainment in RoI has been estimated to be at least 97 % and, based on international indicators, is considered to be high in NI.21,22
In this analysis, the 18 most commonly diagnosed cancers were considered as follows: head and neck (ICD10 C01–C14, C30–32), esophagus (C15), stomach (C16), colorectal (C18–21), pancreas (C25), lung (C34), malignant melanoma (C43), non-melanoma skin (C44), breast (C50; considered for women only), ovarian (C56), corpus uteri (C54), cervix (C53), prostate (C61), bladder (C67), kidney (C64–65), brain and central nervous system (CNS) (C70–72), non-Hodgkin’s lymphoma (C82–85) and leukaemia (C91–95). Details of cases diagnosed at these sites during 1995–2007 were extracted from the registry databases and amalgamated into a single dataset. Multiple primary cancers were excluded based upon international rules.23
Cases were assigned to the smallest geographic unit for which population data is available (wards in NI and electoral divisions (EDs) in RoI), based on the address at diagnosis. In NI, this process was undertaken using a postcode-to-electoral ward lookup file (central postcode directory).24 In RoI, this was done by matching address information to the GeoDirectory database,25 which provides a list of official postal addresses and location details for every property in the country, supplemented with manual coding. For some RoI cases (4.4 %), the address was not detailed enough to pin-point the precise ED to which it belonged; these cases were allocated at random to one of the small number of possible EDs, with the probability of assignment weighted by the population of the possible EDs. In addition, for some cases, the ward/ED was entirely unknown (NI 2.7 %; RoI 3.6 %). In order not to underestimate overall incidence, for these registrations, a fraction of the cases of each cancer type was allocated in proportion to each ED/ward weighted by population.
Annual population estimates for NI wards for the years 1995–2007 were derived from annual age–sex-specific population estimates for district councils, using the 2001 census (the only census during the study period) as the basis for the splits by ward.26,27 In RoI, annual population estimates for EDs were obtained by linear interpolation of the ED populations provided in the 1996, 2002 and 2006 censuses, constrained by the age–sex-specific annual national population estimates.28,29
Since there is no standard definition of urban and rural areas in Ireland, we used population density as an ordinal indicator of the degree of urbanisation of a geographical area. Three categories were created, each containing approximately one-third of the 1995–2007 total population: “rural” (<1 person/hectare, 36 % of total population; average population 2,004,451), “intermediate” (1–15 persons/hectare, 29 %; 1,644,792) and “urban” (>15 persons/hectare, 35 %; 1,940,844).
Markers of socioeconomic status for each geographical area were obtained from NI 2001 and RoI 2002 census data. Three measures had sufficient compatibility in their definitions between the NI and RoI censuses and were therefore included as follows: (1) unemployment—the proportion of the economically active population aged 16–74 resident in the area who were unemployed, (2) educational attainment—the proportion of people aged 16–74 resident in the area who had a university degree and (3) elderly living alone—the proportion of people aged 75 and older resident in the area who lived alone. Among the elderly, who have the highest rates of cancer, living alone is a marker of poverty and experiencing multiple types of enforced deprivation (e.g. inability to afford adequate heating, new clothes or a meal out).30 Following convention, for each of these socioeconomic variables, wards and EDs were grouped into quintiles, each containing approximately 20 % of the total 1995–2007 population, and ranging from lowest (quintile 1) to highest (quintile 5).
Statistical Analysis
A count of the observed number of cancer cases by site and sex was generated for each ward/ED. Expected numbers were calculated by applying all-Ireland incidence rates for each 5-year age group to population counts. Relative risks (RR), with 95 % confidence intervals, were estimated for categories of population density, using negative binomial regression to account for over-dispersion in the data.31 Rural areas formed the reference group, and the RRs for intermediate and urban areas were adjusted for age, country (NI/RoI) and the three markers of socioeconomic status (unemployment, educational attainment and elderly living alone). The inclusion of country in the model was intended, in part, to adjust for systematic differences between countries in incidence, which might have resulted, for example, from the different healthcare systems. The Spearman rank correlation coefficients between the three markers of socioeconomic status were relatively low (unemployment and education, 0.197; unemployment and elderly living alone, 0.147; education and elderly living alone, 0.008), giving confidence that including all three in the model would be unlikely to result in over-adjustment for socioeconomic status.
To aid in summarising the findings and in interpretation, the results for the 18 cancer sites are presented in three groups as follows: (1) cancers where incidence is generally considered to be positively associated with socioeconomic status (non-melanoma skin cancer, malignant melanoma and breast and prostate cancer), (2) cancers where incidence is generally considered to be negatively associated with socioeconomic status (head and neck, esophageal, stomach, colorectal, pancreatic, lung, bladder, kidney and cervical cancer) and (3) cancers where incidence does not vary by socioeconomic status, or associations with socioeconomic status are inconsistent (ovary, uterus and brain and CNS cancers, and non-Hodgkin’s lymphoma and leukaemia).32–38 In a secondary analysis, we distinguished between colon and rectal cancers.
Results
The average annual number of cases of each cancer is shown in Table 1. For Ireland as a whole, the most common cancer in both sexes was non-melanoma skin cancer. In males, this was followed by cancers of the prostate, colorectum and lung. In females, it was followed by cancers of the breast, colorectum and lung. In males, the percentage of all cases which were diagnosed in NI residents ranged from 25 (for prostate cancer) to 34 % (for lung and stomach cancer). In females, the percentage of cases occurring in NI was lowest for brain and CNS cancer (28 %) and highest for head and neck cancer (38 %).
Table 1.
Average annual numbers of cancers diagnosed in Ireland, by site, country and sex, 1995–2007
| Cancer site (ICD10 code) | Ireland | Republic of Ireland | Northern Ireland | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Head and neck (C01-C14,C30-C32) | 438 | 170 | 294 | 105 | 144 | 65 |
| Esophagus (C15) | 301 | 182 | 202 | 122 | 98 | 60 |
| Stomach (C16) | 442 | 278 | 294 | 181 | 148 | 97 |
| Colorectal (C18-C21) | 1,631 | 1,307 | 1,114 | 849 | 517 | 458 |
| Pancreas (C25) | 269 | 272 | 189 | 192 | 81 | 80 |
| Lung (C34) | 1,602 | 1,000 | 1,052 | 649 | 551 | 351 |
| Malignant melanoma (C43) | 285 | 421 | 201 | 298 | 84 | 123 |
| Non-melanoma skin cancer (C44) | 4,294 | 3,777 | 3,080 | 2,666 | 1,215 | 1,111 |
| Breast (C50) | – | 2,965 | – | 1,990 | – | 975 |
| Cervix (C53) | – | 289 | – | 205 | – | 84 |
| Uterus (C54) | – | 403 | – | 258 | – | 145 |
| Ovary (C56) | – | 479 | – | 319 | – | 159 |
| Prostate (C61) | 2,550 | – | 1,900 | – | 649 | – |
| Kidney (C64-C65) | 310 | 188 | 214 | 123 | 96 | 65 |
| Bladder (C67) | 479 | 193 | 331 | 133 | 147 | 60 |
| Brain and CNS (C70-C72) | 234 | 174 | 166 | 125 | 68 | 49 |
| Non-Hodgkin’s lymphoma (C82-C85) | 392 | 354 | 265 | 224 | 127 | 130 |
| Leukaemia (C91-C95) | 348 | 243 | 256 | 172 | 92 | 71 |
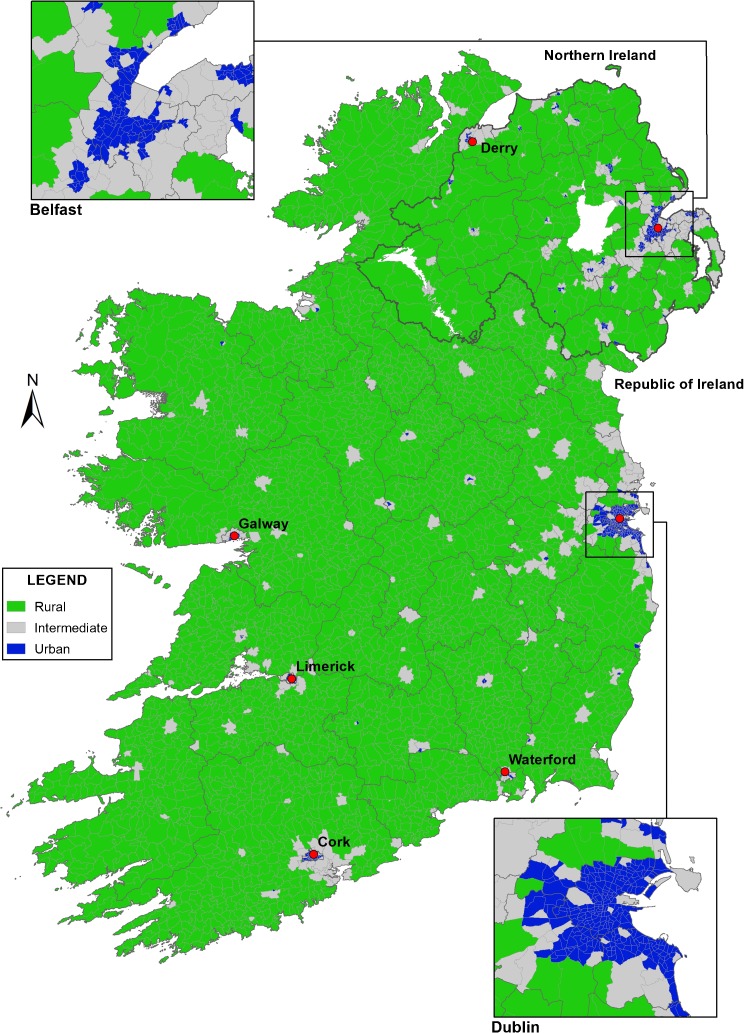
The majority of the land mass of the island was designated rural (population density <1 person/hectare; Fig. 1). The areas designated urban broadly corresponded to cities and larger towns. Intermediate areas were mainly in the periphery of cities or encompassed moderate-sized towns.
FIGURE 1.
Distribution of urban, intermediate and rural areas, Ireland, 1995–2007 and locations of the largest towns and cities.
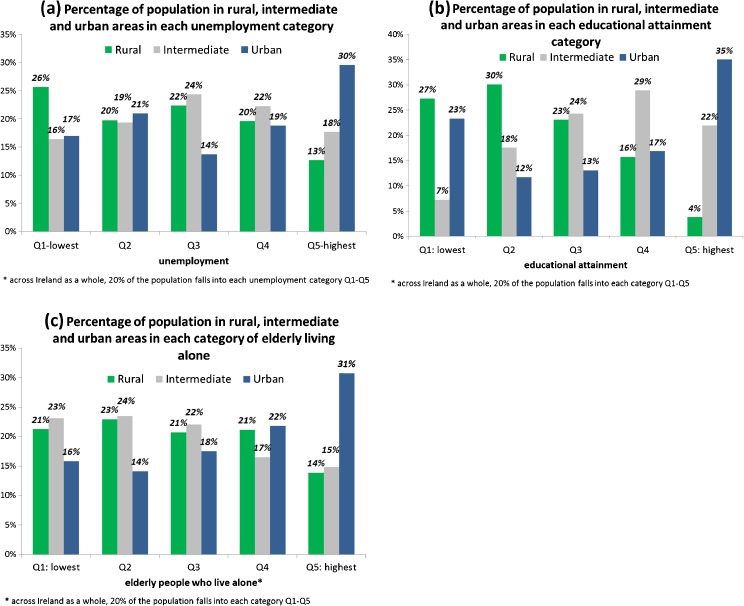
While across the entire island, 20 % of the population fell into each unemployment quintile, this varied significantly between urban, intermediate and rural areas (Fig. 2a; chi-square p < 0.001). In rural areas, 13 % of the population was in the highest unemployment category, compared to 30 % in urban areas; in rural areas, 26 % of the percentage was in the lowest unemployment category compared to 17 % in urban areas. There were also strong, albeit somewhat different, associations between urban–rural status and educational attainment (Fig. 2b; chi-square p < 0.001) and elderly living alone (Fig. 2c; chi-square p < 0.001). Thirty-five percent of people residing in urban areas were in the highest educational attainment category, compared to 4 % in rural areas. In contrast, similar proportions in urban and rural areas were in the lowest educational attainment category (23 and 27 %). In terms of elderly living alone, 14 % of people resident in rural areas were in the category with the highest percentage of elderly people living alone compared to 31 % in urban areas.
FIGURE 2.
Associations between markers of socioeconomic status (unemployment, educational attainment and elderly living alone) and urban–rural status in the population, males and females, Ireland, 1995–2007.
In total, across the entire study period, there were 129,380 cancers in people resident in urban areas, 90,597 in people resident in intermediate areas and 121,810 in people resident in rural areas. Table 2 shows, for the four cancers which are usually positively associated with socioeconomic status, RRs adjusted for age, country and the three socioeconomic markers for people resident in intermediate and urban areas, compared to those resident in rural areas. For two sites (non-melanoma skin and breast cancer), risk increased with increasing urbanisation; although the RRs for intermediate and urban areas were only moderately raised, all were statistically significantly different from unity. For malignant melanoma, for both sexes, risk was slightly higher in urban than rural areas, but this did not reach statistical significance; for males, but not for females, risk was significantly higher in intermediate than in rural areas. For prostate cancer, RRs fell with increasing urbanisation; men resident in urban areas had a statistically significant 6 % lower risk of being diagnosed with prostate cancer than men in rural areas.
Table 2.
Associations between urban–rural residence and cancer risk: adjusted relative risks (RR)a, with 95 % confidence intervals, by cancer site and sex
| Cancer site | Males | Females | ||||
|---|---|---|---|---|---|---|
| Rural | Intermediate | Urban | Rural | Intermediate | Urban | |
| Cancer sites positively associated with socioeconomic status | ||||||
| Malignant melanoma | 1.0 (ref) | 1.20 (1.10–1.31) | 1.08 (0.99–1.18) | 1.0 (ref) | 0.99 (0.92–1.07) | 1.06 (0.98–1.14) |
| Non-melanoma skin cancer | 1.0 (ref) | 1.10 (1.06–1.14) | 1.13 (1.10–1.17) | 1.0 (ref) | 1.11 (1.07–1.15) | 1.23 (1.19–1.27) |
| Breast | – | – | – | 1.0 (ref) | 1.05 (1.02–1.08) | 1.09 (1.05–1.12) |
| Prostate | 1.0 (ref) | 0.97 (0.93–1.01) | 0.94 (0.90–0.97) | – | – | – |
| Cancer sites negatively associated with socioeconomic status | ||||||
| Head and neck | 1.0 (ref) | 1.20 (1.11–1.30) | 1.53 (1.42–1.64) | 1.0 (ref) | 1.08 (0.96–1.22) | 1.29 (1.15–1.45) |
| Esophagus | 1.0 (ref) | 1.07 (0.98-1.17) | 1.21 (1.11–1.31) | 1.0 (ref) | 1.10 (0.98–1.24) | 1.21 (1.08–1.35) |
| Stomach | 1.0 (ref) | 1.15 (1.07–1.25) | 1.36 (1.27–1.46) | 1.0 (ref) | 1.06 (0.96–1.16) | 1.19 (1.08–1.30) |
| Colorectal | 1.0 (ref) | 1.07 (1.03–1.12) | 1.14 (1.09–1.18) | 1.0 (ref) | 1.01 (0.97–1.06) | 1.04 (1.00–1.09) |
| Pancreas | 1.0 (ref) | 0.96 (0.88–1.06) | 0.93 (0.85–1.02) | 1.0 (ref) | 1.07 (0.98–1.18) | 1.06 (0.97–1.17) |
| Lung | 1.0 (ref) | 1.25 (1.19–1.31) | 1.54 (1.47–1.61) | 1.0 (ref) | 1.35 (1.27–1.44) | 1.74 (1.65–1.84) |
| Bladder | 1.0 (ref) | 1.18 (1.10–1.27) | 1.30 (1.21–1.39) | 1.0 (ref) | 1.15 (1.03–1.29) | 1.31 (1.17–1.46) |
| Kidney | 1.0 (ref) | 1.06 (0.97–1.15) | 1.05 (0.96–1.14) | 1.0 (ref) | 1.10 (0.99–1.23) | 1.13 (1.01–1.26) |
| Cervix | 1.0 (ref) | 1.39 (1.26–1.52) | 1.48 (1.35–1.62) | |||
| Cancer sites not associated with socio-economic status | ||||||
| Brain and CNS | 1.0 (ref) | 1.01 (0.91–1.11) | 1.03 (0.94–1.14) | 1.0 (ref) | 1.03 (0.92–1.16) | 1.13 (1.01–1.26) |
| Ovary | – | – | – | 1.0 (ref) | 1.02 (0.96–1.10) | 1.02 (0.96–1.09) |
| Uterus | – | – | – | 1.0 (ref) | 0.97 (0.90–1.05) | 0.94 (0.87–1.01) |
| Non-Hodgkin’s lymphoma | 1.0 (ref) | 0.99 (0.92–1.07) | 1.05 (0.98–1.13) | 1.0 (ref) | 0.95 (0.88–1.03) | 1.04 (0.97–1.13) |
| Leukaemia | 1.0 (ref) | 0.96 (0.89–1.04) | 0.94 (0.86–1.01) | 1.0 (ref) | 0.98 (0.89–1.08) | 0.99 (0.90–1.09) |
aAdjusted for age, country, unemployment, educational attainment, and elderly living alone; RRs shown in bold are statistically significantly different from 1 (p≤O.05)
Of the nine cancers which are usually negatively associated with socioeconomic status, one—pancreatic cancer—showed no relationship with urban–rural residence (Table 2). For the eight other sites (head and neck, esophagus, stomach, colorectum, lung, bladder, kidney and cervix), risk was significantly higher in urban than rural residents, in at least one sex. The strongest associations (RRs raised by around 50 % or more in urban areas) were seen for cancers of the head and neck (in males), lung (males and females) and cervix. Risks for urban residents were also significantly raised, albeit more modestly (approximately 20–40 %), for females with head and neck cancer and for both males and females with esophagus, stomach and bladder cancer. Associations were less strong (risk raised by 4–14 %) for colorectal and kidney cancers. For males, for all sites with the exception of esophagus, pancreas and kidney, people residing in intermediate areas also had significantly higher risk than those in rural areas, although the RR was closer to unity than that for urban areas. For females with lung, bladder and cervical cancer, risk estimates in intermediate areas were also significantly raised compared to rural areas, but were lower than for urban areas. When colon and rectal cancers were considered separately, for colon cancer, the risk was significantly raised for males in urban areas (RR = 1.14, 95 % CI 1.09–1.20) but not for females (RR = 1.00, 95 % CI 0.95–1.05); for rectal cancer, in both sexes, the risk estimate was modestly higher in urban areas (males RR = 1.13, 95 % CI 1.06–1.19; females RR = 1.13, 95 % CI 1.05–1.22).
Of the five cancers not usually associated with socioeconomic status, risks for four (ovary and uterus cancer, non-Hodgkin’s lymphoma and leukaemia) did not vary significantly by urban–rural residence (Table 2). Risk of cancers of the brain and CNS was significantly higher in urban than rural areas, but this was seen only in females, and the risk estimate was only modestly raised (13 %).
Discussion
We found urban–rural variations in the incidence of 12 of 18 of the most common cancers in Ireland, after adjusting for various markers of socioeconomic status. For 11 of these sites, risk was significantly higher in people residing in urban compared to rural areas. The exception was prostate cancer, for which incidence was significantly lower in urban than rural areas.
Cancer Registration
In any analysis of routinely collected data, the possibility must be considered that the patterns observed are an artifact of the data collection process. The two registries from which the data was abstracted follow systematic protocols for identification and registration of cancer cases, which are based on best international practise, and implemented by trained staff. Registrations are actively sought in hospitals and community organizations (e.g. nursing homes) in both urban and rural areas from a variety of data sources, including pathology reports, oncology and radiotherapy clinic records, hospital administration systems and discharge records. The data collected are strictly quality controlled both internally and at the time of submission to international data repositories. As a result of these thorough and rigorous processes, levels of completeness of registration are considered likely to be very high.21,22 While the possibility cannot be entirely excluded that the proportion of “missed” registrations is higher in people residing in rural areas, it seems very unlikely that there are systematic geographical variations in cancer registration to such an extent that they would cause the urban–rural differences in incidence reported here. A proportion of cases could not be assigned with confidence to a specific ED/ward, typically because the patient address lacked sufficient detail. A fraction of these cases was assigned to each ED/ward to avoid underestimating overall incidence. It is possible that cases from rural areas may more often have non-specific addresses, so this process may have meant that we slightly underestimated incidence in rural areas. However, the proportion of such cases was low and would not explain the observed associations. There must, therefore, be other explanations for the patterns observed.
Adjusting for Socioeconomic Status
One of our objectives was to document urban–rural variations in cancer risk, after adjusting for socioeconomic status. The only available markers of socioeconomic status were area-based and derived from census information. Our lack of information on individual-level socioeconomic measures (such as income or occupation) was a limitation, but applies to almost all studies based on routinely collected data. It is also always possible that any marker of socioeconomic status, whether at the area-level or individual-level, may not accurately reflect actual socioeconomic status. These two issues are likely to have resulted in some misclassification of cancer cases and residual confounding. Two observations suggest that this cannot be the sole explanation for the observed associations between urban–rural residence and cancer, however. Firstly, the patterns of association between cancer risk and unemployment and educational attainment in this dataset39 were generally consistent with relationships with socioeconomic status (measured at the area- and individual-level) reported in other developed countries.32–38 Secondly, incidence was higher in urban than rural areas for cancers which are positively (breast and non-melanoma skin cancer) and cancers which are negatively (for example, lung and head and neck cancer) associated with socioeconomic status.
Variations in Access to Screening and Diagnostic Services
Geographical differences in the incidence of some cancers could potentially be explained by area-level variations in access to health services. In this study, country-level variations in healthcare access are also possible, since NI has mainly publically-funded healthcare whereas the RoI system involves a mixture of public and private provision. However, the urban–rural risk estimates were adjusted for country, so this will have attenuated any such systematic country-level differences.
In both the UK (of which NI is part) and RoI, rates of health service utilization in primary care are lower in rural than urban areas.40–42 This provides a possible explanation for the observed urban–rural variations in risk of non-melanoma skin cancers, which are relatively asymptomatic and rarely fatal and many of which can be managed by a general practitioner (GP) without referral to hospital services.
Prostate cancer incidence rates are driven by prostate-specific antigen (PSA) testing,43 most of which is conducted in primary care. PSA testing is widespread in Ireland.44 In NI, PSA testing levels are higher among GPs in rural practises45 and in RoI, although urban–rural variations have not been specifically investigated, there are striking variations between GPs in propensity to undertake PSA testing of asymptomatic men.46 The threshold for onward referral for prostate biopsy in men whose PSA level is “raised” is also a major determinant of prostate cancer incidence,44 and GPs in urban and rural areas may differ in this regard. The observed higher rural prostate cancer risk suggests that, although men resident in rural areas may be less likely to attend their GP, once there, they may be more likely to have a PSA test and, if their PSA level is raised, to be referred onwards for biopsy.
In Ireland, in common with many other countries, women are encouraged to attend for cervical and breast cancer screening. Effective cervical screening reduces cancer incidence (and mortality) in the population, thus areas with higher screening prevalence might be expected to have lower cancer incidence. Organized cervical screening with regular call–recall has been in place in NI since the late 1980s, and commenced on a nationwide basis in RoI in 2008. While many cervical smears were taken annually before the screening programme started in RoI (i.e. opportunistically), little is known about which women had smears and how often. In the UK, once London is discounted, there are no differences in screening uptake between urban and rural areas.47 Given this, and the fact that most smears are taken by GPs, and there is higher GP utilization in urban areas, it seems unlikely that differential uptake of cervical screening could explain the higher cervical cancer risk in urban areas. In terms of breast cancer, urban–rural variations in uptake of organized screening might be expected to have a short-term impact on incidence of invasive cancers (during the prevalent screening round), and a longer-term effect on mortality (which was not considered in this study). In RoI, mammographic breast screening was rolled-out gradually during 2000–2007, starting in Dublin, the capital city. In NI, screening was introduced in 1993 and, consistent with patterns elsewhere in the UK, uptake is lower in and around Belfast, the most densely-populated area.48 All in all, therefore, it is unlikely that the urban–rural difference in breast cancer incidence is due to variations in screening uptake.
Variations in Cancer Attitudes and Help-Seeking
Rural cancer patients have lower expectations of cancer care and are less demanding than urban patients.49 Such findings suggest there may be differences in cancer beliefs, attitudes and help-seeking behaviour between urban and rural residents. If these differences exist, however, they would be expected to manifest as urban–rural variations in stage at diagnosis (which have been shown in some studies; see for example50–52), rather than in overall incidence; the investigation of urban–rural variations in stage at diagnosis was beyond the scope of this paper.
Urban–rural variations could also arise as a result of people with cancer symptoms moving to more urban areas to seek management in specialised cancer centers (which tend to be located in cities). Since we only had information on each individual’s address at the time of cancer diagnosis, we could not explore this. However, it seems unlikely that such mobility would be extensive enough to produce the patterns seen here.
Variations in Cancer Risk Factors
Smoking is a firmly established risk factor for all six cancers where risk was at least 20 % higher in people residing in urban compared to rural areas (head and neck, esophagus, stomach, lung, bladder and cervix).53 Notably, for two of the cancers for which the association with urban residence was strongest—lung and head and neck cancer—the attributable risk for smoking is large.54,55 In 2010, there were regional-level variations in current smoking prevalence in NI and RoI,36 and analysis of data from the 2007 SLAN survey in RoI,56 conducted by the authors of the current paper, revealed that the prevalence of ever and current smoking was slightly—but significantly—higher in towns, cities and mixed urban/rural areas than in entirely rural areas (ever 50.0 vs. 45.7 %, p < 0.001; current 30.0 vs. 24.9 %, p < 0.001). While cancer arises from past exposure to tobacco, and such data are lacking for Ireland, it seems very likely that urban–rural variations in tobacco use contribute to the variations in cancer risk observed here.
Some risk factors are particularly relevant for specific cancers. For cervical cancer, persistent infection with certain “high-risk” strains of HPV is considered a necessary cause.57 There are variations in HPV prevalence across the UK and RoI, albeit at a large geographical scale,58,59 and these are likely to explain, at least in part, urban–rural variations in cervical cancer incidence. For malignant melanoma, exposure to UV, particularly intermittent or recreational, is the dominant risk factor.60 It has been suggested in other countries that urban residents are likely to have greater access to air travel and hence take more foreign holidays.61,62 Consistent with this, RoI census data shows that Dublin residents are most likely to travel abroad.6 In addition, in NI, there are sociodemographic variations in skin cancer knowledge and sun protection practises.63 Moreover, in RoI, use of sunbeds is higher in urban than rural areas.64 Thus, variations in exposure to natural and artificial UV sources most probably account for the slightly elevated risk of melanoma in urban areas.
One of the most obvious differences between urban and rural areas is in levels of air pollution. Some large cohort studies have suggested that outdoor air pollution may increase risk of lung cancer, especially in combination with other risk factors such as smoking and occupation exposures,65 but there does not seem to be much plausible evidence for the role of air pollution in other cancers. Prevalence of exposure to some other established cancer risk factors has been shown to vary geographically in some countries (see for example, alcohol in Scotland,66 obesity in Finland,67 HRT in Denmark,68) but similar data is lacking for Ireland. Nonetheless, it is plausible that urban–rural variations in these and other established risk factors—such as physical activity, diet and occupational exposures—contribute to the observed variations in cancer incidence. However, even allowing for the possibility of geographical differences in exposure to known risk factors, there remains considerable uncertainty about what underlies many of the associations observed in this analysis; this is partly a reflection of the limited understanding of the aetiology of several common cancers.
Strengths and Limitations
The major strengths of this study are that it was population-based, used high-quality cancer incidence data and considered multiple cancer sites. However, since it was an area-based analysis, it is possible that the results are due to ecological fallacy. In this instance, such a fallacy could arise from socioeconomic differences in people in urban and rural areas, but the analysis was adjusted for socioeconomic markers, albeit at an area-level.
The only measure of urban–rural status that is available for Ireland as a whole is population density. Inspection of maps confirmed that the most densely populated areas coincided with the centers of the cities and largest towns, intermediate areas coincided with the periphery of cities and moderate-sized towns. Therefore, is seems plausible that population density provides a reasonably measure of urban–rural residence. Our definition of “urban” was a density of at least 15 people per hectare which in some other countries might not be considered particularly densely populated. There may be gradients of cancer risk with increasing population density within areas defined here as “urban”, but we had too few cases resident in very densely populated areas to be able to investigate this.
Implications
Our findings have several implications for cancer surveillance and control. Firstly, investigating and monitoring urban–rural variations should be recognized as an important part of cancer surveillance activities at the population-level. Nowadays, many cancer registries in developed countries routinely describe and monitor socioeconomic variations in cancer as part of a responsibility to explore (in)equalities in cancer; our study suggests that similar attention should be paid to documenting the extent to which cancer risk varies spatially. Secondly, analyses of urban–rural variations in cancer incidence and mortality should routinely control for socioeconomic status, to ensure that the independent effects of the two concepts are characterised. However, as this study shows, different socioeconomic measures can have different patterns of associations with urbanization: in Ireland as a whole, urban areas tended to have both higher levels of unemployment and higher levels of educational attainment. Moreover, patterns of association may differ in different countries: in NI, urban areas and areas of socioeconomic deprivation tend to coincide, whereas in RoI, some of the most deprived areas are rural. This suggests that care needs to be taken in each setting to adequately and appropriately control for socioeconomic status. Thirdly, attention should be paid in investigating the explanations for the types of urban–rural variations described here and elsewhere; improved understanding of these is needed if strategies/interventions to eliminate disparities are to be successful.
Conclusions
During 1995–2007, there were striking urban–rural variations in the incidence of 12 of 18 of the most common cancers in Ireland; these were evident after adjusting for various markers of socioeconomic status. For one cancer—prostate—risk was higher in people resident in rural areas, an association likely to be driven by geographical variations in PSA testing and prostate biopsy. For the remaining 11 sites—which comprised a mixture of cancers positively and negatively associated with socioeconomic status—risk was significantly higher in people residing in urban areas. For some cancers, these raised risks are likely to be explained by variations in healthcare utilization and known risk factors, most notably smoking and HPV infection. For others, there are no obvious explanations and, in the interests of greater equity, further investigation is warranted.
Acknowledgments
We are grateful to the tumour registration officers and the data processing staff in the two registries who collected the data which formed the basis of this paper. We thank the Ordnance Survey of Ireland and Ordnance Survey Northern Ireland for the maps of electoral divisions and wards. Ordnance Survey Ireland map is reproduced under OSI licence number NCRI/03/05. The Northern Ireland map is Crown Copyright and is reproduced with permission of Land and Property Services under delegated authority from the Controller of Her Majesty’s Stationery Office.
The Northern Ireland Cancer Registry is funded by the Public Health Agency and the National Cancer Registry Ireland by the Department of Health. The funders had no role in study design; data collection, analysis and interpretation; writing the report; or the decision to submit the paper for publication.
References
- 1.International Agency for Research on Cancer. CI5. Cancer Incidence in Five Continents. 2013 Available at URL: http://ci5.iarc.fr/ [Accessed March 26, 2013]
- 2.Pukkala E. Cancer maps of Finland: an example of small area-based mapping. Rec Res Cancer Research. 1989;114:208–215. doi: 10.1007/978-3-642-83651-0_20. [DOI] [PubMed] [Google Scholar]
- 3.Draper G. The geographical epidemiology of childhood leukaemia and non-Hodgkin’s lymphoma in Great Britain 1966–83. London: Office of Population Censuses and Surveys; 1991. [Google Scholar]
- 4.Siesling S, van der Aa MA, Coebergh JW, Pukkala E. The Working Group of The Netherlands Cancer Registry. Time-space trends in cancer incidence in the Netherlands in 1989–2003. Int J Cancer. 2008;122:2106–2014. doi: 10.1002/ijc.23358. [DOI] [PubMed] [Google Scholar]
- 5.Aragones N, Perez-Gomez B, Pollan M, et al. The striking geographical pattern of gastric cancer mortality in Spain: environmental hypotheses revisited. BMC Cancer. 2009;9:316. doi: 10.1186/1471-2407-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carsin AE, Sharp L, Comber H. Geographical, urban/rural and socioeconomic variations in nonmelanoma skin cancer incidence: a population-based study in Ireland. Br J Dermatol. 2011;164:822–829. doi: 10.1111/j.1365-2133.2011.10238.x. [DOI] [PubMed] [Google Scholar]
- 7.Tenbensel T, Eagle S, Ashton T. Comparing health policy agendas across 11 high income countries: islands of difference in a sea of similarity. Health Policy. 2011;106:29–36. doi: 10.1016/j.healthpol.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Ritsatakis A. Equity and the social determinants of health in European cities. J Urban Health. 2013; 90(Suppl 1): 92–104. [DOI] [PMC free article] [PubMed]
- 9.Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. J Rural Health. 1992;8:212–220. doi: 10.1111/j.1748-0361.1992.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson D, Cameron K. Cancer and cancer risk in South Australia: what evidence of a rural–urban health differential? Aust J Rural Health. 2004;12:61–66. doi: 10.1111/j.1038-5282.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 11.Dey S, Soliman AS, Hablas A, et al. Urban–rural differences in breast cancer incidence in Egypt (1999–2006) Breast. 2010;19:417–423. doi: 10.1016/j.breast.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riaz SP, Horton M, Kang J, Mak V, Luchtenborg M, Moller H. Lung cancer incidence and survival in England: an analysis by socioeconomic deprivation and urbanisation. J Thorac Oncol. 2011;6:2005–2010. doi: 10.1097/JTO.0b013e31822b02db. [DOI] [PubMed] [Google Scholar]
- 13.Yang CY, Hseih YL. The relationship between population density and cancer mortality in Taiwan. Jap J Cancer Research. 1998;89:355–360. doi: 10.1111/j.1349-7006.1998.tb00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smailyte G, Kurtinaitis J. Cancer mortality differences among urban and rural residents in Lithuania. BMC Public Health. 2008;8:56. doi: 10.1186/1471-2458-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obertova Z, Brown C, Holmes M, Lawrenson R. Prostate cancer incidence and mortality in rural men—a systematic review of the literature. Rural Remote Health. 2012;12:2039. [PubMed] [Google Scholar]
- 16.Singh GK, Siahpush M, Williams SD. Changing urbanisation patterns in US lung cancer mortality, 1950–2007. J Comm Health. 2012;37:412–420. doi: 10.1007/s10900-011-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp L, Foll P, Deady S, O’Ceilleachair A, Buicke C, Carsin AE. Where do people with lung cancer die and how is this changing? A population-based study. Ir Med J. 2010;103:262–264. [PubMed] [Google Scholar]
- 18.Ó Céilleachair A, Finn C, Deady S, Carsin AE, Sharp L. Have developments in palliative care services impacted on place of death of colorectal cancer patients in Ireland? A population-based study. Ir J Med Sci. 2011;180:91–96. doi: 10.1007/s11845-010-0607-y. [DOI] [PubMed] [Google Scholar]
- 19.Kogevinas M, Pearce N, Susser M, Boffetta P. Social inequalities and cancer. IARC Scientific Publications no. 138. Lyon: International Agency for Research on Cancer; 1997. [Google Scholar]
- 20.Gartner A, Farewell D, Roach P, Dunstan F. Rural/urban mortality differences in England and Wales and the effect of deprivation adjustment. Soc Sci Med. 2011;72:1685–1694. doi: 10.1016/j.socscimed.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Registry. Data Quality and Completeness at the Irish National Cancer Registry. Cork: National Cancer Registry; 2012.
- 22.Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994–2004: a comprehensive report. Belfast/Cork: Northern Ireland Cancer Registry/National Cancer Registry; 2009. [Google Scholar]
- 23.Ferlay J, Burkhard C, Whelan S, Parkin DM. Check and conversion programs for cancer registries. IARC/IACR tools for cancer registries. Lyon: International Agency for Research on Cancer; 2005. [Google Scholar]
- 24.Northern Ireland Statistics and Research Agency. Central Postcode Directory. 2013. Available at URL: http://www.nisra.gov.uk/geography/postcode.htm [Accessed March 26, 2013]
- 25.An Post. GeoDirectory. 2013. Available at URL: http://www.geodirectory.ie/ [Accessed March 26, 2013]
- 26.Northern Ireland Statistics and Research Agency. Mid-year population estimates. 2013. Available at URL: http://www.nisra.gov.uk/demography/default.asp17.htm [Accessed March 26, 2013]
- 27.Northern Ireland Statistics and Research Agency. 2001 Census. 2013. Available at URL: http://www.nisra.gov.uk/Census/2001Census.html [Accessed March 26, 2013]
- 28.Central Statistics Office. Census 2002, 1996 Results and Earlier Censuses. 2013. Available at URL: http://www.cso.ie/en/census/census20021996resultsandearliercensuses/ [Accessed March 26, 2013]
- 29.Central Statistics Office. Population. 2013. Available at URL: http://www.cso.ie/en/releasesandpublications/population/ [Accessed March 26, 2013]
- 30.Central Statistics Office. Survey on Income and Living Conditions (SILC) Thematic Report on the Elderly, 2004 and 2009. 2011 Available at URL: http://www.cso.ie/en/media/csoie/releasespublications/documents/silc/2009/Thematic%20Report%20on%20the%20Elderly%202004%20and%202009.pdf [Accessed September 13, 2013]
- 31.Breslow NE. Extra-Poisson variation in log-linear models. Appl Stat. 1984;33:38–44. doi: 10.2307/2347661. [DOI] [Google Scholar]
- 32.Faggiano F, Partanan T, Kogevinas M, Boffetta P. Socioeconomic differences in cancer incidence and mortality. IARC Scientific Publication. 1997;138:65–176. [PubMed] [Google Scholar]
- 33.Pukkala E, Weiderpass E. Time trends in socioeconomic differences in incidence rates of cancers of the breast and female genital organs (Finland, 1971–1995) Int J Cancer. 1999;81:56–61. doi: 10.1002/(SICI)1097-0215(19990331)81:1<56::AID-IJC11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Cancer incidence by deprivation, England, 1995–2004. London: National Cancer Intelligence Network Coordinating Centre; 2008. [Google Scholar]
- 35.Aarts MJ, Lemmens VE, Louwman MW, Kunst AE, Coebergh JW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46:2681–2695. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Doherty VR, Brewster DH, Jensen S, Gorman D. Trends in skin cancer incidence by socioeconomic position in Scotland, 1978–2004. Br J Cancer. 2010;102:1661–1664. doi: 10.1038/sj.bjc.6605678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilligan T. Social disparities and prostate cancer: mapping the gaps in our knowledge. Cancer Causes Control. 2005;16:45–53. doi: 10.1007/s10552-004-1291-x. [DOI] [PubMed] [Google Scholar]
- 38.Menvielle G, Kunst A. Social inequalities in cancer incidence and cancer survival: lessons from Danish studies. Eur J Cancer. 2008;44:1933–1937. doi: 10.1016/j.ejca.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Registry/Northern Ireland Cancer Registry. All-Ireland Cancer Atlas 1995–2007. Cork/Belfast: National Cancer Registry/Northern Ireland Cancer Registry; 2011.
- 40.Farmer J, Iverson L, Campbell NC, Guest C, Chesson R, Deans G, et al. Rural/urban differences in accounts of patients’ initial decisions to consult primary care. Health Place. 2006;12:210–221. doi: 10.1016/j.healthplace.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Nolan A. A dynamic analysis of GP visiting in Ireland: 1995–2001. Health Econ. 2007;16:129–143. doi: 10.1002/hec.1149. [DOI] [PubMed] [Google Scholar]
- 42.Morrissey K, Clarke G, Ballas D, Hynes S, O’Donoghue C. Examining access to GP services in rural Ireland using microsimulation data. Area. 2008;40:354–364. doi: 10.1111/j.1475-4762.2008.00844.x. [DOI] [Google Scholar]
- 43.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. J Am Med Assoc. 1995;273:548–552. doi: 10.1001/jama.1995.03520310046028. [DOI] [PubMed] [Google Scholar]
- 44.Carsin AE, Drummond FJ, Black A, et al. Impact of PSA testing and prostatic biopsy on cancer incidence and mortality: comparative study between the Republic of Ireland and Northern Ireland. Cancer Causes Control. 2010;21:1523–1531. doi: 10.1007/s10552-010-9581-y. [DOI] [PubMed] [Google Scholar]
- 45.Gormley GJ, Catney D, McCall JR, Reilly PM, Gavin AT. Prostate-specific antigen testing: uncovering primary care influences. BJU Int. 2006;98:996–1000. doi: 10.1111/j.1464-410X.2006.06481.x. [DOI] [PubMed] [Google Scholar]
- 46.Drummond FJ, Carsin AE, Sharp L, Comber H. Factors prompting PSA-testing of asymptomatic men in a country with no guidelines: a national survey of general practitioners. BMC Fam Pract. 2009;10:3. doi: 10.1186/1471-2296-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGahan CE, Blanks RG, Moss SM. Reasons for variation in coverage in the NHS cervical screening programme. Cytopathol. 2001;12:354–366. doi: 10.1046/j.1365-2303.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 48.Kinnear H, Rosato M, Mairs A, Hall C, O’Reilly D. The low uptake of breast screening in cities is a major public health issue and may be due to organisational factors: a census-based record linkage study. Breast. 2011;20:460–463. doi: 10.1016/j.breast.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Bain NSC, Campbell NC. Treating patients with colorectal cancer in rural and urban areas: a qualitative study of the patients’ perspective. Fam Pract. 2000;17:475–479. doi: 10.1093/fampra/17.6.475. [DOI] [PubMed] [Google Scholar]
- 50.Baade P, Dasgupta P, Aitken J, Turrell G. Geographic remoteness and risk of advanced colorectal cancer at diagnosis in Queensland: a multilevel study. Br J Cancer. 2011;105:1039–1041. doi: 10.1038/bjc.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baade P, Turrell G, Aitken JF. Geographical remoteness, area-level socioeconomic disadvantage and advanced breast cancer: a cross-sectional, multilevel study. J Epidemiol Comm Health. 2011;65:1037–1043. doi: 10.1136/jech.2010.114777. [DOI] [PubMed] [Google Scholar]
- 52.Tracey EA, Roder DM, Currow DC. What factors affect the odds of NSW cancer patients presenting with localised as opposed to more advanced cancer? Cancer Causes Control. 2012;23:255–262. doi: 10.1007/s10552-011-9873-x. [DOI] [PubMed] [Google Scholar]
- 53.Secretan B, Straif K, Baan R, et al. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 54.IARC monographs on the evaluation of carcinogenic risks to humans. Volume 83. Tobacco smoke and involuntary smoking. Lyon: International Agency for Research on Cancer; 2004. [PMC free article] [PubMed] [Google Scholar]
- 55.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan K, McGee H, Watson D, et al. SLÁN 2007: survey of lifestyle, attitudes & nutrition in Ireland. Main Report. Dublin: Department of Health & Children; 2008. [Google Scholar]
- 57.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McInerney J, Pilkington L, Keegan H, et al. Human papillomavirus prevalence and genotype distribution in the Irish cervical screening population. J Cytopathol. 2008;19(Suppl 2):13. [Google Scholar]
- 59.Anderson L, O’Rorke M, Jamison J, Wilson R, Gavin A, on behalf of the HPV Working Group members. Prevalence of human papillomavirus in women attending cervical screening in the UK and Ireland: new data from Northern Ireland and a systematic review and meta-analysis. J Med Virol. 2013; 85: 295–308. [DOI] [PubMed]
- 60.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Eklund G, Malec E. Sunlight and incidence of cutaneous malignant melanoma. Effect of latitude and domicile in Sweden. Scand J Plast Reconstruct Surg. 1978;12:231–241. doi: 10.3109/02844317809012999. [DOI] [PubMed] [Google Scholar]
- 62.Agredano YZ, Chan JL, Kimball RC, Kimball AB. Accessibility to air travel correlates strongly with increasing melanoma incidence. Melanoma Res. 2006;16:77–81. doi: 10.1097/01.cmr.0000195696.50390.23. [DOI] [PubMed] [Google Scholar]
- 63.Gavin A, Boyle R, Donnelly D, Donnelly C, Gordon S, McElwee G, et al. Trends in skin cancer knowledge, sun protection practises and behaviours in the Northern Ireland population. Eur J Public Health. 2012;22:408–412. doi: 10.1093/eurpub/ckr087. [DOI] [PubMed] [Google Scholar]
- 64.Sun Smart. Barometer Research. Conducted by Behaviour & Attitudes Marketing Research. Dublin: Irish Cancer Society; 2007. [Google Scholar]
- 65.Vineis P, Husgafvel-Pursiainen K. Air pollution and cancer: biomarker studies in human populations. Carcinogen. 2005;26:1846–1855. doi: 10.1093/carcin/bgi216. [DOI] [PubMed] [Google Scholar]
- 66.Hart C, Ecob R, Smith GD. People, places and coronary heart disease risk factors: a multilevel analysis of the Scottish Heart Health Study archive. Soc Sci Med. 1997;45:893–902. doi: 10.1016/S0277-9536(96)00431-5. [DOI] [PubMed] [Google Scholar]
- 67.Lahti-Koski M, Taskinen O, Simila M, Mannisto S, Laatikainen T, Kneckt P, et al. Mapping geographical variation in obesity in Finland. Eur J Public Health. 2008;18:637–643. doi: 10.1093/eurpub/ckn089. [DOI] [PubMed] [Google Scholar]
- 68.Løkkegaard E, Lidegaard O, Møller LN, Agger C, Andreasen AH, Jørgensen T. Hormone replacement therapy in Denmark, 1995–2004. Acta Obstet Gynecol Scand. 2007;86:1342–1351. doi: 10.1080/00016340701505523. [DOI] [PubMed] [Google Scholar]