Abstract
Background
Cartilage repair (CR) procedures are widely accepted for treatment of isolated cartilage defects at the knee joint. However, it is not well known whether these procedures prevent degenerative joint disease.
Hypothesis/Purpose
CR procedures prevent accelerated qualitative and quantitative progression of meniscus degeneration in individuals with focal cartilage defects.
Study Design
Cohort Study; Level of evidence 2b
Methods
A total of 94 subjects were studied. CR procedures were performed on 34 patients (n=16 osteochondral transplantation, n=18 microfracture); 34 controls were matched. An additional 13 patients received CR and anterior cruciate ligament (ACL) reconstruction (CR&ACL) and 13 patients received only ACL reconstruction. 3.0T MRI with T1ρ mapping and sagittal fat-saturated intermediate-weighted fast spin echo (FSE) sequences was performed to analyze menisci quantitatively and qualitatively (Whole-Organ Magnetic Resonance Imaging Score, WORMS). CR and CR&ACL patients were examined 4 months (n=34; n=13), 1 (n=21; n=8) and 2 (n=9; n=5) years post CR. Control subjects were scanned at baseline and after 1 and 2 years, ACL patients after 1 and 2 years.
Results
At baseline, global meniscus T1ρ values were higher in individuals with CR (14.2±0.6ms; P=0.004) and in individuals with CR&ACL (17.1±0.9ms; P<0.001) when compared to controls (12.8±0.6ms). After two years, there was a statistical difference between T1ρ at the overlying meniscus above cartilage defects (16.4±1.0ms) and T1ρ of the subgroup of control knees without cartilage defects (12.1±0.8ms; P<0.001) and a statistical trend to the CR group (13.3±1.0 ms; P=0.088). At baseline, 35% of subjects with CR showed morphological meniscus tears at the overlying meniscus; 10% of CR subjects showed an increase of WORMS meniscus score within the first year, none progressed in the second year. Control subjects with (without) cartilage defects showed meniscus tears in 30% (5%) at baseline; 38% (19%) increased within the first, and 15% (10%) within the second year.
Conclusions
This study identified more severe meniscus degeneration after CR surgery compared to controls. However, progression of T1ρ values was not observed from 1 to 2 years after surgery. These results suggest, that CR may prevent degenerative meniscus changes.
Keywords: Cartilage repair, meniscus, T1ρ, WORMS, 3.0T MRI
INTRODUCTION
Articular cartilage defects have limited potential to regenerate and are associated with an early onset of osteoarthritis (OA) 8. Over the past decade, cartilage repair (CR) has been increasingly used to treat focal cartilage defects of the knee 35. The most common technique is microfracture (Mfx), which is used for smaller lesions not affecting the subchondral bone. For larger regions, besides autologous chondrocyte implantation (ACI), osteochondral transplantation (OCT) is the procedure of choice 41.
Noninvasive magnetic resonance imaging (MRI) is the most important diagnostic tool for monitoring the post-operative course of these patients 43, 48. 1.5T MRI of the knee is the current standard in clinical practice 20, 29, but evidence suggests that 3.0T MRI may be more advantageous. 3.0T MRI yields a stronger magnetic field strength, allowing for thinner sections, higher plane spatial resolution, and increased signal-to-noise ratio. In addition, 3.0T MRI is more sensitive to diagnosing meniscus pathology, a known contributor to early onset of OA 1, 40. Quantitative T1ρ relaxation time measurements reflect early degenerative changes in the biochemical composition of cartilage such as proteoglycan loss and increase in water content 1,24,25, 40. It has recently also been applied to quantitatively and noninvasively detect meniscus degeneration 5, 39. Although the exact factors that contribute to a higher meniscus T1ρ in subjects with osteoarthritis and a lower meniscus T1ρ in healthy subjects are not clear yet, one study found a positive correlation between cartilage degeneration and increased T1ρ values in the meniscus 38.
Multiple studies have examined results after CR on a descriptive level A challenge . remains to demonstrate that CR can prevent joint degeneration, beyond others, by clinical validating imaging outcomes 48. Few studies have used 3.0T MRI for follow-up, but little is known about meniscus degeneration as an outcome parameter after CR. However, evaluation of the impact of meniscus degeneration is crucial to ensure quality control and the development of future treatment guidelines in patients post CR.
While T1ρ relaxation time measurements of cartilage repair tissue have been described previously 14, the purpose of this study was to evaluate meniscus degeneration, as measured by morphological assessment and quantitative T1ρ meniscus measurements, at multiple longitudinal time points in patients who received CR surgery compared to controls. We hypothesized that patients who underwent CR would have higher meniscus T1ρ values, indicating more degenerative meniscus changes at baseline, but no further meniscus degeneration during follow-up.
MATERIALS AND METHODS
Subjects
A total of 94 subjects was analyzed in this study. A total of 34 subjects was treated with cartilage repair procedures (CR group) for isolated posttraumatic or degenerative full thickness cartilage defects at the knee (graded III and IV according to the International Cartilage Repair Society (ICRS) classification cite). A control cohort (n=34) was recruited, that was matched for Kellgren/Lawrence (KL) score 18 and gender (control group). Thirteen additional subjects received both cartilage repair as well as anterior cruciate ligament (ACL) reconstruction (CR&ACL group). A final additional 13 subjects received only ACL reconstruction (ACL group). The study was approved by the local Institutional Review Board and conducted in accordance with the Committee for Human Research at our institution. All subjects gave written informed consent prior to participation in the study. Data were prospective and nonrandomized.
Surgery
The indication for CR surgery was made in consultation with the patient and confirmed during arthroscopy of the affected knee joint. Exclusion criteria for CR procedures were uncontained large defects of several joint regions, significant degenerative changes of the affected joint (KL >2), non-correctable ligamentous instability, varus or valgus malalignment of >5°, muscle loss, presence of inflammatory or metabolic disorders, obesity (Body mass index (BMI) >30 kg/m2) and age >55 years. Additional exclusion criteria for this study were MR contraindications and retropatellar CR. None of the patients received CR at two sites nor did any receive revision surgery during the observation period. The indication for ACL reconstruction was subacute, complete ACL rupture by clinically diagnosed anterior-posterior laxity (Lachman grades 2 to 3) with confirmation by MRI.
All procedures were performed by one surgeon. Mfx was used for a smaller lesions (<3cm2). For mostly larger defects, patients received OCT. The cartilage defect area was debrided until its edges were completely surrounded by healthy cartilage. Mfx surgery was performed during arthroscopy as described previously 2,47. The cartilage defects treated by OCT (OATS, Arthrex, Naples, FL, USA) were assessed arthroscopically and subsequently treated by an arthrotomy of the knee 2. Osteochondral grafts were harvested from the non-weight bearing non-articulating intercondylar notch region of the trochlea of the same knee during surgery. A mean number of 2.0 ±1.0 transplanted cylinders was used. ACL reconstruction was performed with single bundle hamstring or patellar tendon graft 22. During the postoperative period, weight bearing was limited to 15 kg for 6 weeks (3 weeks if only ACL reconstruction), and was gradually increased to reach full weight bearing after 8 – 12 weeks. Subjects underwent physiotherapy to strengthen the joint during follow-up.
Imaging
Standard standing anteroposterior plain radiographs of the knee were obtained in all subjects at baseline. All subjects were scanned with a 3.0T General Electric (GE) Signa HDx MR scanner (General Electric Healthcare, Milwaukee, WI, USA) using an 8-channel phased array transmit/receive knee coil (Invivo, Orlando, FL, USA). For semi-quantitative Whole-Organ Magnetic Resonance Imaging Score (WORMS) assessment 37, an intermediate-weighted (IW) fat-saturated FSE sequence (TR/TE =4300/51 ms, FOV = 14 cm, matrix =512×256, slice thickness =2.5 mm, gap = 0.5 mm) was used. Sagittal 3D T1ρ sequences were used to quantify the meniscus relaxation time 6,27,39. A spin-lock technique was followed by a SPGR acquisition using transient signals evolving towards steady state 26 with the following parameters: TR/TE =9.3/3.7 ms, time of recovery =1500 ms, FOV =14 cm, matrix =256 × 192, slice thickness =3 mm, BW = 31.25 kHz, views per segment =48, time of spin-lock (TSL) =0/10/40 ms, frequency of spin-lock (FSL) =500 Hz. Parallel imaging with array spatial sensitivity technique (ASSET) was performed with an acceleration factor of 2.
At the clinically important time points 4 months (4.0 ±1.1 months; 34/34 CR subjects, n=16 OCT, n=18 Mfx; 13/13 CR&ACL subjects, n=2 OCT, n=11 Mfx) and 1 year after CR (11.8 ±2.8 months; 21/34 CR subjects, n=10 OCT, n=11 Mfx; 8/13 CR&ACL subjects, n=2 OCT, n=6 Mfx), images were obtained. In 9/34 CR subjects (n=5 OCT, n=4 Mfx) and 5/13 CR&ACL subjects (n=2 OCT, n=3 Mfx) MR studies were obtained 2 years (24.6 ±1.2 months) after surgery. Control subjects without ACL reconstruction (n=34) were scanned at baseline, and after 1 and 2 years. Patients with only ACL reconstruction (n=13) were scanned 1 and 2 years after surgery (see Appendix).
Image analysis
Images were evaluated by two musculoskeletal radiologists separately (P.M.J., 4 years of experience; L.N., 6 years of experience); if scores were not identical consensus reading by both radiologists and another independent radiologist (T.M.L., 22 years of experience) was performed. Images were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ). Regarding plain knee radiographs, subjects presenting with a KL score of more than 2 were excluded from CR surgery and from this study. A UCSF modified WORMS system was used to assess morphological abnormalities as presented in Table 1 38. The medial and lateral menisci were separated into the following compartments: anterior horn, meniscal body, and posterior horn. For prevalence analysis, “no meniscus defect”, “simple tear” and “complex tear” were differentiated. For progression analysis, any increase of entire meniscus WORMS score was considered as “progression”. Controls were divided into subjects with or without morphological cartilage defect. Bone marrow lesions (BMLs) of the compartment with cartilage repair were graded according to the WORMS score and separated into BMLs ≤2cm (WORMS score ≤2) and BMLs >2cm (WORMS score 3).
Table 1.
Morphological meniscus (A) and cartilage (B) grading based on WORMS scoring with its according definitions.
| A: MENISCUS | |
|
WORMS Grade of Meniscus parts
Individually assessed for anterior horn, posterior horn, body of each meniscus |
Definition |
|
| |
| 0 | normal meniscus |
| 1 | intra-substance abnormalities |
| 2 | non-displaced meniscus tear |
| 3 | displaced or complex tear |
| 4 | complete meniscus destruction/ maceration |
|
WORMS entire Meniscus grade
Assessed for medial and lateral meniscus separatly |
Grade of Meniscus parts |
|
| |
| 0 | Grade 0 in all meniscus parts |
| 1 | No grade > 1 in any part |
| 2 | Grade 2 in 1 part |
| 3 | Grade 2 in >1 part |
| 4 | Grade 3 in 1 or more parts |
| 5 | Grade 4 in 1 part |
| 6 | Grade 4 in >1 part |
| Prevalence analysis | Definition |
|
| |
| No meniscus tear | entire meniscus grade <2 (no tear) |
| Simple meniscus tear | Entire meniscus grade =2 (non-displaced tear) |
| Complex meniscus tear | Entire meniscus grade >2 (complex tear) |
| Progression analysis | Definition |
|
| |
| No progression | No increase of the entire meniscus grade over time |
| Progression | Increase of the entire meniscus grade over time |
| B: CARTILAGE | |
| WORMS cartilage score | Definition |
|
| |
| 0 | No cartilage abnormality |
| 1 | Intrasubstance cartilage abnormalities |
| >1 | Morphological cartilae lesion with volume loss |
| Group | Definition |
|
| |
| No cartilage defect | WORMS 0 or 1 |
| Cartilage defect | WORMS >1 |
T1ρ sequences were transferred to a remote workstation (SPARC; Sun Microsystems, Mountain View, CA) and analyzed by using software developed at our institution with an interactive display language (IDL; Research Systems, Boulder, CO) environment. Segmentation of the anterior and posterior horn of the medial and lateral meniscus in every section was performed by one radiologist and supervised by a senior radiologist 39. Sagittal imaging precluded the meniscus body segmentation. T1ρ maps were reconstructed by fitting the T1ρ images pixel by pixel using a Levenberg Marquardt mono-exponential fitting algorithm developed in-house 53.
Reproducibility measurements
Reproducibility was calculated in a randomly selected sample of 10 image data sets for each compartment. For WORMS measurements, each subregion of the images was graded twice by two radiologists on two separate occasions. Linear weighted Cohens Kappa’s values were calculated. Inter-observer kappa was 0.89 for cartilage defects. Intra-observer kappa was 0.91 and 0.95. For bone marrow abnormalities, inter-observer kappa was 0.80, intra-observer kappa was 0.81 and 0.87. Inter-observer kappa was 0.80 for meniscus defects. Intra-observer kappa was 0.89 and 0.95. The mean coefficient of variation (CV, %), determined for T1ρ measurements of the meniscus in our laboratory was 4.1% 5,53.
Statistical analysis
Mean T1ρ values were calculated for both menisci and globally (mean of the value for medial and lateral meniscus) from the segmented regions of interest. Statistical processing was performed with JMP software Version 9 (SAS Institute, Cary, NC, USA). Statistics were obtained applying multivariate regression models, that adjusted in one model for KL score, gender and age, by adding these variables as covariates for each of the analyses. For T1ρ values measurements, one-way analysis of variance (ANOVA) and two-way Students t-test were applied. For morphological analysis Mann-Whitney-U test was used. Results were considered as significantly different if P<0.05. Mean values are presented ± Standard Error of the Mean (SEM), if not stated otherwise.
RESULTS
Subject characteristics
Of a total of 94 subjects in this study, 34 subjects were treated with only CR (21 male, 13 female), 13 with CR and ACL reconstruction, and 13 only with ACL reconstruction, respectively. The CR and control group (n=34) were gender and KL score matched (Table 2). Age was significantly different between the CR and control group (35 ±11 (Standard deviation (SD)) years versus 47 ±11 (SD) years). CR was performed 20/34 times at the medial femoral condyle, 10/34 times at the lateral femoral condyle and 4/34 times at the trochlea (only Mfx). Screening controls for cartilage defects revealed that 13/34 control subjects presented cartilage defects (medial 7/34; lateral 6/34) at baseline.
Table 2.
Epidemiological data of the analyzed groups. SD = Standard deviation; KL = Kellgren / Lawrence; ACL = Anterior cruciate ligament; CR = Cartilage Repair.
| Parameter | Overall | Controls | CR | ACL | ACL&CR |
|---|---|---|---|---|---|
| N (total Number of patients) | 94 | 34 | 34 | 13 | 13 |
| Gender (male : female) | 53 : 41 | 19 : 15 | 21 : 13 | 6 : 7 | 7 : 6 |
| Age ±SD (years) | 40 ±12 | 47 ±11 | 35 ±11 | 37 ±9 | 32 ±11 |
| Side (right : left) | 56 : 38 | 20 : 14 | 20 : 14 | 5 : 8 | 11 : 2 |
| KL score (0:1:2) | 29 : 52 : 13 | 11 : 18 : 5 | 9 : 22 : 3 | 4 : 8 : 1 | 5 : 4 : 4 |
Baseline meniscus T1ρ analysis
At baseline, patients without surgery (control group) presented the lowest T1ρ values (global T1ρ: 12.8 ±0.6 ms; Table 3; Figure 1). Considering both menisci separately, the medial meniscus showed slightly higher values than the lateral meniscus in controls at baseline (13.1 ±0.6 ms versus 12.5 ±0.6 ms, P=0.073). The CR group showed significantly higher global T1ρ value of 14.2 ±0.5 ms 4 months after surgery (P=0.004 versus controls). The CR&ACL group showed the highest T1ρ values 4 months after surgery (17.1 ±0.9 ms; P<0.001 versus controls).
Table 3.
Global meniscus T1ρ relaxation time values ± SEM (ms) after cartilage repair (CR), anterior cruciate ligament reconstruction and CR (CR&ACL) versus control without surgery (P overall (ANOVA), P=0.002).
| Surgery | n | Global T1ρ | P (verus “No surgery”) |
|---|---|---|---|
| No surgery | 34 | 12.8 ±0.6 | |
| CR | 34 | 14.2 ±0.5 | 0.004* |
| CR&ACL | 13 | 17.1 ±0.9 | <0.001* |
Figure 1.
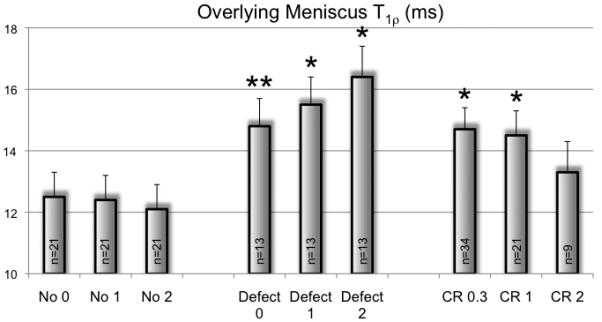
Global meniscus T1ρ values 0.3, 1 and 2 years after cartilage repair (CR) compared to subjects 0.3, 1 and 2 years after combined CR and anterior cruciate ligament reconstruction (CR&ACL) and to controls at baseline and after 1 and 2 years. *P<0.05, compared to the control group at the according timepoint.
Comparing T1ρ values of the overlying meniscus above the cartilage repair regions with the overlying meniscus above untreated cartilage defect regions in the control subgroup with cartilage defects at baseline, no significant difference was detected at baseline (14.7 ±0.7 ms versus 14.8 ±0.9 ms; Figure 2). However, differences with a statistical trend were found between the control subgroup with cartilage defects and the global meniscus T1ρ of the control subgroup without cartilage defects (12.5 ±0.8 ms; P=0.055) and a significant difference was found between the CR group and the control subgroup without cartilage defects (P=0.001). In contrast, T1ρ, for each of the four separately segmented meniscus parts, the difference between the CR group and the control group was not significant at baseline, due to different CR locations.
Figure 2.
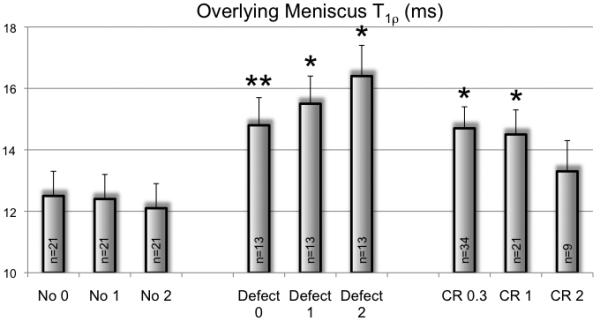
Global meniscus T1ρ values of the control subgroup without cartilage defect at baseline and after 1 and 2 years, compared to meniscus T1ρ at the overlying meniscus above the cartilage defect in the control subgroup with cartilage defect (Defect) and compared to meniscus T1ρ at the overlying meniscus above the cartilage repair region in the cartilage repair group (CR) 0.3, 1 and 2 years after surgery. *P<0.05, compared to the control subgroup without cartilage defect (No) at the according timepoint. **=0.055.
Meniscus T1ρ at follow-up
Global Meniscus T1ρ in the CR group did not increase in the first (14.1 ±0.7 ms) and second year (13.2 ±0.9 ms) after surgery (Figure 1). Global T1ρ values in the control group were stable during follow-up with 13.0 ±0.6 ms after 1 year and 13.1 ±0.6 ms after 2 years. The CR&ACL group did not show a further increase over time, but still showed a significant difference to the control group after 2 years (P=0.037).
In the subgroup of controls without cartilage defects (n=21), global T1ρ values were stable over time and there was no significant difference to T1ρ of the overlying meniscus above the cartilage repair region (n=9) after two years (12.1 ±0.8 ms versus 13.3 ±1.0 ms; P=0.112; Figure 2). However, there was a statistical trend for a difference between T1ρ of the overlying meniscus above the cartilage repair region and T1ρ of the overlying meniscus above the untreated cartilage defect (16.4 ±1.0 ms) after two years (P=0.088) and a statistical difference between T1ρ of the overlying meniscus above the untreated cartilage defect and global T1ρ of the control subgroups without cartilage defects after two years (P<0.001).
Absolute T1ρ progression was also calculated between the different timepoints and showed the same trend, but no statistically significant difference (P>0.05).
Comparison of Mfx and OCT in the CR group
Subjects with Mfx (n=18) showed higher T1ρ at the overlying meniscus than OCT subjects (n=16; Figure 3). T1ρ decreased at the 1 and 2 year time-point in subjects with Mfx. In subjects with OCT T1ρ only decreased in the second year of follow-up. However, these results did not show any significant difference.
Figure 3.
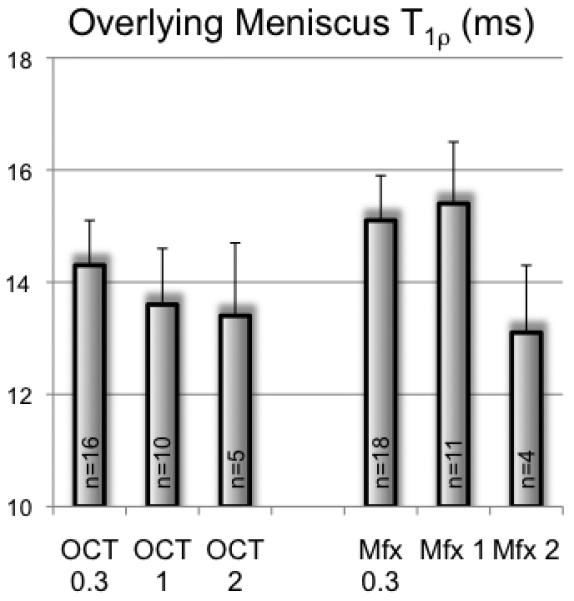
T1ρ values of the overlying meniscus above the repair region in subjects with osteochondral transplantation (OCT) and subjects with microfracture procedure (Mfx) 0.3, 1 and 2 years after surgery. Differences between the groups were not significant (P>0.05).
Correlation of bone marrow lesions with meniscus T1ρ
At baseline, CR subjects with BMLs ≤2 cm (correlating with a WORMS score ≤2) showed slightly higher T1ρ values at the overlying meniscus (15.5 ±1.3 ms; 14/34) than CR subjects with BMLs >2cm (14.1 ±1.0 ms; 20/34; P=0.088). However, after two years BMLs ≤2 cm (n=4) were associated with lower meniscus T1ρ values than BMLs >2cm (n=5; 12.1 ±1.8 ms versus 14.5 ±2.1 ms; P=0.095). Presence of large BMLs 1 year after CR was not significantly associated with meniscus T1ρ after 2 years (P=0.203).
ACL reconstruction
Individuals with only ACL reconstruction (ACL group, n=13) had a global meniscus T1ρ value of 14.3 ±0.8 ms 1 year after surgery (P=0.031 versus control group). The medial meniscus showed lower T1ρ values than the lateral meniscus (13.8 ±0.8 ms versus 14.6 ±0.8 ms, P=0.204). In individuals with only ACL reconstruction, the medial and lateral meniscus both showed higher T1ρ values at the 2 year follow-up time-point compared to the 1 year time-point (medial: 15.7 ±1.0 ms; P=0.027; lateral: 15.7 ±0.9; P>0.05). The absolute difference of the T1ρ values between the two time-points was higher in the medial meniscus. At the 2 year time-point, global meniscus T1ρ in the ACL group (n=13) was significantly higher than in the CR group (n=34; P=0.009).
Morphological meniscus lesions at baseline
At baseline 15 % (5/34) and 20 % (7/34) of the CR group presented with simple and complex morphological meniscus tears, respectively, at the overlying meniscus (Figure 4 and Table 4). The control subgroup without cartilage defects (21/34) showed significantly less meniscus tears (0 % (0/21) simple tears; 5 % (1/21) complex tears; P=0.001). The control subgroup with untreated cartilage defects (13/34; 15 % (2/13) simple tears; 15 % (2/13) complex tears) showed no significant difference of meniscus tears to the CR group (P=0.838), but significantly more meniscus tears than the control subgroup without cartilage defects (P=0.006). The CR&ACL group showed more meniscus tears than all other groups (medial meniscus 92 % (12/13); lateral meniscus 69 % (9/13)). Subjects in the ACL group (only ACL reconstruction) had medial meniscus tears in 35 % (5/13) and lateral meniscus tears in 31 % (4/13)) at 1 year after surgery.
Figure 4.
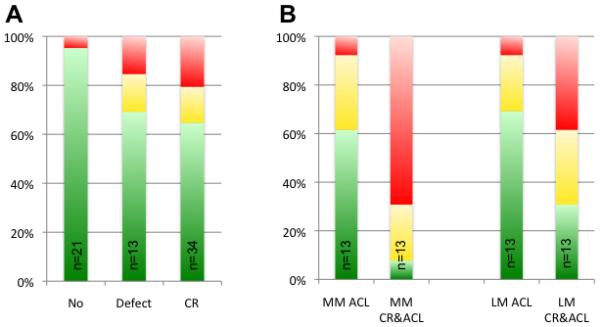
Prevalence of morphological meniscus lesions. Green color indicates no meniscus lesion, yellow simple and red complex meniscus lesions. A: Lesions at the overlying meniscus above cartilage repair regions in the cartilage repair group (CR) and above cartilage defects in the control subgroup with cartilage defect (Defect) were compared to the control subgroup without cartilage defect (No). B: Lesions at the medial (MM) and lateral meniscus (LM) in the group with reconstruction of the anterior cruciate ligament (ACL; 1 year after surgery) and the group with combined CR and ACL surgery (CR&ACL), respectively.
Table 4.
Incidence of morphological meniscus defects in different groups. In the cartilage repair (CR) group, the overlying meniscus above the cartilage repair region was analyzed. In the control subcohort with cartilage defect, the overlying meniscus above the defect was analyzed. In the control subcohort without cartilage defect, the meniscus with the higher WORMS score was considered.
| Group | n | No defect | Simple tear | Complex tear |
|---|---|---|---|---|
| Cartilage Repair | 34 | 65 % | 15 % | 20 % |
| No surgery (all controls) | 34 | 85 % | 6 % | 9 % |
| Control subgroup with cartilage defects | 13/34 | 70 % | 15 % | 15 % |
| Conrol subgroup without cartilage defect | 21/34 | 95 % | 0 % | 5 % |
Progression of morphological meniscus lesions
Any increase in the entire WORMS score was considered as progression of morphological meniscus lesions (Table 5). During follow-up, 10 % of the CR group (2/10) showed an increase at the overlying meniscus in the first postoperative year; none progressed in the second postoperative year (0/9). Within the subgroup of control subjects without cartilage defects 19 % (4/21) increased in the first, 10 % (2/21) in the second year. Within the subgroup with untreated cartilage defects 38 % (5/13) increased in the first, 15 % (2/13) in the second year. The differences between the groups were however not significant (P>0.05). In the CR&ACL group, 14 % and 29 % of subjects showed an increase at the medial and lateral meniscus, respectively, within the first postoperative year. Within the second year, 20 % showed an increase at the medial and also 20 % at the lateral meniscus. An increase of morphological meniscus lesions was found in 19 % of subjects with ACL surgery between the first and second postoperative year.
Table 5.
Morphological meniscus changes measured by WORMS scoring between two time-points (in years). Percentage of subjects, which show an increase in the entire meniscus grading for the overlying meniscus above the cartilage repair region or the cartilage defect region, respectively. In controls without cartilage defect any increase of either meniscus was considered as progression.
| Group; time span (years) | N | Progression |
|---|---|---|
| CR; 0-1 | 21 | 10 % |
| CR; 1-2 | 9 | 0 % |
| Control; 0-1 | 34 | 26 % |
| Control; 1-2 | 34 | 12 % |
| Control subgroup with defect; 0-1 | 13 | 38 % |
| Control subgroup with defect; 1-2 | 13 | 19 % |
| Control subgroup without defect; 0-1 | 21 | 15 % |
| Control subgroup without defect; 1-2 | 21 | 10 % |
DISCUSSION
This longitudinal study analyzed meniscus degeneration as an outcome parameter after cartilage reconstruction procedures. Noninvasive MRI is used for monitoring the post-operative course of these patients to ensure quality control and development of future treatment guidelines. In this study, evaluation of the menisci was performed semi-quantitatively by morphological analysis (WORMS) and quantitatively by meniscus T1ρ relaxation time measurements at several time-points during a two-year 3.0T MRI follow-up. Four months after surgery, patients with CR and ACL reconstruction had a significantly higher meniscus T1ρ than controls, while T1ρ was the highest in subjects who received both surgeries. During follow-up, individuals with only ACL reconstruction and controls with cartilage defects showed a further increase in T1ρ values, while T1ρ values in CR subjects did not (Figure 5). Being aware of the methodological limitations, these findings suggest that individuals with CR surgery may benefit from this procedure as it appears to prevent meniscus degeneration and potentially early onset of OA.
Figure 5.
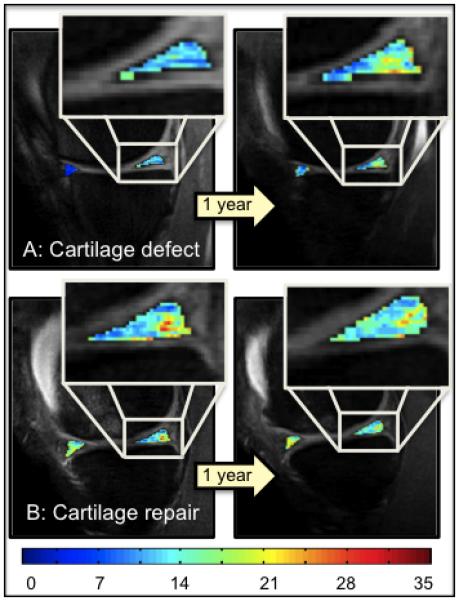
T1ρ color maps of the anterior and posterior horn of the medial meniscus of 1 year and 2 year follow-up time-points, overlaid with the first-echo images. Superior: Control subject with cartilage defect at the medial femoral condyle, who did not receive a cartilage repair (CR) procedure. Inferior: CR subject with osteochondral transplantation at the medial femoral condyle. Blue color indicates low, red color high meniscus T1ρ values. Subjects with untreated cartilage lesion showed a higher increase in T1ρ values over time compared to the subject with CR.
Apart from autologous chondrocyte implantation 16,34, Mfx and OCT are two alternatively applied CR procedures 2,41. However, the outcome after these procedures with respect to prevention of further degenerative changes and early onset of OA of the knee is unclear. Most studies have evaluated clinical outcomes, and few have considered utilizing MRI as follow-up 4,48, 50. However, meniscus evaluation remains an important parameter with respect to evaluation of progression of early and advanced OA 45. In fact, previous studies have used meniscus evaluation to assess the risk of OA as an outcome after surgery, particularly ACL reconstruction 19,51. By evaluating the menisci, we confirmed previously reported findings that there exists an increased presence of more simple and complex meniscus defects in individuals with ACL and CR surgery 10, 30. Cartilage defects usually coincide with degeneration of the overlying meniscus as confirmed in our study 45; individuals with cartilage defects or individuals after repair of cartilage defects presented more meniscus lesions. Additionally, in our study we detected higher meniscus T1ρ values in individuals with CR, ACL reconstruction and untreated cartilage defects. Meniscus degeneration can be quantified and continuously monitored by T1ρ mapping. T1ρ has recently been used for non-invasive biochemical analysis of not only cartilage, but also for detection and monitoring of meniscus degeneration 9, 39. T1ρ measurements of cartilage repair areas have been performed and results have been reported previously 14. Studies have shown that cartilage degeneration correlates with meniscus degeneration 53. Even though T2 relaxation time remains the more widely used technique in biochemically assessing cartilage integrity, studies have shown that T1ρ relaxation time better correlates with cartilage pathology 36, 42. For meniscus evaluation both T2 and T1ρ measurements appear to be useful 53. Rauscher et al found that high meniscus T1ρ values were associated with osteoarthritic knees 39. In our current study, we found that subjects who underwent CR procedures showed no further increase of T1ρ values in the meniscus over time. Consequently, if meniscus T1ρ correlates with cartilage degeneration, it suggests that CR surgery may halt the progression of intrameniscal degeneration and further joint degeneration.
Follow-up time-points at 4 months, 1 year and 2 years post CR surgery were chosen due to their clinical relevance. Most failures occur during the early post-operative period 31,49. The healing process, involving cell proliferation, matrix production and matrix remodeling is especially crucial within the first post-operative year 17. This outcome is also reflected by meniscus T1ρ values: These show a particular improvement not within the first, but within the second year. On the contrary, clinically, many patients only report a benefit of the intervention for two years 32,44. Although OCT is a more invasive, open procedure including arthrotomy of the knee joint, clinically, a better outcome up to 3 years after surgery was reported for OCT compared to Mfx 12. Interestingly, for OCT, we found a slight decrease of T1ρ values already within the first year of follow-up, while Mfx only lead to a decrease of T1ρ values within the second year of follow-up. This finding is concordant with clinical findings 23. MRI has been found to correlate with clinical outcome after Mfx 32.
Although there is no definite correlation between CR histology and clinical outcome, there is evidence that in patients with good histological results, there is less therapy failure 21. In case of OCT, preexisting cartilage is transplanted. This technique showed an improvement in MR findings between 4 months an 1 year postoperatively 28. However, some histological changes to fibrous cartilage and modification of the repair area have been observed. In the case of CR procedures, which involve bone marrow stimulation, meniscus status improved over time. This is consistent with the literature, since a technique of bone marrow stimulation has been described, which allows a better healing of meniscus after meniscus repair 11.
BMLs did not correlate with meniscus T1ρ 4 months or 1 year after surgery. However persisting huge BMLs 2 years after surgery showed a trend for a statistical correlation with a higher meniscus T1ρ. This supports the presumption, that a persisting BMLs is associated with outcome after CR 52. However, BMLs after 1 year did not predict 2 year meniscus T1ρ.
Meniscus integrity is crucial for proper knee joint functioning and shock-absorption. An influence of meniscus lesions on further OA progression has been observed 33. Meniscus defects and OA have also been associated with higher and less homogenous cartilage T2 relaxation time values 15. Our study showed that patients with CR demonstrated improved meniscus T1ρ values over time, which suggests that CR plays a role in halting OA progression as a postoperative outcome. In this context, particularly the findings regarding control individuals with and without morphological cartilage defects seem important. Individuals with untreated morphological cartilage defects had higher T1ρ values at follow-up time points than at baseline, while controls without defects and CR subjects did not.
Meniscus T1ρ in subjects with only ACL reconstruction (ACL group) increased over time. It is known, that although reconstructed, kinematics may not be completely restored. Anterior-posterior tibial translation is usually well restored, while rotational stability was observed to be still pathological after ACL reconstruction 13,46. Therefore ACL reconstructed subjects still suffer from increased degeneration of the knee as detected by T1ρ in our study. In the ACL group, 1 year after surgery in particular the lateral meniscus showed higher T1ρ values, which is consistent with previous T 53 1ρ findings, as well as clinical MR findings that result from the kissing bone bruise. However, during the second year post ACL reconstruction, T1ρ showed a larger increase at the medial meniscus than at the lateral meniscus, indicating a faster degeneration of the medial meniscus after ACL reconstruction, probably due to rotational instability 3. In control patients, the medial meniscus showed slightly higher T1ρ values and more morphological meniscus lesions, which is consistent with prior studies 45. We found that patients with combined ACL and CR procedures showed the highest T1ρ values, with slightly lower values at follow-up. This supports previously reported findings of reasonable outcomes for combined surgery 7.
There are several limitations of this present study. First, not all patients came back for 1 and 2 year follow-up. The low follow-up was due to a young patient clientele with high mobility, who are leaving the area and are not able to present for follow-up visits, as well as limited scan times at our institution and missing out or not reaching the right follow-up time-point. Second, age has been significantly different between both groups; since it is known as one of the most important risk factors for OA, results were adjusted for this parameter. Third, hamstring and patellar tendon grafts (bone-tendon-bone, BTB) were not differentiated, since the focus of this study was on CR and the number of subjects in each group would have been too small for further analysis. In the ACL group 9 Pat received BTB grafts and 4 patients hamstring grafts; in the CR&ACL group 4 patients received BTB grafts and 9 patients hamstring grafts. It may be interesting to evaluate the influence of different techniques in future studies. Fourth, results were not adjusted for lower limb alignment, which could potentially also slightly influence the results. However, subjects with an axis deviation >5° were excluded. Last, only one clinical sequence was used, due to scan-time limitations.
In conclusion, in this study we used 3.0T MRI meniscus T1ρ relaxation time measurements along with morphological meniscus assessment in a cross-sectional and two-year longitudinal analysis of individuals who underwent cartilage resurfacing procedures and compared the findings to normal controls. Meniscus T1ρ values were higher in individuals with CR or cartilage defects at baseline compared to individuals without defects. While T1ρ did not increase at the follow-up time-points in CR patients, increasing T1ρ values were detected in patients with untreated cartilage defects or ACL reconstruction. Morphological meniscus defects showed a lower progression during the second year of follow-up after CR, compared to controls. These results suggest, that individuals with focal cartilage defects may benefit from cartilage repair procedures with regard to prevention of further meniscus matrix degeneration and consequently prevention of early OA at the knee.
What is known about the subject
Although cartilage repair procedures are widely applied for localized cartilage defects, and multiple studies have examined results on a descriptive level, a challenge remains to demonstrate that CR can prevent joint degeneration, by clinical validating imaging outcomes. Noninvasive MRI is the most important diagnostic tool for monitoring the post-operative course of these patients. Since few studies have used MRI for follow-up, little is known about meniscus degeneration as an outcome parameter after CR. However, evaluation of the impact of meniscus degeneration is crucial to ensure quality control and the development of future treatment guidelines in patients post CR.
What this study adds to existing knowledge
The longitudinal study represents a qualitative (WORMS) and quantitative (T1ρ) 3.0T MRI meniscus analysis of 94 individuals of whom 34 underwent cartilage repair procedures for isolated cartilage defects of the knee, 34 were Kellgren-Lawrence score and gender matched controls. Additionally, 13 subjects with ACL reconstruction and 13 subjects with both, ACL reconstruction and cartilage repair surgery were analyzed. We were able to identify higher meniscus T1ρ values and more severe morphological meniscus lesions at four months after CR surgery compared to controls. However, in CR patients progression of T1ρ values was not observed from 1 to 2 years after surgery, while in control subjects with cartilage defects T1ρ values increased significantly. These results suggest, that individuals with focal cartilage defects may benefit from cartilage repair procedures with regard to prevention of further meniscus matrix degeneration and consequently prevention of early OA at the knee.
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health grant R01 AR46905, K25 AR053633, P50 AR060752 and UO1 AR059507 and the OAI (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262).
Appendix
N numbers of subjects included in this study. Four different groups were analyzed. The cartilage repair group (CR) as well as the group that had CR and reconstruction of the anterior cruciate ligament (CR&ACL) contained a subgroup that received osteochondral transplantation (OCT) and a subgroup that received microfracture (Mfx). The control group was subdivided into a subgroup with untreated morphological cartilage defects and a subgroup without cartilage defects. The group with only reconstruction of the anterior cruciate ligament (ACL) had no subgroups. Evaluated time-points were (1) baseline (controls) or 0.3 years post surgery (CR, CR&ACL group), respectively, (2) 1 year follow-up and (3) 2 year follow-up.
| Group | Subgroup | N (baseline/ 0.3 years) |
n (1 year) | n (2 year) |
|---|---|---|---|---|
| CR | All CR subjects | 34 | 21 | 9 |
| Subgroup OCT | 16 | 10 | 5 | |
| Subgroup Mfx | 18 | 11 | 4 | |
| Controls | All control subjects | 34 | 34 | 34 |
| With cartilage defects | 13 | 13 | 13 | |
| Without cartilage defects | 21 | 21 | 21 | |
| CR&ACL | All CR&ACL subjects | 13 | 8 | 5 |
| CR&ACL subgroup OCT | 2 | 2 | 2 | |
| CR&ACL subgroup Mfx | 11 | 6 | 3 | |
| ACL | All ACL subjects | 0 | 13 | 13 |
Footnotes
CONFLICT OF INTEREST
No conflict of interest for any authors.
REFERENCES
- 1.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rhorelaxation of articular cartilage at 4T. Magn Reson Med. 2001;46(3):419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 3.Bellabarba C, Bush-Joseph CA, Bach BR., Jr. Patterns of meniscal injury in the anterior cruciate-deficient knee: a review of the literature. Am J Orthop (Belle Mead NJ) 1997;26(1):18–23. [PubMed] [Google Scholar]
- 4.Benthien JP, Schwaninger M, Behrens P. We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):543–552. doi: 10.1007/s00167-010-1271-5. [DOI] [PubMed] [Google Scholar]
- 5.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43(11):782–788. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 7.Brophy RH, Zeltser D, Wright RW, Flanigan D. Anterior cruciate ligament reconstruction and concomitant articular cartilage injury: incidence and treatment. Arthroscopy. 2010;26(1):112–120. doi: 10.1016/j.arthro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am. 2011;19(2):249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr J, Rawal A, Marberry KM. Concomitant meniscal allograft transplantation and autologous chondrocyte implantation: minimum 2-year follow-up. Am J Sports Med. 2007;35(9):1459–1466. doi: 10.1177/0363546507301257. [DOI] [PubMed] [Google Scholar]
- 11.Freedman KB, Nho SJ, Cole BJ. Marrow stimulating technique to augment meniscus repair. Arthroscopy. 2003;19(7):794–798. doi: 10.1016/s0749-8063(03)00695-9. [DOI] [PubMed] [Google Scholar]
- 12.Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834–842. doi: 10.1007/s00167-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 13.Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. Knee. 2011 doi: 10.1016/j.knee.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzman DJ, Theologis AA, Carballido-Gamio J, Majumdar S, Li X, Benjamin C. T(1rho) and T(2) quantitative magnetic resonance imaging analysis of cartilage regeneration following microfracture and mosaicplasty cartilage resurfacing procedures. J Magn Reson Imaging. 2010;32(4):914–923. doi: 10.1002/jmri.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph GB, Baum T, Carballido-Gamio J, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls - data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13(5):R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P. Autologous Chondrocyte Implantation for Treatment of Cartilage Defects of the Knee: What Predicts the Need for Reintervention? Am J Sports Med. 2011 doi: 10.1177/0363546511423522. [DOI] [PubMed] [Google Scholar]
- 17.Kalson NS, Gikas PD, Briggs TW. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64(10):1444–1452. doi: 10.1111/j.1742-1241.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 18.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 20.Kijowski R, Blankenbaker DG, Davis KW, Shinki K, Kaplan LD, De Smet AA. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250(3):839–848. doi: 10.1148/radiol.2503080822. [DOI] [PubMed] [Google Scholar]
- 21.Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 22.Kothari A, Haughom B, Subburaj K, Feeley B, Li X, Ma CB. Evaluating rotational kinematics of the knee in ACL reconstructed patients using 3.0Tesla magnetic resonance imaging. Knee. 2012 doi: 10.1016/j.knee.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of fullthickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Cheng J, Lin K, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Han ET, Newitt D, Majumdar S. T1rho relaxation quantification using spiral imaging: a preliminary study. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1032–1035. doi: 10.1109/IEMBS.2004.1403339. [DOI] [PubMed] [Google Scholar]
- 28.Link TM, Mischung J, Wortler K, Burkart A, Rummeny EJ, Imhoff AB. Normal and pathological MR findings in osteochondral autografts with longitudinal follow-up. Eur Radiol. 2006;16(1):88–96. doi: 10.1007/s00330-005-2818-6. [DOI] [PubMed] [Google Scholar]
- 29.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17(5):1135–1146. doi: 10.1007/s00330-006-0453-5. [DOI] [PubMed] [Google Scholar]
- 30.Magnussen RA, Mansour AA, Carey JL, Spindler KP. Meniscus status at anterior cruciate ligament reconstruction associated with radiographic signs of osteoarthritis at 5- to 10-year follow-up: a systematic review. J Knee Surg. 2009;22(4):347–357. doi: 10.1055/s-0030-1247773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17(1):13–17. doi: 10.1055/s-0030-1247141. [DOI] [PubMed] [Google Scholar]
- 32.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidencebased systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 33.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19(11):1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Niemeyer P, Kostler W, Salzmann GM, Lenz P, Kreuz PC, Sudkamp NP. Autologous chondrocyte implantation for treatment of focal cartilage defects in patients age 40 years and older: A matched-pair analysis with 2-year follow-up. Am J Sports Med. 2010;38(12):2410–2416. doi: 10.1177/0363546510376742. [DOI] [PubMed] [Google Scholar]
- 35.Niemeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36(11):2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 36.Oneto JM, Ellermann J, LaPrade RF. Longitudinal evaluation of cartilage repair tissue after microfracture using T2-mapping: a case report with arthroscopic and MRI correlation. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1545–1550. doi: 10.1007/s00167-010-1161-x. [DOI] [PubMed] [Google Scholar]
- 37.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauscher I, Stahl R, Cheng J, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 41.Salzmann GM, Niemeyer P, Steinwachs M, Kreuz PC, Sudkamp NP, Mayr HO. Cartilage repair approach and treatment characteristics across the knee joint: a European survey. Arch Orthop Trauma Surg. 2011;131(3):283–291. doi: 10.1007/s00402-010-1047-x. [DOI] [PubMed] [Google Scholar]
- 42.Salzmann GM, Paul J, Bauer JS, et al. T2 assessment and clinical outcome following autologous matrix-assisted chondrocyte and osteochondral autograft transplantation. Osteoarthritis Cartilage. 2009;17(12):1576–1582. doi: 10.1016/j.joca.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Sanders TG, Mentzer KD, Miller MD, Morrison WB, Campbell SE, Penrod BJ. Autogenous osteochondral “plug” transfer for the treatment of focal chondral defects: postoperative MR appearance with clinical correlation. Skeletal Radiol. 2001;30(10):570–578. doi: 10.1007/s002560100371. [DOI] [PubMed] [Google Scholar]
- 44.Solheim E, Hegna J, Oyen J, Austgulen OK, Harlem T, Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17(1):84–87. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 47.Theologis AA, Schairer WW, Carballido-Gamio J, Majumdar S, Li X, Ma CB. Longitudinal analysis of T(1rho) and T(2) quantitative MRI of knee cartilage laminar organization following microfracture surgery. Knee. 2011 doi: 10.1016/j.knee.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee--a review. Eur Radiol. 2009;19(7):1582–1594. doi: 10.1007/s00330-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 49.Trattnig S, Millington SA, Szomolanyi P, Marlovits S. MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol. 2007;17(1):103–118. doi: 10.1007/s00330-006-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: A long-term follow-up study. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 51.Wegrzyn J, Chouteau J, Philippot R, Fessy MH, Moyen B. Repeat revision of anterior cruciate ligament reconstruction: a retrospective review of management and outcome of 10 patients with an average 3-year follow-up. Am J Sports Med. 2009;37(4):776–785. doi: 10.1177/0363546508330141. [DOI] [PubMed] [Google Scholar]
- 52.Welsch GH, Zak L, Mamisch TC, Resinger C, Marlovits S, Trattnig S. Three-dimensional magnetic resonance observation of cartilage repair tissue (MOCART) score assessed with an isotropic three-dimensional true fast imaging with steadystate precession sequence at 3.0 Tesla. Invest Radiol. 2009;44(9):603–612. doi: 10.1097/RLI.0b013e3181b5333c. [DOI] [PubMed] [Google Scholar]
- 53.Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18(11):1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


