Abstract
Purpose
To identify mechanisms of disease in a child born to consanguineous parents, who presented with Omenn syndrome (OS) and was found to carry a heterozygous RAG1 mutation in peripheral blood DNA.
Methods
Mutation analysis was performed on whole blood and buccal swab DNA. Recombination activity of the mutant RAG1 protein and diversity of T cell repertoire were tested.
Results
Apparent heterozygosity for a novel, functionally null RAG1 mutation in peripheral blood DNA from a patient with OS was shown to be secondary to true somatic reversion. Analysis of T cell repertoire demonstrated expression of various TCRBV families, but an overall restricted pattern.
Conclusions
This is the first case of true somatic reversion of a RAG1 mutation in a patient with OS. The reversion event likely occurred at a stage where only a limited pool of T cell progenitors capable of performing V(D)J recombination could be generated. This work emphasizes the importance of performing functional studies to investigate the significance of novel genetic variants, and to consider somatic reversion as a possible disease modifier in SCID.
Keywords: Omenn syndrome, RAG1, somatic reversion, severe combined immune deficiency, T cell repertoire
Introduction
Omenn syndrome (OS) is a combined immunodeficiency characterized by generalized erythroderma, lymphadenopathy and/or organomegaly, elevated IgE, eosinophilia, and expansion of oligoclonal, autologous and activated T cells1. OS may be sustained by various genetic defects that compromise T (and often, B) cell development, with mutations of the RAG1/RAG2 genes being the most common. Such mutations usually retain enough V(D)J recombination activity to allow the development of a small number of T cell clones that undergo peripheral expansion and acquire a Th2 phenotype, thus contributing to the clinical manifestations of the disease [1]. Here we report the first case of an infant with OS due to a homozygous RAG1 mutation and true somatic reversion in peripheral blood cells.
Methods
RAG1 sequencing
The RAG1 coding sequence was amplified from whole blood genomic DNA and from buccal swab DNA using RAG1-specific primers (available upon request) and Phusion Hot Start II High Fidelity DNA Polymerase (new England BioLabs, Ipswich, MA, USA), followed by capillary sequencing.
Analysis of T cell repertoire
Expression of TCRBV families in CD4+ and CD8+ cells was detected by using fluorochrome-conjugated monoclonal antibodies specific for 24 families, according to the manufacturer's specifications (Beckman Coulter, Brea, Calif).
RAG1 recombinase activity
The recombinase activity of wild type RAG1 and of the L411P RAG1 mutant - with and without polymorphisms detected in the family was measured by flow cytometry as described [2].
Results
A male infant born to consanguineous parents of Indian Sikh ethnicity developed severe generalized erythroderma, alopecia, and poor weight gain shortly after birth. No lymphadenopathy or hepatomegaly were present. At five months of age, the patient developed multiple skin abscesses and an episode of Pneumocystis jiroveci pneumonia, prompting laboratory investigations for possible immunodeficiency.
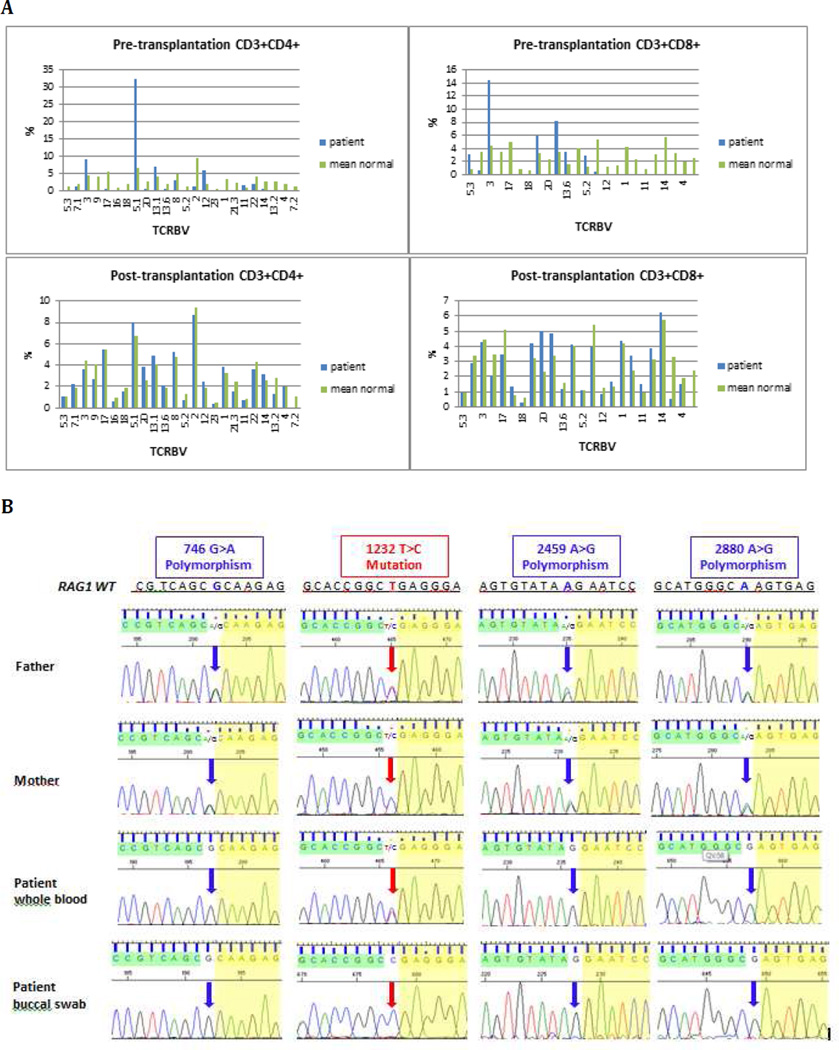
Normal levels of serum IgG and IgA, slightly low level of IgM, and marked elevation of IgE were demonstrated (Table 1). Peripheral eosinophilia was present. The total lymphocyte count was elevated, with expansion of both CD4+ and CD8+ T lymphocytes, absent B cells, and normal number of NK cells. Virtually all CD4+ and CD8+ cells expressed activation markers (Table 1). Flow cytometric analysis of expressed TCRBV families demonstrated a restricted T cell repertoire (Fig. 1A). In vitro lymphocyte proliferation to both PHA and anti-CD3 was markedly reduced (Table 1). The patient met current criteria for OS [3], and treatment with intravenous immunoglobulin (IVIG), cotrimoxazole prophylaxis and cyclosporine (to control erythroderma) was started.
Table 1.
Laboratory evaluation pre- and post-transplantation
| At presentation |
15 months post- transplantation |
|
|---|---|---|
| ALC (×10^9/L) | 28.35 | 2.06 |
| Eosinophils (×10^9/L) | 0.9 | 0.05 |
| CD3 (cells/uL) | 25930 | 1280 |
| CD4 (cells/uL) | 6880 | 760 |
| CD8 (cells/uL) | 17190 | 450 |
| CD4+CD45RA+CD62L+/CD4+ (%) | 0.3 | 57 |
| CD4+CD45RA−/CD4+ (%) | 99.6 | 37.8 |
| CD8+CD45RA+CD62L+/CD8 (%) | 0.3 | 68 |
| CD8+CD45RA−/CD8+(%) | 99.6 | 26.6 |
| CD3+ TCRab+ (% of CD3+) | 99.9 | 94.7 |
| TCRab+/CD4−CD8−(% of CD3+) | 7.3 | 1.5 |
| CD3+ TCRab−(% of CD3+) | 0 | 5.3 |
| CD19 (cells/uL) | 0 | 80 |
| CD16+56 (cells/uL) | 530 | 640 |
| IgG (mg/dl) | 6.9 | 3.3 |
| IgA (mg/dL) | 0.11 | 0.25 |
| IgM (mg/dl) | 0.19 | 0.5 |
| IgE (kU/L) | 29464 | 464 |
| Proliferative response to PHA (unstimulated/stimulated), expressed as % of CD45+ lymphocytes that were EDU+ (control: 0.3/66.0) | 0.1/1.9 | 0.1/58.3 |
| Proliferative response to anti-CD3 (unstimulated/stimulated) expressed as % of CD45+ lymphocytes that were EDU+ (control: 0.3/28.9) | 0.7/0.1 | 0.2/20.7 |
Fig. 1.
A TCRBV repertoire pre- and post-transplantation. Flow cytometric analysis of the percentage of CD4+ (left panels) and CD8+ (right panels) lymphocytes expressing the various TCRBV families before (top panels) and after (bottom panels) hematopoietic cell transplantation in the patient (blue bars), as compared to mean values from healthy age-matched controls (green bar)
B Results of RAG1 sequencing in the patient and his parents Chromatograms of Sanger sequencing demonstrating apparent heterozygosity for the 1232T>C mutation in patient’s whole blood and in both parents. Homozygosity for the same mutation is present in patient’s buccal swab DNA. Analysis of three known RAG1 polymorphisms demonstrates homozygosity in the patient’s samples, and heterozygosity in both parents.
Sequencing of the RAG1 and RAG2 genes on whole blood genomic DNA revealed apparent heterozygosity for a novel RAG1 mutation (c.1232 T>C), predicted to cause p.Leu411Pro amino acid substitution (Fig. 1B) in the nonamer-binding region of RAG1, a domain that is crucial for RAG1 DNA binding and recombination activity. Both parents were shown to be heterozygous for this mutation. We initially speculated that apparent heterozygosity for the RAG1 Leu411Pro mutation could reflect maternal T cell engraftment. However, sequencing of the RAG1 gene on patient’s whole blood genomic DNA also revealed homozygosity for three known RAG1 polymorphisms (c.746A>G; c.2459 A>G; c2880 A>G), for which both parents were found to be heterozygous (Fig. 1B). We then considered the possibility of true somatic reversion. Indeed, homozygosity for c.1232 T>C mutation was demonstrated in genomic DNA from a patient’s buccal swab sample. Using a recently described flow cytometry-based assay [2], we demonstrated that the RAG1 Leu411Pro mutant had virtually undetectable (0.06% of wild-type) recombination activity, which was not modified upon introduction of the RAG1 polymorphisms that had been identified in the patient (Supplementary Table 1).
At 7 months of age, the patient received a haploidentical peripheral blood stem cell (PBSC) transplantation from his father, following reduced intensity conditioning with Busulfan, Fludarabine and anti-thymocyte globulin. He engrafted on day 12, but remained profoundly T and B lymphopenic. Top-up transfusion of paternal PBSC was given 3.5 months after transplantation. Lung and liver graft versus host disease was successfully treated with corticosteroids. Chimerism studies performed 13 months after transplantation showed 19% donor cells in whole blood, and 99% donor T cells in the CD3+ fraction. The patient is currently well 15 months post-transplantation, with T cell counts at the lower limit of normal for age, normal proportions of naive T cells for age, and polyclonal pattern of expression of TCRBV families (Table 1 and Fig. 1A). In vitro T cell proliferation to PHA and anti-CD3 has normalized. Although his B cell counts remain low (0.08 ×10 ^9/L), IVIG has been ceased and his IgG and IgA are within reference range for age (IgG 3.3, IgA 0.24 and IgM 0.33 g/L) (Table 1).
Discussion
Somatic mosaicism due to reversion of gene mutations or to second-site compensatory DNA changes that partially or fully restore protein expression and function has been described in several primary immunodeficiency diseases [4], and may affect multiple cell populations (B cells, T cells or T cell subpopulations) or be restricted to a single cell lineage. A previous case of somatic reversion due to multiple second-site mutations has been reported in a patient with OS [5]. However, the patient reported here is the first case of OS associated with true RAG1 somatic reversion.
Reversion mosaicism in patients with SCID has been variably associated with functional improvement and restoration of a diversified T cell repertoire [6], delayed-onset phenotype [7, 8], or limited increase of T cell count and persistence of a restricted T cell repertoire with impaired in vitro T cell proliferation [9]. Somatic reversion in RAG deficiency is expected to confer selective advantage only if it occurs at an early stage in lymphocyte development, before RAG expression is physiologically extinguished. The recombination activity of the RAG1 Leu411Pro mutant was extremely low, in the range observed for mutants identified in patients with a SCID phenotype [2]. Moreover, similar height of the chromatogram peaks corresponding to the mutant and the wild-type nucleotide at position c.1232 indicates that the majority of T cells carried the revertant allele, suggesting that somatic reversion significantly contributed to residual development of T cells. The observation that multiple TCRBV families were expressed, and yet the T cell repertoire was severely restricted, strongly suggests that the reversion occurred at a stage where only a limited pool of revertant T cell progenitors with productive V(D)J rearrangements could be generated, thereby allowing production of few T cell clonotypes that underwent clonal expansion causing an OS phenotype. Unavailability of pre-transplantation stored samples did not allow us to investigate whether the somatic reversion also involved other blood cell lineages, however lack of circulating B cells indicates that it is unlikely that it occurred at an early stage in lymphoid development, prior to separation of T vs. B lineage commitment. In summary, we have reported the first case of true somatic reversion in RAG1 deficiency. Demonstration of apparent heterozygosity for a novel RAG1 mutation in whole blood DNA could have easily been dismissed as a coincidental finding of no clinical significance for the patient reported here. Our data emphasize the importance of performing careful functional studies to investigate the functional effect of novel genetic variants, and to consider somatic reversion as a possible disease modifier in SCID.
Supplementary Material
Acknowledgements
This work was supported by NIH grant 1P01AI076210, by March of Dimes grant 1-FY-13-500 and by a grant from the Jeffrey Modell Foundation (to LDN). E.C. is the recipient of T32 fellowship grant AI007512.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunology. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 2.Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunology. 2013 Nov 28; doi: 10.1016/j.jaci.2013.10.007. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: The Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2013 Nov 27; doi: 10.1016/j.jaci.2013.09.044. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada T, Candotti F. Somatic mosaicism in primary immune deficiencies. Cur Opin Allergy Clin Immunol. 2008;8:510–514. doi: 10.1097/ACI.0b013e328314b651. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Toma T, Okamoto H, Kasahara Y, Koizumi S, Agematsu K, et al. Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood. 2005;106:2099–2101. doi: 10.1182/blood-2005-03-0936. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Saito M, Nishikomori R, Yasumi T, Izawa K, Murakami T, et al. Multiple reversions of an IL2RG mutation restore T cell function in an X-linked severe combined immunodeficiency patient. J Clin Immunol. 2012;32:690–697. doi: 10.1007/s10875-012-9684-1. [DOI] [PubMed] [Google Scholar]
- 7.Hirschhorn R, Yang DR, Puck JM, Huie ML, Jiang CK, Kurlandsky LE. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat Genet. 1996;13:290–295. doi: 10.1038/ng0796-290. [DOI] [PubMed] [Google Scholar]
- 8.Moncada-Velez M, Velez-Ortega A, Orrego J, Santisteban I, Jagadeesh J, Olivares M, et al. Somatic mosaicism caused by monoallelic reversion of a mutation in T cells of a patient with ADA-SCID and the effects of enzyme replacement therapy on the revertant phenotype. Scand J Immuno9l. 2011;74:471–481. doi: 10.1111/j.1365-3083.2011.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan V, Wahn V, Le Deist F, Dirksen U, Broker B, Muller-Fleckenstein I, et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.