Abstract
Background
Serum albumin concentration has been recognized as a marker of nutrition, severity of inflammation, and hepatic function in patients with various chronic diseases. The purpose of this study was to investigate the impact of pretransplant serum albumin concentration on post-transplant outcome in heart transplant recipients.
Methods and Results
Preoperative laboratory variables, including albumin concentration and donor-related information, were obtained from 822 consecutive patients undergoing heart transplant at Columbia University Medical Center between 1999 and 2010. The association between pretransplant albumin concentration and post-transplant 1-year survival was analyzed. Available data from the United Network for Organ Sharing (n=13 671) were also analyzed to evaluate the impact of preoperative albumin levels on post-transplant outcome. In our cohort, multivariable analysis revealed that preoperative albumin (mg/dL; hazard ratio, 0.46; P<0.0001) and preoperative total bilirubin (mg/dL; hazard ratio, 1.26; P=0.0002) were associated with post-transplant 1-year mortality. This implied that for every 1 mg/dL increase in albumin concentration, the post-transplant 1-year mortality rate decreased by 54%. The Kaplan–Meier analysis based on our patients cohort and the United Network for Organ Sharing dataset showed lower survival rate at 1-year post-transplant in patients with albumin levels ≤3.5 mg/dL compared with those with >3.5 mg/dL (our patients, 91.3 versus 72.4%; P<0.0001; United Network for Organ Sharing, 88.4 versus 84.8%; P<0.0001).
Conclusions
Pretransplant serum albumin concentration is a strong prognostic marker for post-transplant survival in heart transplant recipients.
Keywords: albumin, heart transplantation, prognosis
Serum albumin concentration has been recognized as a marker of nutrition, inflammation, hepatic function, and overall catabolic state.1–3 Hypoalbuminemia has been reported to be associated with poor prognosis in patients with chronic disease, such as end-stage renal disease,4 malignancy,5 and heart failure (HF).6,7 In addition, there is a well-documented association between preoperative hypoalbuminemia and increased postoperative mortality in patients undergoing cardiac and non-cardiac surgery.8–10 Hypoalbuminemia, indeed, is a part of Acute Physiology and Chronic Health Evaluation IV prognostic system, which predict critically ill medical and surgical patients.11
Heart transplantation (HTx) provides a considerable survival benefit for patients with advanced HF.12,13 In response to remarkable improvements in immunosuppression regimens and patient management, post-transplant survival has been significantly improved, with 1 and 5 years survival exceeding 85% and 70%, respectively.12 Post-transplant outcomes have been reported to be associated with a number of pretransplant recipient comorbidities12,14; however, an impact of pretransplant serum albumin concentration on outcome after HTx has not been fully elucidated. Therefore, we investigated the association between pretransplant serum albumin concentration combined with other pre- and perioperative factors and post-transplant survival in patients undergoing HTx.
Methods
The present study was approved by the institutional review board of Columbia University Medical Center.
Participants and Measurements
Data on a total of 822 consecutive patients who underwent HTx at Columbia University Medical Center between November 1999 and September 2010 were retrospectively reviewed, and 1-year post-transplant survival outcome of patients was analyzed. Pretransplant clinical characteristics of patients and laboratory examinations, including serum albumin concentration within 30 days before the surgery, were obtained from medical records. For patients with multiple laboratory measurements before the transplants, the results obtained at the closest date to the surgery were used for the present study. Laboratory variables of each patient used in the analysis were measured at the same day. Pretransplant clinical characteristics reviewed in the present study included age, sex, status at the United Network for Organ Sharing (UNOS), race, body mass index, primary heart disease, with or without left ventricular assist device (LVAD), and serology of cytomegalovirus at the time of HTx. Donor-related information, including donor age, sex, cytomegalovirus serology, and the number of donor/recipient HLA mismatches, was also obtained.
Analysis of the UNOS Dataset
Standard transplant analysis and research files with follow-up were provided by the UNOS. A total number of 42 803 patients of the UNOS dataset undergoing HTx between October 1987 and February 2010 were obtained of whom only 13 671 (31.9%) had pretransplant albumin levels and were included in the present analysis. Post-transplant 1-year survival of patients with preoperative hypoalbuminemia was compared with those with normal albumin concentration as described above using the same cutoff value derived from our patients’ cohort.
Statistical Analysis
Data are presented as mean±SD and frequency (percentage). Normality was evaluated for each variable on the basis of normal distribution plots and histograms and by the Kolmogorov–Smirnov test. Clinical characteristics and laboratory data were compared between groups using Student unpaired 2-tailed t test or χ2 analysis. For continuous variables which were not normally distributed, the Mann–Whitney U test was performed to test group differences. The cutoff value of 3.5 mg/dL of pretransplant albumin levels was used, as it is the lower limit of the reference value at out institution. Specifically, albumin levels of ≤3.5 mg/dL were defined as hypoalbuminemia and albumin levels >3.5 mg/dL were defined as normal albumin concentration throughout the study. A multivariable Cox proportional hazards model was built using a stepwise selection method with an entry significance level of 0.25 and a stay significance level of 0.15 to assess the independent effect of each variable on 1-year mortality after transplant. Pretransplant variables included into the stepwise selection methods were age, sex, UNOS status (1A=1, other status=0), race (black=1, others=0), cytomegalovirus serology before transplant (positive=1, negative=0), pretransplant albumin, sodium, hematocrit, creatinine, and total bilirubin concentrations. Peritransplant variables included in the analysis were ischemic time, donor age, donor sex, cytomegalovirus serology mismatch (positive donor to negative recipients=1, other combination=0), HLA mismatch (6 or 5 mismatch=1, <5 mismatch=0). The proportionality assumption in the Cox proportional hazards model was checked with time-dependent covariate, martingale residual plot and Schoenfeld residual plot. Survival up to 1-year after HTx was compared between patients with hypoalbuminemia and normal albumin using Kaplan–Meier analysis and log-rank test. In all analyses, the value 0.05 was used as the significance level.
All statistical analyses were performed using JMP version 7.0 software (SAS Institute).
Results
Analysis of Clinical Factors Associated with Post-transplant Mortality
Pretransplant clinical characteristics, laboratory variables, and donor-related information derived from the single-center Columbia University database are summarized in Table 1 along with the results from univariate Cox proportional hazards regression analysis of their risks on 1-year survival after transplantation. In univariate analysis, body mass index, pretransplant albumin, sodium, total bilirubin and direct bilirubin, hematocrit, donor sex, and HLA mismatch were significantly associated with 1-year survival.
Table 1.
Clinical Characteristics, Laboratory, and Donor-Related Information
| All Patients (N=822) | 1-Y Survival After Transplant
|
||
|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | ||
| Clinical characteristics at the time of transplant | |||
| Age, y | 53.0±12.3 | 1.016 (1.000–1.033) | 0.054 |
| Sex (men, %) | 637 (77.5%) | 0.673 (0.446–1.017) | 0.060 |
| Race (black, %) | 140 (17.0%) | 1.302 (0.822–2.062) | 0.261 |
| BMI, kg/m2 | 26.1±4.9 | 0.919 (0.883–0.957) | <0.0001 |
| Primary disease (ischemic, %) | 367 (44.7%) | 1.040 (0.714–1.516) | 0.838 |
| UNOS status (1A, %) | 317 (38.6%) | 1.352 (0.927–1.972) | 0.118 |
| LVAD requirement, % | 268 (32.6%) | 1.300 (0.882–1.916) | 0.184 |
| CMV serology (positive, %) | 496 (61.7%) | 0.726 (0.495–1.064) | 0.101 |
| Pretransplant laboratory examination | |||
| Albumin, mg/dL | 3.9±0.6 | 0.452 (0.339–0.602) | <0.0001 |
| Sodium, mEq/L | 137.5±7.3 | 0.952 (0.931–0.974) | <0.0001 |
| Potassium, mEq/L | 4.3±0.5 | 0.857 (0.595–1.235) | 0.409 |
| BUN, mg/dL | 29.4±15.7 | 1.005 (0.993–1.016) | 0.444 |
| Cre, mg/dL, n=820 | 1.4±0.7 | 0.988 (0.762–1.282) | 0.929 |
| Total bilirubin, mg/dL | 1.2±1.0 | 1.222 (1.074–1.390) | 0.002 |
| Direct bilirubin, mg/dL | 0.4±0.5 | 1.640 (1.310–2.054) | <0.0001 |
| AST, IU/L | 34.8±56.6 | 1.0 (0.997–1.003) | 0.838 |
| ALT, IU/L | 32.5±52.7 | 1.0 (0.997–1.003) | 0.897 |
| Total cholesterol, mg/dL (n=814) | 145.7±48.0 | 0.997 (0.993–1.002) | 0.219 |
| Triglyceride, mg/dL (n=820) | 124.1±64.6 | 1.0 (0.997–1.003) | 0.904 |
| Hematocrit, % | 36.3±5.9 | 0.966 (0.935–0.997) | 0.034 |
| Perioperative and donor-related information | |||
| Ischemic time, min (n=503) | 192.8±56.4 | 1.003 (0.998–1.007) | 0.289 |
| Donor age, y | 34.2±13.3 | 1.012 (0.997–1.027) | 0.105 |
| Donor sex, men, % | 453 (58.2%) | 0.619 (0.421–0.911) | 0.015 |
| CMV serology (D+/R− %), n=807 | 178 (22.1%) | 1.432 (0.933–2.199) | 0.100 |
| HLA mismatch (≥5, %), n=763 | 227 (29.8%) | 1.834 (1.229–2.736) | 0.003 |
| Survival after transplant, d | 1649±1188 | … | … |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; CMV, cytomegalovirus; D+/R−, serology-positive donor to serology-negative recipients; HLA, histocompatibility locus antigen; LVAD, left ventricular assist device; and UNOS, United Network for Organ Sharing.
Comparison of Patients With and Without Hypoalbuminemia
Patients were divided into 2 groups: those with preoperative hypoalbuminemia (<3.5 mg/dL) and those with normal albumin (≥3.5 mg/dL). In our cohort, 208 patients (25.3%) showed hypoalbuminemia preoperatively, including 21 patients (2.6%) with an albumin concentration <2.5 mg/dL. A total of 614 patients showed normal albumin levels (>3.5 mg/dL). The comparison of pre- and perioperative factors between the groups is shown in Table 2. Body mass index was lower, the proportions of patients at UNOS Status 1A and those requiring LVAD support at the time of HTx were larger in patients with preoperative hypoalbuminemia than those with normal albumin concentration. Preoperative sodium concentration was lower, total and direct bilirubin concentration was higher, total cholesterol and triglyceride levels were lower, and hematocrit was lower in patients with hypoalbuminemia than those with normal albumin concentration. Perioperative variables were not significantly different between patients with and without hypoalbuminemia. Overall survival duration was longer in patients with normal albumin than those with hypoalbuminemia.
Table 2.
Comparison of Pre- and Peritransplant Factors Between Patients With Hypoalbuminemia and Those With Normal Albumin
| Hypoalbuminemia (n=208) | Normal Albumin (n=614) | P Value | |
|---|---|---|---|
| Clinical characteristics at the time of transplant | |||
| Age, y | 54.2±11.5 | 52.6±12.5 | 0.097 |
| Sex (men, %) | 156 (75.0%) | 481 (78.3%) | 0.319 |
| Race (black, %) | 40 (19.2%) | 100 (16.3%) | 0.329 |
| BMI, kg/m2 | 23.1±4.7 | 27.1±4.5 | <0.0001 |
| Primary disease (ischemic, %) | 101 (48.6%) | 266 (43.3%) | 0.189 |
| UNOS status (1A, %) | 117 (56.3%) | 200 (32.6%) | <0.0001 |
| LVAD requirement, % | 95 (45.7%) | 173 (28.2%) | <0.0001 |
| CMV serology (positive, %) | 130 (63.7%) | 366 (61.0%) | 0.489 |
| Perioperative and donor-related information | |||
| Albumin, mg/dL | 3.1±0.4 | 4.2±0.4 | <0.0001 |
| Sodium, mEq/L | 130.0±6.1 | 140.1±5.7 | <0.0001 |
| Potassium, mEq/L | 4.3±0.5 | 4.3±0.5 | 0.792 |
| BUN, mg/dL | 27.3±16.3 | 30.0±15.4 | 0.031 |
| Cre, mg/dL (n=820) | 1.3±0.8 | 1.4±0.7 (n=612) | 0.234 |
| Total bilirubin, mg/dL | 1.4±1.5 | 1.1±0.8 | 0.009 |
| Direct bilirubin, mg/dL | 0.5±0.7 | 0.3±0.4 | <0.0001 |
| AST, IU/L | 40.7±96.1 | 32.8±33.9 | 0.082 |
| ALT, IU/L | 39.3±85.5 | 30.3±35.1 | 0.033 |
| Total cholesterol, mg/dL (n=814) | 133.4±48.5 | 149.9±47.1 (n=606) | <0.0001 |
| Triglyceride, mg/dL (n=820) | 114.2±57.1 | 127.5±66.6 (n=612) | 0.010 |
| Hematocrit, % | 32.6±5.4 | 37.5±5.5 | <0.0001 |
| Perioperative and donor-related information | |||
| Ischemic time, min (n=503) | 183.3±56.7 (n=122) | 195.9±56.4 (n=381) | 0.032 |
| Donor age, y | 35.6±13.7 | 33.7±13.2 | 0.080 |
| Donor sex (men, %) | 114 (58.2%) | 339 (58.2%) | 0.997 |
| CMV serology (D+/R−, %), n=807 | 41 (20.3%; n=202) | 137 (22.6%; n=605) | 0.486 |
| HLA mismatch (≥5, %), n=763 | 66 (34.2%; n=193) | 161 (28.3%; n=570) | 0.118 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CMV, cytomegalovirus; D+/R−, serology-positive donor to serology-negative recipients; HLA, histocompatibility locus antigen; LVAD, left ventricular assist device; and UNOS, United Network for Organ Sharing.
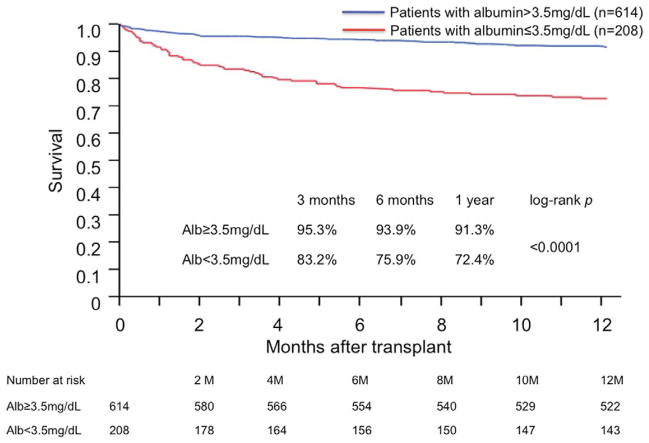
There were a total of 223 post-transplant deaths, 134 (21.8%) in the normal albumin group and 89 (42.8%) in the hypoalbuminemia group. Among the 223 patients who died, 109 deaths occurred during the first year after transplantation with 52 patients (8.5%) in the normal albumin group and 57 (27.4%) in the hypoalbuminemia group. The remaining 114 deaths occurred after 1-year after transplantation, and the mortality rates were similar between the 2 groups, with 82 (15.7%) in the normal albumin group and 32 (22.4%) in the hypoalbuminemia group (P=0.07). Kaplan–Meier survival curves of patients with and without hypoalbuminemia revealed that patients with normal albumin concentration showed better survival compared with those with hypoalbuminemia (1 and 5 years; 91.3% with SE 0.01 versus 72.4% with SE 0.03; 79.5% with SE 0.02 versus 58.0% with SE 0.04, respectively; P<0.0001). Figure 1 illustrates the survival of both groups of patients ≤1 year after transplant. The death in patients with hypoalbuminemia occurred most commonly in the early postoperative period in our cohort. Only limited data were available on the specific causes of death. However, on the basis of an obtainable data, the leading cause of death within 1 year after transplant was infection and the second leading cause was primary graft failure, which was not significantly different between patients with hypoalbuminemia and those with normal albumin concentration (24/57, 42.1% versus 17/51, 33.3% for infection; 6/57, 10.5% versus 4/51, 7.8% for primary graft failure, respectively; P=NS). The 1-year morality rates after transplant in patients with hypoalbuminemia and normal albumin concentration were 27.6% with SE 0.031 versus 8.7% with SE 0.012 (P<0.0001), respectively.
Figure 1.
Kaplan–Meier survival curves of patients with and without pretransplant hypoalbuminemia. Blue line indicates patients with normal albumin concentration defined as >3.5 mg/dL, and red line indicates patients with hypoalbuminemia (≤3.5 mg/dL).
Analysis of Factors Associated with Post-transplant 1-Year Mortality
A multivariable analysis for pretransplant factors associated with 1-year post-transplant mortality was performed by stepwise selection analysis, which selected age, sex (men=1, women=0), race (black=1, others=0), cytomegalovirus serology (positive=1, negative=0), preoperative albumin, and total bilirubin concentration for the inclusion in a subsequent Cox proportional hazards models. Among the variables, negative cytomegalovirus serology, lower albumin and higher total bilirubin concentration were found to be significantly associated with post-transplant mortality within a year (Table 3). The factor showing the most significant association with 1-year post-transplant mortality was identified as preoperative serum albumin concentration (hazard ratio, 0.461; 95% confidential interval, 0.341–0.623; P<0.0001). This implied that for every 1 mg/dL increase in albumin concentration, the post-transplant mortality rate decreased by >54%.
Table 3.
Multivariable Analysis for Preoperative Factors Associated With Post-transplant 1-Year Mortality
| HR (95% CI) | P Value | |
|---|---|---|
| Age, y | 1.018 (1.001–1.036) | 0.039 |
| Sex (men=1, women=0) | 0.729 (0.476–1.117) | 0.147 |
| Race (black=1, others=0) | 1.385 (0.859–2.235) | 0.182 |
| CMV serology (positive=1, negative=0) | 0.621 (0.420–0.920) | 0.017 |
| Preoperative albumin, mg/dL | 0.461 (0.341–0.623) | <0.0001 |
| Preoperative total bilirubin, mg/dL | 1.264 (1.115–1.431) | 0.0002 |
CI indicates confidential interval; CMV, cytomegalovirus; and HR, hazard ratio.
Multivariable analysis for combination of pre- and peritransplant variables associated with post-transplant mortality was also performed. The stepwise selection methods selected sex (men=1, women=0), cytomegalovirus serology (positive donor to negative recipient=1, other combination=0), preoperative albumin, and total bilirubin concentration for the inclusion in a subsequent Cox proportional hazards models. Among the variables, lower albumin concentration and higher total bilirubin concentration were found to be associated with 1-year post-transplant mortality (Table 4). Donor-related factors were not found to have a significant association with mortality. Neither martingale residual plots nor Schoenfeld residual plots showed a specific trend; therefore, the linearity of the effect of albumin levels on survival and the proportionality of the Cox model were confirmed.
Table 4.
Multivariable Analysis for Pre- and Perioperative Factors Associated With Post-transplant 1-Year Mortality
| HR (95% CI) | P Value | |
|---|---|---|
| Sex (men=1, women=0) | 0.741(0.483–1.135) | 0.168 |
| CMV serology (D+/R−=1, other combination=0) | 1.471 (0.956–2.262) | 0.079 |
| Preoperative albumin, mg/dL | 0.445 (0.330–0.599) | <0.0001 |
| Preoperative total bilirubin, mg/dL | 1.193 (1.050–1.355) | 0.007 |
CI indicates confidential interval; CMV, cytomegalovirus; D+/R−, serology-positive donor to serology-negative recipients; and HR, hazard ratio.
Impact of Hypoalbuminemia on Post-transplant Survival of Patients in the UNOS Dataset
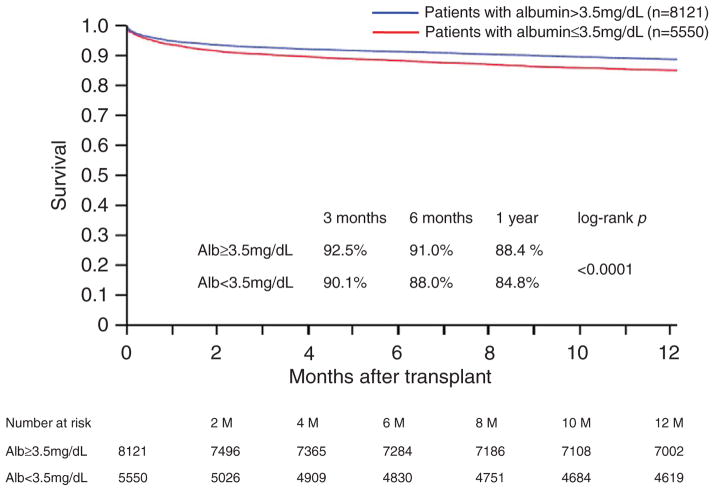
Post-transplant survival of patients in the UNOS dataset with and without hypoalbuminemia was also analyzed using the same cutoff value of pretransplant albumin concentration obtained from our patients cohort (3.5 mg/dL). In a total of 13 671 patients eligible for analysis, 5550 patients (40.6%) had hypoalbuminemia before HTx. In a comparable manner to our single-center cohort, Kaplan–Meier survival curves based on the presence or absence of hypoalbuminemia in the UNOS dataset revealed that patients with normal albumin showed better survival compared with those with hypoalbuminemia (1 and 5 years; 88.4% with SE 0.004 versus 84.8% with SE 0.005, 75.0% with SE 0.005 versus 71.1% with SE 0.007, respectively; P<0.0001). Figure 2 illustrates the survival of both groups of patients in the UNOS data set ≤1 year after transplant.
Figure 2.
Kaplan–Meier survival curves of patients with and without pretransplant hypoalbuminemia based on the United Network for Organ Sharing dataset. Blue line indicates patients with normal albumin concentration defined as >3.5 mg/dL, and red line indicates patients with hypoalbuminemia (≤3.5 mg/dL).
Discussion
In the present study, we have demonstrated that (1) pretransplant hypoalbuminemia is strongly associated with 1-year post-transplant mortality by multivariable analysis including pre- and perioperative factors and (2) the association of hypoalbuminemia and reduced 1-year post-transplant survival was also seen in the UNOS database.
Albumin is a hepatic protein, and its concentration is controlled by several factors, including rate of synthesis, catabolic state, and distribution between the intra- and extravascular spaces in the body. Many conditions, such as malnutrition, catabolism, liver, and renal dysfunction, as well as systemic infection, reduce serum albumin concentrations.1–4,15 Hypoalbuminemia has been reported to be associated with poor prognosis in patients with chronic disease states, such as renal disease, malignancy, and HF.6,16–18 In patients with HF, the prevalence of hypoalbuminemia has been reported to be ≈20% to 30% and it has emerged as an independent risk factor of death.6,19–21 The association of preoperative hypoalbuminemia and postoperative mortality in patients undergoing cardiac surgery has also been reported.8,9,22 The present study, for the first time, describes the impact of pretransplant hypoalbuminemia on 1-year post-transplant survival in HTx recipients. In addition, our investigation revealed that preoperative hypoalbuminemia remained to be a risk factor for postoperative mortality within a year by multivariable analysis on the basis of preoperative and perioperative factors. The death in patients with hypoalbuminemia occurred mostly in the early postoperative period when we dichotomized patients on the basis of albumin levels of ≤3.5 or >3.5 mg/dL (Figure 1). We speculate that preoperative hypoalbuminemia was related to preoperative severity of HF which was also linked to direct causes of early postoperative death in HTx recipients.12,14 Indeed, patients with preoperative hypoalbuminemia had more severe HF compared with those with normal albumin concentration in our cohort; the proportion of patients with UNOS status 1A and those with concomitant LVAD placements was larger in patients with hypoalbuminemia. In addition, previously described prognostic markers in patients with HF, such as low sodium, high total bilirubin, low total cholesterol, and anemia,23–25 were also found in our patients with hypoalbuminemia.
Hypoalbuminemia has also been linked to systemic inflammation, sepsis, and infection.4,26,27 Serum albumin has been shown to selectively inhibit tumor necrosis factor-α–induced vascular cell adhesion protein-1 expression, monocyte adhesion, and nuclear factor-κ B activation in endothelial cells suggesting a role in anti-inflammatory biological mechanisms.26 Therefore, patients with preoperative hypoalbuminemia may present with a persistent proinflammatory state even after cardiac transplant, which might contribute to early postoperative death. A continuous inflammatory state in patients with preoperative hypoalbuminemia increases susceptibility to infection,27 primary graft failure,28 and early development of transplant coronary artery disease,29 which are well-known causes of early postoperative death.12 In addition, patients with preoperative hypoalbuminemia have poor wound healing, which further increases the risk of infection.30 Malnutrition, as reflected by low albumin concentration, may also play a role in poor post-transplant survival. We could only obtain limited information about the causes of death; therefore, we could not demonstrate an actual comparison analysis of causes of death between patients with and without hypoalbuminemia. However, although our data were limited, the infection rate which was a leading cause of death within a year after transplant tended to be higher in patients with hypoalbuminemia.
Albumin has been incorporated into a number of risk stratification models to predict outcomes after LVAD implantation as well.31,32 Although these models cannot be validated in the transplant population to predict the specific clinical outcome because some of the transplant recipients were already treated with LVADs for bridge-to-transplantation, preoperative serum albumin levels are an useful marker for risk stratification in patients with advanced HF. Of note, albumin levels are routinely obtained preoperatively, but it is not considered a biomarker while it has distinct prognostic power for predicting the outcome of patients with advanced HF undergoing surgical interventions. Multiple factors contribute to impaired outcome of patients with hypoalbuminemia and our analysis cannot determine whether albumin is truly an independent risk factor for prognosis because of its interdependency on other outcome factors, such as malnutrition, inflammation, and liver dysfunction. The serum albumin level is, however, a simple integrative factor with apparent clinical usage.
Our observation was based on single-center, retrospective analysis. One of the limitations of this study was the lack of pretransplant hemodynamic data. We could define whether hypoalbuminemia in our cohort was driven by congestion or a low cardiac output state. Further study, especially serial measurements of albumin concentration in patients undergoing LVAD as a bridge-to-transplantation, would provide greater clinical relevance whether modifying hemodynamics could further improve hypoalbuminemia and post-transplant outcome. The association between pre-transplant hypoalbuminemia and post-transplant survival was also found in the available UNOS data set which included >13 000 transplant recipients with reported pretransplant albumin levels. The analysis derived from the UNOS dataset contains limitations. Detailed pre- and post-transplant clinical information were not available for all patients because of incomplete listings in the data set. Our analysis only includes the subset of 31.9% of all patients in the actual UNOS database whose preoperative albumin levels were reported. This may cause a smaller difference of survival of patients with and without hypoalbuminemia in the UNOS dataset compared with our single-center analysis in which we evaluated all patients in a consecutive manner. Another limitation of the study was that the albumin level is a time-variant value, which may not be an ideal parameter to be included in the proportional hazard model. However, we used the value obtained closest to the surgery in the present study, and we think that this level would reflect the overall preoperative condition of a patient. Attention to correct nutrition, inflammation, and hepatic function to normalize serum albumin concentration before transplant could be an effective way to improve post-transplant survival.
In conclusion, we found that pretransplant albumin concentration was independently associated with post-transplant survival of HTx recipients. Our finding may be useful in discussing the risks and timing of transplant listing for patients with advanced HF.
CLINICAL PERSPECTIVE.
Low serum albumin levels have been recognized as a marker of malnutrition, severity of inflammation, and hepatic dysfunction in patients with various chronic diseases. We investigated the impact of pretransplantation serum albumin levels on post-transplantation outcome in heart transplant recipients on the basis of 822 consecutive patients undergoing heart transplant at Columbia University Medical Center. Further, we tested the impact of low serum albumin levels on survival on the dataset from the United Network for Organ Sharing (n=13 671). In our single-center cohort, multivariable analysis revealed that preoperative albumin (mg/dL; hazard ratio, 0.46; P<0.0001) and preoperative total bilirubin (mg/dL; hazard ratio, 1.26; P=0.0002) were associated with post-transplantation 1-year mortality. The Kaplan–Meier analysis based on our patient cohort and the United Network for Organ Sharing dataset showed lower survival at 1-year post-transplantation in patients with albumin levels ≤3.5 mg/dL compared with those with levels >3.5 mg/dL (both P<0.0001). Therefore, pretransplantation serum albumin concentration is a strong prognostic marker for survival after heart transplant.
Acknowledgments
We thank Norio Sugimoto (Sugimoto Data Analysis Service) for his statistical review of the article.
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, and HL073029) and the Herbert and Florence Irving Scholar Award to Dr Schulze.
Footnotes
Disclosures
None.
References
- 1.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 2.Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–1264. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 3.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 4.Kaysen GA, Dubin JA, Müller HG, Rosales L, Levin NW, Mitch WE HEMO Study Group NIDDK. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 2004;65:1408–1415. doi: 10.1111/j.1523-1755.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 6.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL., Jr Serum albumin and mortality in acutely de-compensated heart failure. Am Heart J. 2010;160:1149–1155. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 8.de la Cruz KI, Bakaeen FG, Wang XL, Huh J, LeMaire SA, Coselli JS, Chu D. Hypoalbuminemia and long-term survival after coronary artery bypass: a propensity score analysis. Ann Thorac Surg. 2011;91:671–6. doi: 10.1016/j.athoracsur.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ, Jr, Couper GS, Allred EN, Cohn LH, Rizzo RJ. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118:866–873. doi: 10.1016/s0022-5223(99)70056-5. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 12.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report–2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Jaski BE, Kim JC, Naftel DC, Jarcho J, Costanzo MR, Eisen HJ, Kirklin JK, Bourge RC Cardiac Transplant Research Database Research Group. Cardiac transplant outcome of patients supported on left ventricular assist device vs. intravenous inotropic therapy. J Heart Lung Transplant. 2001;20:449–456. doi: 10.1016/s1053-2498(00)00246-1. [DOI] [PubMed] [Google Scholar]
- 14.Cimato TR, Jessup M. Recipient selection in cardiac transplantation: contraindications and risk factors for mortality. J Heart Lung Transplant. 2002;21:1161–1173. doi: 10.1016/s1053-2498(02)00428-x. [DOI] [PubMed] [Google Scholar]
- 15.De Feo P, Lucidi P. Liver protein synthesis in physiology and in disease states. Curr Opin Clin Nutr Metab Care. 2002;5:47–50. doi: 10.1097/00075197-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 17.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 18.Read JA, Choy ST, Beale PJ, Clarke SJ. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer. 2006;55:78–85. doi: 10.1207/s15327914nc5501_10. [DOI] [PubMed] [Google Scholar]
- 19.Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB CHARM Investigators. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170–177. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL., Jr Serum albumin and mortality in acutely de-compensated heart failure. Am Heart J. 2010;160:1149–1155. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Yanagisawa S, Miki K, Yasuda N, Hirai T, Suzuki N, Tanaka T. Clinical outcomes and prognostic factor for acute heart failure in nonagenarians: impact of hypoalbuminemia on mortality. Int J Cardiol. 2010;145:574–576. doi: 10.1016/j.ijcard.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 22.Dimaria-Ghalili RA. Nutrition risk factors in older coronary artery bypass graft patients. Nutr Clin Pract. 2008;23:494–500. doi: 10.1177/0884533608323428. [DOI] [PubMed] [Google Scholar]
- 23.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M EVEREST Investigators. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. doi: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O’Connor CM, She L, Yancy CW, Young J, Fonarow GC OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–8. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 25.Vrtovec B, Radovancevic R, Delgado RM, Radovancevic B, Bracey AW, Gregoric ID, Frazier OH. Significance of anaemia in patients with advanced heart failure receiving long-term mechanical circulatory support. Eur J Heart Fail. 2009;11:1000–1004. doi: 10.1093/eurjhf/hfp110. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55:820–829. doi: 10.1016/s0008-6363(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 27.Sun HY, Singh N, Cacciarelli TV, Wannstedt C, Wagener MM, Steele C. Dysregulated expression of T-helper cell responses and susceptibility to infections in high-risk liver transplant recipients. Transpl Immunol. 2008;20:68–72. doi: 10.1016/j.trim.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Potapov EV, Wagner FD, Loebe M, Ivanitskaia EA, Müller C, Sodian R, Jonitz B, Hetzer R. Elevated donor cardiac troponin T and procalcitonin indicate two independent mechanisms of early graft failure after heart transplantation. Int J Cardiol. 2003;92:163–167. doi: 10.1016/s0167-5273(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 29.Raichlin E, Bae JH, Kushwaha SS, Lennon RJ, Prasad A, Rihal CS, Lerman A. Inflammatory burden of cardiac allograft coronary athero-sclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–1286. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Otranto M, Souza-Netto I, Aguila MB, Monte-Alto-Costa A. Male and female rats with severe protein restriction present delayed wound healing. Appl Physiol Nutr Metab. 2009;34:1023–1031. doi: 10.1139/H09-100. [DOI] [PubMed] [Google Scholar]
- 31.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 32.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]