Abstract
Aims
Thiazide diuretics are recommended as first line antihypertensive treatment, but may contribute to new onset diabetes. We aimed to describe change in fasting glucose (FG) during prolonged thiazide treatment in an observational setting.
Methods
We conducted an observational, non-randomized, open label, follow-up study of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) and PEAR-2 studies. We enrolled previous participants from the PEAR or PEAR-2 studies with at least six months of continuous treatment with either hydrochlorothiazide (HCTZ) or chlorthalidone. Linear regression was used to identify associations with changes in FG after prolonged thiazide and thiazide-like diuretic treatment.
Results
A total of 40 participants were included with a mean 29 (range 8–72) months of thiazide treatment. FG increased 6.5 (SD 13.0) mg/dL during short-term thiazide treatment and 3.6 (SD 15.3) mg/dL FG during prolonged thiazide treatment. Increased FG at follow-up was associated with longer thiazide treatment duration (beta=0.34, p=0.008) and lower baseline FG (beta=−0.46, p=0.02). β blocker treatment in combination with prolonged thiazide diuretic treatment was also associated with increased FG and increased two-hour glucose obtained from OGTT.
Conclusions
Our results indicate that prolonged thiazide treatment duration is associated with increased FG and that overall glycemic status worsens when thiazide/thiazide-like diuretics are combined with β blockers.
Keywords: hypertension, hydrochlorothiazide, chlorthalidone, glucose, diabetes, thiazide diuretics, β blockers
Introduction
Thiazide diuretics are recommended as first-line treatment for uncomplicated hypertension. [1] However, strong evidence from randomized clinical trials indicates that thiazide and thiazide-like diuretics contribute to adverse metabolic effects (AMEs), such as hyperglycemia and diabetes. [2–4] Diabetes increases the risk for adverse cardiovascular (CV) outcomes in hypertensive patients. [5–7] Since thiazide diuretics contribute to hyperglycemia and diabetes, their benefit in a hypertensive patient, including CV risk reduction, could be offset by these important AMEs. [3] Consensus clinical guidelines for hypertension now recommend caution when prescribing thiazide diuretics due to risk of metabolic effects. [8, 9]
The effects of prolonged thiazide diuretic treatment on new onset diabetes and fasting glucose (FG) have been studied in randomized controlled trials. [10–12] However, only limited data are available describing the effects of prolonged thiazide and thiazide-like diuretic treatment on FG levels and overall glycemic status in a contemporary, observational patient cohort. [11] Characterization of FG after prolonged thiazide treatment in such a cohort might be useful in predicting adverse metabolic effects (AMEs) during prolonged thiazide treatment and clarifying whether duration of thiazide treatment is a risk factor for increased fasting glucose. Therefore, we conducted an observational, non-randomized, open label, follow-up study of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) and PEAR-2 studies, for which detailed metabolic and drug response data was available, to assess effect of prolonged exposure to thiazide diuretic on glucose homeostasis.
Materials and Methods
PEAR and PEAR-2 Study Population
Details of the PEAR study, which investigated genetic influences of the effects of hydrochlorothiazide (HCTZ), atenolol, and their combination on BP and AMEs have been previously published. [13] PEAR-2 similarly investigated genetic influences on BP and AMEs after administration of the thiazide-like diuretic chlorthalidone and the beta blocker metoprolol. Both PEAR and PEAR-2 participants were age 17 through 65 years with mild to moderate essential hypertension but without a history of heart disease or diabetes. Inclusion criteria required newly diagnosed, untreated, or known hypertension currently treated with 1 or 2 antihypertensive drugs. Participants were included if they had an average home diastolic blood pressure (DBP) between 86 and 110 mmHg and office DBP between 91 and 110 mmHg at the end of an average 4 week washout period, if on antihypertensive therapy. Study sites included the University of Florida (Gainesville, FL), Emory University (Atlanta, GA), or the Mayo Clinic (Rochester, MN).
PEAR Follow-Up Study Population
The PEAR Follow-Up Study was an observational, non-randomized, open label, follow-up study of participants previously enrolled in PEAR or PEAR-2. Participants were eligible for the study if they 1) previously participated in a PEAR study at a University of Florida site and had HCTZ or chlorthalidone response data collected, 2) participated in their final PEAR or PEAR-2 study visit at least six months prior to the PEAR Follow-Up Study visit, and 3) were treated with a thiazide or thiazide-like diuretic continuously during the period between the end of PEAR participation and the PEAR Follow-Up Study visit. Participants were eligible if they were 17–75 years of age and not pregnant. All studies were approved by the University of Florida Institutional Review Board and all participants provided written informed consent for study procedures. The PEAR Follow-Up Study is registered on ClinicalTrials.gov (NCT01409434).
PEAR and PEAR-2 Study Design
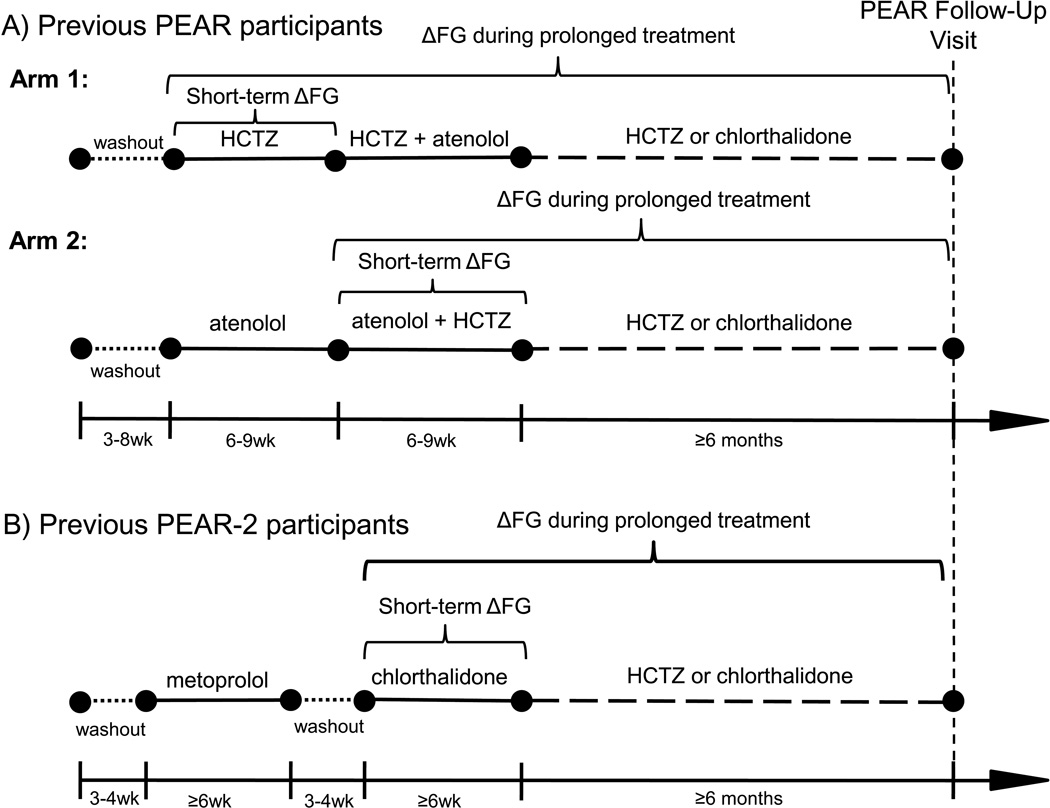
After a washout period, PEAR participants were randomly assigned to receive HCTZ 12.5 mg (Arm 1) or atenolol 50 mg (Arm 2) daily, followed by dose titration, for a total of nine weeks. (Figure 1A) The alternate agent was then added, with similar dose titration over an additional nine weeks. In Arm 1, change in FG was defined as the difference in FG from the baseline visit to the end of HCTZ monotherapy. In Arm 2, change in FG was defined as the difference in FG from the start of HCTZ add-on therapy to the end of the trial.
Figure 1.
Progression of participants through PEAR (A) or PEAR-2 (B) to the PEAR Follow-Up Study over time. Antihypertensive treatments are described above timelines. Laboratory assessment study visits are represented by black circles. Short dashes indicate washout periods. Long dashes indicate the follow-up period. Definitions of change in FG (ΔFG) during short-term and prolonged thiazide and thiazide-like diuretic treatment are indicated by brackets for PEAR Arm 1, PEAR Arm 2, and PEAR-2. HCTZ indicates hydrochlorothiazide; PEAR, Pharmacogenomic Evaluation of Antihypertensive Responses; wk, weeks.
PEAR-2 investigated genetic influences on BP and AMEs after administration of the thiazide-like diuretic chlorthalidone and the β blocker metoprolol. After washout, participants were treated with metoprolol, with dose titration, for eight weeks. (Figure 1B) Participants then underwent another washout period, followed by chlorthalidone 15 mg once daily with dose titration to 25 mg. Short-term change in FG during eight weeks of chlorthalidone was defined as the difference from the start of chlorthalidone monotherapy to the end of the trial. Additional information regarding PEAR (NCT00246519) and PEAR-2 (NCT01203852) methodology can be found in the Supplemental Materials.
PEAR Follow-Up Study Design
The PEAR Follow-Up Study consisted of a single study visit after a minimum 8 hour fast. Medication use data and a social history were obtained, which included weekly alcohol consumption and cigarette smoking status. Anthropomorphic measurements collected during the visit included height, weight, and waist and hip circumference. A blood sample was obtained for measurement of glucose, insulin, HbA1c, a lipid panel, uric acid, and potassium. An oral glucose tolerance test (OGTT) was then performed, whereby each participant drank a 75-gram glucose solution (Azer Scientific, Morgantown, PA). Blood was then collected one hour and two hours after ingestion of the glucose solution. Glucose was measured in whole blood using a YSI 2300 STAT Plus™ (YSI, Yellow Springs, OH). Additional information regarding PEAR Follow-Up Study methodology can be found in the Supplemental Materials.
Statistical Analysis
Participant characteristics at baseline, defined as start of thiazide treatment in PEAR studies, and at the PEAR Follow-Up Study visit were compared using McNemar’s tests and paired t-tests as appropriate. Whole blood glucose measurements from the PEAR Follow-Up Study were converted to plasma-adjusted glucose measurements using a multiplication factor of 1.11, [14] for comparison to fasting plasma glucose measurements collected during PEAR studies. A participant’s change in FG during prolonged thiazide treatment was defined as the difference between FG at start of thiazide treatment (PEAR baseline studies) and FG at the PEAR Follow-Up Study visit. Homeostatic model assessment (HOMA) was calculated as the product of fasting insulin and FG divided by 405. [15]
Linear regression was used to determine associations with change in FG during prolonged thiazide treatment in univariate analyses. Specific variables used in univariate analyses are described in the Supplemental Materials. For univariate linear regressions, 40 participants gave us 87 percent power to detect a parameter estimate of 0.5, assuming a standard deviation (SD) of 15 for both dependent and independent variables with two-sided alpha=0.05. Variables were utilized in stepwise regressions if p≤0.20 in univariate analyses. In stepwise regressions, variables entered the model at p≤0.20 and were retained in the model at p≤0.05. Spearman’s rho partial correlations were used to test correlations. Participants treated with anti-diabetic medication were excluded from analyses to eliminate confounding of anti-diabetic treatment and diabetes. All statistical analyses were performed using SAS 9.2 software (SAS, Cary, NC) and data were stored using REDCap software. [16]
Results
A total of 44 participants participated in the PEAR Follow-Up Study. (Figure S1 in Supplemental Materials) Forty participants were included in this analysis, including 29 (73%) from PEAR and 11 (27%) from PEAR-2. (Table 1) The remaining four participants were excluded from analysis due to treatment with anti-diabetic medication. The mean age of PEAR Follow-Up Study participants was 49 (SD 10) years and the mean follow-up period, indicating length of thiazide treatment following PEAR or PEAR-2, was 29 (SD 19) months (range 8–72 months). PEAR Follow-Up Study population characteristics at baseline and at follow-up are summarized in Table 1. Concomitant pharmacotherapy during the follow-up period and glycemic characteristics at follow-up are summarized in Table 2.
Table 1.
Characteristics of PEAR Follow-Up Study Participants at Baseline and at Follow-up
| Characteristica | Baselineb (n=40) |
Follow-up (n=40) |
P valuec |
|---|---|---|---|
| PEAR participants | 29 (73%) | - | - |
| Arm 1 (HCTZ monotherapy) | 13 (33%) | - | - |
| PEAR-2 participants | 11 (27%) | - | - |
| Gender (female) | 24 (60%) | - | - |
| Race (Caucasian) | 29 (73%) | - | - |
| Weight (kg), mean (SD) | 89.8 (17.7) | 91.7 (17.3) | 0.09 |
| Body mass index (kg/m2), mean (SD) | 31.1 (5.6) | 31.8 (5.4) | 0.09 |
| Waist circumference (cm), mean (SD) | 98 (14) | 102 (16) | 0.002 |
| Abdominal obesityd | 22 (55%) | 27 (68%) | 0.03 |
| SBP, mean (SD)e | 142 (16) | 131 (14) | 0.003 |
| DBP, mean (SD)e | 89 (9) | 83 (10) | 0.02 |
| Consumes alcoholic beverages | 18 (45%) | 22 (55%) | 0.18 |
| Drinks per week, mean (SD) | 5 (7) | 8 (10) | 0.04 |
| Current smoker | 10 (23%) | 7 (16%) | 0.08 |
| Statin treatment | 4 (9%) | 9 (23%) | 0.06 |
| Fasting Glucose (mg/dL), mean (SD) | 91 (12) | 94 (13) | 0.16 |
| Total cholesterol (mg/dL), mean (SD) | 198 (40) | 200 (40) | 0.63 |
| LDL cholesterol (mg/dL), mean (SD) | 122 (36) | 117 (36) | 0.35 |
| HDL cholesterol (mg/dL), mean (SD) | 45 (14) | 52 (17) | <0.0001 |
| Triglycerides (mg/dL), mean (SD) | 154 (121) | 157 (89) | 0.59 |
| Serum potassium (mEq/L), mean (SD) | 4.49 (0.48) | 4.18 (0.49) | 0.003 |
| Uric acid (mg/dL), mean (SD) | 5.9 (1.5) | 6.6 (1.6) | <0.0001 |
| Insulin (µU/mL), mean (SD) | 15.8 (12.6) | 15.2 (10.2) | 0.85 |
| HOMA, mean (SD) | 2.73 (1.79) | 3.32 (2.58) | 0.15 |
| Impaired fasting glucosef | 11 (28%) | 11 (28%) | 0.99 |
SD indicates standard deviation; kg, kilograms; kg/m2, kilograms per meter squared; cm, centimeters, SBP, systolic blood pressure; DBP, diastolic blood pressure; mg/dL, milligrams per deciliter; LDL, low density lipoprotein; HDL, high density lipoprotein; mEq/L, milliequivalents per liter; microunits per milliliter, µU/mL; HOMA, homeostatic model assessment.
Values indicate no. (%) unless otherwise stated.
Baseline is defined as start of thiazide diuretic treatment during PEAR or PEAR-2.
P value indicates paired t-tests or McNemar’s tests for difference between baseline and follow-up.
Abdominal obesity defined as waist circumference ≥88.9 centimeters for females or ≥101.6 centimeters for males.
Average of home BP measurements for baseline and three clinic BP measurements for follow-up study visit.
Impaired fasting glucose defined as fasting glucose ≥ 100 mg/dL.
Table 2.
Characteristics of PEAR Follow-Up Study Participants during Follow-up Period.
| Characteristic at follow-up | Number of participantsa (n=40) |
|---|---|
| Drug Treatment Characteristics | |
| Duration of thiazide treatment (months), mean (SD) | 29 (19) |
| Hydrochlorothiazide treatment | 30 (75%) |
| Dose (mg), median (IQR) | 25 (25–25) |
| Chlorthalidone treatment | 10 (25%) |
| Dose (mg), median (IQR) | 25 (25-25) |
| Thiazide monotherapy | 10 (25%) |
| Beta blocker + thiazide | 12 (30%) |
| ACEI + thiazide | 13 (33%) |
| Beta blocker + ACEI + thiazide | 5 (13%) |
| Statin treatment | 9 (23%) |
| Potassium supplementation | 5 (13%) |
| SSRI treatment | 3 (8%) |
| Glycemic Characteristics | |
| HbA1c (%, (mmol/mol)), mean [SD] | 5.7 [0.4], (38.6 [4.8]) |
| 1-hour OGTT glucose (mg/dL), mean (SD)b | 157 (47) |
| 2-hour OGTT glucose (mg/dL), mean (SD)b | 132 (51) |
| OGTT AUC (mg/dL•h), mean (SD)b | 273 (74) |
| IGT (2-hour OGTT glucose 140–199 mg/dL)b | 16 (40%) |
| EGI (1-hour OGTT glucose ≥ 155 mg/dL)b | 18 (45%) |
| New onset IFGc | 6 (15%) |
SD indicates standard deviation; ACEI, Angiotensin I converting enzyme inhibitor; SSRI, selective serotonin reuptake inhibitor; IQR, interquartile range; OGTT, oral glucose tolerance test; mg/dL, milligrams per deciliter; AUC, area under the curve; IGT, impaired glucose tolerance; EGI, elevated glucose intolerance; IFG, impaired fasting glucose.
Represented as number (percentage) unless otherwise noted.
Data acquired during 2 hour oral glucose tolerance test after 75 gram glucose load
Number of participants with impaired fasting glucose (fasting glucose ≥100 mg/dL) at follow-up who did not have impaired fasting glucose at baseline
Among PEAR Follow-Up Study participants, mean FG was 91 (SD 12) mg/dL at baseline, 97 (SD 16) mg/dL after short-term thiazide treatment (after PEAR), and 94 (SD 13) mg/dL at the PEAR Follow-Up Study visit. This constituted a significant 6.5 (SD 13.0) mg/dL increase (p=0.005) during short-term thiazide treatment and a non-significant 3.6 (SD 15.3) mg/dL increase (p=0.16) during prolonged thiazide treatment relative to baseline. No significant correlation was observed between change in FG during short-term and prolonged thiazide treatment (r=0.16, p=0.38). (Figure S2 in Supplemental Materials)
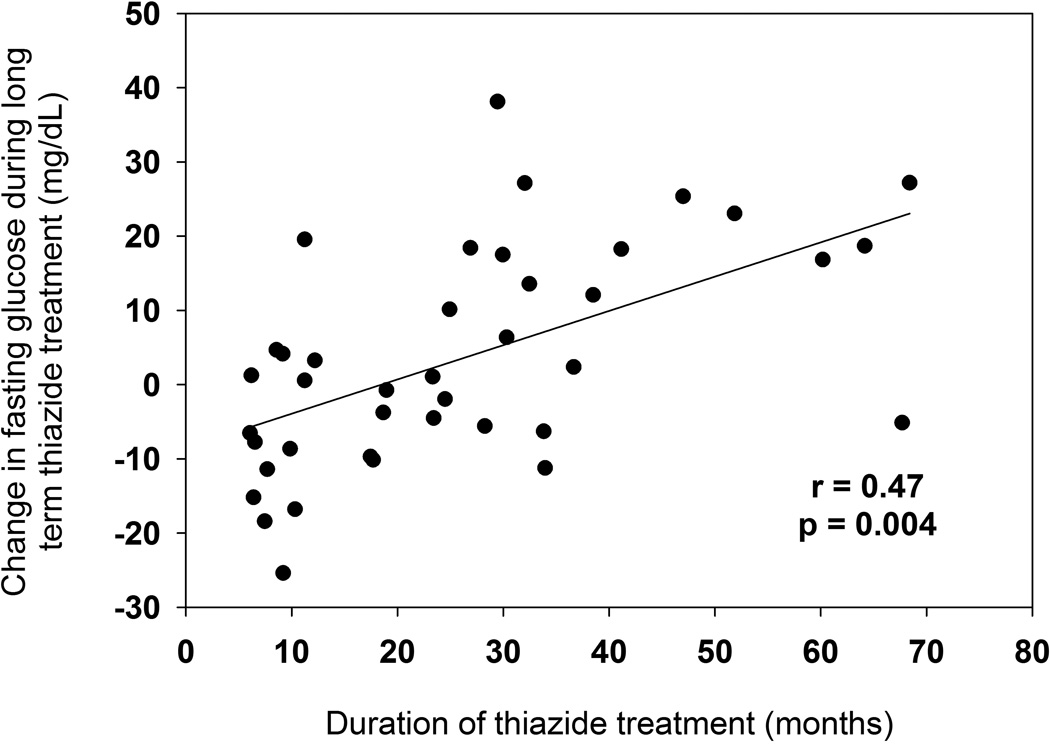
Univariate associations with change in FG during prolonged thiazide treatment are listed in Table 3. FG during prolonged thiazide treatment increased with longer duration of thiazide treatment, with FG increasing by 0.34 mg/dL for each additional month of thiazide treatment (β=0.34, p=0.008). FG during prolonged thiazide treatment decreased by 0.46 mg/dL with each 1 mg/dL increase in baseline FG (β=−0.46, p=0.02), indicating that there was not as much FG increase in those with high baseline FG compared with those with low baseline FG. Change in FG was also positively correlated with duration of thiazide treatment (r=0.47, p=0.004), consistent with the positive parameter estimate in stepwise regressions. (Figure 2) In multivariate analysis, the full model R2 for change in FG during prolonged thiazide treatment was 0.45. Stepwise regression analyses for FG at the follow-up visit and changes in other lab values during prolonged thiazide treatment are summarized in Table S1 and S2 in the Supplemental Materials.
Table 3.
Variables Associated with FG Changes during Prolonged Thiazide Treatment
| Independent variable | Parameter estimate (β)a |
p valueb |
|---|---|---|
| Univariate associations (p≤0.20) | ||
| Baseline FG, mg/dL | −0.70 | 0.003 |
| Change in FG during short-term thiazide treatment, mg/dL | 0.28 | 0.16 |
| Gender (female) | −6.56 | 0.20 |
| Duration of thiazide treatment, months | 0.48 | 0.0002 |
| Beta blocker treatment | 9.76 | 0.05 |
| ACEI treatment | −6.74 | 0.18 |
| Current smoker | −9.37 | 0.15 |
| Family history of T2Dc | 8.11 | 0.11 |
| Baseline SBP, mmHg | 0.23 | 0.17 |
| Stepwise Results (R2=0.45) | ||
| Duration of thiazide treatment, months | 0.34 | 0.008 |
| Baseline FG, mg/dL | −0.46 | 0.02 |
FG indicates fasting glucose; mg/dL, milligrams per deciliter; ACEI, angiotensin I converting enzyme inhibitor; T2D, type 2 diabetes; SBP, systolic blood pressure.
Units for parameter estimates for fasting glucose changes are mg/dL.
P values determined using linear regression excluding participants with anti-diabetic treatment.
Family history of type 2 diabetes in a first-degree relative
Figure 2.
Change in fasting plasma glucose during prolonged thiazide diuretic treatment versus duration of follow-up. P value and r calculated using Spearman partial correlation adjusted for baseline fasting glucose.
In participants treated with a β blocker in addition to a thiazide during the follow-up period, a significant 8.8 (SD 14.8) mg/dL increase in FG was observed compared to baseline (p=0.02). After prolonged administration, FG was also significantly increased in β blocker plus thiazide-treated participants compared to those treated with a thiazide alone (p=0.05). β blocker treatment was associated with a 33 mg/dL increase in two hour OGTT plasma-adjusted glucose compared to participants without β blocker treatment (p=0.03). No significant change was observed in FG in participants treated with ACEIs. Long term change in FG was not significantly different by type of thiazide treatment, HCTZ vs chlorthalidone, (p=0.29), ACEI treatment status (p=0.18), or statin treatment status (p=0.30). Fasting glucose levels at baseline and at follow-up by drug treatment status are summarized in Table S3 in the Supplemental Materials.
Discussion
In the PEAR Follow-Up Study, longer duration of thiazide treatment and lower baseline FG were associated with increased FG after prolonged thiazide treatment. In participants treated with the combination of a β blocker and a thiazide, mean FG during follow-up and two hour glucose following OGTT were higher than in those who were not treated with the combination.
Observations made over a wide range of follow-up time (8–72 months) suggest that FG increased with duration of thiazide treatment in an observational setting. Thiazide treatment duration was strongly associated with an increased FG during the follow-up period. The association of treatment duration with increasing FG may have clinical implications since antihypertensive therapy with thiazide diuretics is typically prolonged and may be life-long. Increased FG as a result of longer thiazide treatment is consistent with previous reports. [10, 12] An increased FG with thiazide treatment is concerning since increased FG, even within the normal range, is strongly associated with increased risk for development of diabetes. [17, 18]
PEAR Follow-Up Study results support an effect of concomitant β blocker treatment on AMEs. Participants treated with both thiazides and β blockers had higher glucose at follow-up than those treated with thiazides alone. β blocker treatment was also associated with increased two hour OGTT glucose. β blocker treatment has been previously associated with diabetes [2] and may impair insulin release through its action on the β1 receptor, [19] causing increases in fasting and post-prandial glucose. Recent observational data from a large health maintenance organization database suggest that hypertensive patients treated with a β blocker plus thiazide diuretic combination had a two-fold increase in risk for development of diabetes compared to those not treated with that combination. [20]
Short-term changes in FG during thiazide treatment were not correlated with changes in FG during prolonged thiazide treatment in the PEAR Follow-Up Study. In addition, the mean FG measured at the end of PEAR was higher than that measured at the end of the follow-up period, which suggests that FG did not increase in a consistently throughout the follow-up period.
This higher FG at the end of PEAR studies may have resulted from increased compliance or increased thiazide dose during the PEAR study period versus observational follow-up period. The strengths of this study are the inclusion of thiazide response data after both short-term and prolonged thiazide treatment in a contemporary hypertensive cohort. The PEAR Follow-Up Study also included detailed information regarding concomitant drug therapy, including antihypertensive, statin therapy, and other medications known to affect glucose homeostasis. Our analysis of data during non-randomized treatment outside of a clinical trial may better reflect actual participant practices and be more generalizable to a broader patient population.
Our study had several limitations worthy of mention. The small sample size of this study may contribute to a lack of significant differences in FG measurements during prolonged thiazide treatment. However, predictors of FG during prolonged thiazide treatment were consistent with previously published models in a much larger patient population. [21] Whole blood FG values were adjusted to reflect plasma FG measurements at follow-up, potentially contributing to variability in the change in FG phenotype. The utilized 11% plasma-adjusted value corresponds well with plasma measurements and is endorsed by the American Diabetes Association. [14] Adherence with drug therapy during follow-up was assessed via in-person interview, which may not reflect actual adherence with chronic medications. However, changes in several objective parameters, including a significant reduction in systolic and diastolic BP, decrease in serum potassium, and increase in serum uric acid strongly suggest medication adherence.
We observed an association between duration of thiazide therapy and increasing FG during prolonged thiazide treatment in the PEAR Follow-Up Study. Concomitant treatment with β blockers was associated with larger mean FG increases and increased two hour OGTT glucose at follow-up, suggesting that the antihypertensive combination of thiazides and β blockers should be avoided in patients with elevated metabolic risk.
Supplementary Material
Acknowledgements
We thank Tomy Mathew and Tim Palmer for processing glucose samples, the PEAR/PEAR-2 research nurses Pamela Connolly, Danielle Poulton, Delores Buffington, and Annette Hall, the PEAR study physicians Drs Kendall Campbell, R Whit Curry, and Seigfried Schmidt, and the participants included in PEAR, PEAR-2, and the PEAR Follow-Up Study.
This work was supported by National Institutes of Health grants U01 GM074492, funded as part of the Pharmacogenomics Research Network, HL086558 (RM Cooper-DeHoff); UL1-TR000064 (University of Florida Clinical and Translational Science Institute), TL1RR029888 from the National Center for Research Resources (JH Karnes), and T32GM007569 from the National Institute Of Child Health & Human Development and National Institute Of General Medical Sciences (JH Karnes).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 3.Karnes JH, Cooper-DeHoff RM. Antihypertensive medications: benefits of blood pressure lowering and hazards of metabolic effects. Expert Rev Cardiovasc Ther. 2009;7:689–702. doi: 10.1586/erc.09.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandeesha H, Pavithran P, Madanmohan T. Effect of antihypertensive therapy on serum lipids in newly diagnosed essential hypertensive men. Angiology. 2009;60:217–220. doi: 10.1177/0003319708316167. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajala U, Qiao Q, Laakso M, Keinanen-Kiukaanniemi S. Antihypertensive drugs as predictors of type 2 diabetes among subjects with impaired glucose tolerance. Diabetes Res Clin Pract. 2000;50:231–239. doi: 10.1016/s0168-8227(00)00189-3. [DOI] [PubMed] [Google Scholar]
- 7.Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999;33:1130–1134. doi: 10.1161/01.hyp.33.5.1130. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 9.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 10.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–988. doi: 10.2337/dc07-1768. [DOI] [PubMed] [Google Scholar]
- 11.Lind L, Pollare T, Berne C, Lithell H. Long-term metabolic effects of antihypertensive drugs. Am Heart J. 1994;128:1177–1183. doi: 10.1016/0002-8703(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 12.Barzilay JI, Davis BR, Cutler JA, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JA, Boerwinkle E, Zineh I, et al. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519–524. doi: 10.1016/j.amjmed.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 19.Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther. 2010;8:793–802. doi: 10.1586/erc.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper-Dehoff RM, Bird ST, Nichols GA, Delaney JA, Winterstein AG. Antihypertensive drug class interactions and risk for incident diabetes: a nested case-control study. J Am Heart Assoc. 2013;2:e000125. doi: 10.1161/JAHA.113.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maitland-van der Zee AH, Turner ST, Schwartz GL, Chapman AB, Klungel OH, Boerwinkle E. Demographic, environmental, and genetic predictors of metabolic side effects of hydrochlorothiazide treatment in hypertensive subjects. Am J Hypertens. 2005;18:1077–1083. doi: 10.1016/j.amjhyper.2005.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.