Abstract
Substance use disorders (SUD) are inheritable and the culprit is hypodopaminergic function regulated by reward genes. We evaluated a natural dopaminergic agonist; KB220 intravenous (IV) and oral variants, to improve dopaminergic function in SUD. Our pilot experiment found a significant reduction of chronic symptoms, measured by the Chronic Abstinence Symptom Severity (CASS) Scale. The combined group (IV and oral) did significantly better than the oral-only group over the first week and 30-day follow-up period. Next, the combination was given to129 subjects and three factors; Emotion, Somatic, and Impaired Cognition, with eigenvalues greater than one were extracted for baseline CASS-Revised (CASS-R) variables. Paired sample t-tests for pre and post-treatment scales showed significant declines (p = .00001) from pre- to post-treatment: t = 19.1 for Emotion, t = 16.1 for Somatic, and t = 14.9 for Impaired Cognition. In a two-year follow-up of 23 subjects who underwent KB220IV therapy (at least five IV treatments over seven days) plus orals for 30+ days: 21 (91%) were sober at six months, 19 (82%) having no relapse; 19 (82%) were sober at one year, 18 (78%) having no relapse; and 21 (91%) were sober two-years post-treatment, 16 (70%) having no relapse. We await additional research and advise caution in interpreting these encouraging results.
Keywords: Chronic Abstinence Symptom Severity (CASS) Scale, dopamine, KB220IV-neuroadaptagen amino-acid therapy (NAAT), reward deficiency syndrome (RDS)
The site of the brain contributing to feelings of well-being is the mesolimbic system. This brain region has been termed the “reward center.” Chemical messengers including serotonin, enkephalins, GABA, and dopamine (DA) work in concert to provide a net release of DA at the nucleus accumbens (NAc), a structure located within the mesolimbic system. It is well known that genes control the synthesis, vesicular storage, metabolism, receptor formation, and neurotransmitter catabolism. The polymorphic-versions of these genes have certain variations that could lead to an impairment of the neurochemical events involved in the neuronal release of DA. The cascade of these neuronal events has been termed the “brain reward cascade” (Blum & Kozlowski 1990) (see Figure 1). A breakdown of this cascade will ultimately lead to a dysregulation and dysfunction of DA. Since DA has been established as the “pleasure molecule” and the “anti-stress molecule,” any reduction in function could lead to reward deficiency and resultant aberrant substance-seeking behavior and a lack of wellness (Blum & Braverman 2000; Blum et al. 1996).
FIGURE 1. Brain Reward Cascade.
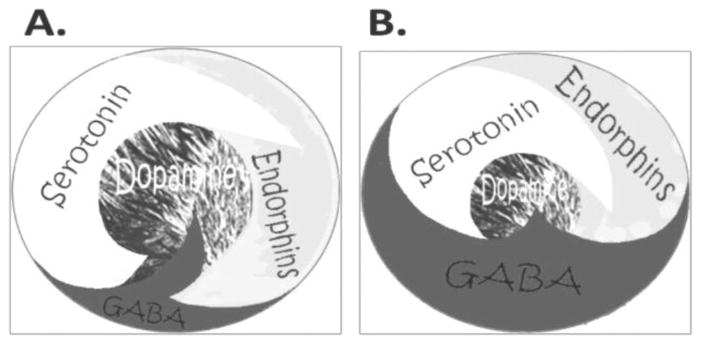
Figure 1(A) represents the normal physiologic state of the neurotransmitter interaction at the mesolimbic region of the brain. Briefly, in terms of the brain reward cascade: serotonin in the hypothalamus stimulates neuronal projections of methionine enkephalin in the hypothalamus, which in turn inhibits the release of GABA in the substania nigra, thereby allowing for the normal amount of dopamine to be released at the nucleus accumbens (reward site of the brain). Figure 1(B) represents hypodopaminergic function of the mesolimbic region of the brain. It is possible that the hypodopaminergic state is due to gene polymorphisms as well as environmental elements including both stress and neurotoxicity from aberrant abuse of psychoactive drugs (e.g., alcohol, heroin, cocaine etc.). Genetic variables could include serotonergic genes (serotonergic receptors [5HT2a]; serotonin transporter 5HTlPR); endorphinergic genes (mu OPRM1 gene; proenkephalin [PENK, PENK polymorphic 3′UTR dinucleotide (CA) repeats]; GABergic gene (GABRB3), and dopaminergic genes (ANKKI Taq A; DRD2 C957T, DRD4 7R, COMT Val/met substation; MAO-A uVNTR; and SLC6A3 9 or 10R). Any of these genetic and/or environmental impairments (over-administration of L-glutamate or IV GABA) could result in reduced release of DA or a reduced number of dopaminergic receptors (Blum et al. 2010a with permission).
It is well established that DA and a number of other linked neurotransmitters are responsible for feelings of well-being; however, attempts to attenuate dysregularities in brain reward circuits using pharmaceutical agents have been met with problematic results (Topol et al. 2010; McCormick et al. 2009) and in some cases suicidal ideation (Faulconbridge et al. 2009). Using powerful neurotransmitter agonists results in down-regulation instead of needed up-regulation of the specific receptors being targeting (Kirsch et al. 2006). Bromocriptine, a powerful DA D2 agonist, has been shown to down regulate DA D2 receptors following chronic administration (Nisoli et al. 1990). L-dopa used for the treatment of Parkinson’s disease has been shown to down regulate DA receptors with associated unwanted side effects (Ulusoy, Sahin & Kirik 2010). The challenge is to find a safe, nonaddicting natural substance that would activate the “brain reward site,” causing up-regulation of DA receptors (D2 in particular) without side effects but having therapeutic value.
Since the 1980s, when our laboratory reported on d-phenylalanine as an anti-alcohol craving substance in rodents (Blum et al. 1987) we have been developing natural dopaminergic agonists. From that time until the present, we have published 23 human clinical outcome benefits (Chen et al. 2011) using a number of tested variants, and we have suggested the adoption of neuroadaptogen amino-acid therapy (NAAT) using the formulations KB220 and KB220Z as an adjunctive treatment for substance use disorder (SUD). As a result of this intensive research, the addiction field has recognized the importance of its use, and since the middle 1980s, many clinics across America have adopted it (Blum & Payne 1991). While terminology for its use has changed from “neuro-nutrient therapy” to “amino acid therapy” to the current nomenclature Neuroadaptagen Amino-Acid Therapy (NAAT), the basic concepts are the same. A quick look at the brain reward cascade (see Figure 1) illustrates the “normal” functioning brain (A) whereby serotonin stimulates enkephalinergic projections in the hypothalamus, which inhibits GABA (an inhibitory neurotransmitter fine tuning the appropriate release of DA) at the substania nigra, leading to the natural physiological release of DA at the NAc. However, as observed in (B), when there are genetic deficits and/or environmental epigenetic elements (including drug toxicity, stress, etc.), there is an impairment of the brain reward cascade, with a resultant lowered DA release leading to the well-known “dopamine depletion hypothesis” (Dackis et al. 1985) and reward deficiency syndrome (RDS) (Comings & Blum 2000).
It is very important to understand that increases in GABA possibly by administration of L-glutamate, known to synthesize GABA in neurons (Belardetti et al. 2009), will induce a hypodopaminergic response and as such chronic administration could induce suicidal ideation as seen in recent studies involving the cannabinoid B1 receptor inhibitor known as Acomplia® (Burch et al. 2009). This is so because the CB1 receptor inhibitor significantly reduces DA release by enhancing GABA neurotransmission (see Figure 1B for depiction). There are very recent studies primarily from our laboratory in conjunction with others showing significant qEEG neuro-regulatory benefits (“normalizing persistent EEG abnormalities”) of using both intravenous (Miller et al. 2010) and oral administration (Blum et al. 2010a) of specific dose-dependent KB220 and KB220Z formulae in protracted abstinent polydrug abusers, psychostimulant abusers, and heroin addicts. These positive qEEG outcomes coupled with unpublished preliminary fMRI studies in China (Blum et al. 2010b) show the direct activation of the NAc with an acute dose of KB220Z. This in part provided the rationale for our incorporating an intravenous (IV) amino-acid formulation to provide adjunctive therapy in a number of polydrug abusing patients seeking treatment in a residential inpatient addiction facility (Bridging the Gaps) located in Winchester, VA.
The goals of intravenous nutritional therapy are in essence the same as goals for oral nutritional therapy. The main difference, obviously, is the time frame, immediacy of the desired nutritional support, and the intended clinical results (see Table 1).
TABLE 1.
Goals of IV Therapy of NAAT
|
Although not tested in this experiment, we are cognizant that other benefits may include removal of destructive free radicals, toxins, metabolites and fat-soluble molecules that invariably build up in the brain and body of an addict; enhancement of immune and liver functions; and accelerated recovery, with reversal of many of the common mental, emotional, and physical symptoms of sobriety.
One major goal is to assist in getting the addicted patient ready and able to not just understand his or her addiction as a disease, but to provide the individual with a clean and sober brain in the shortest time frame so that the healing can begin. In this regard, severity of symptoms during and following treatment with the neuroadaptogen amino-acid therapy (NAAT) in intravenous form was evaluated for over a seven-day and 30-day period as well a two-year follow-up.
MATERIAL AND METHODS
In the second experiment, subjects were administered either oral-only treatment or IV treatment with NAAT variant [KB220] along with other vitamin and mineral nutrients. The subjects were polydrug abusers and in all cases drank alcohol to excess. The basic patented (US patent #6,132,734) formula for NAAT Variant [KB220] further modified by AminoStream, Inc. (Indianapolis, IN) included amino acid precursors such as L-phenylalanine, l-tyrosine, L-tryptophan, 5-hydroxytryptophane, L-glutamine, a serotonin concentrating substance chromium, an enkephalinase inhibitor D-phenylalanine, a neurotransmitter synthesis promoter vitamin B6, as well as both methionine and leucine. The amounts of these ingredients varied according to individualized assessment and responses to the Neurotransmitter Questionnaire (Miller & Miller 2008), which identified potential impairment of serotonergic, endorphinergic, and dopaminergic systems. While each patient was provided a form of oral amino acids and nutrients, the actual ingredients and in many cases the quantities varied and as such the altered formula was generic (Brown, Blum & Trachtenberg 1990; Blum et al. 1988, Trachtenberg & Blum 1988). We have divided the groups into IV and oral (Group A) and oral only (Group B).
IV Group
For Group A, just prior to IV infusion, the client’s vital signs were taken and recorded; the as yet unpublished Chronic Abstinence Symptom Severity (CASS) Scale was completed and oral nutrients were administered. The IV administration was a four-hour infusion once a day, over seven days (preferably consecutive days, but not necessarily), a total of six IV treatments were performed. Due to the short half-life of glutathione most but not all patients, had glutathione added to other ingredients during the last 20 minutes of infusion. Immediately at the completion of IV therapy, each patient was post-tested with the CASS scale and clinically assessed for any behavioral symptoms, positive or negative. Collection of the data and subsequent analyses was developed by MM.
Oral Group
For the Oral Therapy protocol, everyone received nutrients including thiamine, riboflavin, niacin, B6, folate, B12, pantothenic acid, magnesium, choline, para-aminobenzoic acid, lecithin, and inositol. In addition, those who met the criteria for being serotonin deficient also received vitamins A, C, E, K, and D, glycine, leucine, DLPA, tyrosine, boron, calcium, biotin, zinc, potassium, methionine, selenium, copper, iodine, and manganese. Those who met the criteria for being DA deficient also received iodine, zinc, copper, selenium, manganese, chromium, potassium, boron, calcium, biotin, and 5-HTP. In addition, an individual’s drug of choice was used to determine the oral protocol. Moreover, we also utilized the Neurotransmitter Questionnaire.
Assessments
The initial CASS included 25 items including questions related to the following: craving/drug or alcohol hunger; craving for sweets/sugar/bread; craving for salt; loss of appetite; overeating/always hungry; anxiety; internal shakiness; restlessness; impulsiveness; difficulty in focusing; head cloudy; memory loss; depression; mood swings; irritability; daytime sleepiness; insomnia; lack of energy; hypersensitive to stress, sound, noise, or pain, and headaches. This CASS form was utilized in the first experiment only. However, the second experiment utilized a revised CASS, which included only 15 items (CASS-R; see Table 2). The inclusion of only 15 of the 25 initial items was based on redundancy and relevance to the addiction treatment field. The Neurotransmitter Questionnaires also were filled out by each patient (Miller & Miller 2008).
TABLE 2.
Chronic Abstinence Symptom Severity Scale-Revised
| Name:____________________ | Date:__________ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Circle the number that best indicates the severity of each symptom you are experiencing today (zero indicates the absence of the symptom, 10 represents an extreme intensity level). Answer each question as honestly as possible. If you do not understand the meaning of the symptom listed, ask the doctor or nurse. | ||||||||||||
| Low Level | High Level | |||||||||||
| 1. | Craving/drug or alcohol hunger | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2. | Sense of emptiness/incompleteness | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 3. | Anxiety | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 4. | Internal shakiness | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 5. | Restlessness | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 6. | Impulsiveness/act before thinking | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 7. | Difficulty concentrating/focusing | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 8. | Memory problems | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 9. | Depression | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 10. | Irritability | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11. | Problems getting to or staying asleep | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 12. | Fatigue/lack of energy | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 13. | Hypersensitivity to stress | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 14. | Hypersensitivity to noise/sight/touch | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 15. | Pain intensity | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| TOTAL | ||||||||||||
Subjects
All subjects entered into the study meeting inclusion criteria and all subjects signed an informed consent statement. The PATH Research Foundation approved the study (NIH registration # 00002334). Moreover subjects not only signed an approved consent form but also agreed to volunteer for a feasibility study. For protection of the patients the data will conform to standard HIPPA practices mandated by law.
In the initial and exploratory experiment in 2005, 49 subjects were evaluated. (There are no demographics for this group). Subjects from Bridging the Gaps, Inc. were assigned, by self-selection, to either Group A which received IV NAAT with oral nutritional therapy or Group B which received only oral nutritional therapy. Each subject was offered the IV regimen. Only those who selected this option were included in the IV group (Group A) and the remaining subjects were placed on orals only (Group B). All were assessed daily for six days and again after 30 days for severity of symptoms commonly experienced in abstinence. There were 21 subjects in Group A and 28 in Group B. It should be noted that those who self-selected for Group A reported a greater severity of symptoms that those in Group B.
The patients were interviewed and a thorough battery of diagnostic tests and questionnaires were given to assess chemical dependence. These included the following: Drug History Questionnaire; physical assessment; urine drug tests; Breathalyzer; complete CBC blood test; and Chronic Abstinence Symptom Severity Questionnaire (25 items). The patients were determined to be substance dependent according to Diagnostic and Statistical Manual-IV criteria. The subjects were detoxified from drugs within the last two months and had symptoms of craving behavior associated with protracted abstinence. Prior to IV administration of the NAAT variant each patient was pre-assessed for type of impaired brain reward circuitry through the Neurotransmitter Questionnaire.
For the second experiment during the period of December, 2005 to December, 2007, our laboratory recruited 149 subjects but based on our inclusion/exclusion criteria. Twenty subjects met the exclusion criteria due to; failure to complete the six IV treatments, low initial CASS-R scores (below 20), and poor compliance and cooperation with study protocol. The included sample consisted of 129 subjects (mean age = 39.1y + 13.7; 65 males, 35 females, 29 gender missing) from Bridging The Gaps Inc. treatment program in Winchester, VA (a 30- to 90-day chemical dependence rehabilitation program). See Table 3 for drug of choice and percentage breakdown by subjects.
TABLE 3.
Percentage Breakdown of Drug of Choice
| N | ||
|---|---|---|
| Alcohol | 45.74% | 59 |
| Cocaine | 1.55% | 2 |
| Crack | 1.55% | 2 |
| Opiates | 1.55% | 2 |
| Combination | 29.46% | 38 |
| Poly Drug | 9.30% | 12 |
| Adderall, Marijuana | 0.78% | 1 |
| Alcohol, Cocaine | 3.10% | 4 |
| Alcohol, Crack | 0.78% | 1 |
| Alcohol, Heroin | 0.78% | 1 |
| Alcohol, Marijuana | 3.88% | 5 |
| Alcohol, Oxycontin | 0.78% | 1 |
| Alcohol, Sedatives | 0.78% | 1 |
| Cocaine, Alcohol | 3.88% | 5 |
| Cocaine, Marijuana | 0.78% | 1 |
| Crack, Alcohol | 3.10% | 4 |
| Heroin, Alcohol | 0.78% | 1 |
| Marijuana, Alcohol | 0.78% | 1 |
| Unknown | 20.16% | 26 |
The 129 patients from Bridging the Gaps, Inc. were interviewed and given the same thorough battery of diagnostic tests and questionnaires to establish a diagnosis of chemical dependence as the subjects in the initial experiment except that they were given the modified 15 item CASS-R questionnaire. The patients were determined to be substance dependent according to Diagnostic and Statistical Manual-IV criteria. The subjects were detoxified from drugs within the last two months and had symptoms of craving behavior associated with protracted abstinence. Prior to the IV administration of the NAAT variant each patient was assessed for type of impaired brain reward circuitry with the Neurotransmitter Questionnaire. Immediately after subjects completed the CASS-R, they received oral and IV therapy. Each of the 15 variables from the abbreviated CASS-R as well as actual changes in symptom severity was evaluated.
In the third experiment, 23 subjects derived from Bridging the Gaps Treatment Center who were given oral and IV therapy were followed-up at six months, one year, and two years post-IV treatment in terms of being sober and not having any relapse during the targeted time frame.
Statistical Analyses
For the first exploratory experiment, the 25 variables of the CASS were summed for a total score. The 49 patients from the oral plus IV (Group A) and the oral only (Group B) were assessed at baseline, seven days, and one month post-treatment and a paired samples t-test was calculated. A change score (measure at one month minus measure at baseline) was calculated for CASS before and after treatment, and independent sample Student’s t-tests were used to compare group A vs. group B on the change score.
For the second experiment, a factor analysis (principal components extraction with varimax rotation) of the 15 CASS-R variables across the 129 subjects was performed, and factors with eigenvalues greater than one were retained and subjected to varimax rotation. Variables with rotated factor loadings >0.50 were regarded as interpretable. Based on this factor analysis, scales for the CASS-R were constructed, consisting of the sum of the variables with significant loadings for each factor. Paired sample t-tests were used to analyze each scale before the first IV treatment and just prior to the sixth (last) treatment. There was no assessment after the sixth treatment reported in this statistical analysis.
The third experiment was primarily descriptive and no statistical analysis was performed.
RESULTS
Experiment 1
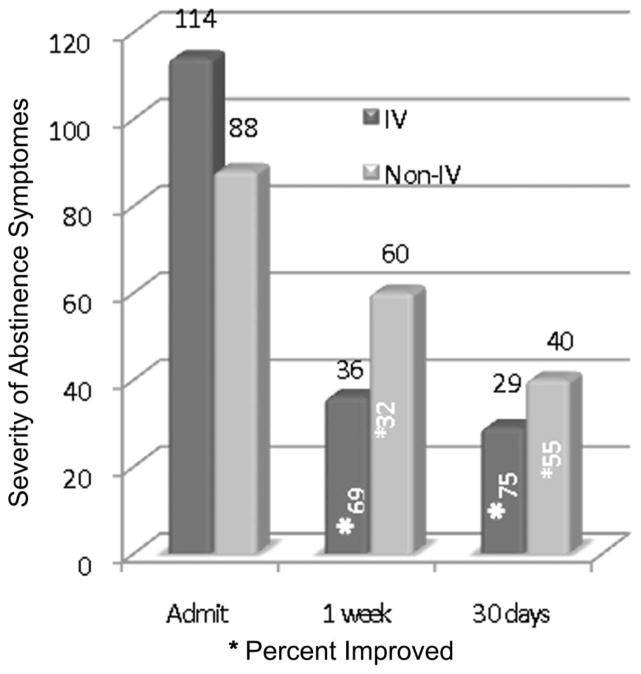
Although the IV group had more severe abstinence symptoms at baseline (113.9 + 37.9) than the oral only group (87.5 + 30.0, t = 2.38, p = .022), no significant differences were found in the oral only group between one week (60.0 + 26.3) and 30 days (40.1 + 25.4) compared to the IV group (35.9 + 26.6 and 29.4 + 21.7, respectively).
Both groups showed significant declines in symptoms from baseline to one week (paired t = 16.2, p = .00001 for IV, paired t = 3.67, p = .003 for oral only, paired t = 9.44, p = .00001 across both) and baseline to 30 days (paired t = 10.61, p = .00001 for IV, paired t = 4.73, p = .0003 for oral only, paired t = 3.01, p = .004 across both). The change score comparing symptoms at 30 days to baseline was significant (t = 2.69, p = .01) with a greater reduction in symptoms for the IV group (−84.4 + 47.5) compared to the oral only group (−47.5 + 38.9) (see Figure 2).
FIGURE 2.
Results of IV and Oral Treatments Compared to Oral Treatment without Combined IV Treatment over a 30-Day Test Period (N = 49)
Experiment 2
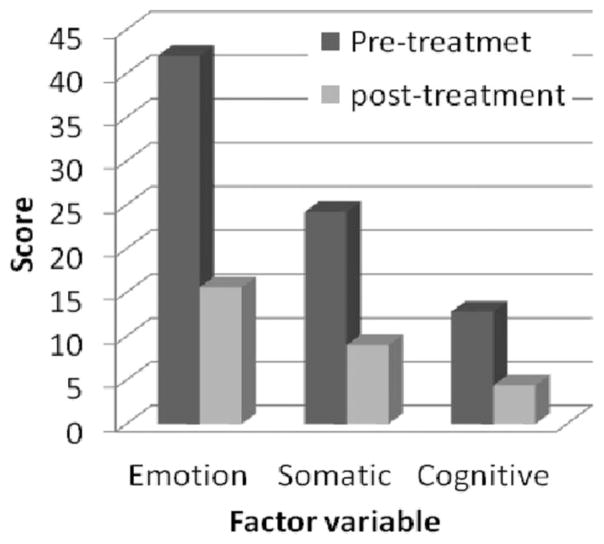
Three factors with eigenvalues greater than one were extracted for the baseline CASS-R variables (Table 4). Factor 1 had significant loadings for Emptiness, Anxiety, Impulsivity, Concentration, Depression, Irritability, Fatigue, and Stress. This factor appears to reflect primarily emotional and affective symptoms in conjunction with fatigue and stress. Factor 2 had significant loadings from Shakiness, Restlessness, Sleep Problems, Stress, and Pain. This factor appears to reflect somatic symptoms. Factor 3 had significant loadings from Concentration, Memory, and Sensitivity to Noise, and appears to assess cognitive complaints. Interestingly, craving—which is so often associated with withdrawal symptoms—did not load on any factor. Three scales were constructed based on this factor analysis: Emotion, Somatic, and Impaired Cognition. Paired sample t-tests between the pretreatment scales and the post-treatment scales were calculated. All three scales showed significant declines (p = .00001) from pre- to post-treatment (Figure 3): t = 19.1 for Emotion, t = 16.1 for Somatic, and t = 14.9 for Impaired Cognition. Means for the three scales before and after treatment are shown in Table 5.
TABLE 4.
Rotated Component Matrix of CASS-R*
| Symptom | Component | ||
|---|---|---|---|
| Crave-Pre | .301 | .375 | .414 |
| Emptiness-Pre | .723 | −.010 | .299 |
| Anxiety-Pre | .657 | .510 | .178 |
| Shakiness-Pre | .358 | .693 | .205 |
| Restless-Pre | .490 | .521 | .202 |
| Impulsivity-Pre | .590 | .142 | .319 |
| Concentration Difficult-Pre | .587 | −.052 | .565 |
| Memory Problems-Pre | .157 | .054 | .724 |
| Depression-Pre | .856 | .136 | .136 |
| Irritability-Pre | .569 | .091 | .411 |
| Sleep problems-Pre | .044 | .629 | .162 |
| Fatigue-Pre | .529 | .462 | .015 |
| Stress-Pre | .586 | .549 | .111 |
| Hypersensitivity noise/sight/touch-Pre | −.005 | .306 | .707 |
| Pain-Pre | −.052 | .647 | .001 |
| 1 | 2 | 3 | |
Extraction Method: Principal Component Analysis. Rotation Method: Varimax with Kaiser Normalization. Rotation converged in eight iterations.
FIGURE 3.
Data Evaluated Pre- and Post- Five IV Treatments
TABLE 5.
Three Factor Eigenvalues
| Scale | Pretreatment | Post-treatment |
|---|---|---|
| Emotion | 42 ± 14.9 | 15.6 ± 12.1 |
| Somatic | 24.2 ± 10.0 | 9.0 ± 7.5 |
| Impaired Cognition | 12.8 ± 5.9 | 4.4 ± 4.3 |
Experiment 3
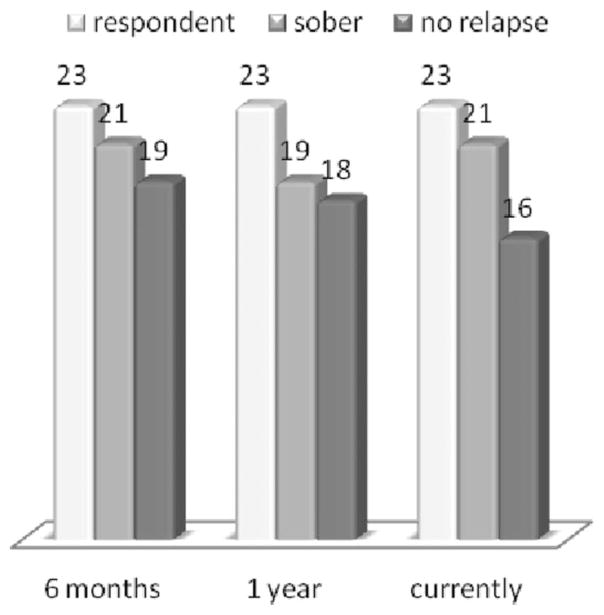
In the third experiment, we carried out follow-up phone interviews conducted by Bridging the Gaps Inc. to determine both sobriety and relapse rates in a small number of clients (n = 23) assessed at six months, one year, and two years post treatment. It is noteworthy, given caveats to this somewhat biased approach (since the interview was not performed by a third party), that in these randomized respondents the following results were obtained: 21 (91%) were sober at six months with 19 (82%) having no relapse; 19 (82%) were sober at one year with 18 (78%) having no relapse; 21 (91%) were sober at two years post treatment with 16 (70%) having no relapse (see Figure 4).
FIGURE 4.
Sobriety and Relapse Rates in 23 Patients Assessed at Six Months, One Year, and Two Years Post Treatment
DISCUSSION
In the present study we have found significant differences between the use of NAAT in both oral and IV form. However, it is important to realize that the most significant finding occurred during the first week whereby the IV form plus orals were significantly more robust in positive outcomes relative to the oral form alone. We are cautiously suggesting that the incorporation of NAAT (KB220IV) may be quite important in terms of successful outcomes. However we encourage others to further confirm these results in a larger population and stringently controlled studies.
We also have validated the CASS-R by performing factor analysis statistics. The factor structure of the CASS-R proved to be quite interesting. The first factor, which accounted for the majority of the variance, appeared to encompass the items on the CASS-R related to emotion. These items included Emptiness, Anxiety, Impulsivity, Concentration, Depression, Irritability, Fatigue, and Stress. The second factor had significant contribution from somatic symptoms, and the third factor reflected cognitive disturbances. The factor scales of Emotion, Somatic, and Cognitive symptoms constructed from those items loading highly on each factor proved very significant following IV treatment. Future research with the CASS-R could take this factor structure into account and utilize the three derived scales, rather than analyzing all 15 items separately.
The major finding in this study relates to the positive effect of the NAAT IV therapy compared to non IV therapy, showing significant difference following the six treatment time frame. This takes on real importance when considering the potentially high rates of those leaving treatment against medical advice (AMA), especially in the first days of treatment. The present findings are in agreement with results of our earlier studies showing significantly reduced AMA rates in polydrug and psychostimulant abusers (Blum et al. 1988; Trachtenberg & Blum 1988). Moreover, findings of the present study showing robust recovery rates and relapse prevention are in agreement with our earlier work using oral NAAT in outpatient DUI offenders (Brown, Blum & Trachtenberg 1990). These results also are in agreement with earlier work from our laboratory utilizing a similar but not identical IV solution in 600 outpatient polydrug abusers with alcohol as the primary drug of choice (Blum et al. 2007). While we are still in the process of exploring NAAT’s mechanism of action, the following brief explanation should shed light on these findings.
Based on neurochemical and genetic evidence, Blum and colleagues (2008) suggested that both prevention and treatment of multiple addictions, such as dependence on alcohol, nicotine, and glucose, should involve a biphasic approach. Thus, acute treatment should consist of preferential blocking of postsynaptic NAc DA receptors (D1–D5), whereas long-term activation of the mesolimbic dopaminergic system should involve activation and/or release of DA at the NAc site. Failure to do so will result in abnormal mood and behaviors, as well as potentially suicidal ideation. Individuals possessing a paucity of serotonergic and/or dopaminergic receptors and an increased rate of synaptic DA catabolism due to high catabolic genotype of the COMT gene are predisposed to self-medicating with any substance or behavior that will activate DA release, including alcohol, opiates, psychostimulants, nicotine, gambling, sex, and even excessive Internet gaming. Acute utilization of these substances and/or stimulatory behaviors induces a feeling of well being. Unfortunately, sustained and prolonged abuse leads to toxic “pseudo” feelings of well being resulting in tolerance, disease or discomfort. Thus, a reduced number of DA receptors, due to carrying the DRD2 A1 allelic genotype, results in excessive craving behavior, whereas a normal or sufficient amount of DA receptors results in low craving behavior (Blum et al. 2008). In fact Dahlgren and colleagues (2011) found an association between the TaqI A1 allele of the DRD2 gene and a substantially increased relapse rate.
In terms of preventing substance abuse, one goal would be to induce a proliferation of DA D2 receptors in genetically prone individuals. While in vivo experiments using a typical D2 receptor agonist induced down regulation, experiments in vitro have shown that constant stimulation of the DA receptor system via a known powerful D2 agonist results in significant proliferation of D2 receptors in spite of genetic antecedents. In essence, D2 receptor stimulation signals negative feedback mechanisms in the mesolimbic system to induce mRNA expression causing proliferation of D2 receptors. Blum and colleagues (2009) have proposed that D2 receptor stimulation can be accomplished via the use of KB220/KB220Z, a natural but therapeutic nutraceutical formulation that potentially induces DA release, causing the same induction of D2-directed mRNA and thus proliferation of D2 receptors in humans. This proliferation of D2 receptors in turn will induce the attenuation of craving behavior. In fact, as mentioned earlier, this model has been proven in research showing DNA-directed compensatory overexpression (a form of gene therapy) of the DRD2 receptors, resulting in a significant reduction in alcohol craving behavior in alcohol preferring rodents (Thanos et al. 2008, 2001). Utilizing natural dopaminergic repletion therapy to promote long term dopaminergic activation may ultimately lead to a common, safe and effective modality to treat RDS behaviors including SUD, attention deficit hyperactivity disorder, obesity, and other reward deficient aberrant behaviors. This concept is further supported by the more comprehensive understanding of the role of DA in the NAc as a “wanting” messenger in the mesolimbic DA system (Blum et al 2012; Davis et al. 2008). There are common genetic mechanisms responsible for both drug effects and subsequent seeking behavior. Past and current treatment of substance-seeking behavior, a subtype of RDS, is considered by most to be inadequate.
The proposed IV NAAT consists of amino-acid neurotransmitter precursors and enkephalinase-inhibition therapy, a patented formulation (US patent 6,132,724 issued 10/ 2000) in brain reward function. The basic formula included amino acid precursors such as L-phenylalanine, l-tyrosine, L-tryptophan, 5-hydroxytryptophane, L-glutamine, a serotonin concentrating substance, chromium, an enkephalinase inhibitor D-phenylalanine, a neurotransmitter synthesis promoter vitamin B6, and as well as both methionine and leucine. The amounts of these ingredients could vary according to individualized assessment. Through a series of both neurogenetic and clinical experiments it is becoming increasingly clear that KB220IV and KB220Z activate the brain reward circuitry. Ongoing research repeatedly confirms that the numerous clinical effects ultimately result in significant benefits for victims having genetic antecedents for addictive, compulsive, and impulsive behaviors classified under the rubric of reward deficiency syndrome.
Preliminary findings in the United States using qEEG, and in China using fMRI, regarding the effects of oral KB220Z Complex™ in addicts on activation of brain reward circuitry provide potentially exciting results. It seems from these preliminary data, utilizing an fMRI 2×2 design at resting state, KB220Z in comparison to placebo shows activation of the caudate-accumbens brain region and potentially a smoothing out of heroin-induced putamen abnormal connectivity. If these results are further confirmed by ongoing studies in China coupled with qEEG results also showing an increase in alpha and increase in low beta, they may have important ramifications for treatment outcomes (Miller et al. 2010; Blum et al. 2010a, b). We must await final analysis of these results from studies still in progress.
It is well known that after prolonged abstinence, individuals who use their drug of choice experience a powerful euphoria that often precipitates relapse. While a biological explanation for this conundrum has remained elusive, we hypothesize that this clinically observed supersensitivity might be tied to genetic dopaminergic polymorphisms. Another therapeutic conundrum relates to the paradoxical finding that the dopaminergic agonist bromocriptine induces stronger activation of brain reward circuitry in individuals who carry the DRD2 A1 allele compared with DRD2 A2 allele carriers. This effect may be related to the potential super sensitivity of D2 receptors due to prolonged denervation of D2 receptors due in part to carrying the A1 allele. Because carriers of the A1 allele relative to the A2 allele of the DRD2 gene have significantly lower D2 receptor density, a reduced sensitivity to DA agonist activity would be expected in the former. Thus, it is perplexing that with low D2 density there is an increase in reward sensitivity with the DA D2 agonist bromocriptine (Lawford et al. 1995).
Moreover, under chronic or long-term therapy with D2 agonists, such as bromocriptine, it has been shown in vitro that there is a proliferation of D2 receptors (Boundy et al. 1995). However, in vivo, the opposite is true whereby bromocriptine down-regulates D2 receptors (Bogomolova et al. 2010). One explanation for this relates to the demonstration that the A1 allele of the DRD2 gene is associated with increased striatal activity of L-amino acid decarboxylase, the final step in the biosynthesis of DA (Laakso et al. 2005). This appears to be a protective mechanism against low receptor density and would favor the utilization of an amino acid neurotransmitter precursor like L-tyrosine for preferential synthesis of DA. There is continuous competition of amino acids including tyrosine, and phenylalanine for brain uptake through the long neutral amino acid (LNAA) complex system. To reduce this competition and allow for appropriate absorption into the brain it is essential that NAAT is taken before meals. The appropriate use of NAAT seems to lead to receptor proliferation to normal levels and results in significantly better treatment compliance only in A1 carriers. We propose that low D2 receptor density and polymorphisms of the D2 gene are associated with risk for relapse of substance abuse, including alcohol dependence, heroin craving, cocaine dependence, methamphetamine abuse, nicotine sensitization, and glucose craving. With this in mind, we have suggested a putative physiological mechanism that may help to explain the enhanced sensitivity following intense acute dopaminergic D2 receptor activation: denervation supersensitivity (Blum et al. 2009).
Rats with unilateral depletions of neostriatal DA display increased sensitivity to DA agonists estimated to be 30 to 100 times (increased sensitivity to agonist therapy) in the 6-hydroxydopamine (6-OHDA; a substance known to deplete DA) rotational model. Given that mild striatal DA D2 receptor proliferation occurs (20%–40%), it is difficult to explain the extent of behavioral supersensitivity by a simple increase in receptor density (LaHoste & Marshall 1992). Thus, the administration of DA D2 agonists would target D2 sensitization and attenuate relapse, especially in D2 receptor A1 allele carriers. This hypothesized mechanism is supported by clinical trials utilizing amino acid neurotransmitter precursors, enkephalinase, and catechol-O-methyltransferase (COMT) enzyme inhibition, which have resulted in attenuated relapse rates in RDS probands (Blum et al. 2009). If future translational research reveals that DA agonist therapy reduces relapse in RDS, it would support the proposed concept, which we term deprivation-amplification relapse therapy (DART). This term couples the mechanism for relapse, which is “deprivation-amplification,” especially in DRD2 A1 allele carriers with natural D2 agonist therapy utilizing amino acid precursors and COMT and enkephlinase inhibition therapy.
The major limitation of the present experiment was the small number of subjects. Furthermore, we are concerned that the utilization of generic amino-acid oral therapy rather than the specific elective amino-acid therapy proven to be efficacious by recent qEEG and on-going fMRI research (Miller et al. 2010; Blum et al. 2010a, b) may have different and more positive initial outcomes. Nevertheless we are encouraged and confident that additional research in a larger sample and double-blinded randomized placebo controlled studies will confirm these results.
It is no surprise that it has taken over four decades to confirm and extend information about the critical role of DA and related genes and gene deficits in the etiology and risk for drug dependence. Hundreds of studies have been reported and many are enabled by neuroscience, neuroimaging, and genetic advances. But, while DA theories have been reported, confirmed, replicated, and replicated again, changes have been slow to move from the bench to the bedside (Dackis et al. 1985).
Thus, acute pharmacological adjunctive treatment should consist of preferential blocking of postsynaptic NAc DA receptors (D1–D5), whereas long-term activation of the mesolimbic dopaminergic system should involve activation and/or release of DA at the NAc site. We proposed that D2 receptor stimulation might be accomplished through the use of NAAT. Basic and clinical testing of such a compound is underway. This natural but therapeutic nutraceutical formulation that induces DA release could cause the induction of D2-directed mRNA and proliferation of D2 receptors in humans. Proliferation of D2 receptors in turn will induce the attenuation of drug-like craving behavior (Blum et al. 2009) and will impact DART thereby reducing relapse.
It is very interesting that other natural substances such as omega-3 fatty acids have been proposed as adjuvant treatment in alcoholism (Le-Niculescu et al. 2011). This is important because it is known that omega-3 fatty acid deficiency results in reduced DA release in the frontal cortex, a relapse site for psychoactive drug abuse. Moreover an omega-3 rich diet may have neuroprotective benefits for dopaminergic neurons (i.e. suppressing cytokine storm, reactive oxygen species from contaminants in crystal methamphetamine (Tanriover et al. 2010)). Moreover, omega-3 fatty acids may regulate the tryptophan hydroxylase gene, which should result in an increased DA release in the reward circuitry of the brain. Thus it has been shown to reduce recurrent unipolar depressant disorder (Nemets, Stahl & Belmaker 2002).
CONCLUSIONS
The present research supports Blum and colleagues’ (2009) proposal that D2 receptor stimulation can be accomplished via the use of KB220IV/KB220Z, a natural but therapeutic nutraceutical formulation that potentially induces DA release, causing induction of D2-directed mRNA and thus proliferation of D2 receptors in humans. This in turn will induce the attenuation of craving behavior. Nonetheless, after 30 years of advances in research on substance use, abuse, and dependence, progress in treatment strategies have been slow to develop. Efforts to integrate known neural mechanisms with other psychotherapeutic treatment options to combat relapse should be encouraged. It is well known that addicts in recovery after prolonged abstinence are particularly vulnerable to relapse. Individuals, who use their drug of choice, after abstinence, experience a powerful euphoria that quickly can precipitate a full-blown relapse. While a biological explanation for this conundrum has remained elusive, it has been hypothesized that this clinically observed “supersensitivity” might be the result of premorbid or state genetic dopaminergic polymorphisms. While treatment with bromocriptine or Bupropion has not appeared to be helpful in psychostimulant abuser outcomes because of potential down-regulation of DA receptors, other approaches might be less provocative (Castells et al. 2010).
Prevention, diagnosis, treatment, and relapse-prevention tactics must be augmented by promoting rigorous five-year outcome research in both outpatient and residential inpatient programs. After over four decades of pharmacological, neuroscience and psychiatric research involving genetics and genomics, the field is poised to embark on large population studies incorporating these newer theories, especially as they relate to dopaminergic targeting of mesolimbic pathways. We encourage academic and clinical scientists to develop studies that will bridge “science to recovery” (Giordano & Blum 2011) yielding novel standard of care guidelines to treat RDS (addictive, impulsive, and compulsive behaviors). Rather than choosing between pharmacological treatments which either block the high or maintain the drug state with less abusable agonists, we suggest changing the focus to treating the underlying, premorbid vulnerabilities, with exercise or meditation, or herbal medications, or nutraceuticals. This may be an approach that can help break the vicious cycle of use, abuse, abstinence, and relapse so common today.
Utilizing exercise, yoga, meditation, careful monitored partial mu receptor agonists (buprenorphine) treatments, and/or natural dopaminergic repletion therapy to promote long-term dopaminergic activation could lead to a common, safe and effective modality to treat RDS. Moreover, it is important that during treatment nutrition education, which empowers individuals to make healthier dietary choices, be an intervention in all comprehensive substance abuse treatment programs. Education about nutrition in schools and in the community (through public health initiatives) can be an invaluable means of preventing and/ or reducing RDS behaviors. In the future coupling nutrition education with potential genetic testing of reward genes in children may provide preventive information to assist parents in their quest for finding ways to prevent/attenuate aberrant substance-seeking behavior.
This concept is further supported by the more comprehensive understanding of the role of DA in the NAc as a “wanting” messenger in the mesolimbic DA system. In fact, aerobic exercise may reduce relapse vulnerability by preventing the increase in cocaine-seeking and associated neuroadaptations in the prefrontal cortex that develop over an abstinence period (Lynch et al. 2010).
We have further suggested that DSM diagnosis should include gene polymorphic testing using candidate gene analysis to assist in individualizing diagnosis, risk, and therapy. By classifying risk severity and striving to remediate these deficits with neuroadaptagens, we could improve future clinical trials (Blum et al. 2010b). Conner and colleagues (2010) in a preliminary study suggested that it is possible to identify children at risk for problematic drug use prior to onset of drug dependence by testing hypodopaminergic genes and identifying personality and environmental elements as predictors. Moreover, the DRD2 A1 allele by itself cannot be used to diagnosis risk by itself because it would result in many false positives. While there are 3,355 (in Pubmed as of 10–21–12) studies on the dopamine D2 receptor gene and associated polymorphisms, additional rigorous research is required to further our understanding of the specific role of these complex polymorphisms in dopaminergic function (i.e. synthesis, receptor binding, receptor responsiveness). Finally, pharmacological therapies have had limited success because these powerful agents have focused on maintenance or interference with drug euphoria rather than correcting or compensating for premorbid DA system deficits. It is well known that powerful D2 agonists like bromocriptine down regulate D2 density (Taylor & Gold 1990). Thus, based on the present results and others (Ross 2002) albeit with limitations, we cautiously suggest that early incorporation (first week of treatment) of NAAT IV therapy may be most beneficial in reducing AMA rates and provide brain neurotransmitter rebalancing and enhanced well-being. We strongly suggest that additional studies conducted by independent researchers and institutions should analyze the efficacy of both oral and IV NAAT.
Acknowledgments
We thank the staff of Bridging the Gaps of Winchester, VA, the staff of AminoStream, Inc., Indianapolis, Indiana, and the staff of PATH Foundation, NY. The writing of this article was funded in part by LifeGen, Inc., San Diego, CA. We also thank James Braly, M.D. for patients’ assessments. Other support came from NIAAA grants R01-AA07112 and K05-AA00219 and the Medical Research Service of the US Department of Veterans Affairs (to MO-B).
Footnotes
The patented NAAT IV therapy has been exclusively licensed to AminoStream and as such the following authors’ disclose financial interest: DM, MM, MM, DM, SS, SS, BWD, AB, MAM, RLW, JG, and KB. All other authors declare that they have no competing interest.
References
- Belardetti F, Ahn S, So K, Snutch TP, Phillips AG. Block of voltage-gated calcium channels stimulates dopamine efflux in rat mesocorticolimbic system. Neurophramacology. 2009;56(6–7):984–93. doi: 10.1016/j.neuropharm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. (Supplement) [DOI] [PubMed] [Google Scholar]
- Blum K, Payne J. Alcohol and the Addictive Brain. New York: The Free Press (Simon and Schuster); 1991. [Google Scholar]
- Blum K, Kozlowski GP. Ethanol and neuromodulator interactions: A cascade model of reward. In: Ollat H, Parvez S, Parvez H, editors. Alcohol and Behavior. Utrecht, Netherlands: VSP Press; 1990. [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Current Pharmaceutical Design. 2012;18(1):113–8. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Giordano J, Morse S, Liu Y, Tan J, Bowirrat A, Smolen A, Waite R, Downs BW, Madigan M, Kerner M, Fornari F, Stice E, Braverman E, Miller D, Bailey JA. Genetic Addiction Risk Score (GARS) analysis: Exploratory development of polymorphic risk alleles in poly-drug addicted males. Integrative Omics and Applied Biotechnology. 2010a;1(2):1–14. [Google Scholar]
- Blum K, Chen TJ, Morse S, Giordano J, Chen AL, Thompson J, Allen C, Smolen A, Lubar J, Stice E, Downs BW, Waite RL, Madigan MA, Kerner M, Fornari F, Braverman ER. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and poly-drug abusers utilizing putative dopamine D2 agonist therapy: Part 2. Postgraduate Medicine. 2010b;122:214–26. doi: 10.3810/pgm.2010.11.2237. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Downs BW, Bowirrat A, Waite RL, Braverman ER, Madigan M, Oscar-Berman M, DiNubile N, Stice E, Giordano J, Morse S, Gold M. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing “deprivation-amplification relapse therapy” (DART) Postgraduate Medicine. 2009;121:176–96. doi: 10.3810/pgm.2009.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen AL, Chen TJ, Braverman ER, Jeinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Theoretical Biology & Medical Modeling. 2008;12:5–24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen TJH, Downs BW, Meshkin B, Blum SH, Martinez Pons M, Mengucci JF, Waite RL, Arcuri V, Varshofsiky M, Braverman ER. Synaptamine (SG8839) an amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of reward deficiency syndrome (RDS) Trends in Applied Sciences Research. 2007;2(2):132–38. [Google Scholar]
- Blum K, Cull JG, Braverman ER, Comings DE. Reward Deficiency Syndrome. American Scientist. 1996;84:132–45. [Google Scholar]
- Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, Cataldie L. Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: Double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol. 1988;5:481–493. doi: 10.1016/0741-8329(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Blum K, Briggs AH, Trachtenberg MC, Delallo L, Wallace JE. Enkephalinase inhibition: Regulation of ethanol intake in genetically predisposed mice. Alcohol. 1987;4(6):449–56. doi: 10.1016/0741-8329(87)90084-x. [DOI] [PubMed] [Google Scholar]
- Blum K, Wallace JE, Briggs AH, Trachtenberg MC. Evidence for the importance of the “genotype” theory in alcohol seeking behavior: A commentary. Alcohol and Drug Research. 1985;6(6):455–61. [PubMed] [Google Scholar]
- Bogomolova EV, Rauschenbach IY, Adonyeva NV, Alekseev AA, Faddeeva NV, Gruntenko NE. Dopamine down-regulates activity of alkaline phosphatase in Drosophila: The role of D2-like receptors. Journal of Insect Physiology. 2010;56:1155–59. doi: 10.1016/j.jinsphys.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Pacheco MA, Guan W, Molinoff PB. Agonists and antagonists differentially regulate the high affinity state of the D2L receptor in human embryonic kidney 293 cells. Molecular Pharmacology. 1995;48(5):956–64. [PubMed] [Google Scholar]
- Brown RJ, Blum K, Trachtenberg MC. Neurodynamics of relapse prevention: A neuronutrient approach to outpatient DUI offenders. Journal of Psychoactive Drugs. 1990;22(2):173–87. doi: 10.1080/02791072.1990.10472542. [DOI] [PubMed] [Google Scholar]
- Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, Woolacott N. Rimonabant for the treatment of overweight and obese people. Health Technology Assessment. 2009;(Suppl 3):13–22. doi: 10.3310/hta13suppl3/03. [DOI] [PubMed] [Google Scholar]
- Castells X, Casas M, Pérez-Mañá C, Roncero Cl, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database of Systematic Reviews (Online) 2010;(2):CD007380. doi: 10.1002/14651858.CD007380.pub3. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, Madigan MA, Waite RL, Bailey JA, Kerner M, Yeldandi S, Majmundar N, Giordano J, Morse S, Miller D, Fornari F, Braverman ER. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: Proposing an addiction candidate gene panel map. Journal of Psychoactive Drugs. 2011;43:108–27. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–41. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Conner BT, Hellemann GS, Ritchie TL, Noble EP. Genetic, personality, and environmental predictors of drug use in adolescents. Journal of Substance Abuse Treatment. 2010;38(2):178–90. doi: 10.1016/j.jsat.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS, Davies RK, Sweeney DR. Bromocriptine treatment for cocaine abuse: The dopamine depletion hypothesis. International Journal of Psychiatry in Medicine. 1985;15(2):125–35. doi: 10.2190/gxpa-98ak-4rr4-hd9b. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K, Hwang R, Kennedy JL. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:620–28. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Wargelius HL, Berglund KJ, Fahlke C, Blennow K, Zetterberg H, Oreland L, Berggren U, Balldin J. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? a pilot study. Alcohol. 2011;46:509–13. doi: 10.1093/alcalc/agr045. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Wadden TA, Berkowitz RI, Sarwer DB, Womble LG, Hesson LA, Stunkard AJ, Fabricatore AN. Changes in symptoms of depression with weight loss: Results of a randomized trial. Obesity. 2009;17(5):1009–16. doi: 10.1038/oby.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano J, Blum K. Probing the mysteries of recovery through nutrigenomic and holistic medicine: “Science meets recovery” through seminal translational research. Counselor Magazine. 2011 Available at http://www.counselormagazine.com/feature-articles-mainmenu-63/28-researchscientific/1106-probing-the-mysteries-of-recovery-through-nutrigenomics-and-holistic-medicine-science-meets-recovery.
- Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vait LD, Hennig J. Imaging gene-substance interactions: The effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neuroscience Letters. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Syvälahti E, Hietala J. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenetics and Genomics. 2005;15(6):387–91. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF. Dopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor density. Synapse. 1992;12(1):14–26. doi: 10.1002/syn.890120103. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Rowell JA, Qualichefski J, Fletcher BH, Syndulko K, Ritchie T, Noble EP. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nature Medicine. 1995;1:337–41. doi: 10.1038/nm0495-337. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Case NJ, Hulvershorn L, Patel SD, Bowker D, Gupta J, Bell R, Nurnberger JI, Tsuang MT, Kuczenski R, Geyer MA, Rodd ZA, Niculescu AB. Convergent functional genomic studies of omega-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. The 9th International Conference Proceedings of Functional Foods in Health and Disease; 2011. pp. 194–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biological Psychiatry. 2010;68(8):774–77. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CG, Henningfield JE, Haddox JD, Varughese S, Lindholm A, Rosen S, Wissel J, Waxman D, Carter LP, Seeger V, Johnson RE. Case histories in pharmaceutical risk management. Drug and Alcohol Dependence. 2009;105(Suppl 1):S42–55. doi: 10.1016/j.drugalcdep.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Miller DK, Bowirrat A, Manka M, Miller M, Stokes S, Manka D, Allen C, Gant C, Downs BW, Smolen A, Stevens E, Yeldandi S, Blum K. Acute intravenous synaptamine complex variant KB220™ “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: Part 1, pilot study with 2 case reports. Postgraduate Medicine. 2010;122:188–213. doi: 10.3810/pgm.2010.11.2236. [DOI] [PubMed] [Google Scholar]
- Miller M, Miller D. Staying Clean & Sober. Salt Lake City, Utah: Woodland Publishing; 2008. [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. American Journal of Psychotherapy. 2002;159:477–79. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Memo M, Missale C, Carruba MO, Spano PF. Repeated administration of lisuride down-regulates dopamine D-2 receptor function in mesostriatal and in mesolimbocortical rat brain regions. European Journal of Pharmacology. 1990;176(1):85–90. doi: 10.1016/0014-2999(90)90135-s. [DOI] [PubMed] [Google Scholar]
- Ross J. The Mood Cure. New York: Penquin Books; 2002. [Google Scholar]
- Taylor WA, Gold MS. Pharmacologic approaches to the treatment of cocaine dependence. Western Journal of Medicine. 1990;152:573–77. [PMC free article] [PubMed] [Google Scholar]
- Tanriover G, Seval-Celik Y, Ozsoy O, Akkoyunlu G, Savcioglu F, Hacioglu G, Demir N, Agar A. The effects of docosa-hexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochemica et Cytobiologica. 2010;48(3):434–41. doi: 10.2478/v10042-010-0047-6. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62(7):481–86. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. Journal of Neurochemistry. 2001;78(5):1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL. CRESCENDO Investigators. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet. 2010;376(9740):517–23. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- Trachtenberg MC, Blum K. Improvement of cocaine-induced neuromodulator deficits by the neuronutrient tropamine. Journal of Psychoactive Drugs. 1988;20(3):315–31. doi: 10.1080/02791072.1988.10472501. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Sahin G, Kirik D. Presynaptic dopaminergic compartment determines the susceptibility to L-DOPA-induced dyskinesia in rats. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13159–164. doi: 10.1073/pnas.1003432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2000;41:32–40. [PubMed] [Google Scholar]