Abstract
Loss of cardiomyocytes plays a critical role in the pathogenesis of heart failure. With fewer myocytes, the heart is unable to sustain efficient contraction. Much attention has been focused on understanding mechanisms of cell death in myocytes with the ultimate goal being to reduce the extent of injury and improve function in the failing myocardium. Both necrosis and apoptosis contribute to loss of myocytes, and this loss of cells is a hallmark of cardiac pathologies, including ischemia/reperfusion, myocardial infarction, and heart failure. Apoptosis is a highly regulated process that is activated via death receptors in the plasma membrane or via permeabilization of the mitochondria. Necrosis is generally viewed as an uncontrolled process that leads to mitochondrial swelling, cell rupture, and subsequent inflammation. However, recent studies have uncovered a signaling pathway that mediate regulated necrosis, or necroptosis. Mitochondria play an important role in both apoptosis and necrosis, and changes in their morphology can affect the cells’ susceptibility to stress. This review focuses on the various modes of cell death in the myocardium and highlights how they contribute to loss of myocytes in response to stress.
Introduction
The myocardium is comprised of terminally differentiated cardiomyocytes that are responsible for contractile function. Thus, the heart exhibits more pronounced pathological effects as a consequence of excessive cell death. Because the heart has a limited capacity to efficiently regenerate, it is important to preserve existing cardiac mass to support proper function. Maintenance of cardiac homeostasis depends on cardiomyocyte death and renewal, and excessive loss of cardiomyocytes has been implicated in many cardiovascular diseases, such as myocardial ischemia/reperfusion (I/R) (1) and congestive heart failure (2). Loss of cells in the heart occurs primarily via apoptosis and necrosis. Studies have shown that both acute substantial cardiomyocyte loss and chronic low levels of apoptosis can contribute to the development of heart failure (3). Necrotic cell death due to impaired Ca2+ regulation has also been implicated in cardiac dysfunction and progression to heart failure (4). Proteins involved in regulating mitochondrial morphology have also been connected to both apoptotic and necrotic death. Much attention has been focused on understanding mechanisms of cell death in order to reduce the extent of injury and improve function in the failing myocardium. This review focuses on the various modes of cell death in the myocardium and how they contribute to loss of myocytes in response to stress.
Apoptosis
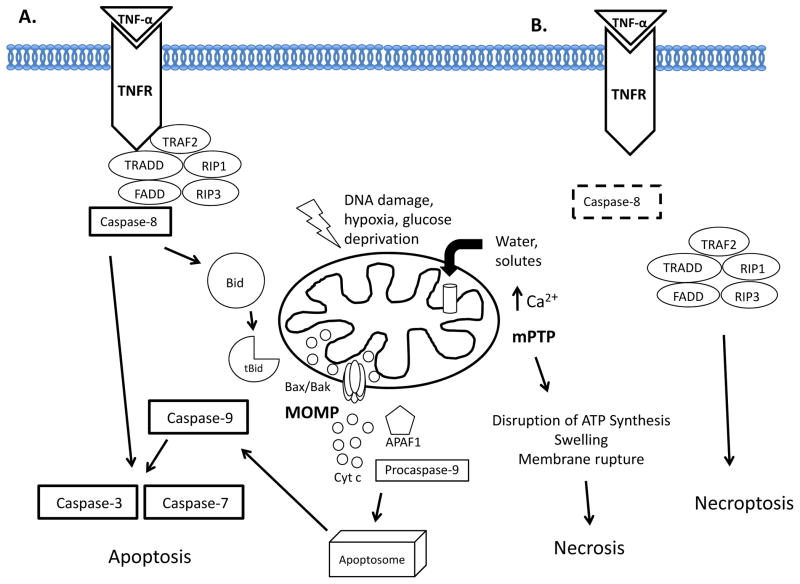
Apoptosis is a highly regulated, evolutionarily conserved, and energy-requiring process by which activation of specific signaling cascades ultimately leads to cell death. An apoptotic cell undergoes structural changes including cell shrinkage, plasma membrane blebbing, nuclear condensation, and fragmentation of the DNA and nucleus. This is followed by fragmentation into apoptotic bodies that are quickly removed by phagocytes, thereby preventing an inflammatory response (5). Two well characterized apoptotic pathways exist in cells: the extrinsic death receptor pathway and intrinsic mitochondrial pathway. These two pathways have distinct modes of activation but converge downstream at the level of effector caspases (Figure 1).
Figure 1.
Cell Death Pathways. A. Apoptosis. In the extrinsic death receptor pathway, binding of an extracellular ligand such as TNF-α to its death receptor TNFR recruits adaptor molecules to form the death-inducing signaling complex (DISC). Caspase-8 cleaves and activates effector caspase-3 and -7. In the intrinsic mitochondrial pathway, intracellular stress signals such as DNA damage, hypoxia, or glucose deprivation activate the pro-apoptotic Bax and Bak. During mitochondrial outer membrane permeabilization, Bax and Bak form pores that allow the release of apoptogens such as cytochrome c into the cytosol. Cytochrome c and APAF1 form the apoptosome which recruits and activates caspase-9 and leads to caspase-3 and -7 activation. Death-receptor mediated caspase-8 activation can also cleave Bid to form truncated Bid (tBid) and activate the intrinsic pathway. B. Necrosis/Necroptosis. Excessive influx of Ca2+ into the mitochondria leads to opening of the mitochondrial permeability transition pore (mPTP) and disruption of the proton gradient and ATP synthesis. The influx of water and solutes into mitochondria leads to swelling and membrane rupture. Regulated necrosis, or necroptosis, occurs in the absence of caspase-3 where the RIP1-RIP3 complex facilitates necroptosis.
Extrinsic Death Receptor Pathway
In the extrinsic pathway, cell death is induced via activation of a death domain-containing receptor located at the plasma membrane. Binding of a ligand, such as FasL or TNF-α, to its death receptor (e.g. Fas Receptor, TNF Receptor [TNFR]), promotes the formation of the death-inducing complex (DISC). The DISC is composed of multiple proteins including adaptor molecules such as the Fas-associated death domain (FADD) or TNF-receptor-associated death domain (TRADD), receptor-interacting protein 1 (RIP1) and caspase-8. Caspase-8 subsequently activates the downstream effector caspases 3 and -7, which are responsible for proteolytic cleavage of critical proteins in the cell (5) (Figure 1A). Studies have demonstrated that death receptor ligands and subsequent activation of their receptors are involved in the development of heart failure. For instance, TNF-α is rapidly released from resident mast cells and macrophages in response to myocardial ischemia (6). TNF-α can also be synthesized and released from cardiomyocytes (7). Moreover, transgenic mice with cardiac-specific overexpression of TNF-α develop dilated cardiomyopathy (8). Activation of the Fas/FasL pathway through adenoviral FasL overexpression increases levels of apoptosis in neonatal cardiomyocytes in vitro, and in adult rat cardiomyocytes in vivo. Similarly, Fas-deficient lpr mice also exhibit reduced infarcts and less apoptosis after myocardial I/R (9). Thus, these studies suggest that activation of death receptors is detrimental to myocytes.
Intrinsic Mitochondrial Pathway
The intrinsic pathway is activated by intracellular stress signals such as loss of growth factors, hypoxia, oxidative stress, and DNA damage. It converges at the mitochondria which undergo permeabilization of the outer membrane to release pro-apoptotic proteins into the cytosol. The mitochondria integrate signals from the anti- and pro-apoptotic Bcl-2 family of proteins. The anti-apoptotic proteins (Bcl-2, Bcl-XL, and Mcl-1) preserve mitochondrial integrity under normal conditions by inhibiting activation of the pro-apoptotic Bcl-2 proteins. The pro-apoptotic Bcl-2 proteins are subdivided into the BH3-only proteins (Bid, Bim, Bad, BNIP3, NIX, BNIP3L, Puma, Noxa) and the multidomain effector proteins Bax and Bak, which share three conserved Bcl-2 homology (BH) domains and are structurally similar to the anti-apoptotic Bcl-2 proteins (10). Pro-apoptotic BH3-only proteins are selectively activated in response to specific intracellular stress signals via transcriptional regulation or post-translational modification. Once activated, they transduce stress signals by translocating from the cytosol to mitochondria, where they neutralize the anti-apoptotic Bcl-2 proteins or directly activate Bax and Bak (11). Bax and Bak oligomerize to form pores in the outer mitochondrial membrane that allow cytotoxic proteins such as cytochrome c, second mitochondria-derived activator of caspases (SMAC)/direct inhibitor of apoptosis-binding protein with low pI (DIABLO), Omi/High temperature requirement protein A2 (HtrA2), endonuclease G, and apoptosis-inducing factor (AIF) to cross from the intermembrane space into the cytosol. Cytochrome c binds to the cytosolic proapoptotic protein apoptotic protease activating factor 1 (APAF1), resulting in formation of the apoptosome that recruits and activates procaspase-9. Active caspase-9 subsequently cleaves and activates the effector caspases 3- and -7 (12). Although the extrinsic and intrinsic pathways respond to distinct signals and have different initiator caspases, they converge at the effector caspases. There is also cross-talk between the extrinsic and intrinsic pathways where caspase-8 cleaves the cytosolic BH3-only protein Bid. Truncated Bid (tBid) translocates to the mitochondria, where it activates Bax and Bak in the outer mitochondrial membrane (13) (Figure 1A).
The Bcl-2 family members play important roles in the myocardium. Activation of BH3-only proteins and the downstream effectors Bax/Bak leads to death of myocytes. For instance, overexpression of the BH3-only protein BNIP3 in myocytes leads to mitochondrial dysfunction and cell death (14). Ablation of the gene encoding for the BH3-only protein Puma diminishes loss of cardiomyocytes in response to I/R injury (15). Similarly, Bax-deficient mice have reduced mitochondrial damage and infarct size after myocardial I/R (16). Transgenic mice overexpressing Bcl-2 in the heart have diminished apoptosis and smaller infarct size after I/R compared to wild type mice (17). Clearly, the Bcl-2 proteins are critical regulators of myocyte viability in response to stress.
Mitochondrial Permeability Transition Pore and Necrosis
In contrast to the tightly regulated apoptotic pathways, necrosis is ATP-independent and is associated with cell rupture and initiation of inflammation (18). Opening of the mitochondrial permeability transition pore (mPTP) is a major contributor to necrotic cell death. Opening of the pore leads to dissipation of the proton gradient responsible for oxidative phosphorylation and ATP synthesis 18. Another consequence of mPTP opening is mitochondrial swelling and rupture due to the sudden influx of water and solutes. Although the exact composition of the mPTP is still unknown, cyclophilin D has been identified as a critical component of the pore. Mice lacking the Ppif gene, which encodes cyclophilin D, show a reduction in infarct size after I/R injury, and isolated mitochondria from their hearts are resistant to Ca2+-induced swelling and permeability transition (19, 20).
Mitochondria are capable of storing large amounts of Ca2+ which can serve to protect against Ca2+-induced cytotoxicity. Also, several important steps in ATP production, such as the TCA cycle, are Ca2+-dependent. The Ca2+ is taken up by the mitochondria via the mitochondrial Ca2+ uniporter (MCU) in the inner membrane. Although the activity of the Ca2+ uniporter was initially observed several decades ago, the molecular identity of the uniporter was just recently identified (21, 22). Excessive influx of Ca2+ into the mitochondria is associated with necrotic cell death due to opening of the mPTP. For instance, myocardial I/R is associated with elevated levels of intracellular Ca2+, and pharmacological inhibition of the MCU prevents mPTP opening and diminishes I/R injury (23).
Apoptosis and Necrosis Crosstalk
Recent evidence has shown that there is crosstalk between the apoptotic and necrotic pathways, facilitated by Bcl-2 family proteins and the mPTP. Bax and Bak are known to play a primary role in activating apoptosis in response to myocardial I/R, and Bax/Bak double knockout mice exhibit reduced infarcts compared to wild type mice (24). Interestingly, Bax/Bak/cyclophilin D triple knockout mice do not show further reduction in infarct size compared to the Bax/Bak double knockout mice. In addition, cells and mitochondria lacking Bax and Bak are resistant to mPTP opening and necrosis, suggesting that Bax and Bak play distinct roles in regulating both apoptosis and necrosis 24.
The endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) are important for Ca2+ storage in cells. Mitochondria are located adjacent to the ER/SR and can therefore absorb much of the Ca2+ that is released by the ER/SR to prevent cytotoxicity. Studies have found that several of the Bcl-2 family proteins are also localized to the ER/SR where they regulate calcium homeostasis. Bcl-2 and Bcl-XL increase calcium stores in the ER by reducing calcium release, whereas Bax and Bak increase the release of ER Ca2+ stores into the cytosol (25). The BH3-only protein Nix is localized to both mitochondria and ER/SR. Interestingly, targeting Nix specifically to mitochondria induces apoptosis via caspase activation in cardiomyocytes, whereas ER/SR-targeted Nix triggers mPTP opening and necrosis by increasing Ca2+ release from the ER/SR (26). Furthermore, cyclophilin D ablation protects cells from ER/SR-targeted Nix-mediated cell death, while caspase inhibition and Bax/Bak ablation protect against cell death mediated by mitochondrial-targeted Nix (27). These findings indicate that the BH3-only proteins can induce either apoptosis or necrosis depending on the cellular localization.
Death Receptors and Regulated Necrosis
Necrosis has been traditionally defined as accidental and uncontrolled cell death, but accumulating evidence suggests that necrosis can also be a tightly regulated process 18. In fact, it is now known that death receptors can activate both apoptosis and a form of necrosis called necroptosis (Figure 1B). Studies have demonstrated that the kinase activity of RIP1 and its interaction with RIP3 after death receptor activation are involved in necroptosis (28). Interestingly, caspase-8 suppresses RIP3-RIP1 kinase complex-dependent necroptosis by proteolytic cleavage (29). Caspase-8 deficient embryos die between embryonic days 10.5 and 11.5 due to activation of RIP3. Kaiser et al. discovered that caspase-8-RIP3 double knockout mice are viable and mature into fertile adults (30). In addition, studies have reported that necroptosis contributes to I/R injury. Administration of Necrostatin-1 (Nec-1), a small molecule inhibitor of RIP1, protects cardiomyocytes against I/R injury in vitro and in vivo (31). Interestingly, the presence of Nec-1 delays opening of the mPTP in isolated rat cardiomyocytes subjected to oxidative stress 31. Nec-1 administration also reduces infarct size in wild type mice but fails to produce a similar effect in CypD−/− mice (32) suggesting that RIP1-RIP3 requires a functional mPTP to induce necroptosis.
The specific signals that activate RIP1-RIP3 and necroptosis, as well as the downstream execution steps are still being uncovered. Several studies suggest that it involves perturbation of mitochondrial function. Recently, Wang et al. reported that the mitochondrial protein phosphatase phosphoglycerate mutase 5 (PGAM5) plays an important role in necroptosis (33). They discovered that PGAM5 interacts with the RIP1-RIP3 complex at the mitochondria, and this interaction results in the recruitment and activation of the mitochondrial fission protein Drp1. Interestingly, knockdown of Drp1 blocks necrosis, indicating that mitochondrial fragmentation via Drp1 is required for necrosis 33.
MicroRNAs and Cell Death
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression. Numerous miRNAs are expressed in the myocardium and they are important for proper cardiac function. Cardiac-specific ablation of Dicer, an endonuclease required to process miRNAs into their mature, active form, results in abnormal contractile protein expression and profound sarcomere disarray, coupled with significantly decreased cardiac function, that rapidly progresses to heart failure and death (34). Interestingly, Dicer protein levels are reduced (34) and expression of specific miRNAs are altered in failing human hearts (35). Numerous miRNAs have been shown to participate in the pathogenesis of cardiovascular disease. For instance, miR-1, miR-133, miR-208, miR-23a, and miR-199b play major roles in the development of cardiac hypertrophy, whereas miR-21, miR-199a, miR-210, and miR-494 are important for myocyte survival during ischemia (36). The miRNAs can directly regulate apoptosis in myocytes by altering expression of critical survival genes. For instance, overexpression of miR-1 in cardiomyocytes reduces expression of antiapoptotic Bcl-2 and leads to increased apoptosis after hydrogen peroxide treatment (37).
Other miRNAs appear to have a beneficial role in the myocardium. Deletion of miR-214 leads to reduction in cardiac contractility and increases apoptosis after I/R injury. miR-214 is upregulated during ischemic injury, and its cardioprotective mechanism is attributed to its regulation of the sodium/calcium exchanger (Ncx1), as well as repression of proteins that mediate cell death (38). A high-throughput functional screen of human miRNAs identified miR-590 and miR-199 to significantly increase proliferation of neonatal and adult cardiomyocytes in vitro and in vivo. Injection of AAV9 vectors expressing the two miRNAs into the peri-infarcted area also reduces infarct size, improves cardiac function, and stimulates cardiac regeneration after MI, demonstrating that administration of miRNAs can promote cardiac recovery (39). In addition to their role in apoptosis, miRNAs can also regulate necroptosis. Liu et al. found that miR-155 increases cardiomyocyte progenitor cell survival upon oxidative stress by targeting RIP1 (40).
Mitochondrial Dynamics and Cell Death
Mitochondria undergo fission and fusion in response to changes in the extracellular and intracellular environments. Dynamin-related protein 1 (Drp1) interacts with fission protein 1 (Fis1) in the outer mitochondrial membrane to promote mitochondrial fission (41). Mitofusins 1 and 2 (Mfn1 and Mfn2) regulate fusion of the outer mitochondrial membrane, and optic atrophy protein 1 (Opa1) regulates fusion of the inner mitochondrial membrane. These proteins are highly expressed in the heart and are implicated in both apoptosis and necrosis.
Many studies have demonstrated that mitochondrial morphology affects cardiac function and susceptibility to injury. For instance, deletion of Drp1 in mice is embryonic lethal, and cardiomyocytes from Drp1 knockout embryos have reduced contractility, indicating Drp1-mediated mitochondrial fission is important for proper heart development (42). Excessive Drp1-mediated mitochondrial fission is also associated with increased susceptibility to cell death in the myocardium. Pharmacological or genetic inhibition of Drp1 decreases cell sensitivity to mPTP opening, and reduces cell death after simulated I/R in vitro. Additionally, administration of the mitochondrial division inhibitor (Mdivi-1) or knockdown of Drp1 using siRNA reduces infarct size in adult mice in vivo (43). Drp1 activity is regulated by posttranslational modifications, including phosphorylation. Dephosphorylation of Drp1 by calcineurin promotes its translocation to mitochondria and its ability to induce mitochondrial fission (44). Recently, Wang et al. reported miR-499 regulates mitochondrial fission and apoptosis in myocytes by targeting calcineurin (45). This study also found that overexpression of miR-499 results in downregulation of calcineurin and abrogation of Drp1-mediated mitochondrial fission in neonatal myocytes exposed to hypoxia. Interestingly, miR-499 is reduced in the area at risk in ischemic heart and in neonatal rat cardiomyocytes exposed to hypoxia, suggesting that downregulation of miR-499 under pathological conditions results in increased Drp1-mediated mitochondrial fission and cell death.
Alterations in mitochondrial fusion proteins also influence cardiac function and susceptibility to cell death. For instance Opa1+/− cardiac mitochondria are enlarged and have reduced sensitivity to Ca2+-induced mPTP opening. Surprisingly, these mice are more sensitive to hemodynamic stress at 3 months of age (46). Although initial mitochondrial and cardiac functions are normal, at 12 months the Opa1+/− mice have impaired cardiac mitochondrial function and develop late-onset cardiomyopathy (47). Moreover, Mfn2 deficiency results in enlarged mitochondria (48), while Mfn1 deficiency leads to formation of small spherical mitochondria (49). Similar to the Opa1+/− mitochondria, both Mfn1 and Mfn2-deficient mitochondria are more resistant to mPTP opening. Mfn2−/− mice are also more resistant to myocardial I/R injury 48. Thus, these studies suggest that the mitochondrial fusion proteins are involved in regulating mPTP and cell death. However, exactly how these proteins influence mPTP opening is still unknown.
Conclusion
Although cardiovascular diseases are very complex and involve multiple cellular processes, it is clear that cell death plays a critical role in the pathogenesis of heart failure. The treatments to control heart diseases have improved, but there is still a great need to develop better therapies for patients with cardiovascular disease. The regulated nature of cell death opens up the possibility of manipulating death pathways to salvage the myocytes. Although proof-of-concept studies in several experimental animal models have revealed that blockade of apoptosis or necrosis is a validated therapeutic strategy, a number of key questions remain to be answered. For instance, it is unclear whether some of the key proteins involved in regulating cell death have physiological roles beyond that of apoptosis or necrosis, and if so, whether this increases the risk for toxicity in patients. This effect was observed in a clinical trial targeting TNF-α in heart failure patients in which treatment with a TNF-α antagonist adversely affected the course of the disease rather than improving the heart failure (50). Another issue is determining which step to target in the pathways. It is uncertain whether preservation of cell survival by inhibiting effector caspases truly results in preservation of cell function since they do not preserve mitochondrial integrity. Mitochondria are responsible for providing the myocytes with ATP and therefore essential for survival. Additionally, miRNA therapy is currently being tested in several clinical trials and appears to be promising. Although this is a novel approach to modulating proteins in the cell death pathways, miRNAs often have multiple targets, many which have not yet been identified. Clearly, an increased understanding of the proteins involved in regulating cell death is necessary before new effective and safe therapies can be developed to prevent or treat cardiovascular disease.
Acknowledgments
ABG is supported by NIH grants R01HL101217, and R01HL092136, and AMO is supported by T32GM007752.
Footnotes
Conflicts of Interest
None
References
- 1.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 3.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003;60:608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, et al. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 8.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 9.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, et al. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 14.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 15.Toth A, Jeffers JR, Nickson P, Min JY, Morgan JP, et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–H60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 16.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 18.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 20.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 21.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–40. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de García-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members. Biochem Pharmacol. 2003;66:1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 26.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YS, Challa S, Moquin D, Genga R, Ray TD, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Wang L, Miao L, Wang T, Du F, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, et al. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 32.Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–43. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, et al. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 38.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, van Mil A, Vrijsen K, Zhao J, Gao L, et al. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med. 2011;15:1474–1482. doi: 10.1111/j.1582-4934.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong SB, Gustafsson AB. New roles for mitochondria in cell death in the reperfused myocardium. Cardiovasc Res. 2012;94:190–196. doi: 10.1093/cvr/cvr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 44.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JX, Jiao JQ, Li Q, Long B, Wang K, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 46.Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94:408–417. doi: 10.1093/cvr/cvs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Liu T, Tran A, Lu X, Tomilov AA, et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc. 2012;1:e003012. doi: 10.1161/JAHA.112.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF, Jr, et al. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012;302:H167–H179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]