Abstract
The wild-type human p53 (TP53) tumor suppressor can be posttranslationally modified at over 60 of its 393 residues. These modifications contribute to changes in TP53 stability and in its activity as a transcription factor in response to a wide variety of intrinsic and extrinsic stresses in part through regulation of protein-protein and protein-DNA interactions. The TP53 gene frequently is mutated in cancers, and in contrast to most other tumor suppressors the mutations are mostly missense often resulting in the accumulation of mutant protein, which may have novel or altered functions. Most mutant TP53s can be posttranslationally modified at the same residues as in wild-type TP53. Strikingly, however, codons for modified residues are rarely mutated in human tumors, suggesting that TP53 modifications are not essential for tumor suppression activity. Nevertheless, these modifications might alter mutant TP53 activity and contribute to a gain-of-function leading to increased metastasis and tumor progression. Furthermore, many of the signal transduction pathways that result in TP53 modifications are altered or disrupted in cancers. Understanding the signaling pathways that result in TP53 modification and the functions of these modifications in both wild-type TP53 and its many mutant forms may contribute to more effective cancer therapies.
Keywords: TP53, p53, phosphorylation, acetylation, methylation, ubiquitylation, transcription
Introduction
In the words of Robert S. McNamara: “Perhaps I don't know how much I don't know, and there is much indeed.”
The human tumor suppressing p53 (hereafter TP53; MIM# 191170) transcription factor can be posttranslationally modified at over 15 percent (∼60) of its 393 amino acid residues (SwissProt # P04637-1) by a myriad of alterations including phosphorylation, acetylation, methylation and ubiquitylation (Supp. Figure S1) in response to a wide variety of stresses and signaling pathways. The physical and metabolic stresses relevant to cancer include oncogene activation, DNA damage, oxidative stress, hypoxia, hyperoxia and nutrient starvation (Fig. 1). Other posttranslational modifications (PTMs) that are less studied include glycosylation, ADP ribosylation, sumoylation, neddylation, cysteine and methionine oxidation, cysteine alkylation and tyrosine nitration; however, little or no information relevant to mutant (MUT) p53 is available for these PTMs.
Figure 1.
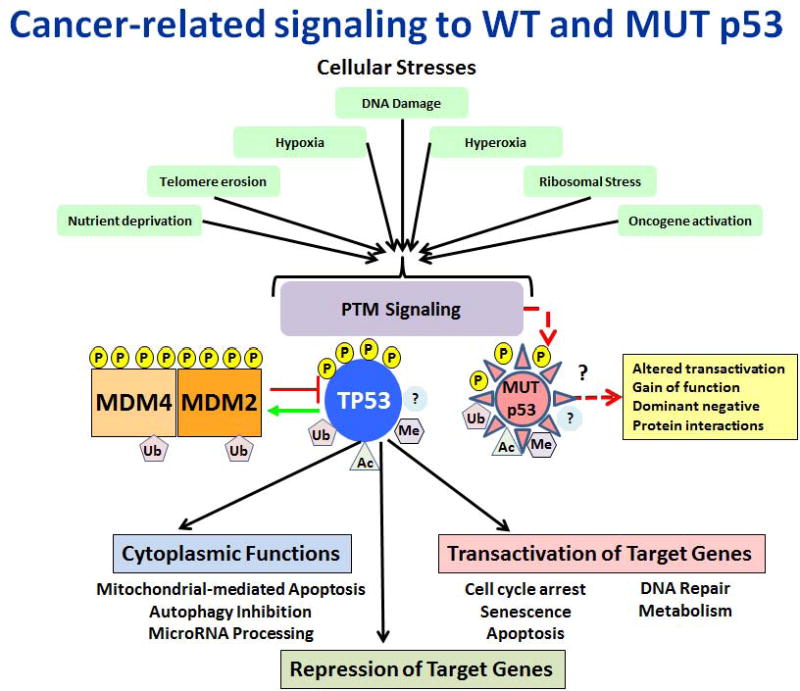
Cancer-related signaling to WT and MUT TP53. A wide variety of stress stimuli activate cellular signaling pathways leading to PTMs of TP53 and many of its interacting partners, resulting in the activation and stabilization of WT TP53. WT TP53 functions as a transcription factor to activate or repress hundreds of target genes; it also has non-transcriptional functions that are primarily cytoplasmic. MUT TP53 is modified in much the same manner but some modifications to MUT TP53 result in a GoF that enhances tumorigenesis.
In normal unstressed cells TP53 is kept at a low level through ubiquitylation by its major negative regulators MDM2 and MDM4 (MDMX) which target TP53 for proteasomal degradation. MDM2/MDM4 also bind to transactivation domain 1 (TAD1) and inhibit the ability of TP53 to interact with transcriptional components, thus preventing transcription. TP53 PTMs modulate TP53 stability as well as its interactions with DNA, chromatin and many cofactors that influence TP53-mediated transcription or repression of target genes. TP53 directly activates the transcription of several hundred genes through binding to response elements near promoters or in enhancers; some genes are also directly repressed (Riley et al., 2008). Many more genes are indirectly repressed by TP53 through the induction of microRNAs or long non-coding RNAs (Rinn and Huarte, 2011). TP53 “activation” is a loosely used term that means different things in different contexts. We use “activation” to mean a change in the functional properties of TP53 that are not due solely to an increase in its intracellular concentration. TP53 also has non-transcriptional, cytoplasmic functions that are only briefly addressed. The possibility that TP53 may have nuclear functions that extend beyond transcriptional regulation is not addressed here.
The majority of TP53 PTMs occur in unstructured regions: i) the amino- (N-)terminal transactivation domains (TAD1 and TAD2); ii) the linker after the sequence-specific DNA binding domain (DBD); and iii) the carboxyl- (C-) terminal regulatory domain that also serves as a DNA binding domain that is not sequence specific. These regions primarily serve as sites for interaction with the many proteins (>300) that interact with TP53. Thus, PTMs primarily control TP53 activity through modulating many of these interactions rather than by changing TP53 structure. Unfortunately, there have been relatively few in-depth studies addressing the effects of PTMs on TP53 protein interactions (see below). A few modifications do occur in the structured DBD (e.g. Fig. 2) and tetramerization (TET) (Fig. 3) domains (Table 1; see the Supporting Information for the color version, Supp. Table S1), but these may also be involved primarily in controlling protein-protein interactions rather than structure per se.
Figure 2.
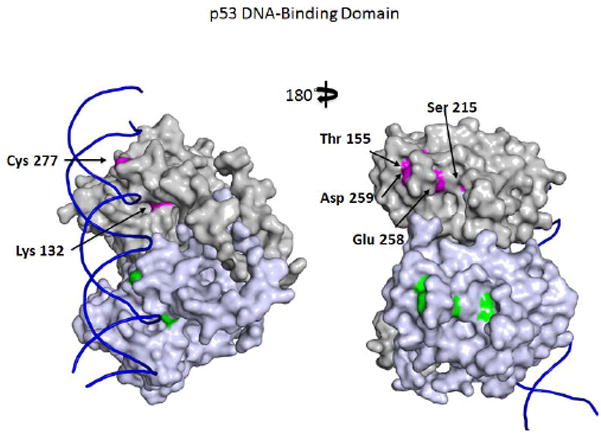
Frequent mutations of modified residues in the TP53 DBD. A space-filling dimer of the TP53 DBD bound to a representation of a response element based on the X-ray structure of Emamzadah et al. (2011) (residues 94-291; PDB 3TS8). The two subunits are colored gray or purple. The six modified residues that are more commonly mutated are shown in color (red or green in the different subunits) and are identified in one subunit. The structure is rotated 180° to give front and back views.
Figure 3.
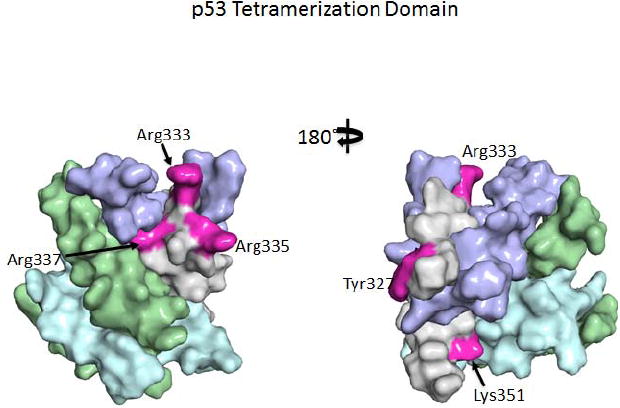
Modified residues in the TET of TP53. A space filling model of the TET (subunits colored purple, blue, green and gray) based on the X-ray structure of Jeffery et al. (1995) is shown (residues 325-356, PDB 1C26); the five residues that can be posttranslationally modified are colored red on one subunit and are labeled. The structure is rotated 180° to give front and back views.
Table 1. Cancer Mutants in Known TP53 PTM Sites.
| Residue | Domain | Modifi- cation |
Enzyme | Signal | Vertebrate Residue Conservation |
TP73 TP63 |
Mutant Residue |
Number Mutations at this Residue |
Mouse Mutant Model |
Cell Line |
|---|---|---|---|---|---|---|---|---|---|---|
| Ser6 | TAD1 | P | JNK2 | DNA Damage | 0.63 | NO | L† | 1 | - | - |
| Ser9 | TAD1 | P | HIPK4, CK1 | DNA Damage | 0.51 | NO | - | 0 | - | - |
| Ser15 | TAD1, NES1 | P | ATM, ATR, NUAK1 | DNA Damage | 0.98 | NO | R† | 1 | S18A | - |
| Thr18 | TAD1, NES1 | P | CK2, VRK1/2 | DNA Damage | 0.84 | NO | - | 0 | - | - |
| Ser20 | TAD1, NES1 | P | CHK1/2, CK1, PLK3 | DNA Damage | 0.74 | NO | - | 0 | S23A | - |
| Asn29 | TAD1 | carboxyl M | PIMT | ? | 0.65 | NO | - | 0 | - | - |
| Asn30 | TAD1 | carboxyl M | PIMT | ? | 0.60 | NO | S† | 1 | - | YES |
| Ser33 | TAD1 | P | p38K, CDK5, CDK9 | DNA Damage | 0.58 | NO | T↓, F† | 3 | - | - |
| Ser37 | TAD1 | P | ATR, PRAK | DNA Damage | 0.53 | NO | T↓, P† | 2 | - | YES |
| Ser46 | TAD2 | P | ATM, p38K, CDK5, DYRK2 | DNA Damage | 0.53 | NO | P↑, F† | 6 | Hupki S46A | - |
| Thr55 | TAD2 | P | TAF1, ERK2 | Unstressed | 0.23 | NO | I† | 1 | - | - |
| Thr81 | TAD2, PRD | P | JNK2 | DNA Damage | 0.35 | NO | I† | 2 | - | - |
| Ser99 | PRD | P | ATM, ATR, DNA-PK | DNA Damage | 0.79 | YES | P↓, F↓ | 4 | - | - |
| Lys101 | NRD | Ub | MDM2 | Unstressed | 0.65 | NO | R†, N↓ | 4 | - | - |
| Ser106 | DBD | P | AURA | ? | 0.40 | NO | R↓, GΔ | 8 | - | YES |
| Lys120 | DBD | Ac | TIP60, hMOF | DNA Damage | 1 | YES | QΔ, EΔ, MΔ, NΔ, R↓ | 16 | K117R 3KR | - |
| Cys124 | DBD | GTN | ? | ? | 0.84 | NO | S†, RΔ, G↓, Y†, W† | 6 | - | YES |
| Lys132 | DBD | Ub | MDM2? | Unstressed? | 1 | YES | QΔ, EΔ, Wø, Lø, TΔ, RΔ, MΔ, NΔ | 205 | - | YES |
| Lys139 | DBD | Ub | MDM2? | Unstressed? | 1 | YES | Q↓, E†, T↓, R†, NΔ | 30 | - | YES |
| Ser149 | DBD | OGN P? | ? | ? | 0.74 | NO | T†, P↓, A†, F↓ | 21 | - | - |
| Thr155 | DBD | P | COP9/CSN5 | Unstressed | 0.53 | NO | PΔ, A†, S†, NΔ, IΔ, Mø | 102 | - | YES |
| Lys164 | DBD | Ac | p300 | ? | 1 | YES | Q↓, EΔ, T↓, R↓, M↓, N↓ | 49 | K161R 3KR | YES |
| Ub | PIRH2 | |||||||||
| Cys182 | DBD | GTN | ? | ? | 0.63 | NO | S†, R↓, Y↓ | 18 | - | - |
| Ser183 | DBD | P | AURB | ? | 0.70 | NO | P†, L† | 9 | - | - |
| Thr211 | DBD | P | AURB | ? | 0.98 | YES | PΔ, A↓, S†, N†, IΔ | 35 | - | YES |
| Ser215 | DBD | P | AURA | ? | 1 | YES | RΔ, GΔ, C↓, NΔ, T↓, IΔ, Kø | 110 | - | YES |
| Glu258 | DBD | ADR | PARP1 | ? | 1 | YES | KΔ, Q↓, A†, GΔ, VΔ, D↓ | 129 | - | YES |
| Asp259 | DBD | ADR | PARP1 | ? | 0.72 | NO | N↓, HΔ, YΔ, Pø, Sø, AΔ, G↓, VΔ, EΔ | 97 | - | YES |
| Ser269 | DBD | P | AURB, DAPK | ? | 0.79 | NO | R↓, G↓, C†, N↓, T↓, I↓ | 26 | - | - |
| Glu271 | DBD | ADR | PARP1 | ? | 1 | YES | K↓, Q↓, Pø, Rø, A↓, G↓, VΔ, D† | 67 | - | YES |
| Cys277 | DBD | ALK | 15d-PGJ2 | ? | 1 | YES | RΔ, GΔ, YΔ, S†, FΔ, WΔ | 100 | - | YES |
| Thr284 | DBD | P | AURB, DAPK? | ? | 0.95 | NO | PΔ, A†, K†, I† | 19 | - | YES |
| Lys291 | DBD | Ub | MKRN1 | Unstressed | 0.93 | NO | Q†, E†, T↑, R†, M†, N† | 23 | - | - |
| Lys292 | DBD | Ub | MKRN1, MDM2, PIRH2 | Unstressed | 0.77 | NO | Q↑, E†, Gø, T†, R†, I†, N† | 24 | - | - |
| Lys305 | LNK | Ac | p300 | DNA Damage | 0.74 | YES | EΔ, T↓, RΔ, MΔ, N↓ | 16 | - | - |
| Ub | MDM2, PIRH2 | Unstressed | ||||||||
| Ser313 | LNK | P | CHK1/2 | DNA Damage | 0.70 | NO | R†, C†, N†, I† | 5 | - | - |
| Ser314 | LNK | P | CHK1/2 | DNA Damage | 0.65 | NO | F† | 1 | - | - |
| Ser315 | LNK | P | AURA, CDK9 | DNA Damage | 0.79 | NO | P†, C†, F† | 4 | Hupki S315A | - |
| S312A | ||||||||||
| Lys319 | LNK, NLS1 | Ac | PCAF | DNA Damage | 0.88 | YES | E↓, R†, N† | 7 | - | - |
| Ub | MDM2 | Unstressed | ||||||||
| Lys320 | LNK, NLS1 | Ub, N8 | E4F1, FBX011, MDM2 | Unstressed? | 0.72 | NO | N↓ | 6 | K317R | YES |
| Lys321 | LNK, NLS1 | N8 | FBX011, MDM2 | Unstressed? | 0.74 | NO | R↑ | 1 | - | - |
| Tyr327 | TET | NO | ? | NO | 0.70 | NO | H†, D†, S†, C† | 4 | - | - |
| Arg333 | TET | Me | PRMT5 | ? | 0.91 | YES | - | 0 | - | - |
| Arg335 | TET | Me2 | PRMT5 | ? | 0.84 | YES | G†, H↑, L† | 3 | - | - |
| Arg337 | TET | Me2 | PRMT5 | ? | 0.95 | NO | CΔ, H↓, PΔ, LΔ | 37 | - | YES |
| Lys351 | TET, NES2 | Ub | MLS2, MDM2 | Unstressed? | 0.72 | NO | N† | 1 | - | - |
| Lys357 | REG | Ac | hMOF | DNA Damage | 0.58 | NO | - | 0 | - | - |
| Ub | MDM2, PIRH2 | |||||||||
| Ser362 | REG | P | IκB2 | DNA Damage | 0.65 | NO | - | 0 | - | - |
| Ser366 | REG | P | IκB2, CHK2 | DNA Damage | 0.74 | NO | A† | 2 | - | - |
| Lys370 | REG | Ac | p300, CBP | DNA Damage | 0.81 | NO | Q† | 1 | K367R K6R, 7KR | - |
| Me1,2 | SMYD2 | |||||||||
| N8 | MDM2 | Unstressed | ||||||||
| Ub | MDM2, PIRH2 | |||||||||
| Ser371 | REG | P | PKCα | ? | 0.58 | NO | - | 0 | - | - |
| Lys372 | REG | Ac | p300, CBP | DNA Damage | 0.70 | NO | - | 0 | K369R K6R, 7KR | - |
| Me1,2 | SET7, SET9 | |||||||||
| N8 | MDM2 | Unstressed | ||||||||
| Ub | MDM2, PIRH2 | |||||||||
| Lys373 | REG | Ac | p300, CBP | DNA Damage | 0.72 | NO | - | 0 | K370R K6R, 7KR | - |
| Me1,2 | G9A, GLP | |||||||||
| N8 | MDM2 | Unstressed | ||||||||
| Ub | MDM2, PIRH2 | |||||||||
| Ser376 | REG | P | GSK3β, PKC | ? | 0.70 | NO | T†, A† | 2 | - | - |
| Thr377 | REG | P | CHK1/2 | DNA Damage | 0.58 | NO | - | 0 | - | - |
| Ser378 | REG | P | CHK1/2, PKC, p38K | ? | 0.84 | NO | - | 0 | - | - |
| Lys381 | REG | Ac | p300, CBP | DNA Damage | 1 | NO | - | 0 | K378R K6R, 7KR | - |
| Ub | MDM2, PIRH2 | Unstressed | ||||||||
| Lys382 | REG | Ac | p300, CBP, hMOZ | DNA Damage | 0.88 | YES | - | 0 | K379R K6R, 7KR | - |
| Me2 | SET8, PR-SET7 | DNA Damage | ||||||||
| Ub | MDM2, PIRH2 | Unstressed | ||||||||
| Lys386 | REG | Ac | p300, CBP | DNA Damage | 0.88 | NO | - | 0 | K383R K6R, 7KR | - |
| SUMO | PIAS, PIASxβ | ? | ||||||||
| Ub | MDM2, PIRH2, COP1 | Unstressed | ||||||||
| Thr387 | REG | P | CHK1 | DNA Damage | 0.26 | NO | - | 0 | - | - |
| Ser392 | REG | P | CDK9, PKR, FACT/CK2 | DNA Damage | 1 | NO | L† | 1 | S389A | - |
Table information was abstracted largely from the p53 IARC TP53 Database R16 http://p53.iarc.fr/ (Petitjean et al., 2007) which describes the properties of 29,575 somatic mutations and 635 germline mutations. The human TP53 sequence is taken from SwissProt # P04637-1 and is known as the ‘canonical’ sequence or ‘p53alpha’; residue 1 is the initiating methionine. The mouse TP53 protein sequence is derived from RefSeq entry NP_035770; Residue 1 corresponds to the first methionine codon which is located three residues 5′ of the first methionine codon in the human cDNA. Residue: Bold indicates a more frequently mutated residue (>90/29,575). Domain: TAD1, Transactivation Domain 1; TAD2, Transactivation Domain 2; PRD, Proline Rich Domain; NRD, N-terminal Repression Domain; DBD, Sequence-specific DNA Binding Domain; LNK, Linker Domain; TET, Tetramerization Domain; REG, Regulatory Domain, non-specific DNA binding domain; NES, Nuclear Export Signal; NLS, Nuclear Import Signal; gray shading indicates the different TP53 domains. Modification: P, phosphorylation; Ac, acetylation; ALK, alkylation; Ub, ubiquitylation; Me1, monomethylation; Me2, dimethylation; Su, sumoylation; N8, neddylation; ADR, ADP-ribosylation; OGN, O-glycosylation; GTN, Glutathionylation; NO, Nitration. Residue conservation data was obtained from TP53 Mut Assessor (Leroy et al., 2013). Vertebrate: Conservation of the residue in vertebrate TP53: Forty-two vertebrate TP53 sequences were aligned using CLUSTAL. For each position from 1 to 393, conservation of each residue of the human TP53 has been ranked from 0 (not found in any other TP53 to 1 (residue conserved in all vertebrate TP53s). Only full-length TP53 sequences were used for this analysis. TP73/TP63: Conservation of the residue in human TP73 and TP63. The human TP53 sequence was aligned to human TP63 and TP73 to identify residues conserved in the three proteins. Yes: residue conserved; No: residue not conserved.
Missense Mutants:transactivation competent;
partial loss of transactivation;
supertransactivator;
loss of transactivation;
not evaluated. Enzyme/Protein Abbreviations: see Supp. Figure S1. Mouse Mutant Model: Mutant models were taken from the literature (see text); the mouse p53 residue is given together with the amino acid (single letter code) encoded by the mutant. Mutation of multiple residues is indicated as per the literature. Cell Line: The availability of a mutant human cell line is indicated as per the IARC TP53 database.
TP53 PTMs show significant redundancy and interdependency. N-terminal phosphorylation was first shown to direct TP53 C-terminal acetylation (Sakaguchi et al., 1998), and several N-terminal phosphorylations were found subsequently to be interdependent (Saito et al., 2003). For example, using transient transfection of TP53 plasmids into H1299 tumor-derived cells, a change in Ser15 to alanine blocked phosphorylation at Ser9, Thr18 and Ser20 after ionizing radiation (IR). Similar interdependencies have been reported for acetylation and methylation sites in the C-terminal region (West and Gozani, 2011). Interdependencies suggest that mutation of some sites could have wider consequences with respect to TP53 activity. Nevertheless, mutations at six sites (see below) that abrogate PTMs are frequent in human cancers (Table 1). Redundancy is clearly demonstrated with mouse knock-in mutants for Ser18 and Ser23. Changing either residue to alanine had relatively minor effects on DNA damage responses, but changes in both residues to alanine abrogated DNA damage-induced apoptosis (Chao et al., 2006a). Several of the enzymes that modify TP53 depend on “docking sites” that are somewhat remote from the site modified (Endicott et al., 2012). While cancer mutations in these remote landing sites might abrogate modification of MUT TP53 proteins in cancer without directly affecting the modification site, TP53 docking sites for only a few modifying enzymes have been elucidated.
TP53 PTMs have been most extensively studied in response to DNA damage, which activates the ATM/ATR DNA damage response pathway (Kruse and Gu, 2009b; Vousden and Prives, 2009). Following the induction of DNA double-strand breaks (DSBs) or the generation of single-stranded DNA from DNA replication stress, telomere uncapping, or other repair processes, ATM and ATR rapidly phosphorylate TP53 on Ser15, Ser37 and possibly on Ser46. ATM and ATR also activate the effector kinases CHK1 and CHK2, which phosphorylate TP53 at additional sites (Table 1, Supp. Figure S1). The DNA damage response can activate a host of other protein kinases (Bensimon et al., 2011), some of which also phosphorylate TP53. Phosphorylation of TP53 N-terminal residues inhibits binding of TP53 by MDM2/MDM4, TP53 ubiquitylation and also promotes binding of the histone acetylases p300/CBP (KAT3B/KAT3A). The p300 protein acetylates mainly C-terminal residues that enhance binding of TP53 to DNA, block TP53 ubiquitylation and further activate TP53, probably by promoting or inhibiting TP53 interactions with binding partner proteins, some of which serve as co-regulators similar to p300. DNA damage also induces an important acetylation of the DNA contact residue Lys120 by TIP60/MOF, as well as methylation of several C-terminal residues by various protein methylases. Methylations can enhance or repress TP53 transcriptional activity (West and Gozani, 2011). The DNA damage response also results in PTM of many of the proteins that regulate TP53, such as MDM2, MDM4 and p300, and independently affects their activity towards TP53.
Contrary to many of the components of the DNA damage response, the TP53 transcriptional response to acute DNA damage, including classical TP53 DNA damage-induced genes (Jiang et al., 2011; Li, et al., 2012), does not appear to be essential for tumor suppression (Christophorou et al., 2006). This finding raises the question of what role PTMs of TP53 play in TP53-mediated tumor suppression (Loughery and Meek, 2013; Meek, 2009; Zilfou and Lowe, 2009). Extensive studies have shown that no single PTM appears to be essential for TP53-mediated tumor suppression. In response to oncogene activation and aberrant proliferative signals, TP53 is activated primarily through the ARF pathway rather than through DNA damage-induced signaling; however, neoplastic cells often display evidence of DNA damage. Few TP53 PTMs have been shown to be caused by induction of ARF, although ARF induction does induce acetylation of TP53 Lys120 (Mellert et al., 2007). Nevertheless, six residues that are subject to PTM-- Lys132, Thr155, Ser215, Glu258, Asp259 and Cys277-- are mutated in tumors significantly more frequently than are other single posttranslationally modified residues (Table 1 and Fig. 2), suggesting that modifications to these residues might contribute to TP53-mediated tumor suppression. Alternatively, changes to these residues, all of which lie in the structured DBD, may contribute to TP53 instability. Surprisingly, modifications of these residues are among the least studied TP53 PTMs.
Additional evidence for the importance of TP53 PTMs in TP53-mediated biology, if not in tumor suppression, comes from the fact that several residues subject to modification, including the six DBD residues listed in the paragraph above, are highly conserved through evolution including TP53 from simple eukaryotes (Lane et al., 2010; MacLaine and Hupp, 2009). A third indication for the importance of PTMs in tumor suppression comes from the mutation or overexpression of TP53 modifiers in cancers with wild-type (WT) TP53. The best known examples are the amplification of MDM2 and overexpression of its protein, the major negative regulator of TP53 in cancers, notably in sarcomas but also in breast and other cancers (Wade et al., 2013), and the overexpression of or a gain-of-function (GoF) mutation of PPMD1 (WIP1), the gene for a TP53-induced phosphatase that dephosphorylates TP53 as well as many other DNA damage response and repair proteins, or WIP1 overexpression (Kleiblova et al., 2013; Lu et al., 2008; Zhu and Bulavin, 2012). However, dephosphorylation of TP53 by WIP1 may be less important for attenuating its activity than is reduction of ATM and/or ATR activation or the removal of inactivating phosphorylations from MDM2/MDM4 by WIP1.
The role of TP53 PTMs in WT TP53-mediated functions has been covered by many extensive reviews, (e.g., Anderson and Appella, 2009; Freeman and Espinosa, 2013; Gu and Zhu, 2012; Jenkins et al., 2012; Loughery and Meek, 2013; Meek and Anderson, 2009; Vousden and Prives, 2009). Here, we review the literature with regard to PTMs that are known to occur to MUT TP53, and we discuss PTMs that influence TP53 binding partners. To the best of our knowledge, no PTMs have been identified in MUT TP53s that do not also occur in WT TP53, but expression of many of the signaling enzymes that modify TP53 and other chromatin components can become dysregulated in cancer, thus potentially modulating normal signaling to TP53. Finally, we raise questions that are relevant to future research on the role of TP53 PTMs in cancer biology.
Posttranslational Modifications of MUT TP53
Alteration of the TP53 gene itself or perturbations in TP53 signaling pathways are the most frequent events in tumorigenesis (Petitjean et al., 2007) and can be considered hallmarks of cancer cells (Pfeifer and Hainaut, 2011). Cancer-associated changes in tumor suppressor genes such as CDKN2A (ARF), RB1 or BRCA1 are generally due to loss of protein due to silencing or deletion. This contrasts with TP53 the normal function of which commonly is altered by direct single nucleotide substitution missense mutations that often give rise to a stable mutant protein.
The vast majority of TP53 mutations in cancers result in loss of DNA sequence-specific binding and the ability to activate transcription of most TP53 target genes and thus loss of tumor suppressive function. Still nearly one-third of cancer-associated mutants retain limited or altered TP53 transcriptional function (Resnick and Inga, 2003). Moreover, loss of function missense MUT TP53 often antagonizes WT TP53 tumor suppressor functions in a dominant negative manner through tetramer formation with WT protein. In addition and totally independent of WT TP53 functions, many MUT TP53 proteins also acquire oncogenic GoF(s) which endow selective growth advantages to cells with the mutations such as transactivation of new target genes or inappropriate interactions with other cellular proteins (Brosh and Rotter, 2009; Goh et al., 2011; Lozano, 2007). Regardless of the general or mechanistic classification of the MUT TP53s, and knowing the role that PTMs have on WT TP53 functionality, it is expected and in some cases demonstrated (discussed below) that PTMs impact the activities of MUT TP53. Although TP53 mutations are found in all coding exons of the TP53 gene, more than 95% of the base substitution mutations are located in exons 4-9, which encode the DBD of the protein. Among the mutations in this domain, about 30% locate within six “hotspot” residues that are frequent in almost all cancer types (Cho et al., 1994; Hollstein et al., 1991), e.g. Arg175, Gly245, Arg248, Arg249, Arg273, and Arg282, but which are not known to be posttranslationally modified.
Similar to WT TP53, a variety of parameters at the posttranslational level contribute to the multifunctionality of MUT TP53 that fine-tune and modify cellular localization, the rate of its degradation and the specificity of TP53 for its target genes, as well as establishing a code for the interaction of TP53 with other proteins. For this review, we approach the relationship between PTMs and MUT TP53 with two simple questions. Are PTM residues on TP53 protein mutated and if so what are the consequences? Do PTMs impact MUT TP53 functions?
If the posttranslationally modified residues are important for the tumor suppressor and genome guardian roles of TP53, one might expect that a mutation at such residues would abolish or compromise those functions and that missense mutations in these PTM residues should be frequently observed in tumors. Analysis of the TP53 tumor mutation databases reveals that the vast majority of residues subjected to PTM are infrequently mutated in human tumors (Table 1). In addition, none of the twenty PTM sites described in the DBD falls in a hotspot for tumor mutations. Nevertheless, the codons for Lys132, Thr155, Ser215, Glu258, Asp 259 and Cys277 have over 90 cancer-associated mutations/codon (Table 1), well above the average of ∼70 mutations per codon for the TP53 DBD (excluding hotspot mutations), although little is known about the role of these mutants in tumor development. The codons for two other PTM sites in the DBD (Lys164 and Glu271) are mutated somewhat less frequently (49 and 67 times, respectively) than the average for the DBD excluding hotspot mutants. Surprisingly, most amino acid changes in these residues only partially compromised TP53 transactivation (Table 1). The Arg337 residue is subject to dimethylation and is considered a hotspot for TP53 germline mutations that present as adrenocortical tumors (Latronico et al., 2001; Ribeiro, et al., 2001). Furthermore, in vitro mutagenesis for each of the known residues that can be posttranslationally modified in TP53 did not lead to significant reduction of its transactivation activities in a yeast based assay (Kato et al., 2003).
As for the second question, little is known about the role of PTMs in the regulation of MUT TP53 activity. Most data comes from in vitro studies using non-physiological expression levels and/or transformed cell lines where several signaling pathways that influence TP53 activities are also altered. Nevertheless, data coming mainly from animal models supports the view that PTMs along with the oncogenic context are crucial to malignant functions of MUT TP53 (Muller et al., 2011). For example, Adorno et al. (2009) showed that activated RAS signaling promotes the phosphorylation of a MUT TP53 Arg280Lys at Ser6 and Ser9 and results in the formation of a MUT TP53/SMAD complex, which in turn inhibits p63 (hereafter TP63) antimetastasis activities. In the following sections, examples are presented of how MUT TP53 is regulated through PTMs. We have focused on three major covalent modifications: phosphorylation, acetylation and ubiquitylation.
Phosphorylation of Serines and Threonines in MUT TP53
Phosphorylation of TP53 is generally considered the first step in TP53 stabilization and the enhancement of TP53 transactivating activities at its target genes. There are ∼30 residues that can be phosphorylated, mostly located in the N and C termini. Several can be targeted by multiple kinases implying some level of redundancy. Yet the residues generally considered critical for TP53 antiproliferative and antiapoptotic activities, including Ser15, Thr18, Ser20, Ser46 and Ser392, are infrequently mutated in cancers. In fact, tumor missense mutations of TP53 at sites subject to phosphorylation are exceedingly rare (Table 1). In agreement with this, knock-in mice with single amino acid replacements that preclude modification of those residues have shown unexpectedly modest effects on TP53 function compared to in vitro results in transfected cell lines that had predicted a more profound impact (Toledo et al., 2006). The exceptions are residues Thr155 and Ser215 located in the DBD (found 99 and 110 times in tumors, respectively (Petitjean et al., 2007, TP53 IARC database R16). Interestingly, Thr155 is one of the few sites for which phosphorylation promotes TP53 degradation. The COP9 signalosome (CSN)-associated kinase complex phosphorylates Thr155, promoting TP53 ubiquitylation by both MDM2 and the papillomavirus E6-AP ligases and subsequent proteasomal degradation (Bech-Otschir et al., 2001). Phosphorylation of Ser215 also promotes the inhibition of TP53 protein activities. In this case, phosphorylation of TP53 by Aurora-A kinase abrogates WT TP53 DNA binding and transactivation activity (Liu et al., 2004). Overexpression in HCT116 TP53-/- cells of mutants mimicking non-phosphorylatable (Ser215Ala) and phosphomimetic (Ser215Asp) forms of this residue resulted in the specific loss of phosphorylation at Ser392 and Ser33, respectively. In addition Thr155 and Ser215 together with two other PTM sites (Ser269 and Glu271) are spatially located far from the TP53-DNA interface but have the potential to induce TP53 protein conformational changes, thus affecting functional activities (Shiraishi et al., 2004).
Like WT TP53, MUT TP53 can be posttranslationally modified in the absence of, or in response to, stress signals although relatively little is known about how these modifications affect MUT TP53 activities. The patterns of phosphorylation on MUT TP53s can differ from WT patterns. Nearly 20 years ago, Ullrich et al. (1993) showed in one of the pioneering studies of PTMs of MUT TP53 that in the absence of stress phosphorylation of specific residues in MUT TP53 were altered compared to WT. Using tumor cells harboring transcriptionally defective MUT TP53s, no changes in phosphorylation of Ser9 were observed, but phosphorylation at Ser15 was reduced and phosphorylation at Ser392 was increased. On the other hand, Minamoto et al. (2001) found that MUT TP53 can be phosphorylated at various sites including Ser15, Thr81 and Ser392 in vitro and in human tumors in vivo. The phosphorylation of residues on MUT TP53 is expected to influence the pattern of other modifications in the protein as well as to promote changes in conformation that would affect activities of MUT TP53 including interactions with other proteins and responses to anticancer drugs. For example, mutations at phosphorylation sites corresponding to Ser15 and 46 but not to Ser20 in the N-terminal region of TP53 result in variations in the radiosensitivity of lung cancer cells (Okaichi et al., 2011). Phosphorylation of the N terminus by JNK or the C terminus by PLK2 enhances MUT TP53 GoF (Valenti, et al., 2011; Zerbini, et al., 2005), whereas phosphorylation at Ser392 promotes MDM2-mediated degradation and reduces the transforming activity of the MUT TP53 protein (Gillotin et al., 2010). Furthermore PML, which interacts constitutively with mutant TP53 and regulates key TP53 PTMs, is required for MUT TP53 GoF (Haupt et al., 2009).
Ser392 is one of the few PTM residues for which detailed information is available as to how PTMs impact MUT TP53 activities and tumorigenesis (reviewed in Matsumoto et al. (2006)). Located near the C terminus, Ser392 is phosphorylated in response to UV radiation by a protein complex containing CK2, hSpt16 and SSRP1 (Keller et al., 2001) which stabilizes the TP53 tetramer (Sakaguchi et al., 1997) and activates the sequence-specific DNA binding activity of TP53 (Hupp and Lane, 1995). In the absence of stress, Ser392 is frequently found to be hyperphosphorylated in several tumor-derived cell lines harboring the Arg248Trp or Arg273His hotspot mutations (Minamoto et al., 2001; Ullrich et al., 1993; Warnock et al., 2011). In human transitional cell carcinomas, ∼60% of the samples harboring TP53 missense mutations showed constitutive phosphorylation of Ser392 (Furihata et al., 2002). Other studies have reported a correlation between high frequency of cells with Ser392 hyperphosphorylation and poor prognosis or advanced tumor stage and tumor grade in TP53-positive cancers including esophageal squamous cell carcinomas and a variety of related skin tumors (Bar et al., 2009; Matsumoto et al., 2004a, 2004b). However, Yap and collaborators (2004) reported that for breast tumors with high expression of MUT TP53, the levels of phosphorylated Ser392 were reduced.
How Ser392 phosphorylation of MUT TP53 might contribute to tumor progression is not known. It has been proposed that Ser392 phosphorylation enhances tetramer formation of certain GoF MUT TP53s, which then become more potent oncoproteins (Bode and Dong, 2004). Also, the Ser392Ala mutation dramatically reduces the half-life of misfolded (structural) TP53 Arg175His protein but not the Arg248Trp mutant which has a native conformation (Gillotin et al., 2010). Yet the Ser392Ala protein enhanced the oncogenic capability of the MUT TP53 Arg175His protein in Ras-transformed rat embryo fibroblasts (Yap et al., 2004).
Another extensively studied phosphorylated residue in TP53 mutants is Ser15, a key to TP53 activation after stress signals (Jenkins et al., 2012). Results of these studies often are contradictory, which may reflect the conditions used and the cells examined. In early observations of DNA binding mutants, a lack of Ser15 phosphorylation was noted (Nagata et al., 1999; Ullrich et al., 1993). But in other studies that included many more MUT TP53 cancer cell lines from different tissue origins, no conclusive correlation between Ser15 modification and abundance, localization, or DNA binding affinity of MUT TP53 were found under stressed or unstressed conditions (Liu et al., 2013; Minamoto et al., 2001; Ray et al., 2012), suggesting that other players influence the impact of this modification on MUT TP53 functions. However, Melnikova et al. (2003) found constitutive Ser15 phosphorylation in UV-induced mouse skin tumors as well as in human breast and pancreatic cancer cell lines expressing Arg280Lys, Leu194Phe, and Arg273His mutants. In addition they found MDM2 overexpression did not alter the level of constitutively phosphorylated MUT TP53, a result which suggests that phosphorylation of MUT TP53 at Ser15 may contribute to increased stabilization and to oncogenic activities. While the levels of Ser15 phosphorylation found in squamous cell carcinoma and skin tumors were low (Matsumoto et al., 2004a, 2004b), phosphorylation of this residue was highly correlated with a high Ki-67 labeling index proliferation marker in basal cell carcinoma; this finding suggests that Ser15 phosphorylation might affect the proliferative activity of skin derived cells. In addition, Zerbini and colleagues (2005) found that Ser15 phosphorylation of MUT TP53 can play a critical role in regulating the oncogenic function of MUT TP53 and cell survival via the NF-κB signaling pathway. Inhibition of NF-κB signaling promoted the stabilization of MUT TP53 (Pro223Leu/Val274Phe) through increased phosphorylation at Ser15 by the JNK kinase. Moreover, it was reported that inhibition of Ser15 or Ser315 phosphorylation partially restored WT function in osteosarcoma SaOs2 cells transfected with TP53 proteins mutated at residues Ala143 and His175 (Sugikawa et al., 1999).
The complexity of regulation of MUT TP53 and role of PTMs is illustrated in a recent study by Valenti et al. (2011) which shows that WT and Arg175His TP53s induce polo-like kinase 2 (PLK2) expression in response to doxorubicin and cisplatin, although by different mechanisms. Whereas WT TP53 binds its consensus sequence in the PLK2 promoter (Burns et al., 2003), the MUT TP53 lacks this capacity and induces PLK2 expression by hitchhiking onto the transcription factor NF-Y at CCAAT-boxes, thus promoting p300 recruitment and histone acetylation. Interestingly, activated PLK2 phosphorylated MUT TP53 at the C terminus, particularly residue Thr377, stimulating TP53 acetylation (Valenti et al., 2011). As a result, this modification enhances the transcriptional activity of TP53 and, in the case of MUT TP53, increases its interaction with the p300 cofactor and MUT TP53 target promoters. The phosphorylated MUT TP53–p300 complex subsequently can interact with NF-Y to induce transcription providing an autoregulatory loop that reinforces MUT TP53 activity which results in enhanced cell growth and chemoresistance to conventional anticancer drugs.
Acetylation of MUT TP53 Lysines
In addition to phosphorylation, acetylation of TP53 has been described as indispensable for many TP53 biological activities (Tang et al., 2008). Eleven lysine residues on TP53 can be acetylated, nine are located in the C terminus while only two are in the DBD (Lys120 and Lys163). TP53 is acetylated by several histone acetyltransferases (Kruse and Gu, 2009b) including the structurally related p300 and CREB-Binding Protein (CBP), p300/CBP-Associated Factor (PCAF), TIP60, hMOF and hMOZ (Table 1 and Supp. Figure S1). The acetylation levels of TP53 are enhanced in response to stress and correlate well with TP53 activation, promoting the recruitment of cofactors for promoter specific TP53 transcriptional activity (Brooks and Gu, 2003; Carter and Vousden, 2009). Acetylation also stimulates TP53 stabilization by inhibiting the ubiquitylation process induced by MDM2/MDM4 repressive complexes (Dai and Gu, 2010).
Although most acetylated lysine sites on TP53 are not altered in human tumors, some residues including Lys120, 164 and 305 are often found mutated in cancer (Table 1). Importantly, the location of Lys120 and 164 in the DBD, the region most often mutated in cancers, suggests that these residues might have physiological roles and non-redundant effects on TP53 function. Acetylation of Lys120 is indispensable for the activation of target genes involved in apoptosis but not cell cycle arrest, while Lys164 acetylation is associated with activation of the majority of TP53 target genes (Tang et al., 2008). Notably, the fact that both residues are conserved in all species containing functional TP53 genes suggests a role for this modification throughout evolution.
Acetylation of several residues in MUT TP53 has been observed in various cancer cell lines. In the absence of stress, the Arg248Trp and Arg273His MUT TP53 proteins were hyperacetylated at Lys320, Lys373 or Lys382 when compared with non-tumorigenic cell lines (Minamoto et al., 2001). A similar observation was found by Warnock et al. (2011) for Lys382 acetylation in colon carcinoma cells harboring Arg273 mutations. These intense patterns of acetylation might facilitate accumulation of MUT TP53 in the nucleus (Bode and Dong, 2004).
As found for other PTMs, the relationship between acetylation and signaling for WT and MUT TP53 proteins remains unclear. One of the major issues contributing to a lack of understanding of the impact of TP53 acetylation is the battle for lysine modifications (Carter and Vousden, 2009). Most of the lysine residues subject to acetylation can also be modified by methylation, ubiquitylation, and neddylation, all of which have inhibitory effects on a variety of TP53 functions (Kruse and Gu, 2009b). For example, acetylation of C-terminal lysine residues stabilizes TP53 and blocks TP53 degradation by inhibiting ubiquitylation of these residues (Li et al., 2002). The picture is even more complex if we consider the crosstalk between phosphorylation and acetylation. Specific phosphorylation patterns can promote acetylation of the C terminus and initiate a phosphorylation-acetylation cascade (Ferreon et al., 2009; Ou et al., 2005; Puca et al., 2009; Sakaguchi et al., 1998). This dynamic process for TP53 PTMs also appears to occur in cells with MUT TP53 as described in the previous section in the experiments by Valenti and colleagues (2011) with PLK2 and MUT TP53.
Recently, Rodriguez et al. (2012) showed that acetylation of MUT TP53 has a profound impact on metabolic and survival activities within cancer cells. Using several MUT TP53 cancer cell lines that included ovarian TOV (TP53Arg175His), breast carcinoma MDA-MB-231 (TP53Arg280Lys), T47D (TP53Lys194Phe), BT20 (TP53Lys132Gln) and pancreatic cancer PANC1 (Arg280Thr), they found that glucose restriction induces acetylation of lysines at the C terminus of MUT, but not WT, TP53 protein. With a C-terminal acetylation-mimicking version of TP53 (Gly245Ala-K6Q) autophagic degradation of TP53 dependent on the acetylation status of the protein was noted. As a result, tumor cells lost regulation of the autophagic process in response to starvation, which resulted in increased autophagy and cell death. Perez and collaborators (2010) found that acetylation of the conformational mutants Arg175His and Gly245Ala by p300/CBP-associated factor (PCAF) or by treatment with deacetylase inhibitors resulted in the restoration of partial WT DNA-binding activity and growth suppression. These results demonstrate that acetylation can influence MUT TP53 activities and also suggest that MUT TP53 affinity for DNA might be influenced by post-acetylation events, such as interaction with cellular factors or by additional PTMs that occur in an acetylation-dependent manner.
Ubiquitylation and Other Modifications of MUT TP53
While phosphorylation and acetylation of TP53 can be involved in activating TP53 biological functions, polyubiquitylation generally is responsible for inactivating TP53 functions by promoting degradation of TP53 protein. However, the effects of other modifications such as neddylation and sumoylation are less clear (Hock and Vousden, 2010). In contrast to polyubiquitylation, monoubiquitylation, neddylation and sumoylation may target TP53 to different cellular locations. With the exception of Lys101, most lysine residues modified by ubiquitylation (Ub) are located in the DBD and in the C terminus. As shown in Table 1, most lysine codons distributed in the C terminus are not mutated in cancers except for Lys305.
Since lysines that are neddylated (320, 321, 370, 372 and 373) or sumoylated (386) have not been found to be altered in human tumors or implicated in MUT TP53 functions, we restrict this discussion to ubiquitylation of MUT TP53. The E3 ubiquitin ligase MDM2 is the main driver of TP53 ubiquitylation, although several other proteins with similar activities including Pirh2, Cop1, TOPORS, and CHIP have been reported to promote the degradation of TP53 independently of MDM2 (Lukashchuk and Vousden, 2007). Notably, MDM2 is a TP53 transcriptional target, establishing a negative feedback loop for TP53 activities (reviewed in (Lee and Gu, 2010)). Additionally, overexpression of MDM2 is observed in many tumor types and results in aberrant inactivation of TP53.
The tight control that exists between MDM2 and TP53 in normal cells is lost in tumors harboring MUT TP53 and results in hyperstability that correlates with MUT TP53 oncogenic activities. The hyperstability is associated with the loss of TP53-mediated transactivation of MDM2 (Midgley and Lane, 1997). Li et al. (2011) characterized the degradation of endogenous MUT TP53, using a panel of randomly chosen human cancer cell lines expressing either WT or MUT TP53. Tumor-derived endogenous MUT TP53 cell lines showed a complete lack of ubiquitylation and exhibited dramatic constitutive TP53 stabilization (10- to 20-fold above WT TP53 cancer cell lines).
The precise mechanisms responsible for deregulating MUT TP53 protein levels in cancer cells are not clear. Additional alterations may be required to stabilize MUT TP53. For example, only the tumor tissue arising in knock-in mice that express MUT TP53 Arg172 (human Arg175) display constitutive stabilization of MUT TP53 (Lang et al., 2004; Olive et al., 2004; Terzian et al., 2008). Lukashchuk and Vousden (2007) provided one of the first studies showing that the regulation of MUT TP53 stability differs from that of WT TP53. Although MDM2 could interact with conformationally altered TP53-DBD mutant proteins, ubiquitylation by MDM2 of MUT TP53 was less efficient than for WT TP53. They proposed that other E3 ligases could be responsible for the degradation of MUT TP53. Two recent studies identified new players in the regulation of MUT TP53 stability. Li et al. (2011) found that stabilization of MUT TP53 in cancer cells can result from inhibition of MDM2 and the chaperone CHIP E3 ligases by the HSP90 chaperone machinery. Wiech et al. (2012) showed that HSP70 together with MDM2 enhances TP53Arg175His aggregation and alters its subcellular distribution resulting in the stabilization of this MUT TP53. Together, these observations suggest a more complex relationship between MDM2 and TP53 and indicate that the degradation of MUT TP53 may be selectively compromised in tumor cells.
Finally, TP53 can undergo other modifications, including methylation, O-linked β-N-acetylglucosamine (O-GlcNAcylation), poly(ADP-ribosyl)ation and nitration, but the significance of these modifications for MUT TP53 functions and tumorigenesis is unclear. Overall, understanding the effects of PTMs on MUT TP53 stability and function may help predict responsiveness to chemotherapy of tumors harboring MUT TP53.
Mouse Knock-in Models of PTM Sites
Knock-in mutations in mice have provided important information about TP53 functions even though significant differences exist between the human and mouse TP53 networks (see (Menendez et al., 2009)). To date, twelve Trp53 knock-in mouse models have been created that contain mutations in residues that are posttranslationally modified, and these are described below (see Table 1). Since there have not been any studies that address the impact of PTMs in mice with MUT TP53, we summarize findings regarding the roles of PTMs themselves on TP53 function, with the idea that these findings will guide future studies on the interaction between PTMs and MUT TP53s. Although some PTM site mutations decreased survival and increased spontaneous tumor formation, none of these mutations were as severe as the Trp53-/- mutation. The median survivals for Trp53-/-, Trp53+/-, and Trp53+/+ mice are 20, 78 and 120 weeks, respectively (Donehower and Lozano, 2009). The mouse models specifically targeted phosphorylation and acetylation residues consisting of serines and lysines, respectively. Most TP53 PTMs were dispensable and the changes did not affect lifespan. Only three studies showed increases in spontaneous tumor formation and decreased lifespan as compared to TP53 WT mice (Armata et al., 2007; Chao et al., 2006a; MacPherson et al., 2004). Whether any of the residues examined are essential when mice are stressed/challenged has not been thoroughly studied. Most of the PTM mutations were found to result in tissue specific defects in response to DNA damage. For a more comprehensive review of engineered mouse models of TP53, see Donehower (this issue).
Changes to Phosphorylated Residues
To address the consequences of loss of phosphorylation, two groups changed the Ser18 codon (equivalent to Ser15 in human TP53) to a non-phosphorylatable Ala codon to create Trp53S18A/S18A mouse models in different genetic backgrounds (Chao et al., 2003; Sluss et al., 2004). The Sluss group went on to report the median lifespan for Trp53S18A S18A mice as 81 weeks (Armata et al., 2007), whereas the lifespan was 98 weeks and 71 weeks for Trp53+/+ and Trp53+/- mice, respectively. Chao and colleagues (2003) reported their mice had no predisposition to spontaneous tumorigenesis. The Trp53S18A/S18A mutation did not affect basal or DNA damage-induced TP53 protein levels nor DNA binding as compared to WT. However, TP53 PTM levels, specifically of Lys379 (human Lys382) acetylation, were reduced in mouse embryonic fibroblasts (MEFs) after DNA damage. Transactivation of apoptotic targets was defective in thymocytes, fibroblasts, and splenocytes after DNA damage. Despite impaired apoptotic responses, Trp53S18A/S18A could not rescue the embryonic lethality phenotype of Mdm2-/- mice and rescued the embryonic lethality of Xrcc4-/- caused by TP53-dependent apoptosis in only a few mice (2/11) (Chao et al., 2003; Sluss et al., 2004). The Trp53S18A/S18A mice developed lymphomas more rapidly than the heterozygous or WT mice following crosses with Eμ-myc transgenic mice (Sluss et al., 2010), but to a lesser extent than Trp53 null mice. Interestingly, Trp53S18A/S18A as compared to WT MEFs exhibited increased levels of ROS and decreased gene expression of the Sestrin family of antioxidants, increased inflammatory cytokines, metabolic stress and defects in glucose homeostasis (Armata et al., 2010).
The Trp53S23A/S23A (corresponding to human Ser20Ala) mice had a decreased lifespan (median was 63 weeks) and developed B-cell lineage lymphomas, while Trp53-/- mice developed thymic lymphomas and sarcomas. Thymocytes and developing cerebellum were defective in apoptosis and had decreased TP53 stabilization following DNA damage (MacPherson et al., 2004).
Combining the Ser18Ala and Ser23Ala mutations led to dramatic changes. In the double homozygous mutant mice Trp53S18A/S18A;S23A/S23A apoptosis was abolished in thymocytes, similar to Trp53-/- thymocytes (Chao et al., 2006a), whereas apoptosis in Trp53S18A/S18A thymocytes was slightly impaired as compared to WT thymocytes. Similar to TP53 null mice, Trp53S18A/S18A;S23A/S23A could rescue embryonic lethality of Xrcc4-/- mice. However, unlike Xrcc4-/- Trp53-/-, the Xrcc4-/- Trp53S18A/S18A;S23A/S23A mice were not prone to spontaneous tumor formation. Trp53S18A/S18A;S23A/S23A mice had a reduced lifespan (median was 80 weeks) as compared to WT mice, and the spectrum of tumors in these mice differed from those in Trp53S23A/S23A animals. It should be noted that the differences in tumor spectrum and lifespan could be due to the different genetic background of the mutant strains (Chao et al., 2006a). The absence of Trp53S23A/S23A mice in this background precludes a conclusion about the relative roles of Ser18 and Ser23 PTMs.
Modification at human TP53 Ser46 was examined in a mouse with a human TP53 replacement (hupki) since this amino acid is not conserved between humans and mice. A knock-in consisting of exons 4 through 9 of mouse Trp53 was replaced with the corresponding human TP53 exons (codons 33-332; p53hki) as well as with a Ser46Ala replacement (Trp53hkiS46A). TP53 stabilization after DNA damage or oxidative stress was reduced in embryonic stem cells (ESCs), fibroblasts and thymocytes of Trp53hkiS46A as compared to the Trp53hki cells. Like the other two TP53 PTM single mutants, Ser18Ala and Ser23Ala, TP53-dependent apoptosis was partially defective after DNA damage, yet the defect could not rescue the embryonic lethality of the Xrcc4‐/‐ mice (Feng et al., 2006). Additionally, apoptosis also was impaired in Ras-expressing MEFs challenged with DNA damage. Possible effects of the Ser46Ala mutation on lifespan or spontaneous tumor formation were not described.
Three mouse models have been described for assessing the consequences of phosphorylation at Ser315. In a Trp53hkiS315A Hupki mouse system (Lin et al., 2005), similar TP53 protein levels compared to Trp53hki were observed after DNA damage. However, the TP53 in ESCs from Trp53hkiS315A mice was hyperphosphorylated at Ser46 and Ser392 as compared to ESCs from Trp53hki mice. Also, TP53 protein levels were increased after retinoic acid induced differentiation in Trp53hkiS315A ESCs as compared to Trp53hki ESCs. Some TP53-dependent transcriptional responses were defective in Trp53hkiS315A ESCs during ESC differentiation, notably repression of the self-renewal factor Nanog.
Two mouse models based on TP53 Ser312Ala (corresponding to human TP53 Ser315) have been created in different backgrounds (Lee et al., 2011; Slee et al., 2010). These mice developed normally and remained tumor free for at least 2 years (Lee et al., 2011; Slee et al., 2010). The TP53 from Trp53S312A/S312A was functionally normal after DNA damage in that it could induce apoptosis and cell-cycle arrest as well as prevent centrosome duplication as compared to WT cells (Lee et al., 2011). Moreover, Trp53S312A/S312A MEFs and thymocytes were still phosphorylated at Ser18 and Ser23 after DNA damage (Lee et al., 2011). The Trp53S312A/S312A in both mouse strains was unable to rescue Mdm2-/- embryonic lethality (Lee et al., 2011; Slee et al., 2010) although, Trp53S312A/S312A Mdm2-/- embryos could be detected at day 13.5 in one of the studies (Slee et al., 2010). Tumorigenesis following irradiation was enhanced in Trp53S312A/S312A mice in the Slee et al. (2010) study, but not in the Lee et al. (2011) experiments. Furthermore, Lee et al. found that tumorigenesis was not increased following crosses with an Eμ-myc mouse. Gene expression profiles from Eμ-mycTg; Trp53S312A/S312A and Eμ-mycTg; Trp53+/+ mice were comparable suggesting that phosphorylation at the Ser312 site is not necessary for TP53 function (Lee et al., 2011).
The lifespan and tumor incidence of Trp53S389A/S389A (corresponding to human Ser392) were similar to that in WT mice (median lifespan is 110 weeks) (Bruins et al., 2004). The MEFs had decreased in vitro DNA binding activity, delayed transcriptional activation of some target genes, and decreased apoptotic response after UV irradiation but not after IR or oncogene activation (Bruins et al., 2004). The Trp53S389A/S389A mice were more susceptible to skin tumors and urinary bladder tumors than WT mice when challenged with two nucleotide excision repair-specific DNA damaging agents, UVB irradiation or 2-acetylaminofluorene, respectively (Bruins et al., 2004; Hoogervorst, et al., 2005), while whole body IR effects on Trp53S389A/S389A mice did not differ from WT (Hoogervorst et al., 2005).
Changes to Acetylated Residues
Changing a lysine to arginine prevents acetylation. The Lys117Arg (corresponding to human Lys120) mutation results in a complete blockage of apoptotic responses in mouse thymocytes, testis, intestine, and spleen after DNA damage (Li et al., 2012). The Trp53K117R/K117R MEFs could not induce Noxa, Dr5 or Puma apoptotic genes, but could still induce cell-cycle arrest and senescence. Li et al. also created Trp533KR/3KR mice with Lys117Arg, Lys161Arg, Lys162Arg mutations (corresponding to the human Lys120, Lys164, and Gln165). Unlike cells from the Trp53K117R/K117R mice, MEFs and thymocytes from the Trp533KR/3KR mice were defective in apoptosis, cell cycle arrest, and senescence following DNA damage, but the Trp533KR/3KR mice retain the ability to regulate metabolic genes. TP53 protein was induced and acetylated on the C-terminal lysines and phosphorylated on Ser18 following DNA damage in MEFs from both Trp53K117R/K117R and Trp533KR/3KR mice (Li et al., 2012). Surprisingly, the lifespan and tumor incidence of Trp53K117R/K117R and Trp533KR/3KR mice were similar to WT mice.
The lifespan of Lys317Arg (corresponding to human Lys320) mice is similar to WT mice (Chao et al., 2006b), and TP53 induction following DNA damage in MUT and WT MEFs and in thymocytes was similar. There was enhanced apoptosis in Trp53K317R/K317R thymocytes and in cells from the small intestine and retinas, but not in MEFs as compared to WT following DNA damage. Oncogene activated (E1A/Ras) Trp53K317R/K317R MEFs exposed to DNA damage also had increased apoptotic responses vs. WT. Trp53K317R/K317R thymocytes had enhanced TP53 transcriptional activity (both activation and repression) following DNA damage as compared to WT, a finding that led to the conclusion that acetylation of Lys317 normally decreases TP53 transcriptional activity (Chao et al., 2006b).
In two mouse models, modifications of C-terminal PTMs are addressed. Feng et al. (2005) mutated six lysine codons while Krummel et al. (2005) mutated seven. The Trp53K6R model corresponds to Lys to Arg changes for the residues 367, 369, 370, 378, 379, 383 (human lysines 370, 372, 373, 381, 382, 386). DNA damage induced TP53 protein and phosphorylation at Ser18 and Ser389 in ESCs and MEFs from Trp53K6R/K6R mice at levels similar to WT cells. TP53 also could be ubiquitylated but at lower levels than WT. Some defects in TP53 transcriptional activity in Trp53K6R/K6R ESCs, but not in MEFs, were observed (Feng et al., 2005). In the Trp537KR model, which in addition to the Trp53K6R changes added Lys384Arg (corresponding to human Thr387), the mice are viable and normal. TP53 protein levels after DNA damage in Trp537KR/7KR MEFs were similar to those in WT cells and Trp537KR/7KR thymocytes, and DNA damage or oncogene activation could induce cell cycle arrest and apoptosis in Trp537KR/7KR MEFs. Interestingly, TP53 protein and target genes were induced in thymocytes from Trp537KR/7KR mice at a faster rate as compared to WT cells after DNA damage (Krummel et al., 2005). We note that the above changes also would prevent methylation, neddylation, or sumoylation of these residues. Arginines can be methylated, but not by the enzymes that normally methylate lysines. Overall, modifications to the seven C-terminal lysines do not appear important, at least in the assays examined.
Like human TP53, mutant forms of mouse TP53 also can be posttranslationally modified; however, little is known about how these modifications affect MUT TP53 activities. Ser18 in MEFs from Trp53R172H or Trp53R270H mice (corresponding to human hotspot residues Arg175 or Arg273, respectively) undergoes phosphorylation after DNA damage and p21 (Cdkn1a) expression was induced (Olive et al., 2004). In contrast, MEFs from the Trp53hkiR248W mouse also were phosphorylated at Ser18 after DNA damage, but induction of common TP53 target genes such as Cdkn1a (p21), Mdm2, Pidd and Bax were greatly decreased (Song et al., 2007). Phosphorylation of Ser18 was only detected in thymoma but not the normal thymus of Trp53R246S mice (corresponding to human Arg249) (Lee et al., 2012). MEFs containing TP53 single point mutations corresponding to human Asn131Tyr, Arg248Trp, Arg249Trp, or Arg273Cys could all be phosphorylated at Ser15 and acetylated at Lys379 even though none of these could induce p21 and Puma protein expression, whereas Gln104Leu MEFs underwent the same PTMs but could induce p21 and Puma expression (Odell et al., 2013).
Overall, while data from knock-in TP53 mice suggest that some PTMs (e.g. phosphorylation of Ser46 or acetylation of Lys120) or combinations of PTMs (phosphorylation of Ser15 and Ser20) contribute importantly to individual TP53 functions, none are crucial for tumor suppression. Most surprising is the lack of effects of the 6KR and 7KR constructs as these residues are highly modified in multiple ways that appear from in vitro studies to be important for individual TP53 functions (Carter and Vousden, 2009).
Dysregulation of Signaling Pathways that Modify TP53 in Cancer
Sequencing of cancer genomes has revealed altered signaling pathways in many human tumors either directly through mutation or indirectly through copy number changes or epigenetic mechanisms (Vogelstein et al., 2013; You and Jones, 2012). Indeed, nearly half the driver genes mutated in cancers encode proteins that directly regulate chromatin through the modification of histones or DNA. Among these are some that also modify TP53. Although a comprehensive analysis is beyond the scope of this review, we summarize below several cancer-associated changes that may affect TP53. For many of these pathways, enzymes or cofactors, the impact on various MUT TP53s remains to be investigated.
As mentioned above, the WIP1 phosphatase and MDM2/MDM4 are overexpressed in several different cancers. While overexpression of these enzymes frequently is associated with WT TP53, overexpression also may occur in tumors with MUT TP53. In contrast to WIP1, the gene for the scaffolding subunit of the PP2A phosphatase, encoded by PPP2R2A, is commonly mutated or deleted in several cancers including prostate and breast (Kurimchak and Graña, 2013). The PP2A phosphatase is abundant and regulates various branches of the DNA damage response pathway. It dephosphorylates several TP53 residues including constitutively phosphorylated Thr55, which is important for TP53 stabilization after stress (Table 1, Supp. Figure S1). Other phosphatases also may become dysregulated in cancers (Stebbing et al., 2013).
ATM is critical for initiation of the DNA damage response, for activating several effector kinases, and for phosphorylating several residues on TP53. ATM is one of the ∼140 mutated driver genes altered in human cancers as is CHEK2 (CHK2), a DNA damage checkpoint effector kinase (Vogelstein et al., 2013). ATM activation also requires TIP60, which is downregulated in several cancers including prostate, breast, and colorectal (Xu et al., 2012). Several of the Polo-like kinases are overexpressed or down regulated in tumors (Strebhardt, 2010). PLK1 is overexpressed and regulates TP53 indirectly through phosphorylation of TOPORS, which inhibits its sumoylation activity and enhances TP53 degradation (Yang et al., 2009). On the other hand, PLK3 is downregulated in human tumors and directly phosphorylates TP53 Ser20, thereby inhibiting MDM2 binding and enhancing p300 binding. MDM2 and MDM4 are amplified or overexpressed in a number of cancers and are driver genes for human cancer (Vogelstein et al., 2013; Wade et al., 2013). Both AURA and AURB are overexpressed in several cancers including breast, colorectal, and prostate (Lens et al., 2010), and many cancers exhibit elevated JNK activity (Sabapathy, 2012).
Among the chromatin-modifying enzymes mutated or dysregulated in cancers that modify TP53, in addition to TIP60, are the histone methyltransferases G9A and PRMT5, the histone demethylase LSD1, and the histone lysine acetyltransferases p300, CBP, and PCAF. The histone deacetylases HDAC2 and SIRT1 also show altered expression or mutation (You and Jones, 2012). Epigenetic cofactors, for example DBC1, an ATM phosphorylation-dependent negative regulator of SIRT1 activity, also are mutated or dysregulated in cancers. Deletion or down-regulation of DBC1 activity activates SIRT1, resulting in the deacetylation of TP53 and a decrease in its activity as a transcription factor (Zannini et al., 2012). The PIN1 proline isomerase, which binds TP53 in a phosphorylation-dependent manner, frequently is overexpressed in breast cancers, and 53BP1, which binds dimethylated TP53 to direct it to repair foci, is downregulated in some cancers.
The above examples are but a few of the ways the mutation and altered expression of genes in cancer might affect the status of MUT TP53 PTMs and TP53's functions in different cancers. However, while some PTMs of MUT TP53 have been shown to contribute to a GoF (e.g., JNK phosphorylation of Ser6, Ser9, and Thr81 described below), relatively little is known about how dysregulation of other modifications might affect the activity of MUT TP53.
TP53 Protein-Protein Interactions Modulated by PTMs
Over the past 30 years more than 300 individual proteins or protein complexes have been reported to interact with TP53 (Collavin et al., 2010), and more recent proteomics approaches have confirmed a large number of interacting partners for WT TP53 (Huang et al., 2012) as well as unique binding partners for cancer-associated MUT TP53 (Coffill et al., 2012). The activities of many enzymes that modify TP53 are affected by binding cofactors. The co-crystal or NMR structures of several TP53 fragments with a binding partner have been determined. Like the tails of histones, the unstructured N- and C-terminal tails of TP53 extend outward to provide interaction and modification surfaces. This similarity with histones may not be entirely accidental; many of the histone writers, readers and erasers also interact with TP53 (Badeaux and Shi, 2013; Patel and Wang, 2013). However, the effects of PTMs on binding partner interactions have been examined in detail for only a handful of proteins. Below, we summarize what is known for a few examples relevant to cancer. While PTMs can modify interactions, little is known about the effects of PTMs on interactions between TP53 partners and MUT TP53 proteins. Dysregulation of signaling pathways that modify TP53 could affect the activities of those MUT TP53s that retain transactivation potential, and alterations of TP53 binding partners could contribute to TP53 GoF.
TP53 Tetramerization and PTMs
Tetramerization of TP53 is required both for stable binding to most response elements and for efficient transcriptional activation of target genes as well as for tumor suppressive activity (Pietenpol et al., 1994). Although the frequency of mutations in the TET domain (∼8%) is lower than that for the DBD, mutations that change more than 70 percent of the residues in the ∼34 residue TET have been reported in human cancers. Furthermore, 17% of germline mutants in people with Li-Fraumeni or Li-Fraumeni-like syndrome reside in the TET. Most TET missense mutants diminish or abrogate the formation of tetrameric peptides in vitro (Kamada et al., 2011).
In addition to TP53-mediated transcriptional activation and repression, tetramerization is required for some PTMs (Warnock et al., 2008) including efficient ubiquitylation by MDM2 (Maki, 1999). Abrogation of tetramerization reduces phosphorylation at Ser6, Ser9, Ser15 (in human TP53 but not at mouse Ser18), Ser46 and Ser315 as well as acetylation at Lys320 (Sakaguchi et al., 1998) and Lys382 (Warnock et al., 2008). Efficient oligomerization of BAK in the mitochondrial apoptosis pathway, which may contribute to TP53-mediated tumor suppression, also requires formation of TP53 tetramers (Pietsch et al., 2007). The TET interacts with several binding partners including the S100 protein family, ARC, cABL, MDM2 and 14-3-3, and these interactions may require tetramer formation or the binding partners may influence tetramerization (see (Kamada et al., 2011)). Phosphorylation of the N terminus and phosphorylation and acetylation of the C terminus of TP53 modulate the affinity of some S100 proteins for the TP53 TET or TAD domains and thus may influence TP53-mediated transcription (van Dieck et al., 2009).
Five amino acids - Tyr327, Arg333, 335, and 337, and Lys351 - in the TET are subject to PTM (Fig. 3). Low, physiological concentrations of nitrous oxide donors can cause nitration of Tyr327, nuclear retention of TP53 and increased DNA binding (Yakovlev et al., 2010). Monomethylation of Arg333, and dimethylation of Arg335 and 337 by PRMT5 in association with the TP53 cofactors, Strap and JMY, also induce nuclear accumulation and oligomerization of TP53 which can stimulate TP53-mediated G1 arrest after DNA damage (Jansson et al., 2008). Depletion of PRMT5 shifts TP53 binding to the promoters of genes connected with apoptosis. The effect of PRMT5-mediated dimethylation on TP53 oligomerization is somewhat puzzling because most cancer-associated mutations that alter one of the three TET Arg residues significantly reduce the ability of a tetramer-domain peptide to form tetramers at physiological concentrations. Lys351, which is located within the alpha helix of the TET, is ubiquitylated by MSL2. MLS2 also ubiquitylates the nearby Lys357 independently of MDM2 and induces cytoplasmic localization of TP53 (Kruse and Gu, 2009a). No cancer-associated mutations have been reported to affect Lys357, and the one mutation that affects Lys351 was still transactivation competent, suggesting this may be a passenger mutation. Possible roles for TET domain PTMs in modulating MUT TP53 function remain to be investigated.
MDM2 and p300/CBP
MDM2 was among the first proteins whose interaction with TP53 was shown to be modulated by TP53 PTMs. In a landmark paper, Shieh et al. (1997) reported that DNA damage-induced phosphorylation of Ser15 and Ser37 reduced the interaction of MDM2 with human TP53. Shortly thereafter, Sakaguchi et al. (1998) provided evidence that phosphorylation of N-terminal TP53 residues enhanced TP53 acetylation at Lys320 and Lys382 by PCAF and p300, respectively, again in response to DNA damage. We now know that the regulation of these interactions is more complex. MDM2 binding to the TP53 N terminus, and probably the binding of MDM4, is inhibited primarily by phosphorylation of Thr18, although the phosphorylation of this residue may depend on prior phosphorylation of Ser15 or Thr81. MDM2 also interacts with TP53 through a weaker site in the DBD of TP53. This interaction, which is needed for MDM2-mediated degradation of TP53, also is modulated by phosphorylation (Kulikov et al., 2006). In contrast to MDM2, the interaction of p300 and CBP with TP53 is enhanced by multisite phosphorylation of the TP53 N terminus (Lee et al., 2010; Teufel et al., 2009). Furthermore, TP53, MDM2 and p300/CBP form a ternary complex that promotes the polyubiquitylation of TP53 (Ferreon et al., 2009). [Readers are referred to several excellent reviews that address PTMs and TP53-MDM2/MDM4-p300/CBP interactions, e.g. (Loughery and Meek, 2013; Wade et al., 2009).] As noted above, the expression of MDM2, MDM4, p300 and CBP can be dysregulated in cancers with both WT and MUT TP53. Overexpression of MDM2/MDM4 may well overcome the inhibitory N-terminal phosphorylation of TP53; nevertheless, some tumors exhibit overexpression of both MDM2 and MUT TP53, and these have poor patient prognosis. Small molecules that inhibit the interaction of MDM2/MDM4 with TP53 are being developed for cancer therapies, but caution has been suggested since their use could stabilize MUT TP53 with GoF, leading to worse outcomes. Efforts also are being made to enhance the degradation of stabilized, MUT TP53s (Muller and Vousden, 2013).
SMAD
Cordenonsi et al. (2007) demonstrated that SMAD2/3 binds to the N terminus of WT TP53 in a manner dependent on the phosphorylation of Ser6/9 by CK1ε/δ (but not phosphorylation of Ser15, Thr18, or Ser20) in response to Ras/MAPK signaling, thus integrating TGF-β signaling with TP53-mediated stress responses. Subsequently, Adorno et al. (2009) showed that activated Ras-mediated phosphorylation of Ser6/9 in MUT TP53 greatly enhanced the formation of a ternary MUT TP53-SMAD-TP63 complex in breast cancer cells which inactivates the ability of TP63 to protect against cell migration and invasion. Oncogenic Ras was required for the CK1ε/δ-mediated phosphorylation of TP53 and for metastatic spread of tumor cells. Thus, in the context of MUT TP53, phosphorylation contributes to GoF through coupling with the TGF-β pathway (for reviews see (Elston and Inman, 2012; Meek and Anderson, 2009)).
Proline Isomerase 1 (PIN1)
PIN1 is a highly conserved prolyl isomerase that specifically recognizes phosphorylated pSer-Pro and pThr-Pro sequences, often in unstructured regions of proteins. PIN1 facilitates conformational changes that create or remove protein interaction sites that serve as switches for regulating a variety of cell processes including cell cycle progression (Liou et al., 2011). PIN1 expression and activity is tightly controlled in a cell type and cell cycle specific manner. It binds to at least 4 of 6 potential binding sites in the human TP53 protein in a phosphorylation-specific manner: pSer33-Pro34, pSer46-Pro47, pThr81-Pro82 and pSer315-Pro316. These sites are phosphorylated in response to DNA damage and/or oncogene activation. Phosphorylation of Thr81 in WT TP53 creates a binding site for PIN1 which induces dissociation of MDM2 through creation of a binding site for CHK2 and phosphorylation of Ser20, thereby preventing poly-ubiquitylation and increasing TP53 stability (Berger et al., 2005) (Fig. 4A). PIN1 binding also increases the association of TP53 with p300 leading to increased acetylation of Lys373 and 382, thus enhancing TP53 association with its response elements. For individuals with the TP53 Pro72 polymorphism that enhances binding of iASPP, a highly evolutionarily conserved inhibitor of TP53-mediated apoptosis and transcriptional activation, phosphorylation of Ser46 and PIN1 binding releases iASPP thereby enhancing apoptosis (Mantovani et al., 2007). For WT TP53, all of these effects contribute to tumor suppression. In contrast, the downstream effects of PIN1 binding to MUT TP53 are very different (Fig. 4B) (Girardini et al., 2011). In MDA-MB-231 breast cancer cells (Arg280Lys) PIN1 enhanced the interaction of MUT TP53 with antimetastatic TP63 and with transcription factors such as Ets-1, NF-Y, Sp1 and VDR, all of which can activate a transcriptional program that increases cell migration and invasiveness. Overexpression of PIN1 in association with MUT TP53 in breast cancer is associated with poor prognosis. PIN1 also is important for enabling a number of other phosphorylation-mediated switches that regulate tumor growth (Theuerkorn et al., 2011).
Figure 4.
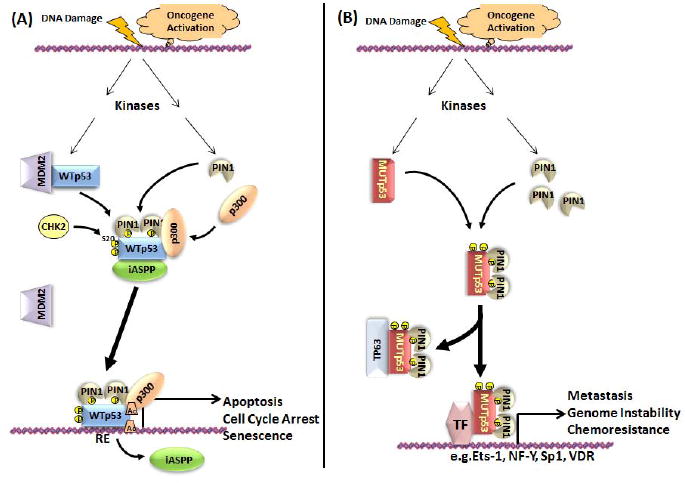
Model for the regulation of WT (A) and MUT (B) TP53 by PIN1. DNA damage and oncogenic signaling activate both WT TP53 (WTp53) and MUT TP53 (Mutp53) through several common kinases, e.g. JNK1/2, p38MAPK, PKCδ, that phosphorylate the same residues in each (Table 1, Supp. Fig. S1) creating binding sites for PIN1. Activation of WT TP53 by PIN1 contributes to the DNA damage response and to mechanisms that may suppress tumor development (see text). In contrast, binding of PIN1 to MUT TP53 leads to MUT TP53-mediated sequestration of TP63 and the interaction of MUT TP53 with transcription factors such as ETS-1, NF-Y, SP1 and VDR. These interactions alter the transcription profile of cells to induce metastasis and activities that contribute to tumor development. PIN1 often is overexpressed in tumors.
Binding Proteins Modulated by TP53 Methylation
Mono- or di-methylation of TP53 at four C-terminal lysine residues by at least six different lysine methyltransferases facilitates binding by TIP60, PHF20, L3MBTL1 and 53BP1 to TP53 (Fig. 5, Table 1, Supp. Figure S1) (Carr et al., 2012; West and Gozani, 2011). SMYD2 (KMT3C) monomethylates Lys370 to repress TP53 activity; SET7/9 (KMT7) monomethylates Lys372 and stabilizes chromatin-bound TP53; the related methyltransferases G9A (KMT1C) and GLP (KMT1D) dimethylate Lys373 and inactivate TP53; and SET8 (KMT5A) monomethylates Lys382 and represses TP53 activity. The enzymes that dimethylate Lys370 and Lys 382 have not yet been identified. SET7/9 monomethylation of Lys372 is enhanced by DNA damage while SET8 is downregulated by DNA damage, and G9A/GLP activity remains unchanged. The demethylase LSD1 removes one methyl moiety from dimethylated Lys370. Additionally, there is crosstalk between the different methylations as well as TP53 acetylation. Although none of these modifications are crucial for tumor suppression based on data from mice having the C-terminal six or seven lysines substituted by arginine (K6R, 7KR) as described above (Feng et al., 2005; Krummel, et al., 2005), the dysregulation of these modifications might well contribute to tumor initiation or progression. The interactions of TIP60, PHF20, L3MBTL1 and 53BP1 (and perhaps others) modulate TP53 stability, its interaction with DNA, and the targeting of TP53 to different locations, and thus also could influence the functionality of MUT TP53.
Figure 5.
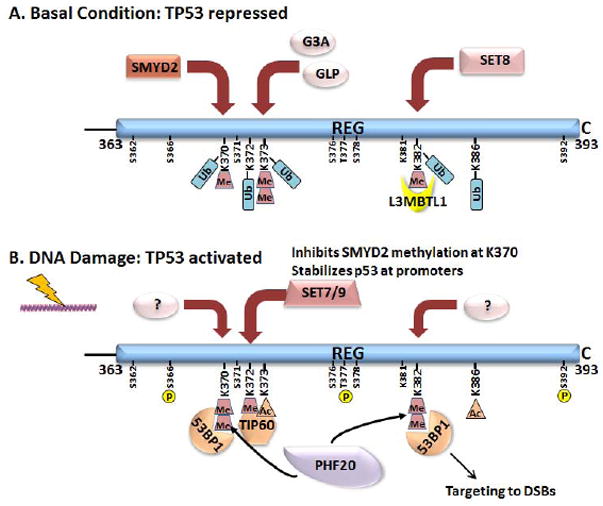
The functions and locations of TP53 are modulated by methylation of lysines at its C terminus and the interactions of these with methyl-binding proteins in basal conditions and after DNA damage. TP53 is mono- or dimethylated at four lysines under basal conditions (Lys370Me1, Lys373Me2, L:ys382Me1) or after DNA damage (Lys370Me2, Lys362Me1, Lys382Me2) by at least six lysine methyltransferases (see text). These lysines as well as others and nearby serines and threonines also are subject to PTMs including ubiquitylation, neddylation (not shown), sumoylation (not shown), and phosphorylation. The choreography of these events is not well known.
TIP60 (KAT5)
The binding of the acetytransferase TIP60 to TP53 was reported to depend on K372 monomethylation by the acetyltransferase SET7/9 (Kurash et al., 2008). However, since more recent studies failed to confirm a role for SET7/9 in regulating TP53 activity (Campaner et al., 2011; Lehnertz, et al., 2011), the role of lysine methylation in recruitment of TIP60 or its cousins hMOF (KAT8) and hMOZ (KAT6B) to TP53 after DNA damage remains to be determined. Nevertheless, these MYST family acetyltransferases contain conserved bromodomains that bind methylated lysines. Several TP53-interacting proteins (e.g. CCDC8, PML, UHRF1) also modulate the acetylation of TP53 by MYST family acetyltransferases (e.g., Dai et al., 2013; Dai et al., 2011; Rokudai et al., 2013). Thus, the relative importance of TP53 methylation for mediating acetylation in normal or tumor cells still needs to be addressed.
PHF20
PHF20 is a transcription factor that transcriptionally activates TP53 expression and also is a component of the hMOF lysine acetyltransferase that acetylates TP53 at Lys120. PHF20 specifically binds TP53 Lys370me2 and Lys382me2 through one of its two Tudor domains. Homodimeric PHF20 simultaneously binds Lys370me2 and Lys382me2 (Fig. 5), greatly strengthening its interaction with TP53 (Cui et al., 2012). Through inhibition of ubiquitylation, PHF20 stabilizes TP53 thereby contributing to increased TP53 expression after DNA damage. However, PHF20 is overexpressed in a number of cancers where phosphorylation on PHF20 Ser291 by AKT/PKB, which frequently is over-activated in human cancer, causes a reduction in TP53 accumulation and inhibition of TP53 transcriptional activity (Li et al., 2013; Park et al., 2012). Thus, PHF20 is postulated to contribute to tumor progression of several cancers through inhibition of TP53, but whether it affects activities of MUT TP53 has yet to be investigated.
L3MBTL1
L3MBTL1 is a transcriptional repressor that binds monomethyl H3K4 or dimethylated H4K20 through its three MBT (malignant brain tumor) domains to effect the compaction of nucleosomes (Trojer et al., 2007). L3MBTL1 interacts with TP53 that is monomethylated at Lys382 by SET8 and is recruited to TP53 target genes to repress TP53-mediated induction under basal conditions (Fig. 5). In vitro, L3MBT1 can bind a dimethylated Lys382 peptide with equal affinity (West et al., 2010). In response to DNA damage, the levels of SET8 and consequently Lys382 monomethylation decline; this reduction would be expected to reduce the association of L3MBTL1 with TP53 target promoters and to alleviate the transcriptional repression of DNA-damage response genes.
53BP1
53BP1 was the first identified TP53 binding protein (Iwabuchi et al., 1994). It is an important component of the DNA damage response machinery that assembles at DNA double-strand breaks (DSBs) where it contributes to the decision to repair by homologous recombination (HR) or by non-homologous end joining (NHEJ) (Noon and Goodarzi, 2011). 53BP1 is recruited to DSBs through an interaction with dimethylated Lys20 of histone H4 in a manner that depends on RNF8- and RNF168-mediated ubiquitylation and removal of L3MBTL1 by the AAA-ATPase VCP/p97 (Acs et al., 2011). 53BP1 binds TP53 dimethylated at either Lys370 or Lys382 (Fig. 5) through its tandem Tudor domains (Huang et al., 2007; Kachirskaia et al., 2008). The dimethylation of Lys382, which is increased in response to DNA damage, stabilizes TP53 and recruits it to sites of DNA damage where TP53 may enhance the fidelity of DNA repair (Roy et al., 2010). 53BP1 expression is lost in some tumors (Bartkova et al., 2007). How the recruitment and release of methyl binding proteins to TP53 is choreographed is largely unknown, but the nearby location of acetylation and phosphorylation sites on TP53 to the methylated residues (Fig. 5) suggests opportunities for regulated binding and/or regulated methylation/demethylation.
Other Interacting Proteins
Other proteins whose interactions with TP53 have been shown to be modulated by PTMs include RPA, 14-3-3, PC4, TAF1 and H1.2 (Supp. Figure S1C), but little is known about the interaction of these proteins with MUT TP53. The RPA70 subunit interacts with TAD2 of TP53, but the RPA32 subunit becomes hyperphosphorylated after DNA damage which leads to dissociation of the two proteins. Serrano et al. (2013) recently reported that the TP53-RPA interaction is collaboratively regulated by DNA-PK, ATM/ATR. Release presumably frees both proteins to carry out their diverse functions.
The 14-3-3 family of proteins was the first phosphoserine/phosphothreonine binding proteins discovered, and they play important roles in cellular signaling (Gardino and Yaffe, 2011). 14-3-3 proteins also were the first TP53 binding partners shown to be modulated by PTMs; dephosphorylation of Ser376 in response to DNA damage creates a consensus 14-3-3 binding site at phospho-Ser378 which enhances TP53 binding to response elements (Waterman et al., 1998). More recently the structure of 14-3-3σ bound to a C-terminal TP53 peptide phosphorylated at Thr387 was solved (Schumacher et al., 2010). Binding of two 14-3-3 dimers to the C-terminal TP53 tails is postulated as a mechanism for enhancing TP53 tetramer formation as well as for inhibiting TP53 ubiquitylation.
The acetylation of TP53 Lys373 and 382 in response to UV irradiation promotes recruitment of the TFIID subunit TAF1 to two sites in the p21 (CDKN1A) promoter through TAF1's bromodomains to promote looping and transcriptional activation (Li et al., 2007). Acetylation of Lys382/381 in a TP53 C-terminal peptide enhances binding of the transcriptional positive coactivator PC4 by about an order of magnitude (Debnath et al., 2011). PC4 binding increases TP53 binding to response elements and activation of transcription, possibly in part through inducing DNA bending. However, PC4 is overexpressed in several human cancers suggesting that it also may function as an oncogene. H1.2 is a linker histone that forms a stable complex with TP53 in normal cells and represses TP53-mediated transcription. Recently, the interaction of H1.2 with TP53 was found to be suppressed by p300-mediated acetylation of the TP53 C-terminal REG and by DNA-PK mediated phosphorylation of H1.2, which triggers rapid activation of TP53-mediated transcription (Kim et al., 2012).
Conclusions and Perspectives
“There are known knowns; there are things we know we know.
We also know there are known unknowns; that is to say we know there are some things we do not know.
But there are also unknown unknowns – the ones we don't know we don't know.” Donald Rumsfeld.
Thirty years of research have illuminated multiple pathways and mechanisms by which the TP53 protein is modified at more than 60 sites. While in vitro studies suggest that a number of these PTMs modulate the ability of TP53 to carry out a still growing list of tasks it performs in normal cells, in vivo studies indicate that none of these PTMs are essential for tumor suppression. Nevertheless, we suggest that many may contribute to tumor suppression in important ways. Until relatively recently, the primary roles of TP53 in tumor suppression were thought to be similar to its main roles in the DNA damage response – cell cycle arrest, providing time for repair, apoptosis and the establishment of senescence. However, recent elegant in vivo studies using several independent approaches (Jiang et al., 2011; Li et al., 2012; Timofeev et al., 2013; Valente, et al., 2013) have uncovered additional ways by which TP53 prevents tumor formation, including mechanisms through metabolic control, amelioration of oxidative stress and DNA repair. Although any single mechanism may not be essential for tumor suppression in most cases, collectively each contributes and backs up the others. As a consequence of this redundancy, mutations that change single modifiable residues that primarily influence one of these mechanisms may show little effect on the overall suppression of spontaneous tumor formation in knock-in mouse models. However, as techniques are refined to allow the study of individual pathways of tumor suppression, the importance of individual modifications for specific pathways may be revealed. Such dissection also should lead to a better mechanistic understanding of roles of each PTM, keeping in mind, however, that not all PTMs may have discernible functional significance.
The role(s) of most PTMs for MUT TP53 are less clear. For the one-third of missense mutants that retain some transactivation function, PTMs may perform roles similar to their roles in modulating WT TP53, but this remains to be determined. For structurally destabilized MUT TP53, recent studies indicate contributions for phosphorylation of Ser6, Ser9 and Thr81 toward a GoF for at least some mutants (Adorno et al., 2009; Girardini et al., 2011). For most other modifications, the jury remains out. We simply do not have a sufficient understanding of their effects, especially for MUT TP53s that may accumulate to many times the concentration attained for WT TP53. We know that structurally destabilized MUT TP53 complexes with its cousin TP63 sequestering it and inhibiting TP63's ability to repress functions that contribute to cell migration, invasion and metastasis, but what other cellular pathways might be disrupted through the sequestering or altered targeting of other proteins? Powerful proteomics approaches are just beginning to be used to probe differences in the interaction partners of WT and MUT TP53 (Coffill et al., 2012), and similar approaches can be used to interrogate the effects of PTMs (Jenkins et al., 2008).
Despite 30 years of intense research, there remains much to be revealed about the PTMs of WT TP53. Although enzymes have been identified in vitro that can perform most PTMs, in many cases, it is not known which enzyme or enzymes respond to specific signals in vivo. Likewise, there is relatively little information about where in the cell PTMs take place or how long they remain. TP53 functions primarily as a tetramer on DNA, but virtually nothing is known about the stoichiometry of modification in a tetramer in vivo. Are the effects of modification of a site additive or must all four sites be modified? How many modifications may coexist on one TP53? As measured in vivo, many sites are sparsely occupied. How does PTM signaling to TP53 differ in different cell types? Which PTMs are important for each TP53 function? How do modifications and pathways differ between humans and model systems or between individual humans?
In addition to these issues, the following are among many important questions to be addressed concerning MUT TP53:
Can PTMs be increased or decreased to modulate MUT TP53 stability or location?
Which PTMs contribute significantly to MUT TP53 GoF?
Can PTMs rescue or inhibit MUT TP53 function?
How does dysregulation of signaling pathways in cancer cells affect MUT as well as WT TP53?
Because cancer is mainly a disease of old age, little if any evolutionary pressure exists to optimize TP53 function in tumor suppression. In most cases, mutation of TP53 is a late event in tumor progression. Thus, one can imagine that someday the willful manipulation of TP53 activity, possibly through control of specific TP53 PTMs, might contribute to cancer prevention, possibly by eliminating precancerous cells or neoplastic cells before they become invasive and establish the metastases that kill most patients.
Supplementary Material
Acknowledgments
We thank K. Bebenek for assistance with structural representations of PTM residues, and D. W. Meek (University of Dundee) and K. Sakaguchi (Hokkaido University) for comments and suggestions. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Disclosure Statement: The authors declare no conflict of interest.
References
- Acs K, Luijsterburg MS, Ackermann L, Salomons FA, Hoppe T, Dantuma NP. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat Struct Mol Biol. 2011;18(12):1345–50. doi: 10.1038/nsmb.2188. [DOI] [PubMed] [Google Scholar]
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell. 2009;137(1):87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Anderson CW, Appella E. Signaling to the p53 tumor suppressor through pathways activated by genotoxic and non-genotoxic stresses. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. Elsevier; 2009. pp. 2185–2204. [Google Scholar]
- Armata HL, Garlick DS, Sluss HK. The ataxia telangiectasia-mutated target site Ser18 is required for p53-mediated tumor suppression. Cancer Res. 2007;67(24):11696–703. doi: 10.1158/0008-5472.CAN-07-1610. [DOI] [PubMed] [Google Scholar]
- Armata HL, Golebiowski D, Jung DY, Ko HJ, Kim JK, Sluss HK. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol. 2010;30(24):5787–94. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. 2013;14(4):211–24. doi: 10.1038/nrm3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar JK, Slomska I, Rabczyńki J, Noga L, Gryboś M. Expression of p53 protein phosphorylated at serine 20 and serine 392 in malignant and benign ovarian neoplasms: correlation with clinicopathological parameters of tumors. Int J Gynecol Cancer. 2009;19(8):1322–8. doi: 10.1111/IGC.0b013e3181b70465. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Hořejší Z, Sehested M, Nesland JM, Rajpert-De Meyts E, Skakkebæk NE, Stucki M, Jackson S, Lukas J, Bartek J. DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours. Oncogene. 2007;26(53):7414–22. doi: 10.1038/sj.onc.1210553. [DOI] [PubMed] [Google Scholar]
- Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, Pollmann C, Dubiel W. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20(7):1630–9. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon A, Aebersold R, Shiloh Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett. 2011;585(11):1625–39. doi: 10.1016/j.febslet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Berger M, Stahl N, Del Sal G, Haupt Y. Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage. Mol Cell Biol. 2005;25(13):5380–8. doi: 10.1128/MCB.25.13.5380-5388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4(10):793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15(2):164–71. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9(10):701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- Bruins W, Zwart E, Attardi LD, Iwakuma T, Hoogervorst EM, Beems RB, Miranda B, van Oostrom CTM, van den Berg J, van den Aardweg GJ, Lozano G, van Steeg H, et al. Increased sensitivity to UV radiation in mice with a p53 point mutation at Ser389. Mol Cell Biol. 2004;24(20):8884–94. doi: 10.1128/MCB.24.20.8884-8894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23(16):5556–71. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campaner S, Spreafico F, Burgold T, Doni M, Rosato U, Amati B, Testa G. The methyltransferase Set7/9 (Setd7) is dispensable for the p53-mediated DNA damage response in vivo. Mol Cell. 2011;43(4):681–8. doi: 10.1016/j.molcel.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Carr SM, Munro S, La Thangue NB. Lysine methylation and the regulation of p53. Essays Biochem. 2012;52:79–92. doi: 10.1042/bse0520079. [DOI] [PubMed] [Google Scholar]
- Carter S, Vousden KH. Modifications of p53: competing for the lysines. Curr Opin Genet Dev. 2009;19(1):18–24. doi: 10.1016/j.gde.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R, Hollstein M, Appella E, Xu Y. Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem. 2003;278(42):41028–33. doi: 10.1074/jbc.M306938200. [DOI] [PubMed] [Google Scholar]
- Chao C, Herr D, Chun J, Xu Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J. 2006a;25(11):2615–22. doi: 10.1038/sj.emboj.7601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C, Wu Z, Mazur SJ, Borges H, Rossi M, Lin T, Wang JYJ, Anderson CW, Appella E, Xu Y. Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol Cell Biol. 2006b;26(18):6859–69. doi: 10.1128/MCB.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265(5170):346–55. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443(7108):214–7. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- Coffill CR, Muller PAJ, Oh HK, Neo SP, Hogue KA, Cheok CF, Vousden KH, Lane DP, Blackstock WP, Gunaratne J. Mutant p53 interactome identifies nardilysin as a p53R273H-specific binding partner that promotes invasion. EMBO Rep. 2012;13(7):638–44. doi: 10.1038/embor.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17(6):901–11. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A, Soligo S, Dupont S, Piccolo S. Integration of TGF-β and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315(5813):840–3. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- Cui G, Park S, Badeaux AI, Kim D, Lee J, Thompson JR, Yan F, Kaneko S, Yuan Z, Botuyan MV, Bedford MT, Cheng JQ, et al. PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53. Nat Struct Mol Biol. 2012;19(9):916–24. doi: 10.1038/nsmb.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16(11):528–36. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Shi D, Gu W. Negative regulation of the acetyltransferase TIP60-p53 interplay by UHRF1 (Ubiquitin-like with PHD and RING Finger Domains 1) J Biol Chem. 2013;288(27):19581–92. doi: 10.1074/jbc.M113.476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Tang Y, Jung SY, Qin J, Aaronson SA, Gu W. Differential effects on p53-mediated cell cycle arrest vs. apoptosis by p90. Proc Natl Acad Sci U S A. 2011;108(47):18937–42. doi: 10.1073/pnas.1110988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath S, Chatterjee S, Arif M, Kundu TK, Roy S. Peptide-protein interactions suggest that acetylation of lysines 381 and 382 of p53 is important for positive coactivator 4-p53 interaction. J Biol Chem. 2011;286(28):25076–87. doi: 10.1074/jbc.M110.205328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehart CJ, Chahal JS, Flint SJ, Perlman DH. Extensive post-translational modification of active and inactivated forms of endogenous p53. Mol Cell Proteomics. 2013 doi: 10.1074/mcp.M113.030254. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA. Insights into wildtype and mutant p53 functions provided by genetically engineered mice. Hum Mutat. 2014;35:xxx–yyy. doi: 10.1002/humu.22507. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer. 2009;9(11):831–41. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- Elston R, Inman GJ. Crosstalk between p53 and TGF-β Signalling. J Signal Transduct. 2012;2012:294097. doi: 10.1155/2012/294097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamzadah S, Tropia L, Halazonetis TD. Crystal structure of a multidomain human p53 tetramer bound to the natural CDKN1A (p21) p53-response element. Mol Cancer Res. 2011;9(11):1493–9. doi: 10.1158/1541-7786.MCR-11-0351. [DOI] [PubMed] [Google Scholar]
- Endicott JA, Noble MEM, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- Feng L, Hollstein M, Xu Y. Ser46 phosphorylation regulates p53-dependent apoptosis and replicative senescence. Cell Cycle. 2006;5(23):2812–9. doi: 10.4161/cc.5.23.3526. [DOI] [PubMed] [Google Scholar]
- Feng L, Lin T, Uranishi H, Gu W, Xu Y. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol. 2005;25(13):5389–95. doi: 10.1128/MCB.25.13.5389-5395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreon JC, Lee CW, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE. Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc Natl Acad Sci U S A. 2009;106(16):6591–6. doi: 10.1073/pnas.0811023106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA, Espinosa JM. The impact of post-transcriptional regulation in the p53 network. Brief Funct Genomics. 2013;12(1):46–57. doi: 10.1093/bfgp/els058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata M, Kurabayashl A, Matsumoto M, Sonobe H, Ohtsuki Y, Terao N, Kuwahara M, Shuin T. Frequent phosphorylation at serine 392 in overexpressed p53 protein due to missense mutation in carcinoma of the urinary tract. J Pathol. 2002;197(1):82–8. doi: 10.1002/path.1082. [DOI] [PubMed] [Google Scholar]
- Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011;22(7):688–95. doi: 10.1016/j.semcdb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillotin S, Yap D, Lu X. Mutation at Ser392 specifically sensitizes mutant p53H175 to mdm2-mediated degradation. Cell Cycle. 2010;9(7):1390–8. doi: 10.4161/cc.9.7.11253. [DOI] [PubMed] [Google Scholar]
- Girardini JE, Napoli M, Piazza S, Rustighi A, Marotta C, Radaelli E, Capaci V, Jordan L, Quinlan P, Thompson A, Mano M, Rosato A, et al. A Pin1/mutant p53 axis promotes aggressiveness in breast cancer. Cancer Cell. 2011;20(1):79–91. doi: 10.1016/j.ccr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Goh AM, Coffill CR, Lane DP. The role of mutant p53 in human cancer. J Pathol. 2011;223(2):116–26. doi: 10.1002/path.2784. [DOI] [PubMed] [Google Scholar]
- Gu B, Zhu WG. Surf the post-translational modification network of p53 regulation. Int J Biol Sci. 2012;8(5):672–84. doi: 10.7150/ijbs.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S, di Agostino S, Mizrahi I, Alsheich-Bartok O, Voorhoeve M, Damalas A, Blandino G, Haupt Y. Promyelocytic leukemia protein is required for gain of function by mutant p53. Cancer Res. 2009;69(11):4818–26. doi: 10.1158/0008-5472.CAN-08-4010. [DOI] [PubMed] [Google Scholar]
- Hock A, Vousden KH. Regulation of the p53 pathway by ubiquitin and related proteins. Int J Biochem Cell Biol. 2010;42(10):1618–21. doi: 10.1016/j.biocel.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hoogervorst EM, Bruins W, Zwart E, van Oostrom CTM, van den Aardweg GJ, Beems RB, van den Berg J, Jacks T, van Steeg H, de Vries A. Lack of p53 Ser389 phosphorylation predisposes mice to develop 2-acetylaminofluorene-induced bladder tumors but not ionizing radiation-induced lymphomas. Cancer Res. 2005;65(9):3610–6. doi: 10.1158/0008-5472.CAN-04-4328. [DOI] [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–8. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jeong JS, Okamura J, Sook-Kim M, Zhu H, Guerrero-Preston R, Ratovitski EA. Global tumor protein p53/p63 interactome: making a case for cisplatin chemoresistance. Cell Cycle. 2012;11(12):2367–79. doi: 10.4161/cc.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupp TR, Lane DP. Two distinct signaling pathways activate the latent DNA binding function of p53 in a casein kinase II-independent manner. J Biol Chem. 1995;270(30):18165–74. doi: 10.1074/jbc.270.30.18165. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S. Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci U S A. 1994;91(13):6098–102. doi: 10.1073/pnas.91.13.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB. Arginine methylation regulates the p53 response. Nat Cell Biol. 2008;10(12):1431–9. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- Jeffrey PD, Gorina S, Pavletich NP. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science. 1995;267(5203):1498–502. doi: 10.1126/science.7878469. [DOI] [PubMed] [Google Scholar]
- Jenkins LMM, Durell SR, Mazur SJ, Appella E. p53 N-terminal phosphorylation: a defining layer of complex regulation. Carcinogenesis. 2012;33(8):1441–9. doi: 10.1093/carcin/bgs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LMM, Mazur SJ, Rossi M, Gaidarenko O, Xu Y, Appella E. Quantitative proteomics analysis of the effects of ionizing radiation in wild type and p53K317R knock-in mouse thymocytes. Mol Cell Proteomics. 2008;7(4):716–27. doi: 10.1074/mcp.M700482-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Brady CA, Johnson TM, Lee EY, Park EJ, Scott MP, Attardi LD. Full p53 transcriptional activation potential is dispensable for tumor suppression in diverse lineages. Proc Natl Acad Sci U S A. 2011;108(41):17123–8. doi: 10.1073/pnas.1111245108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachirskaia I, Shi X, Yamaguchi H, Tanoue K, Wen H, Wang EW, Appella E, Gozani O. Role for 53BP1 tudor domain recognition of p53 dimethylated at lysine 382 in DNA damage signaling. J Biol Chem. 2008;283(50):34660–6. doi: 10.1074/jbc.M806020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada R, Nomura T, Anderson CW, Sakaguchi K. Cancer-associated p53 Tetramerization Domain Mutants: QUANTITATIVE ANALYSIS REVEALS A LOW THRESHOLD FOR TUMOR SUPPRESSOR INACTIVATION. J Biol Chem. 2011;286(1):252–8. doi: 10.1074/jbc.M110.174698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, Ishioka C. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci U S A. 2003;100(14):8424–9. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7(2):283–92. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Kim K, Jeong KW, Kim H, Choi J, Lu W, Stallcup MR, An W. Functional interplay between p53 acetylation and H1.2 phosphorylation in p53-regulated transcription. Oncogene. 2012;31(39):4290–301. doi: 10.1038/onc.2011.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiblova P, Shaltiel IA, Benada J, Ševčík J, Pecháčková S, Pohlreich P, Voest EE, Dundr P, Bartek J, Kleibl Z, Medema RH, Macurek L. Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint. J Cell Biol. 2013;201(4):511–21. doi: 10.1083/jcb.201210031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel KA, Lee CJ, Toledo F, Wahl GM. The C-terminal lysines fine-tune P53 stress responses in a mouse model but are not required for stability control or transactivation. Proc Natl Acad Sci U S A. 2005;102(29):10188–93. doi: 10.1073/pnas.0503068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JP, Gu W. MSL2 promotes Mdm2-independent cytoplasmic localization of p53. J Biol Chem. 2009a;284(5):3250–63. doi: 10.1074/jbc.M805658200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009b;137(4):609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikov R, Winter M, Blattner C. Binding of p53 to the central domain of Mdm2 is regulated by phosphorylation. J Biol Chem. 2006;281(39):28575–83. doi: 10.1074/jbc.M513311200. [DOI] [PubMed] [Google Scholar]
- Kurash JK, Lei H, Shen Q, Marston WL, Granda BW, Fan H, Wall D, Li E, Gaudet F. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol Cell. 2008;29(3):392–400. doi: 10.1016/j.molcel.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Kurimchak A, Graña X. PP2A counterbalances phosphorylation of pRB and mitotic proteins by multiple CDKs: potential implications for PP2A disruption in cancer. Genes Cancer. 2013;3(11-12):739–48. doi: 10.1177/1947601912473479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP, Cheok CF, Brown C, Madhumalar A, Ghadessy FJ, Verma C. Mdm2 and p53 are highly conserved from placozoans to man. Cell Cycle. 2010;9(3):540–7. doi: 10.4161/cc.9.3.10516. [DOI] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119(6):861–72. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Pinto EM, Domenice S, Fragoso MCBV, Martin RM, Zerbini MC, Lucon AM, Mendonca BB. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab. 2001;86(10):4970–3. doi: 10.1210/jcem.86.10.7957. [DOI] [PubMed] [Google Scholar]
- Lee CW, Ferreon JC, Ferreon ACM, Arai M, Wright PE. Graded enhancement of p53 binding to CREB-binding protein (CBP) by multisite phosphorylation. Proc Natl Acad Sci U S A. 2010;107(45):19290–5. doi: 10.1073/pnas.1013078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Kang SU, Jeon Y, Park JW, You JS, Ha SW, Bae N, Lubec G, Kwon SH, Lee JS, Cho EJ, Han JW. Protein L-isoaspartyl methyltransferase regulates p53 activity. Nat Commun. 2012;3:927. doi: 10.1038/ncomms1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010;17(1):86–92. doi: 10.1038/cdd.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Teoh WW, Phang BH, Tong WM, Wang ZQ, Sabapathy K. Cell-type, dose, and mutation-type specificity dictate mutant p53 functions In vivo. Cancer Cell. 2012;22(6):751–64. doi: 10.1016/j.ccr.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tong WM, Wang ZQ, Sabapathy K. Serine 312 phosphorylation is dispensable for wild-type p53 functions in vivo. Cell Death Differ. 2011;18(2):214–21. doi: 10.1038/cdd.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz B, Rogalski JC, Schulze FM, Yi L, Lin S, Kast J, Rossi FMV. p53-dependent transcription and tumor suppression are not affected in Set7/9-deficient mice. Mol Cell. 2011;43(4):673–80. doi: 10.1016/j.molcel.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Lens SMA, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10(12):825–41. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- Leroy B, Fournier JL, Ishioka C, Monti P, Inga A, Fronza G, Soussi T. The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res. 2013;41(Database issue):D962–9. doi: 10.1093/nar/gks1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AG, Piluso LG, Cai X, Gadd BJ, Ladurner AG, Liu X. An acetylation switch in p53 mediates holo-TFIID recruitment. Mol Cell. 2007;28(3):408–21. doi: 10.1016/j.molcel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, Moll UM. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res. 2011;9(5):577–88. doi: 10.1158/1541-7786.MCR-10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem. 2002;277(52):50607–11. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Park J, Piao L, Kong G, Kim Y, Park KA, Zhang T, Hong J, Hur GM, Seok JH, Choi SW, Yoo BC, et al. PKB-mediated PHF20 phosphorylation on Ser291 is required for p53 function in DNA damage. Cell Signal. 2013;25(1):74–84. doi: 10.1016/j.cellsig.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7(2):165–71. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Liou YC, Zhou XZ, Lu KP. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci. 2011;36(10):501–14. doi: 10.1016/j.tibs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, Shi XB, deVere White RW, Gao AC. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate. 2013;73(4):418–27. doi: 10.1002/pros.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279(50):52175–82. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- Loughery J, Meek D. Switching on p53: an essential role for protein phosphorylation? BioDiscovery. 2013;1(8) doi: 10.7750/BioDiscovery.2013.8.1. [DOI] [Google Scholar]
- Lozano G. The oncogenic roles of p53 mutants in mouse models. Curr Opin Genet Dev. 2007;17(1):66–70. doi: 10.1016/j.gde.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lu X, Nguyen TA, Moon SH, Darlington Y, Sommer M, Donehower LA. The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008;27(2):123–35. doi: 10.1007/s10555-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol. 2007;27(23):8284–8295. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany NY) 2009;1(5):490–502. doi: 10.18632/aging.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D, Kim J, Kim T, Rhee BK, van Oostrom CT, DiTullio RA, Jr, Venere M, Halazonetis TD, Bronson R, De Vries A, Fleming M, Jacks T. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J. 2004;23(18):3689–99. doi: 10.1038/sj.emboj.7600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki CG. Oligomerization is required for p53 to be efficiently ubiquitinated by MDM2. J Biol Chem. 1999;274(23):16531–16535. doi: 10.1074/jbc.274.23.16531. [DOI] [PubMed] [Google Scholar]
- Mantovani F, Tocco F, Girardini J, Smith P, Gasco M, Lu X, Crook T, Del Sal G. The prolyl isomerase Pin1 orchestrates p53 acetylation and dissociation from the apoptosis inhibitor iASPP. Nat Struct Mol Biol. 2007;14(10):912–20. doi: 10.1038/nsmb1306. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Furihata M, Kurabayashi A, Ohtsuki Y. Phosphorylation state of tumor-suppressor gene p53 product overexpressed in skin tumors. Oncol Rep. 2004a;12(5):1039–43. [PubMed] [Google Scholar]
- Matsumoto M, Furihata M, Kurabayashi A, Sasaguri S, Araki K, Hayashi H, Ohtsuki Y. Prognostic significance of serine 392 phosphorylation in overexpressed p53 protein in human esophageal squamous cell carcinoma. Oncology. 2004b;67(2):143–50. doi: 10.1159/000081001. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Furihata M, Ohtsuki Y. Posttranslational phosphorylation of mutant p53 protein in tumor development. Med Mol Morphol. 2006;39(2):79–87. doi: 10.1007/s00795-006-0320-0. [DOI] [PubMed] [Google Scholar]
- Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9(10):714–23. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol. 2009;1(6):a000950. doi: 10.1101/cshperspect.a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellert H, Sykes SM, Murphy ME, McMahon SB. The ARF/oncogene pathway activates p53 acetylation within the DNA binding domain. Cell Cycle. 2007;6(11):1304–6. doi: 10.4161/cc.6.11.4343. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Santamaria AB, Bolshakov SV, Ananthaswamy HN. Mutant p53 is constitutively phosphorylated at Serine 15 in UV-induced mouse skin tumors: involvement of ERK1/2 MAP kinase. Oncogene. 2003;22(38):5958–66. doi: 10.1038/sj.onc.1206595. [DOI] [PubMed] [Google Scholar]
- Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9(10):724–37. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- Midgley CA, Lane DP. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene. 1997;15(10):1179–89. doi: 10.1038/sj.onc.1201459. [DOI] [PubMed] [Google Scholar]
- Minamoto T, Buschmann T, Habelhah H, Matusevich E, Tahara H, Boerresen-Dale AL, Harris C, Sidransky D, Ronai Z. Distinct pattern of p53 phosphorylation in human tumors. Oncogene. 2001;20(26):3341–7. doi: 10.1038/sj.onc.1204458. [DOI] [PubMed] [Google Scholar]
- Muller PAJ, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. J Cell Biol. 2011;192(2):209–18. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PAJ, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Anan T, Yoshida T, Mizukami T, Taya Y, Fujiwara T, Kato H, Saya H, Nakao M. The stabilization mechanism of mutant-type p53 by impaired ubiquitination: the loss of wild-type p53 function and the hsp90 association. Oncogene. 1999;18(44):6037–49. doi: 10.1038/sj.onc.1202978. [DOI] [PubMed] [Google Scholar]
- Noon AT, Goodarzi AA. 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair (Amst) 2011;10(10):1071–6. doi: 10.1016/j.dnarep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Odell AF, Odell LR, Askham JM, Alogheli H, Ponnambalam S, Hollstein M. A novel p53 mutant found in iatrogenic urothelial cancers Is dysfunctional and can be rescued by a second-site global suppressor mutation. J Biol Chem. 2013;288(23):16704–14. doi: 10.1074/jbc.M112.443168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaichi K, Nose K, Kotake T, Izumi N, Kudo T. Phosphorylation of p53 modifies sensitivity to ionizing radiation. Anticancer Res. 2011;31(6):2255–8. [PubMed] [Google Scholar]
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119(6):847–60. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ou YH, Chung PH, Sun TP, Shieh SY. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA-damage-induced C-terminal acetylation. Mol Biol Cell. 2005;16(4):1684–95. doi: 10.1091/mbc.E04-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim D, Dan HC, Chen H, Testa JR, Cheng JQ. Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53. J Biol Chem. 2012;287(14):11151–63. doi: 10.1074/jbc.M111.333922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Patel DJ, Wang Z. Readout of epigenetic modifications. Annu Rev Biochem. 2013;82:81–118. doi: 10.1146/annurev-biochem-072711-165700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RE, Knights CD, Sahu G, Catania J, Kolukula VK, Stoler D, Graessmann A, Ogryzko V, Pishvaian M, Albanese C, Avantaggiati ML. Restoration of DNA-binding and growth-suppressive activity of mutant forms of p53 via a PCAF-mediated acetylation pathway. J Cell Physiol. 2010;225(2):394–405. doi: 10.1002/jcp.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–9. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Hainaut P. Next-generation sequencing: emerging lessons on the origins of human cancer. Curr Opin Oncol. 2011;23(1):62–8. doi: 10.1097/CCO.0b013e3283414d00. [DOI] [PubMed] [Google Scholar]
- Pietenpol JA, Tokino T, Thiagalingam S, el-Deiry WS, Kinzler KW, Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci U S A. 1994;91(6):1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch EC, Leu JIJ, Frank A, Dumont P, George DL, Murphy ME. The tetramerization domain of p53 is required for efficient BAK oligomerization. Cancer Biol Ther. 2007;6(10):1576–83. doi: 10.4161/cbt.6.10.4719. [DOI] [PubMed] [Google Scholar]
- Puca R, Nardinocchi L, Givol D, D'Orazi G. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene. 2009;29(31):4378–87. doi: 10.1038/onc.2010.183. [DOI] [PubMed] [Google Scholar]
- Ray D, Murphy KR, Gal S. The DNA binding and accumulation of p53 from breast cancer cell lines and the link with serine 15 phosphorylation. Cancer Biol Ther. 2012;13(10):848–57. doi: 10.4161/cbt.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MA, Inga A. Functional mutants of the sequence-specific transcription factor p53 and implications for master genes of diversity. Proc Natl Acad Sci U S A. 2003;100(17):9934–9. doi: 10.1073/pnas.1633803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, DeLacerda L, Rabin M, Cadwell C, Sampaio G, Cat I, Stratakis CA, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98(16):9330–5. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Huarte M. To repress or not to repress: This is the guardian's question. Trends Cell Biol. 2011;21(6):344–53. doi: 10.1016/j.tcb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, Reynoso K, Bargonetti J, Wellstein A, Albanese C, Avantaggiati ML. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle. 2012;11(23):4436–46. doi: 10.4161/cc.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A. 2013;110(10):3895–900. doi: 10.1073/pnas.1300490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Musselman CA, Kachirskaia I, Hayashi R, Glass KC, Nix JC, Gozani O, Appella E, Kutateladze TG. Structural insight into p53 recognition by the 53BP1 tandem Tudor domain. J Mol Biol. 2010;398(4):489–96. doi: 10.1016/j.jmb.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy K. Role of the JNK pathway in human diseases. Prog Mol Biol Transl Sci. 2012;106:145–69. doi: 10.1016/B978-0-12-396456-4.00013-4. [DOI] [PubMed] [Google Scholar]
- Saito S, Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace AJ, Jr, Appella E, Anderson CW. Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem. 2003;278(39):37536–44. doi: 10.1074/jbc.M305135200. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12(18):2831–41. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K, Sakamoto H, Lewis MS, Anderson CW, Erickson JW, Appella E, Xie D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry. 1997;36(33):10117–10124. doi: 10.1021/bi970759w. [DOI] [PubMed] [Google Scholar]
- Schumacher B, Mondry J, Thiel P, Weyand M, Ottmann C. Structure of the p53 C-terminus bound to 14-3-3: implications for stabilization of the p53 tetramer. FEBS Lett. 2010;584(8):1443–8. doi: 10.1016/j.febslet.2010.02.065. [DOI] [PubMed] [Google Scholar]
- Serrano MA, Li Z, Dangeti M, Musich PR, Patrick S, Roginskaya M, Cartwright B, Zou Y. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013;32(19):2452–62. doi: 10.1038/onc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–34. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Kato S, Han SY, Liu W, Otsuka K, Sakayori M, Ishida T, Takeda M, Kanamaru R, Ohuchi N, Ishioka C. Isolation of temperature-sensitive p53 mutations from a comprehensive missense mutation library. J Biol Chem. 2004;279(1):348–55. doi: 10.1074/jbc.M310815200. [DOI] [PubMed] [Google Scholar]
- Slee EA, Benassi B, Goldin R, Zhong S, Ratnayaka I, Blandino G, Lu X. Phosphorylation of Ser312 contributes to tumor suppression by p53 in vivo. Proc Natl Acad Sci U S A. 2010;107(45):19479–84. doi: 10.1073/pnas.1005165107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss HK, Armata H, Gallant J, Jones SN. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol Cell Biol. 2004;24(3):976–984. doi: 10.1128/MCB.24.3.976-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss HK, Gannon H, Coles AH, Shen Q, Eischen CM, Jones SN. Phosphorylation of p53 serine 18 upregulates apoptosis to suppress Myc-induced tumorigenesis. Mol Cancer Res. 2010;8(2):216–22. doi: 10.1158/1541-7786.MCR-09-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hollstein M, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9(5):573–80. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- Stebbing J, Lit LC, Zhang H, Darrington RS, Melaiu O, Rudraraju B, Giamas G. The regulatory roles of phosphatases in cancer. Oncogene. 2013 doi: 10.1038/onc.2013.80. in press. [DOI] [PubMed] [Google Scholar]
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9(8):643–60. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- Sugikawa E, Yazaki N, Tsunoda S, Nakanishi N, Ohashi M. Inhibition of mutant p53 phosphorylation at serine 15 or serine 315 partially restores the function of wild-type p53. Biochem Biophys Res Commun. 1999;261(2):256–63. doi: 10.1006/bbrc.1999.1019. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–26. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22(10):1337–44. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel DP, Bycroft M, Fersht AR. Regulation by phosphorylation of the relative affinities of the N-terminal transactivation domains of p53 for p300 domains and Mdm2. Oncogene. 2009;28(20):2112–8. doi: 10.1038/onc.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuerkorn M, Fischer G, Schiene-Fischer C. Prolyl cis/trans isomerase signalling pathways in cancer. Curr Opin Pharmacol. 2011;11(4):281–7. doi: 10.1016/j.coph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Timofeev O, Schlereth K, Wanzel M, Braun A, Nieswandt B, Pagenstecher A, Rosenwald A, Elsässer HP, Stiewe T. p53 DNA binding cooperativity is essential for apoptosis and tumor suppression In vivo. Cell Rep. 2013;3(5):1512–25. doi: 10.1016/j.celrep.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–23. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- Trojer P, Li G, Sims RJ, 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, Wang YH, Reinberg D. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell. 2007;129(5):915–28. doi: 10.1016/j.cell.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Ullrich S, Sakaguchi K, Lees-Miller S, Fiscella M, Mercer W, Anderson C, Appella E. Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumors is altered compared to wild-type p53. Proc Natl Acad Sci U S A. 1993;90(13):5954–8. doi: 10.1073/pnas.90.13.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente LJ, Gray DHD, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, Janic A, Strasser A. p53 efficiently suppresses tumor development in the complete absence of Its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013;3(5):1339–45. doi: 10.1016/j.celrep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Valenti F, Fausti F, Biagioni F, Shay T, Fontemaggi G, Domany E, Yaffe MB, Strano S, Blandino G, Di Agostino S. Mutant p53 oncogenic functions are sustained by Plk2 kinase through an autoregulatory feedback loop. Cell Cycle. 2011;10(24):4330–40. doi: 10.4161/cc.10.24.18682. [DOI] [PubMed] [Google Scholar]
- van Dieck J, Teufel DP, Jaulent AM, Fernandez-Fernandez MR, Rutherford TJ, Wyslouch-Cieszynska A, Fersht AR. Posttranslational modifications affect the interaction of S100 proteins with tumor suppressor p53. J Mol Biol. 2009;394(5):922–30. doi: 10.1016/j.jmb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2009;20(5):299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock LJ, Knox A, Mee TR, Raines SA, Milner J. Influence of tetramerisation on site-specific post-translational modifications of p53: comparison of human and murine p53 tumor suppressor protein. Cancer Biol Ther. 2008;7(9):1481–9. doi: 10.4161/cbt.7.9.6473. [DOI] [PubMed] [Google Scholar]
- Warnock LJ, Raines SA, Milner J. Aurora A mediates cross-talk between N- and C-terminal post-translational modifications of p53. Cancer Biol Ther. 2011;12(12):1059–68. doi: 10.4161/cbt.12.12.18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman MJF, Stavridi ES, Waterman JLF, Halazonetis TD. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19(2):175–8. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- West LE, Gozani O. Regulation of p53 function by lysine methylation. Epigenomics. 2011;3(3):361–9. doi: 10.2217/EPI.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West LE, Roy S, Lachmi-Weiner K, Hayashi R, Shi X, Appella E, Kutateladze TG, Gozani O. The MBT repeats of L3MBTL1 link SET8-mediated p53 methylation at lysine 382 to target gene repression. J Biol Chem. 2010;285(48):37725–32. doi: 10.1074/jbc.M110.139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech M, Olszewski MB, Tracz-Gaszewska Z, Wawrzynow B, Zylicz M, Zylicz A. Molecular mechanism of mutant p53 stabilization: the role of HSP70 and MDM2. PLoS One. 2012;7(12):e51426. doi: 10.1371/journal.pone.0051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xu C, Price BD. Mechanistic links between ATM and histone methylation codes during DNA repair. Prog Mol Biol Transl Sci. 2012;110:263–88. doi: 10.1016/B978-0-12-387665-2.00010-9. [DOI] [PubMed] [Google Scholar]
- Yakovlev VA, Bayden AS, Graves PR, Kellogg GE, Mikkelsen RB. Nitration of the tumor suppressor protein p53 at tyrosine 327 promotes p53 oligomerization and activation. Biochemistry. 2010;49(25):5331–9. doi: 10.1021/bi100564w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li H, Zhou Z, Wang WH, Deng A, Andrisani O, Liu X. Plk1-mediated phosphorylation of Topors regulates p53 stability. J Biol Chem. 2009;284(28):18588–92. doi: 10.1074/jbc.C109.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap DBS, Hsieh JK, Zhong S, Heath V, Gusterson B, Crook T, Lu X. Ser392 phosphorylation regulates the oncogenic function of mutant p53. Cancer Res. 2004;64(14):4749–54. doi: 10.1158/0008-5472.CAN-1305-2. [DOI] [PubMed] [Google Scholar]
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22(1):9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannini L, Buscemi G, Kim JE, Fontanella E, Delia D. DBC1 phosphorylation by ATM/ATR inhibits SIRT1 deacetylase in response to DNA damage. J Mol Cell Biol. 2012;4(5):294–303. doi: 10.1093/jmcb/mjs035. [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Wang Y, Correa RG, Cho JY, Libermann TA. Blockage of NF-κB induces serine 15 phosphorylation of mutant p53 by JNK kinase in prostate cancer cells. Cell Cycle. 2005;4(9):1247–53. doi: 10.4161/cc.4.9.1966. [DOI] [PubMed] [Google Scholar]
- Zhu YH, Bulavin DV. Wip1-dependent signaling pathways in health and diseases. Prog Mol Biol Transl Sci. 2012;106:307–25. doi: 10.1016/B978-0-12-396456-4.00001-8. [DOI] [PubMed] [Google Scholar]
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


