Abstract
Liposomes are spherical-enclosed membrane vesicles mainly constructed with lipids. Lipid nanoparticles are loaded with therapeutics and may not contain an enclosed bilayer. The majority of those clinically approved have diameters of 50–300 nm. The growing interest in nanomedicine has fueled lipid–drug and lipid–protein studies, which provide a foundation for developing lipid particles that improve drug potency and reduce off-target effects. Integrating advances in lipid membrane research has enabled therapeutic development. At present, about 600 clinical trials involve lipid particle drug delivery systems. Greater understanding of pharmacokinetics, biodistribution, and disposition of lipid–drug particles facilitated particle surface hydration technology (with polyethylene glycol) to reduce rapid clearance and provide sufficient blood circulation time for drug to reach target tissues and cells. Surface hydration enabled the liposome-encapsulated cancer drug doxorubicin (Doxil) to gain clinical approval in 1995. Fifteen lipidic therapeutics are now clinically approved. Although much research involves attaching lipid particles to ligands selective for occult cells and tissues, preparation procedures are often complex and pose scale-up challenges. With emerging knowledge in drug target and lipid–drug distribution in the body, a systems approach that integrates knowledge to design and scale lipid–drug particles may further advance translation of these systems to improve therapeutic safety and efficacy.
Keywords: drug delivery systems, lipids, liposomes, phospholipids, micelle, disposition, nanotechnology, nanoparticles, pegylation
INTRODUCTION
It begins in the late 1950s with the discovery by Saunders and Thomas1 and Bangham and Horne2 that simple hydration of dry lipid film coated on a glass surface produces spherical vesicles or liposomes. This basic observation not only enabled the exploration of lipid–drug and lipid–protein interactions, but it spurred the development of liposomes and lipid nanoparticles as drug carriers to enhance therapeutic benefits. Today, liposomes or lipid vesicles are a pivotal biocompatible and biodegradable drug delivery and formulation platform. They are typically constructed with a synthetic lipid bilayer membrane, a biomimetic of cell membranes, to entrap drug inside an aqueous core. Under the protection of the lipid membrane, a well-subscribed early concept was that drug inside the aqueous compartment could be transported to tissue, cell, or intracellular targets. Incorporating drug molecules in these particles was proposed to shield healthy bystander tissues and cells from drug toxicity while the drug is en route to sites of pharmacological action or disease (effect) sites. In theory, water-soluble (hydrophilic) agents can be encapsulated in the aqueous core enveloped by the lipid membrane or attached on the membrane surface with lipid conjugated to soluble agents. The potential to carry both hydrophobic and hydrophilic compounds has made liposomes one of the favorite research topics in drug carrier research for scientists across disciplines. However, it was soon discovered that each liposome and lipid nanoparticle (constructed with different lipid mixtures) exhibits distinct physical stability, distribution, and patterns of elimination time course in the body. Many years passed before scientists began to appreciate the challenges of premature liposome degradation and clearance, and found lipid compositions that produce stable liposomes that circulate for a sufficient amount of time in the body. Together, physical stability (in storage and in the body) and pharmacokinetics (time-course study) of liposomes intended to reduce rapid elimination or clearance are some of the keys to successful translation of liposome drug delivery systems into therapeutic products.
Depending on design, liposomes may contain a single or multiple (onion-like concentric) bilayered lipid membrane composed of natural or synthetic lipids, with diameters ranging from tens of nanometers to micrometers.3 However, not all lipid nanoparticles have a contiguous bilayer that would qualify them as lipid vesicles or liposomes. For example, some lipid nanoparticles may have up to 33% of drug bound to lipid molecules.4 Although these lipid nanoparticles may be physically stable, the membrane with high densities of drug molecules may not behave as a liposomal membrane capable of encapsulating aqueous contents. Thus, we qualify this variability by discussing both liposomes and lipid nanoparticles in this manuscript. In some cases, lipid–drug aggregates may assume micelle-like structures. Micelles are thermodynamically stable multimeric nanoaggregate structures of amphipathic lipidic molecules in solution about 5–10 nm in diameter. Typical micelles contain a hydrophobic core; however, inverted micelles have a small hydrophilic interior. Other lipid nanoparticles of lipid–drug complexes may be prepared as water-in-oil or oil-in-water emulsions and conform into colloidal dispersions. Lipids and derivatives exhibiting a range of biochemical and biophysical properties (size, charge, and surface structure) can be synthesized and engineered to develop drug carriers for specific therapeutic applications. This potential flexibility and associated potential number of variations in lipid–drug combinations (because of the unique lipid–drug interactions) and therapeutic target design result in wide-ranging lipid–drug compositions. Thus, with no two liposomes or lipid nanoparticles being identical, it makes rigorous manufacturing control imperative.
Since their discovery, liposomes have enjoyed significant attention in laboratory and pharmaceutical research because of a number of attributes. The bilayer membrane could protect drug from hydrolysis or oxidative degradation, thereby minimizing toxicity (i.e., improving the therapeutic index). Prolonged drug circulation or residence time in the body may increase drug bioavailability (reduce clearance) and provide sufficient time for drug molecules to arrive at disease targets. Other potential advantages include the ability to carry multiple drugs at once; the addition of targeting moieties, such as antibodies; and the bio-degradable and tunable drug release in response to temperature, pH, or other environmental inputs.
It took about 35 years after the late 1950s discovery to realize the first clinical liposome application in drug delivery. In 1995, Doxil (PEGylated liposome-encapsulated doxorubicin) became the first liposome drug delivery system approved for human use by the US Food and Drug Administration (FDA).5,6 Today, Doxil and other liposomal doxorubicin and daunorubicin are widely used to treat ovarian cancer and Kaposi's sarcoma (over 300,000 patients are treated each year), and to protect patients from anthracycline cardiotoxicity.7 Moreover, Doxil was reported to improve doxorubicin levels in Kaposi's sarcoma tissues by as much as 22-fold compared with healthy normal skin tissues.8,9 Several drugs and molecules, such as anticancer and antibacterial agents, imaging and probing agents, peptide hormones, proteins, enzymes, vaccines, and genetic material, have been loaded into the aqueous compartment or lipid phases of liposomes.
As shown in Table 1, about 15 liposome and lipid-based drug formulations are approved for human use. Six are treatments intended for cancers; others are for fungi, microbes, preventive vaccination, analgesia, macular degeneration, and hormone replacement. Select lipid-based drug candidates in late-stage (Phase II/III) clinical trials are presented in Table 2. Currently, all human clinical trials intended for product licensing approval by the FDA must be registered with ClinicalTrials.gov, a US Department of Health and Human Services sponsored clinical trial registry. According to ClinicalTrials.gov, there are 589 interventional drug studies with a liposome platform as of May 2013. Interestingly, no FDA-licensed liposome or lipid nanoparticle is coated with ligands or targeting moieties for homing drug to target tissues, cells, or subcellular organelles. Such targeted therapeutics (with or without precise and controlled drug release) are an emerging area of research. These ligand-coated particles, often referred to as actively targeted liposomes, are a challenge to reproduce and manufacture at clinically meaningful scales, even if validated in small animals. Optimization of physiochemical properties involved in stability, toxicity, and immune surveillance, and the development of robust scale-up and manufacturing processes could be challenging in some cases. Although the first-generation liposome and lipid nanoparticle therapeutic products proved this platform to be safe and effective for delivery of drugs and vaccines, their use for nucleic acid and gene therapeutics continues to be explored.
Table 1.
Marketed Liposomal and Lipid-Based Products
| Trade Name (Company) | Lipid Platform | Drug | Size | Indication |
|---|---|---|---|---|
| Anticancer | ||||
| Doxil/Caelyx (Janssen) | PEG–liposome | Doxorubicin | 100 nm | Kaposi's sarcoma, ovarian cancer, breast cancer, combination with bortezomib in multiple myeloma |
| DaunoXome (Galen) | Liposome | Daunorubicin | 45–80 nm | Kaposi's sarcoma |
| DepoCyt (Pacira) | Liposome | Cytarabine | 20 μm | Malignant lymphomatous meningitis |
| Marqibo (Talon) | Liposome | Vincristine | 100 nm | Acute lymphoblastic leukemia |
| Myocet (Cephalon) | Liposome | Doxorubicin | 80–90 nm | Combination therapy with cyclophosphamide in breast cancer |
| Antifungal | ||||
| Abelcet (Sigma–Tau) | Lipid drug particles | Amphotericin B | 2–5 μm | Aspergillosis |
| AmBisome (Astellas) | Liposome | Amphotericin B | <100 nm | Antifungal, leishmaniasis |
| Amphotec (Alkopharma) | Micelle | Amphotericin B | 115 nm | Aspergillosis |
| Vaccine | ||||
| Epaxal (Crucell) | Virosome | Hepatitis A antigen | 150 nm | Hepatitis A |
| Inflexal V (Crucell) | Virosome | Influenza antigen | 150 nm | Influenza |
| Analgesics | ||||
| Diprivan (Fresenius Kabi) | Lipid emulsion | Propofol | 180 nm | Anesthesia |
| DepoDur (Pacira) | MV liposome | Morphine | 17–23 μm | Postsurgical pain |
| Exparel (Pacira) | MV liposome | Bupivacaine | 24–31 μm | Analgesia |
| Other | ||||
| Visudyne (QLT) | Liposome | Verteporfin | – | Age-related macular degeneration |
| Estrasorb (Medicis) | Micelle | Estradiol | 600 μm | Menopausal therapy |
MV, multivesicular.
Table 2.
Select Lipid-Based Products in Clinical Development
| Therapeutic | Product Name | Sponsor | Indication | Trial Phase |
|---|---|---|---|---|
| BLP-25a | Stimuvax | Merck | Nonsmall cell lung cancer | Phase III |
| Cytarabine | CPX-351 | Celator | Acute myeloid leukemia | Phase III |
| MHC Ib | Allovectin-7 | Vical Inc. | Metastatic melanoma | Phase III |
| Cisplatin | Lipoplatin | Regulon | Nonsmall cell lung cancer | Phase III |
| SPI-77 | NYU | Ovarian cancer | Phase II | |
| Aroplatin | NYU | Malignant mesothelioma | Phase II | |
| Doxorubicin | ThermoDox | Celsion | Primary hepatocellular carcinoma | Phase III |
| Refractory chest wall breast cancer | Phase II | |||
| Colorectal liver metastases | Phase II | |||
| 2B3-101 | To-BBB | Brain metastases and glioma | Phase II | |
| Meningeal carcinomatosis | Phase II | |||
| MPL/QS21c | RTS,S/ASO1B | GSK | Malaria | Phase II |
| Oxaliplatin | MBP-426 | Mebiopharm | Gastrointestinal adenocarcinoma | Phase II |
| Paclitaxel | LEP—ETU | Insys | Breast cancer | Phase II |
| EndoTAG-1 | MediGene | Breast cancer | Phase II | |
| PNU-91934 | MSKCC | Esophageal cancer | Phase II | |
| SN38d | CPX-1 | Celator | Colorectal cancer | Phase II |
| LE-SN38 | C&L Grp B | Metastatic colorectal cancer | Phase II | |
| MM-398 | Merrimack | Gastric and pancreatic cancer | Phase II |
The BLP-25 lipopeptide is a 25-amino-acid protein sequence (STAPPAHGVTSAPDTRPAPGSTAPP) containing a palmitoyl lysine residue at the carboxy terminal. BLP-25 provides specificity of the mucin 1 (MUC1) integral membrane protein to stimulate an anti-MUCl immune response.
Allovectin-7 is a cancer immunotherapeutic formulated as a plasmid/cationic lipid complex containing DNA sequences encoding HLA-B7 and beta-2-microglobulin—the heavy and light chains of the major histocompatibility complex (MHC) class I, respectively.
RTS,S/AS01B is a recombinant hybrid peptide malaria candidate vaccine formulated as a liposome adjuvant system with immunostimulants monophosphoryl lipid A (MPL) and QS21 (a natural saponin that is the purification fraction 21 from the bark of the South American tree Quillaja saponaria).
SN38 (7-ethyl-10-hydroxy-camptothecin) is the active metabolite of prodrug irinotecan (CPT-11), converted through carboxylesterase enzymes.
C&L Grp B, Cancer and Leukemia Group B; GSK, GlaxoSmithKline; MSKCC, Memorial Sloan-Kettering Cancer Center; and NYU, New York University School of Medicine.
Since our last review on liposome drug delivery systems,10 research continues to fuel development of liposomes and lipid nanoparticles that improve the pharmacokinetics and therapeutic index of drugs by extending their margin of safety and efficacy. This manuscript discusses the emerging research and clinical developments in liposome and lipid nanoparticle delivery of therapeutics. We highlight opportunities for value-added clinical translation of compounds based on this platform. To do so, we first discuss physiochemical properties that are key to characterize and optimize prior to in vivo scaling.11 As recent reviews focus on biophysical and chemical aspects of liposome preparation, characterization, targeting, and optimization, we briefly discuss basic properties of liposomes and lipid nanoparticles.3,11–14 We next discuss scale-up considerations then in vivo delivery and current advances in passive and active drug targeting. This is followed by applications of liposomes and lipid nanoparticles as multifunctional carriers, vaccines, gene therapeutics, and oral drug delivery systems. We conclude with a highlight on future directions and innovations in liposome and lipid nanoparticle therapeutics.
BASIC PROPERTIES OF LIPOSOMES AND LIPID NANOPARTICLES
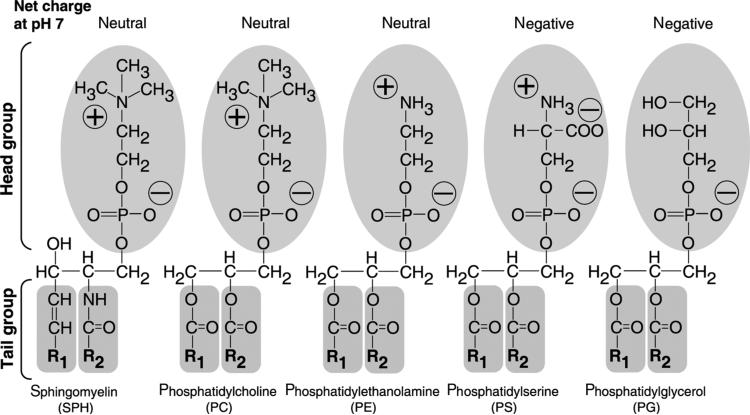
Lipid vesicles or liposomes are colloidal particles composed of phospholipid molecules that form contiguous membrane bilayers able to entrap solute. Although liposomes and lipid nanoparticles may be prepared with nonphospholipid molecules such as cardiolipin and other synthetic derivatives, to date most all core lipids derive from a phospholipid backbone structure. Lipid nanoparticles, on the contrary, may have a significant fraction of drug and other lipid-bound molecules such that thermodynamically stable lipidic nanoparticles are formed. They may or may not stably encapsulate a solute within the aqueous compartment. Although the specific composition and constituents for each liposome or lipid nanoparticle varies, most pharmaceutical formulations use synthetic products of natural phospholipids and their derivatives. Some of the major phospholipids typically used in pharmaceutical applications are presented in Figure 1. Liposome and lipid nanoparticle-based therapeutic drugs approved for humans typically contain phosphatidylcholine (PC; neutral charge) as a major membrane building block, with fatty acyl chains of varying lengths and saturation (Table 3). In some cases, cholesterol (~30 mol % of total lipid) is included to increase rigidity and reduce serum-induced membrane instability because of serum protein binding.15 Cellular and physiological mechanisms may also influence lipid particle surface charge, membrane fluidity, surface hydration, size, and distribution and clearance of lipid-associated drug from the body.
Figure 1.
A schematic presentation of commonly used phospholipids. Most of the commonly used lipids are presented with hydrophobic R1 and R2 fatty acyl tail groups and a hydrophilic head group carrying a net charge at neutral pH 7. The head group determines the charge of a phospholipid, whereas the lipid tail group contributes no charge. The lipids with head groups (oval shape shaded area) for sphingomyelin (SPH), phosphatidylcholine, and phosphatidylethanolamine exhibit neutral net charge. Phosphatidylserine and phosphatidylglycerol carry a negative net charge at neutral pH 7. The tail groups (R1 and R2) for each phospholipid can have various lengths (typically C14–C18) and degrees of saturation. SPH contains a sphingoid base backbone (unshaded) and the other four phospholipids contain a glycerol backbone (unshaded). In addition, R1 of SPH is a C15-saturated carbon chain and R2 is a fatty acid residue connected to the sphingoid base backbone through an amide functional group. The fatty acid residues for the other four phospholipids are attached to the glycerol backbone via an ester functional group. The detailed effects on the physical properties of phospholipids because of charge and variation in R1 and R2 are described in Table 3.
Table 3.
Attributes of Head and Fatty Acyl (Tail) Groups for Commonly Used Phospholipids
| Phospholipid Property | Effect on Liposome Membrane and Nanoparticle Characteristics | Functional Attributes |
|---|---|---|
| Head group | ||
| SPH/choline: –(CH2)2–N(CH2)3+ | Some surface hydration | Neutral charge |
| Ethanolamine: –(CH2)2–NH3+ | Minimal surface hydration | Neutral charge |
| Serine: –CH2–CH(COO–)–NH3+ | Some surface hydration | Negative charge |
| Glycerol: –CH2–CH(OH)–CH2OH | Some surface hydration | Negative charge |
| PEG (ethanolamine): –(CH2)2–NH-PEGa | Enhanced surface hydration and steric effect | Negative charge |
| Tail group—fatty acyl chains: R1 and R2 (C14–18 in length) | ||
| Increase in the degree of saturation | More rigidity; less fluidity | Elevated Tc |
| Increase in the chain length of R1 and R2 | Increased thickness of bilayer | Elevated Tc |
| Varying degree of saturation and chain length on R1 and R2 | Decreased order of membrane packing | Reduced Tc (compared with phospholipid with two identical fatty acyl tails) |
PEG: –[O–(CH2)2]n–OH.
Tc, lipid-phase transition temperature.
Depending on lipid composition, preparation methods, and physical structure, lipidic particles may assume a configuration other than liposomes. As schematically shown in Figure 1, lipids and phospholipids contain a charged or hydrophilic domain and two fatty acyl chains (tails) typically 14–18 carbons in length. In solution, phospholipids and adjacent lipid molecules interact and align to form contiguous bilayer sheets. The bi-layer sheets in solution form enclosed vesicles analogous to cells with a spherical membrane. Depending on the fatty acyl chain length of lipids and lipid structure, each lipid bilayer or lamellae assumes a thickness of 3–6 nm. Liposomes can also have more than one lipid bilayer—multilamellar vesicles (or MLVs) consist of several concentric (multiple onion-like) bilayers and have spherical diameters of 500–5000 nm. Multivesicular liposomes (MVLs)—the lipid platform for DepoDur and Exparel (Table 1)—are structurally distinct from multilamellar liposomes. They are aggregates of hundreds of water-filled polyhedral compartments separated by lipid bilayer septa and are 5000–50,000 nm in diameter.16,17 These large MVLs are also known as DepoFoam.
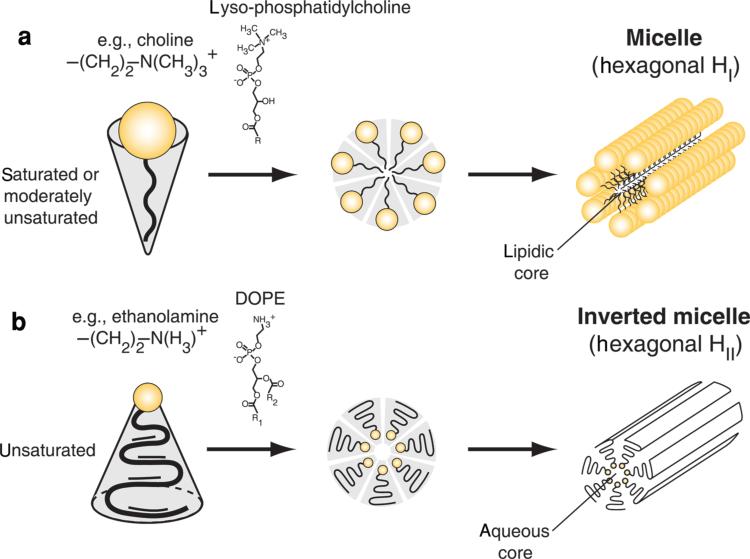
Micelles, on the contrary, are lipid aggregates with a lipophilic core and polar surface (Fig. 2a). In some cases, micelles may contain a small polar core and lipophilic surface exposed to aqueous environments as the thermodynamically most favorable aggregates (Fig. 2b). These inverted micelles are formed by phospholipids with a smaller head group, such as phosphatidylethanolamine (PE; compared with PC with a larger head group diameter), and a moderately unsaturated fatty acid tail. In solution, inverted micelles tend to form higher-order tube-like aggregates constructed of sheets of extended parallel stacks. These structures are known as the hexagonal (HII) lipid polymorphic phase.18 Although liposomes can serve as a drug carrier for tissue, cell, and intracellular targeted delivery, micelles may act as a solubilizer for water-insoluble drugs. Micelles enable injectable preparations of otherwise insoluble drugs into a colloidal emulsion or solution suitable for human administration. These small lipid nanoparticles, while physically stable, may not necessarily have a lipid membrane, nor enclosed aqueous or lipophilic core. Instead, they may exist as a lipid matrix of one or several lipid monolayers or bilayers, within or encapsulating other materials such as polymers, quantum dots, gold, iron oxide, or silica.
Figure 2.
A schematic presentation of lipids and derivatives that form micelles and inverted micelles. (a) Lipidic micelles (hexagonal HI) are formed because of a large hydrophilic head group, such as a lyso-phosphatidylcholine with a choline head group and a saturated fatty acid. They form stable molecular aggregates that resemble sheets of tubes with an internal lipidic core. (b) Inverted micelles (hexagonal HII) are formed because of phospholipid with a neutral and small head group, such as phosphatidylethanolamine, with unsaturated fatty acyl tails that tend to form inverted cone structures in solution (e.g., 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine or DOPE). They form stable molecular aggregates that resemble sheets of tubes with an internal aqueous space.
Regardless, it suffices to say that most liposome and lipid nanoparticle formulations use synthetic products of natural phospholipid carrying fatty acyl chains of various lengths and degrees of saturation. Although a mammalian cell membrane contains about 500–1000 different lipid species,19 liposome therapeutic products are constructed with one or two phospholipids in the final composition to simplify characterization and scale-up preparation of licensed products. A simple and minimalist approach to selecting a lipid composition is necessary for clinical translation. The key consideration is to select a set of physical characteristics that provide optimal liposome and lipid nanoparticle stability in storage as well as specified clinical pharmacokinetic (disposition in vivo, particularly plasma clearance) characteristics. Such a focused approach has proved successful for developing therapeutic products based on this drug delivery system.
Surface Charge
Depending on the lipid composition and the head group of lipids, liposomes and lipid nanoparticles may carry a negative, neutral, or positive net charge (Fig. 1, Table 3). The overall net charge of the particles is typically expressed as surface or zeta potential. Particles without charge have higher tendency to aggregate than those with net charge. In solution, surface charge of particles depends on the lipid head group composition, salt, and pH. At physiologic pH 7.4, therapeutic liposomes and lipid nanoparticles composed of sphingomyelin (SPH), PC, or PE carry a neutral net charge, whereas phosphatidylserine (PS) and phosphatidylglycerol (PG) exhibit one negative net charge (Fig. 1).
The nature and density of the surface charge may impact stability, pharmacokinetics, biodistribution, and cellular affinity and drug internalization. Upon entering the circulation, negatively charged liposomes are subjected to opsonin protein binding (liposome opsonization). Although opsonization of bacteria and viruses (which often carry a negative net charge) reduces the electrostatic surface repulsion between invading microbes and phagocytic cells (macrophages) of the mononu-clear phagocyte system (MPS), whether this mechanism is key to the observation that negative charge enhances cellular uptake in vivo is not clear. Nevertheless, negatively charged particles containing PS or PG have been shown to enhance cellular uptake through endocytosis at a faster rate and to a greater extent than neutral counterparts.20,21 Moreover, negative surface charge is recognized by receptors found on a variety of cells, including macrophages.20,22 Inclusion of glycolipids, such as the ganglioside GM1 or phosphatidylinositol significantly reduces uptake by macrophages and MPS cells, resulting in prolonged blood circulation times. A small amount of negatively charged lipids may stabilize neutral liposomes against an aggregation-dependent phagocytic uptake mechanism.23 On the contrary, when positive charges are not fully neutralized by negatively charged DNA, cationic liposomes and lipid nanoparticles with net positive charge have a tendency to interact with proteins in serum. These interactions may potentially lead to compliment activation by certain serum proteins adsorbing to the particle surface. In some instances, this process may also enhance uptake by the MPS and cause eventual clearance by the lung, liver, or spleen.24
Recently, it was reported that macrophage uptake of polysaccharide nanoparticles with 150 nm diameter increases when negative and positive charge density increases; however, up-take of particles with positive charge appeared to be nearly twofold higher than negative particles.25 Thus, for equivalent and larger particles, carrying net positive charge tends to enhance macrophage and other phagocytic uptake. At high lipid doses, cationic liposomes activate the classical complement pathway, and negatively charged liposomes activate the alternate (lectin) pathway.26,27 Interestingly, complement activation is sensitive to the negative charge on the phosphate head group and appeared to be linked to the charge on phosphate. Negative liposomes without a phosphate group failed to induce complement activation.28,29 Thus, not all negatively charged liposomes have complement-activating potential. Taken together, positively charged liposomes increase plasma protein adsorption and exhibit higher tendency for untoward effects because of a higher rate of nonspecific cellular uptake. Negatively charged lipid particles are common to most FDA-approved therapeutic lipid–drug formulations.
Fluidity of Lipid Membrane and Lipid Nanoparticles
Organized in a thermodynamically most stable bilayer structure, lipid molecules in liposomes and lipid nanoparticles may exhibit a well ordered or gel phase below the respective lipid phase transition temperature (Tc), and a disordered or fluid phase above Tc. The Tc is measured by a number of methodologies including fluorescence probe polarization, calorimetry, and electron spin resonance of membrane spin probes. The Tc is sometimes referred to as the lipid melting temperature or Tm. At the Tc, equal proportions of the two phases coexist. Because of the formation of segregated gel and fluid domains within the bilayer at Tc, a maximum in liposome leakiness is observed.30 Overall, the phase behavior of a liposome membrane determines permeability, aggregation, protein binding, and to a lesser degree, fusion of liposomes. As outlined in Table 3, the Tc of each lipid molecule depends on the length and nature (saturated or unsaturated) of its fatty acid chains. Thus, by selection and appropriate combinations of lipids, the fluidity of lipid bilayers can be predicted for physiological temperature (37°C). For instance, liposomes with distearoylphosphatidylcholine (DSPC; Tc = 55°C) with its 18-carbon fatty acyl chains would exhibit the gel phase, whereas dimyristoylphosphatidyl choline (DMPC; Tc = 24°C) with two symmetrical 14-carbon fatty acyl chains would be in the fluid phase at physiological temperature. The intermediate 16-carbon saturated fatty acyl chain containing dipalmitoylphosphatidylcholine (DPPC; Tc = 41°C) would form mostly the gel phase at 37°C. Introduction of a double bond or unsaturated fatty acid to DPPC, that is, dioleoylphosphatidylcholine (DOPC) with its two oleoyl C18:1c9, reduces the Tc to –17°C. Incorporation of other lipidic molecules such as cholesterol (up to 30% of the total amount of membrane PC) into a PC bilayer may lead to an increase in membrane fluidity and broaden the temperature range in which the lipid membrane goes into transition.31 In other words, such addition has a buffering effect on the Tc. More recently, additional derivatives of cholesterol including chimera cholesterol– PC derivatives have been reported to further improve membrane stability.32
Phase transition behavior of lipid bilayers has been exploited to enhance liposome aggregation, lipid transfer, and drug release. It is important to note that while desirable, fusion between liposomes and cells requires high activation energy because of membrane-bound water. Thus, fusion is a rare event without the help of fusion proteins or significant energy input such as pH, temperature, or other environmental sources. In contrast, liposome aggregation (requiring a lower energy) could mediate membrane destabilization that leads to the release of encapsulated drug. Following administration, the temperature of gel phase liposomes or lipid nanoparticles accumulated in local tissue can be raised to Tc with external heat sources such as infrared, microwave, ultrasound, or lasers. However, such strategies must account (compensate as necessary) for the Tc depression because of drugs bound to lipid membranes or protein-bound lipid membranes. In some instances, drug binding may abrogate the phase transition behavior altogether.33,34 Additionally, binding of serum proteins may influence the phase transition behavior and also the premature release rate of drug trapped within the aqueous compartment of liposomes.35 Moreover, fluidity, in particular liposomes that exhibit phase transition behavior at or near physiologic temperatures (37°C), may enhance the activity of cell surface phospholipases that degrade lipids and generate lysophospholipids (by deacylation at the A1 or A2 positions of phospholipids). In another scenario, the formation of micelles within the lipid bilayer because of increasing concentrations of lysophospholipids may accelerate the drug release rate because of the surfactant property of lysolipid micelles. Intrathecally administered lysophospholipids have been shown to elicit neurobehavioral toxicity in rats.36 Collectively, appropriate lipid compositions that provide fluidity necessary to maintain lipid structure, as well as physical properties at physiological temperature, are key considerations in designing liposome and lipid nanoparticle drug delivery systems.
Surface Hydration or Steric Effect
It has been known for quite some time that the degree of hydration on the membrane surface plays a role in liposome aggregation. Increasing the hydration shell on the membrane tends to reduce liposome aggregation and phagocytic cell uptake. Thus, in the 1980s, attempts were made to increase membrane hydration to reduce aggregation and avoid recognition of the MPS by coating the membrane surface with hydrophilic polymers. Initial efforts used glycolipids and gangliosides, such as GM1 or lipids that are chemically conjugated to hygroscopic or hydrophilic polymers, including various lengths and branching of polyethylene glycol (PEG) and polymeric glycosytic chains. It was later found that lipid-conjugated PEGs of varying lengths with a long-standing human safety profile are cost-effective, and provide a sufficient degree of surface hydration for pharmaceutical product development. The technology using PEG-modified liposomes and lipid nanoparticles is similar to protein PEGylation. For liposome incorporation, PEG can be conjugated to the terminal amine of PE, instead of conjugating PEG to therapeutic proteins such as adenosine deaminase (Alderase, for treatment of severe combined immunodeficiency syndrome) to reduce immune recognition and rapid clearance.37 PEG can also be conjugated to molecules such as cholesterol that anchor into the lipid bilayer, which has been explored for folate targeting.38 These PEG–cholesterol derivatives and PEGylated lipids are commercially available from several suppliers. PEGylated liposomes, sometimes referred to as sterically stabilized or stealth liposomes, were first described by Allen and Chonn.39 PEGylated liposomes greatly reduced macrophage binding and recognition as foreign particles, as well as phagocytic clearance by cells of the MPS through spleen and liver elimination. Systematic study results indicate the optimum PEG polymer size and the density of PEG is MWavg = 1000–2000 (Ref. 40) and 5–10 mol % total lipid. Depending on the length and density of the PEG polymer, PEG on the liposome membrane occupies an additional 5-nm surface hydration thickness41 without significantly modifying the overall charge property of liposome membranes.
PEGylated liposomes have greatly increased the plasma half-life of doxorubicin and have consequently allowed development of the liposomal doxorubicin product Doxil for cancer. The extended circulation plasma half-life achieved with PEG in lipid membranes allows the encapsulated drug, doxorubicin, to eventually accumulate in tumors through leaky blood vessels that supply tumor targets,42–44 a phenomenon known as the enhanced permeability and retention (EPR) effect.45 It should be noted that the EPR effect is not uniformly present in all tumors and has significant heterogeneity within and between tumor types. When present, it is a slow process that requires liposomal drug to be in the blood circulation for extended times. Without extension of the plasma lifetime of liposome drugs with PEG, the utility of the EPR effect would have been missed. Other water-soluble polymers46–51 have been explored to increase circulation time by resisting protein adsorption. However, PEG polymers appear to be more robust with acceptable safety data essential for product development considerations. Indeed, the long-standing human safety data on the use of PEG as an excipient for parenteral preparations are one of the key advantages of using PEG-conjugated lipid. Initially, there were concerns regarding heterogeneity of long-chain PEG polymers, purified from petroleum products. However, this issue has been solved with availability of synthetic homogeneous PEG polymer by Shearwater.52 One should be aware, however, that extremely large PEG polymers may exhibit slow renal clearance and thus could accumulate in the liver and remain in the body for quite some time.53 There are a number of reports that have raised concerns about the immunogenicity of PEGylation54 and associated accelerated blood clearance effect.55,56 However, many of these studies are carried out with liposome-bound PEG with various molecular weights and branching structures to elicit immunogenic response in animals. Also, well-validated assays for anti-PEG antibodies are lacking. Therefore, at present, it is difficult to draw definitive conclusions on the immunogenicity of PEG and potential clinical impacts.57 Regardless, surface hydration of liposomes and lipid nanoparticles with extended plasma half-lives has provided a clear direction that allows these particles to avoid premature phagocytic uptake and provide sufficient time in blood to passively navigate to target cells and tissues.
Impact of Size and Structure
It is well documented that the size of liposomes influences pharmacokinetics, tissue distribution, and clearance. Hepatic up-take and accumulation, tissue extravasation, tissue diffusion, and kidney excretion may depend heavily upon particle size. Only liposomes of a particular size (≤100–150 nm) are able to exit or enter fenestrated vessels in the liver endothelium or tumor microenvironment.58 Liposomes in blood vessels do not easily escape out of capillaries that perfuse tissues such as the lung, heart, and kidney if they are within the diameter range of 100–150 nm (Table 4). Liposomes and particles, 100– 200 nm in diameter, may distribute to bone marrow, spleen, and liver sinusoids, and to some extent may escape through discontinuous leaky capillaries within these organs. Although lung alveoli could trap particles of several micrometers in size, the pulmonary capillary barrier pore size is estimated to be around 35 nm, a value twofold to threefold lower than that of the pores within the endothelial lining of capillaries in the kidney. Islet tissues in the pancreas and glomerulus in the kidney have smaller pores with diameters reported to be around 10– 15 nm (Table 4). These tissue and capillary pore size data59–61 provide a context of why most liposome preparations of 50– 200 nm do not easily escape from continuous blood capillaries in their intact form. However, when extravasated from blood vessels (typically through discontinuous capillaries in the liver, spleen, bone marrow, and to some extent in the lung), liposomes greater than 100–150 nm are often taken up by phagocytes or remain in the tissues for an extended time. The majority of phagocytes with liposomes accumulate in the spleen and liver for eventual elimination. Once in a tissue, liposomes may be retained because of the pore size or interstitial dimensions of the tissue (Table 4).59,61–67
Table 4.
Estimated Pore Size of Capillaries and Organs
| Organ | Physiological Structure | Estimated Pore Size (nm) | Species |
|---|---|---|---|
| Capillary | Fenestrated (diaphragmed) (endocrine glands) | 6–12 | Human61 |
| Fenestrated (nondiaphragmed) (kidney glomerulus) | 10–15 | Human61 | |
| Discontinuous/leaky | 50–180 | Human/rabbit61 | |
| Heart | Left ventricle microvessels | 5 | Human62 |
| Lung | Pulmonary endothelium | 8–35 | Dog63 |
| Liver | Hepatic sinusoids | 110 | Human64 |
| Spleen | Splenic sinusoids | 200 | Rat/mouse59 |
| Kidney | Glomerular endothelium | 80–130 | Rabbit/mouse65 |
| Basement membrane | 3 | Rat66 | |
| Podocytes | 32 | Human67 |
Cellular internalization—phagocytosis, macropinocytosis, caveolin- and clathrin-dependent endocytosis, and caveolinand clathrin-independent endocytosis—may also be influenced by particle size.68–70 Caveolin- and clathrin-dependent and caveolin- and clathrin-independent endocytosis are most relevant to liposomes of 50–150 nm in diameter.71 Particles less than 10 nm undergo renal filtration through the glomerular capillary wall and are not reabsorbed.72 In mice, reduction of liposome size to 50 nm diameter or below greatly reduced MPS-mediated clearance73 and achieved a plasma half-life similar to those achieved with PEGylated liposomes 100–150 nm in diameter.74,75 In addition, in vivo MPS cell uptake can be saturated with high doses of liposomes with drug that inhibits phagocytic activity or by predosing with large quantities of control liposomes. However, these strategies may not be practical for clinical application because of the adverse effects related to the impairment of phagocytic functions in the MPS (a natural mechanism to clear microbe invasions).
Thus, to avoid MPS uptake and to prolong blood circulation time, most therapeutic liposomes and lipid nanoparticles are designed within 50–100 nm diameters. For example, DaunoXome—a liposomal cancer therapeutic—consists of 45– 80 nm diameter particles intended to reduce MPS uptake. Serum protein binding and related complement-dependent activation are shown to be dependent on liposome size and together, these two mechanisms increase the rate of clearance in vivo. In sum, liposome and lipid nanoparticle diameter less than 50–80 nm enjoy significantly lower MPS-dependent clearance in humans. With PEGylation, particles with diameters less than 100–150 nm exhibit reduced plasma protein binding, MPS and hepatic uptake, and longer blood circulation times.
SCALE-UP FROM LABORATORY TO CLINICAL PREPARATIONS—TRANSITIONING FROM PRECLINICAL TO CLINICAL STUDIES
Since the first FDA approval of a liposome-based doxorubicin pharmaceutical product in 1995, liposome and lipid–drug particle research activities that progress from in vitro and in vivo preclinical animal testing to clinical trials have increased dramatically. There are at least 107 active (out of 589 interventional) clinical trials containing the terms “liposome” or derivatives. It is essential that novel liposomal drug preparations, initially tested in the laboratory setting on a microliter scale, are adaptable and can maintain the same characteristics when prepared in liter volumes or more for preclinical and clinical testing. Large volumes are necessary to evaluate lipid particle preparations in appropriate animal models, such as efficacy and safety evaluations in rodents, nonrodents, and in some cases primates, which support regulatory submission for product licensing. Industrial-scale production of liposomal and lipid nanoparticle products for pharmaceutical purposes requires not only the ability to produce sufficient quantities, but also requires reproducibility and rigorous adherence to quality standards as described in the Good Manufacture Practice guidelines.
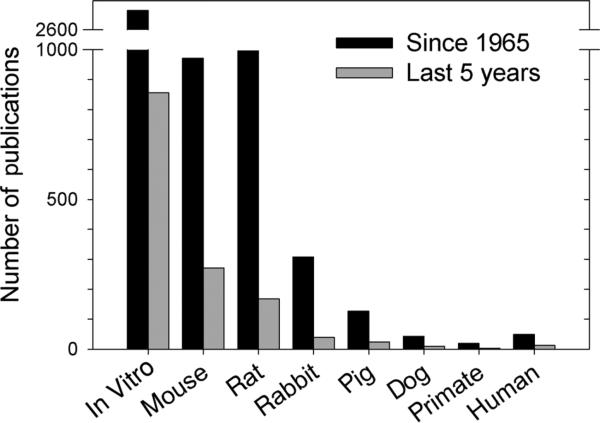
The development of suitable, scalable methods for liposome and lipid–drug particle production has posed a challenge for many laboratory scientists and innovators when it comes to translate their products from bench-top testing to in vivo studies and eventual clinical trials. One can gauge this difficulty by analyzing the published manuscripts for novel formulations tested in vitro in cell culture systems that progress to mice, rats, and nonrodent larger animals such as rabbits, pigs, dogs, and primates. An analysis of published reports since 1965 and the last 5 years is summarized in Table 5 and plotted in Figure 3. It is apparent that a majority of reports are either in vitro or utilize mouse models and a diminishing number of reports in the literature progress to primate and eventual human testing. These data suggest that less than 1% of reported novel liposomal formulations are likely to enter human clinical trials.
Table 5.
Number of Publications on Liposome Research In Vitro and In Specific Animal Species
| Search Terms in PubMed Liposome AND “(Term Below)” Search Date 7/25/13 | Publications Since 1965 | Publications Since 2008 | ||
|---|---|---|---|---|
| Number | Total (%) | Number | Total (%) | |
| In vitro | 2610 | 50.9 | 856 | 61.9 |
| Mouse | 972 | 19.0 | 271 | 19.6 |
| Rat | 996 | 19.4 | 168 | 12.2 |
| Rabbit | 308 | 6.0 | 39 | 2.8 |
| Pig | 127 | 2.5 | 24 | 1.7 |
| Dog | 43 | 0.8 | 9 | 0.7 |
| Primate | 19 | 0.4 | 3 | 0.2 |
| Clinical Trial | 49 | 1.0 | 12 | 0.9 |
Figure 3.
Number of publications on liposome research in vitro and in specific animal species. Data recorded in the PubMed database were identified with the search terms “liposome AND (‘in vitro’ or specific animal species).” For “human,” the term “clinical trial” was used for the search query. Data were compiled and plotted as a bar graph for the number of publications since 1965 and the last 5 years (2008–2013). A summary of numerical data is presented in Table 5.
Although the decision to advance a project through in vivo studies is complex, all projects moving into clinical development must be scaled from laboratory to clinical volumes and must meet a number of challenging criteria. The final product must be: (1) within the uniformity specification, (2) reproducible within a defined size range, (3) sterile in the case of injectable formulations, (4) devoid of any potentially harmful additives, and (5) stable in storage (with adequate shelf-life). These are some criteria relevant to injectable liposome and lipid nanoparticle products and are in addition to other quality control measures essential for licensing approval of injectable drugs, discussed in detail elsewhere.76,77 Also, the preparation process must be time-efficient and cost-effective if it is to be industrially viable.
At all stages of development, it is critical to envision a diagnostic, therapeutic, or vaccine product for which the preparation method is adaptable to industrial scale production. Even if a novel concept proves promising, a complicated preparation procedure that cannot be adapted to a larger scale for pre-clinical testing drastically diminishes the translational potential. Thus, one must consider designing a scalable or adaptable method early in research and development so that the liposome characteristics of the large-scale product will be similar to its small-scale counterpart. For preparations with only a fraction of drug encapsulated or incorporated into lipid particles, removal of free drug through additional purification steps, although necessary, may add significant cost, time, and risk of contamination. In what follows, we briefly review liposome and lipid particle preparation procedures and highlight their potential for commercial scale-up.
Because of its simplicity, most laboratory investigations use the lipid thin-film hydration method, first described in 1965, followed by size reduction to prepare small unilamellar liposomes.78 The hydrated lipid film produces large MLVs or liposomes. Then, a sonication, homogenization, or extrusion procedure is used to reduce the particle size and form unilamellar structures. Variations of this laboratory method are still widely used for liposome preparation on a micro- to milliliter scale. A number of attempts have been made using this method to produce liposomes on a several-hundred milliliter scale for preclinical testing, including that reported by Asmal et al.79 to evaluate the antiviral efficacy of liposome-encapsulated antithrombin-III in primates. However, thin-film hydration has a number of drawbacks. As the capacity of the drying vessel is dependent on the final liposome volume, large-scale production would require expansive equipment with a large surface area over which to coat the lipid film. This problem could potentially be overcome by spray drying and other industrial procedures.
Another disadvantage of thin-film hydration is that it produces large MLVs. In contrast, a majority of liposomal drug products are smaller particles that require significant size reduction from several microns to 50–200 nm in diameter. The ultrasonic technique, typically using bath- or probe-type sonicators that disrupt MLVs, is convenient for small-scale preparation but is not suitable for scale-up production because of several technical challenges. It is difficult to provide uniform ultrasonic energy input over a large volume of material, the risk of oxidation and degradation of phospholipid is high, and metal leaching from the sonicator probe is well documented.80,81 Although attempts have been made to control “cycles per burst” and duration to improve sonication procedures, significant hurdles remain.
Homogenization techniques rely on high-velocity collisions to reduce particle size. Mayhew et al.82 have developed a microemulsifier that splits a sample of large, heterogeneous lipid particles into two streams and recombines them in a continuous, multicycle, high-velocity, high shear-force collision, leading to the production of monodisperse liposomes less than 100 nm in diameter.82 A number of high-pressure homogenization instruments based on the concept of high-velocity collision are available, including Microfluidic's HC series (Newton, Massachusetts) and Avestin's Emulsiflex homogenizers (Ottawa, Canada). The ability to run as a continuous-flow process means that large-scale homogenization does not necessarily require massive equipment, making it technically appealing. By controlling formulation, concentration, pressure, and number of homogenization cycles, homogenization becomes a controllable, scalable, and reproducible size-reduction method.83 New high-pressure homogenization technologies and process control procedures are available to control product degradation and temperature. Although small (~50 nm diameter) particles can be uniformly produced by this method, intermediate to large particles cannot be made with this approach without assistance with other filtration/extrusion technology. Although these high-pressure continuous-flow instruments provide high-throughput potential, scalability, and reproducible size reduction efficiency, a significant capital investment and measurable volume loss during production could pose significant barriers for researchers with limited materials.
Low-pressure extrusion of liposomes through a series of filters with defined pore diameters to reduce particle size could provide preclinical and clinical scale materials with less volume loss compared with homogenizers. Typically, these instruments can be used to produce a few milliliters to greater than 10 L of product. The advancements in filter matrices, such as those made from polycarbonate, have enabled innovations in the production of filters with uniform pore diameters as small as 35 nm with little variation. The lipid particles are extruded serially through a polycarbonate filter (e.g., Nucleopore with a defined pore size) to produce lipid nanoparticles with a relatively uniform size distribution. There are several commercial extruders available, including the Lipex (Northern Lipids, Burnaby, Canada), Maximator HPE 12.0–100 (CPL Sachse, Berlin, Germany), and LiposoFast (Avestin, Ottawa, Canada). Stable liposomes in volumes up to 0.5 L have been produced aseptically with a Lipex extruder for clinical studies and for in vivo studies in non-human primate models.79,84,85 However, large-scale extrusion is hindered by the difficulty of controlling the temperature of large extrusion volumes as well as the tendency of lipid to deposit on the filter membrane, causing slow flow rates and clogging of the pores. Filter clogging may be addressed by innovative cross-flow designs, such as continuous low-pressure extrusion through a hollow-fiber membrane with tangential flow to reduce clogging.86
Instead of thin-film hydration and size reduction, liposomes can be produced by mixing the organic phase containing the dissolved lipid with the aqueous phase at defined conditions. Reverse-phase evaporation procedures are based on this strategy, creating an emulsion between the organic and aqueous phases and subsequently removing the organic solvent by evaporation to form liposomes.87 An alternative but more robust approach is to rapidly inject the lipids dissolved in organic solvent into an excess of aqueous solution. First described by Batzri and Korn,80 ethanol injection involves dissolution of the lipids in ethanol followed by rapid injection of the ethanol mixture into the heated aqueous phase. Upon injection, the lipids immediately form bilayer vesicles that encapsulate aqueous content.80 By adjusting parameters such as injection temperature and the ethanol–water ratio, liposome size can be well controlled.88,89 Ethanol injection methods and their derivatives, such as those employing a membrane through which the ethanol is injected, are capable of producing liposomes with average diameters less than 100 nm and low polydispersity.89,90 In an effort to make a fully scalable system, Wagner et al.76,91 developed a cross-flow injection module in which the aqueous phase is pumped from its starting vessel to a collecting vessel, and the ethanolic phase is injected mid-way at an injection module. This could be run in a continuous fashion with scaling solely dependent on the size of the attached vessels.76,91 Variations of the ethanol injection method have been used to produce a number of liposomal pharmaceutical products. Some modifications may be needed for certain lipid–drug formulations because not all lipids and drugs are soluble in ethanol and inadequate dissolution or mixing could result in heterogeneous composition and size of liposomal drug products.92 However, solvent injection techniques may be an ideal procedure for lipid compositions that are soluble in pharmaceutically compatible solvent such as ethanol because of the simplicity, versatility, and scalability of the process.
Some proteins and oligonucleotides are sensitive to denaturation in organic solvent and require gentler handling. Detergent dialysis or depletion is a potentially scalable procedure that may be more suitable for these agents. Lipids are mixed with a surfactant or detergent in aqueous solution to produce micelles, and subsequent dilution or removal of the detergent produces liposomes with the ability to encapsulate proteins and oligonucleotides in their native form.95 Detergent depletion incorporating capillary dialysis has been used to produce sterile liposomes (d = 50 and 200 nm) in quantities up to 5 L for clinical application.94 Detergent depletion is simple, flexible, mild, and potentially scalable, but has several significant disadvantages. Encapsulation of hydrophobic compounds is poor using the dilution method, but methods used to remove the detergent may also remove hydrophilic compounds. The multistep process can also be time-consuming.92,93 These hindrances, particularly the challenge of removing residual trace amounts of detergent, make detergent dialysis and depletion methods more costly for industrial-scale preparations.
There are other laboratory procedures described for liposome and lipid particle preparation including double emulsion, freeze–thaw, dehydration–rehydration, fast-extrusion, and recently, the use of supercritical carbon dioxide. Pressurized carbon dioxide acts as a solvent into which the lipids are initially dissolved. Rapid depressurization with simultaneous mixing of the precipitating lipids into the aqueous phase results in the spontaneous generation of liposomes.95 Supercritical carbon dioxide has garnered particular interest in the biotechno-logical community because of its antimicrobial properties and potential as a sterilizing agent, which could be beneficial in the production of liposomes for clinical use.96,97 Although some of these methodologies appear to be robust for small-scale production, and some have been tested on a larger scale, they are still in the exploratory and developmental stage for large-scale preparation.
In summary, there are several large-scale liposome and lipid particle preparations that are available to produce pharmaceutical products. When possible, scale-up issues should be considered early in the course of developing new lipid–drug formulations intended for pharmaceutical application. Relevant advantages and disadvantages of the techniques discussed above are summarized in Table 6. Although ethanol injection and high-pressure homogenization are proven methods to produce clinical products of lipid nanoparticles on a large scale, detergent depletion techniques may be more gentle and suitable for protein therapeutics and gene therapeutics.
Table 6.
Select Methods of Liposome Preparations and Their Advantages and Disadvantages in Scale-Up Procedures
| Basic Technique | Advantages for Scaling | Disadvantages for Scaling | Scalability Potential | |
|---|---|---|---|---|
| Formulation method | ||||
| Thin film hydration | Solvent evaporation followed by rehydration in aqueous phase | Simple | Requires size reduction Equipment size is volume dependent |
Suitable for small to mid-size batches |
| Reverse-phase evaporation | Mixing of immiscible solvent with aqueous phase to form emulsion followed by evaporation of solvent | Simple | Multistep process Size reduction required |
Suitable for small to mid-size batches |
| Solvent injection | Injection of miscible solvent (generally ethanol) into aqueous phase | Single-step process Continuous processing |
Presence of solvent without postremoval Not all lipids/drugs dissolve in ethanol |
Very good |
| Detergent depletion (dialysis) | Mixed-micelle formation with detergent followed by detergent dilution or removal | Gentle | Presence of detergent Multistep process |
Good for sensitive proteins and oligonucleotides |
| Supercritical fluid | Solvation of lipids in supercritical carbon dioxide followed by injection into low-pressure aqueous phase | No organic solvent Sterility |
Expensive equipment | Good potential |
| Size reduction | ||||
| Sonication | Ultrasonic energy to disrupt vesicles | Simple | Poor reproducibility Polydisperse population |
Suitable for small batches only |
| High-pressure homogenization | High-velocity collisions mechanically disrupt vesicles | Monodisperse population Reproducible Continuous processing |
Volume loss Limited size control |
Very good |
| Low-pressure extrusion | Forcing through a filter of defined pore size | Monodisperse population Reproducible Continuous processing |
Clogging of membrane Difficult to maintain temperature |
Good for small to mid-size batches |
DISPOSITION OF LIPOSOMES AND LIPID NANOPARTICLES IN VIVO: PRECLINICAL AND CLINICAL INSIGHTS
As with any drug development, the intended therapeutic target drives the final lipid composition of lipid–drug particles. As a result, mechanisms of biodistribution, disposition, and pharmacokinetic parameters measured in vivo vary with lipid composition, size, charge, and degree of surface hydration/steric hindrance. In some cases, the degree of drug binding to lipid and membrane structure may also influence the overall disposition profile. In addition, drug administration routes may determine the rate and extent of target and off-target tissue exposure. Intravenously (i.v.) administered liposomal drug formulations, for example, gain immediate access to blood and rapidly distribute to highly perfused tissues such as the liver, kidney, and spleen that regulate drug elimination. Intravenously administered lipid–drug particles may also expose or bind immediately to plasma proteins. In contrast, intramuscularly (i.m.) administered liposomal drug may gain access to the blood much slower, providing sustained but lower levels of plasma drug concentration over time. Depending on lipid composition and particle size, subcutaneously (s.c.) administered lipid–drug particles may provide extended but lower plasma drug levels than the i.m. route; in some cases, they could circulate as lipid–drug complexes in the lymphatic system before drug finds its way to the blood. Although some success in topical and oral routes of liposomal drug application has been reported, to date there is no liposomal therapeutic product given orally. Therefore, our discussion focuses on the application of liposome and lipid nanoparticle drug delivery systems designed for systemic—i.v., i.m., and s.c.—dosage forms.
Regardless of any route of administration, drug encapsulated or associated to lipid particles traverse to target and off-target tissues through the blood or lymphatic circulation. Most often, the blood carries free drug, lipid-associated drug, or the mixture of both forms into tissues through capillary perfusion. Drug-carrying particles composed of lipid and lipid membranes may interact with plasma proteins in blood that include albumin, lipoproteins (i.e., HDL, LDL, etc.), and other cell-associated proteins. Although it is possible that both the amount and identities of proteins on the particle surface have a direct effect on the biodistribution of nanoparticles, the precise mechanism of protein binding is not well understood, nor is it known how the amount of protein binding triggers a biological response.98 Approximately 20 (Refs. 99,100) of roughly 3700 proteins that make up the plasma proteome101,102 have been associated with lipid particles. Some of these proteins (e.g., apolipoprotein A-I of HDL via the reverse cholesterol transport pathway) may remove phospholipids and fatty acids (such as oleic acids in some liposome compositions) in the lipid bilayer, thereby destabilizing the liposome and membranes.103–105 As a result, encapsulated or lipid-associated drug may leave or dissociate from the complex prematurely. In addition, in the case of acid- or pH-responsive liposomes containing fatty acid derivatives or acid-responsive lipids, protein binding may abrogate the pH sensitivity of liposomes. Lipid–protein interactions may also explain the drastically reduced transfection activity of DNA–cationic lipid complexes in vivo. Also, plasma protein binding has been shown to modify the gel-to-fluid phase transition of phospholipids with a saturated fatty acyl chain, such as DPPC (Tc = 41°C).106 Aside from modifying the drug release from liposomes, protein binding, particularly to cationic lipids, may also lead to immunologic consequences such as complement activation because of the nonspecific cationic lipid binding in rats.107 Whether complement activation is a significant issue in delivery of DNA in humans with cationic lipids remains to be addressed.
Nevertheless, there is a need to account for the role of complement activation and opsonization on clearance when designing liposome and lipid nanoparticle formulations.108 Lipid–protein interactions may increase the phagocytic activity and nonspecific cell uptake in tissues leading to rapid liposome and lipid nanoparticle clearance in the spleen and liver and to some extent in the kidney, the major elimination organs. Liposomes and lipid nanoparticles coated with hydrophilic polymers such as PEG and glycolipids have reduced protein binding and phagocytic-mediated rapid clearance. Although inclusion of PEGylated lipids has greatly reduced MPS-mediated clearance, drugs in liposomes and lipid nanoparticles are typically and eventually cleared by the liver and disposed through biliary elimination. A fraction of drug in these particles may distribute to the target sites of action (e.g., where rapid tumor growth occurs). Also, a small fraction of liposomes may distribute to skin and extremities, and clear from these tissues at a much slower rate. The drug levels in these off-target sites may accumulate with repeated- or multi-dosing regimens. Although enhanced doxorubicin localization of liposome-formulated drug to the skin, for example, may provide therapeutic benefits for Kaposi's sarcoma skin disease, it may also produce dermal lesions in cancer patients, which is referred to as hand and foot syndrome (Palmer–Plantar erythrodysesthesia syndrome). It has been proposed that infection and tumor growth induce inflammation, leading to vasculature permeability (EPR effects), which thereby enhances the accumulation of liposome-associated or liposome-encapsulated drugs to these sites of inflammation.109 In this scenario, PEGylation prevents “first-pass” hepatic clearance of lipid particles, which is a fast process, and thus provides lipid nanoparticles sufficient time in the blood for the slower tissue penetration kinetics to catch up; the net result is a higher degree of lipid-associated drug accumulated in target (e.g., tumors or infection) sites.
Following subcutaneous or intramuscular injection, large MVLs may become trapped at the injection site and serve as a drug depot.17,110 Smaller liposomes primarily disperse from the injection site through lymph vessels and arrive at a draining lymph node. If small enough, liposomes and lipid nanoparticles (especially smaller micelles) proceed through the lymphatic system and enter into the blood. Uptake into the lymphatics and movement from nodes into the lymph vessels is predominantly size dependent.111 Particles 10–80 nm in diameter administered s.c. readily enter and exit the lymphatic system.112 In dogs and rabbits, the estimated upper size limit for particles to pass through lymph nodes and proceed through the lymphatic circulation is 20–30 nm.113,114 Therefore, particles greater than 40–50 nm in diameter are retained in nodes.115,116 However, because of their size, these particles are confined to lymph node sinuses.117 These properties may be leveraged to accumulate liposomes in the lymph nodes. This could serve to halt the metastatic lymphatic progression of cancers.118,119 Size-dependent particle distribution in the lymphatic system can also be used to attack the high viral loads that persist in lymphoid tissues of HIV-infected patients despite multidrug therapy eliminating virus in the blood.120–123 Our research indicates that when s.c. administered in primate macaques, liposomes and lipid-associated drug nanoparticles containing HIV protease inhibitors accumulate in lymph nodes throughout the body at levels fivefold to 30-fold higher than in blood and beyond levels achievable by orally administered drugs.4,124
Below, we will briefly discuss the collective experience of the in vivo behavior of liposomes and lipid nanoparticles with appropriate circulation lifetimes (passive targeting) and liposomes conjugated to ligands with specific affinity for receptors within a tissue, cell, or intracellular target (active targeting).
Passive Targeting to Tissues and Cells
Improved understanding of how physiochemical characteristics of liposomes and lipid nanoparticles relate to their time course of distribution and elimination in the body has confirmed the ability to modulate the pharmacokinetics of a drug either encapsulated within or physically associated to a lipidic drug delivery system. Clearly, not all drugs must be present in the blood a long time to be therapeutically useful. However, some may require chronic exposure to tissues, cells, or blood. Unlike micellar drug formulations where the drug in the particle dissociates soon after diluting in the blood, liposomes and lipid nanoparticles are by design not susceptible to dilution effects, concentration-dependent drug release, or disintegration.
Taking advantage of the understanding of the large particle uptake potential of phagocytic cells, liposome-encapsulated and lipid-associated antifungal amphothericin B were designed with the intent to enhance drug accumulation in phagolysosomes within the same phagocytes that harbor the fungi. As these phagocytes traffic to and accumulate in the spleen, the antifungal drug amphotericin B (formulated in liposomes and lipid nanoparticles AmBisome, Abelcet, and Amphotec) gains direct access to the intravesicular sites (i.e., phagolysosomes within macrophages and phagocytes) of fungal growth without having to resort to ligand–receptor interactions. This strategy that exploits cellular and physiological processes and a basic understanding of particle clearance mechanisms is called passive targeting. In the case of amphotericin B, which exhibits renal toxicity because of drug aggregation and accumulation in renal tissues, lipid-formulated drug reduces renal toxicity, and thus in the process, reduces off-target (renal) drug accumulation and toxicity.
For drugs that require sustained blood and tissue levels for chronic conditions such as cancer and pain, rapid drug clearance into cells or tissues of drug elimination may become a barrier to clinical translation. In this case, avoidance of phagocytic uptake or clearance by the cells of the MPS is desirable. As mentioned previously, circulation time can be increased by reduction of lipid particle size and modifying the surface/steric effect with membrane hydration through PEG derivatives. Prolonged circulation times indirectly enhance the accumulation of lipid-associated or lipid-encapsulated drugs by allowing slow penetration into cancer-laden tissues (a slow process that takes time). Most, if not all, of the currently approved liposomal and lipid-based therapeutics (Table 1) are passively targeted nanomedicines. The EPR effect is the tendency for small nontargeted particles (<400 nm) circulating in the blood to accumulate in the interstitial space of tumors and inflamed tissues because of abnormal leaky (new or neo) vasculature and impaired lymphatic drainage, a hallmark of many cancer pathologies.125,126 By prolonging drug circulation time and the ability of lipid-associated drug particles of 50–150 nm diameter to eventually accumulate in the neovasculature found in a tumor mass, an enhanced drug accumulation is achieved. For example, when daunorubicin is encapsulated in PEGylated liposomes (Doxil), which enables long circulation times, doxorubicin concentrations in Kaposi's sarcoma lesions in AIDS patients have been shown to be 10–20 times those in normal skin.127 Compared with free daunorubicin, liposomal daunorubicin (DaunoXome), which also enables long circulation times, produced almost a 10-fold increase in tumor uptake in a murine lymphosarcoma model (P-1798).128 However, the EPR effect is a heterogeneous phenomenon and is limited to some solid tumors larger than approximately 4.6 mm in diameter.129,130 Nascent tumors and nonvascularized disease sites are unlikely to benefit from this EPR effect. Moreover, there are questions regarding EPR in real human tumors that involve concerns that this effect is an artifact of animal models.131 Even if one accepts that EPR might occur in humans, there are clearly physiological differences within and between tumors and patients. Regardless, through prolonged and sustained plasma drug levels and by steering drug away from off-target accumulation, liposome and lipid nanoparticle formulations may significantly reduce drug toxicity even if only a small fraction of lipid–drug particles eventually accumulate at target sites. Hence, the passive targeting of drug using liposome and lipid nanoparticle formulations could enhance the therapeutic index sufficiently to justify clinical progression of drugs that may otherwise be unsuitable for development. Passive drug targeting with liposomes and lipid nanoparticles could also be considered for repurposing drugs that may exhibit significant off-target drug accumulation because of cell and tissue membrane binding; lipid-bound drugs may substantially reduce this off-target drug accumulation potential.
Active Drug Targeting to Tissues, Cells, and Organelles
Active targeting is intended to home drug exclusively to a specific tissue, cell, or intracellular organelle. Certain drug delivery applications may need rapid responses through a fast and active homing drug delivery system. In theory, a rapid or immediate drug action could be achieved by deploying a delivery system that can facilitate binding to a select cell type (i.e., pathogenic tissue) within a given tissue. This way, the lipid and lipid particles will associate with cells upon contact and provide enriched local drug concentration. The visionary Paul Ehrlich referred to such targeted therapies as a “magic bullet.”132 Unfortunately, the complex molecular underpinnings of cancer have limited the efficacy of anticancer agents targeted to an individual molecular entity.133 The first description of targeted liposomes was with immunoliposomes or liposomes coated with targeted antibody.134,135 Through an improved understanding of HIV and cancer biology—including signaling pathways, microenvironment functions, and metastatic evolution—we now have a range of target receptors to attack, including those for angiogenesis, epidermal growth factor, matrix metalloproteinase, cell migration, transferrin, and CD4+ T cells.136 Recent comprehensive cancer-associated phenotype or marker antigens have been reported for several cancers.137–141
Active drug targeting can be organized into three categories, namely primary, secondary, and tertiary levels. Primary targeting involves delivering drug to select tissues and organs. Only a fraction of total drug that is metabolized and enters the blood will get into these tissues and organs. Secondary targeting involves getting drugs into the cells within these tissues and organs. Even a smaller fraction of total drug may get to this stage. Finally, tertiary targeting involves localizing drug to subcellular organelles. One can imagine only a small fraction of drug that gets into the cells will get into organelles. Because intracellular drug targeting is considerably challenging, tertiary targeting is an emerging science.142
Tertiary targeting depends on cellular internalization (pinocytosis, endocytosis, and phagocytosis). Pinocytosis involves fluid uptake of soluble drug, whereas endocytosis and phagocytosis are often involved in drug particle uptake. Nearly all uptake pathways lead to the endosomal/lysosomal degradative pathway unless a particle has mechanisms to escape this fate. The four mechanisms of cellular uptake and subcellular localization of particles are: (1) caveolin-dependent endocytosis (~60 nm particles), (2) clathrin-dependent endocytosis (~120 nm particles), (3) caveolin- and clathrin-independent endocytosis (~90 nm particles), and (4) macropinocytosis (>1μm particles).71 Caveolin-dependent endocytosis may be induced by ligands such as folic acid143,144 and albumin.145 Clathrin-dependent endocytosis may be triggered by the protein transferrin146,147 and ligands for glycosylated receptors.148 It is one of the best characterized pinocytosis pathways. To avoid drug degradation in lysosomes filled with degradative enzymes, the liposome membrane can be engineered to release drug content or undergo membrane fusion at pH 5.0–5.5. As endosomal pH is recorded at 5.0–5.5, destabilization of the liposome membrane has been shown to enable drug and other molecules to escape from endosomes before entering the lysosomal pathway.149 Caveolin- and clathrin-independent pathways are not well understood but are known to involve cholesterol-rich microdomains (lipid rafts). Macropinocytosis is also caveolin- and clathrin-independent and similar to phagocytosis it is an actin-driven process that nonspecifically internalizes larger particles. Although considering these mechanisms of cellular internalization, it is important to note that unless a significant fraction of administered lipid particles are found in target tissues and cells, efforts to target drugs to intracellular organelles would not have any measurable impact in vivo.
Thus, the general role of targeting ligands is to direct a significant fraction of drug to and retain it in the right tissue, cells, or organelles, and avoid significant exposure to off-target sites. Surface ligands such as antibodies, aptamers, peptides, or small molecules that recognize antigens specific to or associated with a tumor microenvironment may be used for active targeting (Table 7). Ligands may also be used to target vascular endothelial cell surfaces for oncology or cardiovascular indications. The amount and density of targeting ligands on the liposome surface are important control parameters. Molecular targets should be selected based on accessibility (cellular surface), specificity, internalization rate, density, and immunogenicity.150 To get drug inside cells, the molecular target must be able to internalize the targeting ligands attached to a liposome. For example, CD19, folate receptor, and human epidermal growth factor receptor 2 (HER-2) are internalizing cellular surface receptors suitable for liposome targeting, whereas CD20 may have a limited internalization rate that is not suitable for intracellular delivery. Another aspect to achieve high targeting efficiency is the selection of highly potent therapeutics to be encapsulated in targeted liposomes. Instead of using approved drugs such as vinblastine and doxorubicin (with effective cytotoxic concentrations EC50 in the 10–7 M range), more potent cytotoxic agents such as DM1 (EC50 ~10–11–10–12 M), a maytansine derivative, and MMAE (monomethyl auristatin E) (EC50 ~10–9–10–11 M), an auristatin analogue, may further improve therapeutic impact of targeted liposomes. Using highly potent drug instead of vinblastine and doxorubicin has significantly improved the therapeutic outcome of parent antibody molecules by several fold.151
Table 7.
Select Tumor Antigens and Their Targeting Ligands
| Disease | Molecular Target | Targeting Ligand | Lipid Used | In Vitro Test | In Vivo Test |
|---|---|---|---|---|---|
| Breast cancer | HER-2 | SP90 | SP90–PEG–DSPE | – | + |
| Estrogen receptor | Estrogen | ES–PEG–DSPE | + | + | |
| HER-2 | mAb fragments | mAb–PEG–DSPE | + | + | |
| Surface nucleosomes | mAb 2C5 | mAb–PEG3400–DSPE | + | + | |
| Ovarian cancer | Gelactinase | Gelactinase peptides | CTT2–PEG3400–DSPE | – | + |
| SSTR2 | Octreotide | OCT–PEG–DSPE | + | + | |
| Alpha(v) beta(3) integrin | RGD peptides | RGD–PEG2000–DSPE | + | + | |
| Lymphoma | CD22 | HB22.7 (mAb) | mAb–DSPE–mPEG | – | + |
| BAFF receptor | mBAFF | mBAFF–PEG–DSPE | + | + | |
| CD19 | Anti-CD19-IgG2a | aCD19–DSPE–mPEG | + | + | |
| Lung cancer | NSCLC cell line | SP5-2 | SP5–PEG–DSPE | – | + |
| LHRH receptor | Analog of LHRH peptide | LHRH–PEG–DSPE | – | + | |
| Murine tumor | Transferrin receptor | Transferrin | Transferrin–DSPE–PEG | + | + |
| FA receptors | Folic acid | FA–PEG–DSPE | – | + | |
| Alpha(v) beta(3) integrin | RGD tripeptides | RGD–PEG–DSPE | – | + | |
| Prostate cancer | Sigma receptor | Anisamide | AA–PEG3400–DSPE | + | + |
| EGFR | Anti-EGFR scFv C10 | scFv–PEG–DSPE | + | – |
+ stands for p < 0.05 or significant.
– stands for p > 0.05, insignificant or unspecified.
2C5, a monoclonal antibody that is a nucleosome-specific nonpathogenic antinuclear antibody; AA, anisamide; aCD19, antibody bound to CD19; CD19, cluster of differentiation 19; CD22, cluster of differentiation-22; CD19, cluster of differentiation-19; DSPE, distearoylphosphatidylcholine; EGFR, epidermal growth factor receptor; FA, folic acid; HER-2, human epidermal growth factor receptor 2; IgG2a, subclass of IgG; LHRH, luteinizing hormone-releasing hormone; mAB, monoclonal antibody; mBAFF, mutant soluble B-cell activating factor; NSCLC, nonsmall cell–lung carcinoma; PEG, polyethylene glycol; RGD, arginine–glycine–aspartate; anti-EGFR scFv C10, a novel anti-EGFR single-chain variable antibody fragment (scFv) generated from screening a phage antibody library; SSTR2, somatostatin receptor 2; SP90, a synthetic targeting peptide with a sequence of SMDPFLFQLLQ; and SP5-2, a synthetic peptide with a sequence TDSILRSYDWTY.
Because of higher stability and purity, ease of synthetic production, and nonimmunogenicity, some suggest that aptamers and small molecule ligands such as peptides, sugars, and other small molecules are preferred over antibodies.152 However, antibodies remain a popular and potent molecule used in ligand-mediated targeting approaches. About 100 antibody molecules have been anchored on a single 200-nm diameter liposome, which allows for multipoint binding to cells expressing various densities of the targeted antigen.153 At present, SGT-53 is the only liposome with a conjugated antibody under clinical investigation (Table 8). FDA-approved monoclonal antibodies, such as Herceptin and Rituxan, have been used as targeting ligands.154 Multivalent ligands, which have multiple binding groups and enhance the therapeutic efficacy of an antibody, can impact cell biology in ways that monovalent ligands cannot. Cellular internalization by pancreatic cells was enhanced when the anti-EGFR (epidermal growth factor receptor) antibody cetuximab was multivalently presented.155 Unfortunately, antibodies randomly orient themselves on the liposome surface because of the variety of reactive groups within a molecule, which could lead to unexpected off-target effects and poor targeting performance.
Table 8.
Ligand-Conjugated Liposomes in Clinical Trials
| Liposome Therapeutic (Name, Therapeutic) | Intended Treatment | Targeting Agent | Molecular Target | Company | Phase |
|---|---|---|---|---|---|
| MBP-426 (oxaliplatin) | Gastroesophageal adenocarcinoma | Transferrin | Transferrin receptor | Mebiopharm Company | Phase II |
| SGT-53 (pDNA with p53 gene) | Solid tumors | Ab derivative (TfRscFv)a | Transferrin receptor | Synergene therapeutics | Phase I |
| 2B3-101 (doxorubicin) | Brain and breast cancer | Glutathione | BBB transporters | To-BBB | Phase II |
The targeting agent for SGT-52 is an antibody derivative [a single-chain variable fragment (scFv)] with binding affinity to the transferrin receptor (TfR).
Ab, antibody; BBB, blood–brain barrier; pDNA, plasmid DNA; TfRscFv, transferrin receptor single-chain antibody variable fragment.
Aptamers (single-stranded short nucleic acid ligands) are a new class of targeting ligand that mimic protein-binding molecules and bind to any antigen target with high affinity, much like antigen–antibody binding. Produced by relatively simple and inexpensive chemical synthesis of oligonucleotide residues, aptamers rival antibodies because of their small size, high-affinity binding, low toxicity and immunogenicity, ease of isolation and scale-up, and control of conjugation orientation. Liposomes expressing an aptamer targeted to E-selectin, which is present in inflamed vasculature in advanced tumors in mice, showed a similar but slightly increased plasma half-life compared with a PEGylated liposome control when tested in mice (32 ± 7 vs. 24 ± 4 h).156 This agrees with a report of liposomes increasing the plasma residence time of aptamers in rats (113 vs. 49 min).157 Another report used an aptamer for nucleolin, a bcl-2 micro RNA (mRNA)-binding protein involved in cell proliferation in breast cancer.158 This nucleolin seeking aptamer-liposome selectively delivered in vitro the potent chemotherapeutic cisplatin to target human breast cancer cells instead of control human prostate cancer cells. Also, complementary DNA of the aptamer acted as an antidote to disrupt the aptamer-mediated targeted drug delivery.
As transferrin receptors are overexpressed on many cancer cells, transferrin is widely used as a targeting ligand. Two liposomal formulations for targeted drug/gene delivery, MBP-426 and SGT-53, currently under Phase II/I clinical trials, use transferrin and an anti-transferrin receptor single-chain antibody variable fragment, respectively, as targeting ligands (Table 8). Folic acid (folate), a vitamin essential for numerous bodily functions including rapid cell division and growth, has been widely used as a targeting ligand for liposomes. However, folic acid supplied through the human diet (cereals, breads, leafy vegetables, egg yolks) can competitively interfere with such targeting. Anisamide, a high-affinity sigma receptor ligand, has been conjugated to liposomes and improves the delivery of chemotherapeutics and small-interfering RNA (siRNA) to tumors.159,160 Another sigma receptor ligand, haloperidol, conjugated to liposomes can increase DNA delivery to breast cancer cells by 10-fold.161
Although active targeting of liposomes and lipid nanoparticles to target cells continues to drive the majority of scientific exploration and research reports, translation of these innovations into therapeutic candidates and products remains challenging because of the difficulty in scaling (complex formulation process) and unforeseen challenges encountered in humans because of protein–ligand interactions. In some cases, targeting moieties may induce immune-related responses, including elicitation of immune response to the integrin (which promotes metastatic cancer cell attachment and spread) targeting moiety RGD (arginine–glycine–aspartate) peptide. Addressing some of these issues could improve the success rate in translating targeted lipid particle drug delivery platforms into therapeutic products. As mentioned, the practicality of a large-scale pharmaceutical preparation of targeted lipid particles must be considered early in the drug design and development process to realize the clinical use of these drug delivery systems to significantly improve the safety and efficacy of highly potent therapeutic compounds.
Despite some of the scale-up challenges with preparation of lipid membranes expressing surface recognition molecules, there are actively targeted liposomal drugs undergoing clinical trials. Three actively targeted liposome therapeutics are summarized in Table 8 according to their molecular target and clinical progression—mainly Phase I and II. Liposomal oxaliplatin, MBP-426, is coated with transferrin and targeted to the transferrin receptor; liposome-encapsulated plasmid DNA (pDNA) designed to express the p53 cancer suppressor gene (SGT-53) is coated with an antibody fragment, scFv, that recognizes the transferrin receptor; and liposomal doxorubicin coated with glutathione (2B3–101) is designed to enhance transport across the blood–brain barrier through the glutathione transporter.
As active targeting requires a targeting moiety to remain lipid associated in biologic environments that undergo metabolic and fluid flows, it is important to consider their stability as well as the ease of preparation, scaling, and functional performance—that is, their ability to bind to target cells after depositing a significant fraction into tissues. Often, the targeting moiety may be water soluble and thus cannot anchor stably on the membrane surface without conjugating to phospholipid or fatty acyl chains. In some cases, lipophilic or helical peptides are used as an anchoring domain for the targeting moiety. To provide a hydrophobic anchor for a targeting moiety, a number of chemical conjugation procedures have been described in the literature with varying degrees of success and complexity. We highlight some of the most common approaches and where appropriate point out the experience in clinical translation. As shown in Figure 4a, in the case of short 3–20-amino-acid peptides, the peptide could be synthesized with a terminal amino acid conjugate to palmitic acid retained at the end of an automated solid-phase peptide synthesis. The well-established solid-phase peptide synthesis technique involves a series of deprotection/coupling cycles that result in the desired sequence of amino acids with a (C16) fatty acylated ly-sine, or palmitoyl lysine, residue at the C-terminus, which is usually removed to obtain a water soluble peptide. By using a 25-amino-acid mucin 1 (MUC1) sequence (a mucinous glycoprotein and tumor-associated antigen) with a palmitoylated terminal lysine residue at its carboxy terminus, scientists were able to produce a liposomal vaccine with MUC1 antigen (BLP-25), which is undergoing Phase II clinical evaluation. Other chemical conjugation approaches include formation of disulfide bridges with terminal cysteine (Cys) on a targeting peptide and a succinimidyl 3-(2-pyridyldithio)propionate lipid anchor (Fig. 4b); or thioether linkage using a meleimide-activated [via succinimidyl-4-(p-maleimidophenyl) butyrate] lipid anchor (Fig. 4c). The two clinical candidates, 2B3-101 and SGT-53, are composed of liposomes with glutathione and an antibody fragment specific to the transferrin receptor, respectively, attached through a thioether linkage using maleimide-activated lipids and Cys–SH on the ligands. On the contrary, MBP-426 conjugation is achieved by a peptide bond, formed between the activated carboxyl group on N-glutaryl phosphatidylethanolamine and the activated amino group on transferrin with reagents ethyl(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (Fig. 4c).
Figure 4.
Some common chemistry for conjugating targeting ligands to lipid anchors. (a) For short peptides containing about 3–20 amino acids, a 16-carbon chain palmitoyl or other fatty acid chains may be attached to the protein through a lysine, cysteine (Cys), or glycine residue on either the N-terminal or C-terminal end of the protein sequence. (b) A sulfhydryl-containing terminal Cys on a targeting peptide ligand can be coupled to succinimidyl 3-(2-pyridyldithio)propionate-activated phosphatidylcholine (PC) lipid anchors, forming disulfide bond linkages. (c) A sulfhydryl-containing terminal Cys on a targeting peptide ligand can be coupled to succinimidyl-4-(p-maleimidophenyl) butyrate-activated PC lipid anchors, forming stable thioether linkages. (d) An activated amino group on a targeting peptide ligand (e.g., transferrin) can be coupled to an activated carboxyl group on a PC lipid anchor [e.g., N-glutaryl phosphatidylethanolamine (NGPE)-PC] through a carbodiimide reaction with ethyl(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS), forming peptide bond linkages. Addition of NHS to EDC reactions increases efficiency. The black dot indicates atoms that form the bond of interest.
There are three general approaches used for attaching ligands and incorporating them into liposomes: ligands are mixed with lipid during liposome preparation, conjugated after liposome formation, or inserted into preformed liposomes. Lipid mixing involves carrying out the reaction between the ligand and lipidic anchor first, purifying the conjugate, then combining the lipidic ligand with other lipids in the liposome preparation. In theory, this allows stoichiometric control of the ligand density, it is a single-step and efficient manufacturing procedure, and could help hydrophobic material embed better within lamellae. For these reasons, mixing has significant advantages for scale-up preparation. However, targeting moieties are distributed both in the inner and outer surfaces of the liposome. If a targeting moiety is a large protein, protein denaturation might be an issue for preparation methods that include heat, shearing, or other energy sources to reduce particle size.
In the case of conjugating a ligand to preformed liposomes or lipid nanoparticles, the lipidic anchor is already included in the liposome bilayer and the coupling reaction occurs on the surface of preformed liposomes. This avoids potential denaturation that may occur during liposome synthesis. In addition, ligands are only attached to the outside surface, conserving material and maximizing encapsulation volume. However, chemical coupling efficiency to preformed liposomes is not 100% and unconjugated ligand must be removed. Also, there may be a risk of altering the structure of the carrier particle or encapsulated drug. A variation to this is to insert the lipophilic targeting moiety, only after formation of liposomes. This approach, so-called postinsertion, has some advantages but requires the conjugated ligand molecule to be in a micellar form or mixed with surfactant. This process may not be 100% efficient. Additional methods and details of ligand coupling reactions and their strengths and challenges have been recently reviewed.162
In summary, active targeting of liposomes, particularly in large-scale preparation, is an emerging science and is in the early stages of development. To encounter the least resistance when it comes time for a targeted nanotherapeutic to be scaled, the first step is to ensure product and content uniformity. That is, reproducible results, batch-to-batch consistency, and stability are crucial. The second step is the selectivity—whereby a targeted ligand enhances the localization of a therapeutic to a biological site, be it tissue, cells, or intracellular organelles. The ability of a targeted strategy to efficiently navigate physiological barriers and deliver a significant fraction of therapeutics to specific cells and cellular organelles are major challenges. Not all target cell recognition ligands coated on lipid particles enhance tumor tissue accumulation; some ligands are designed to promote cellular uptake. For ligand attachment, one of the more stable and commonly used covalent linkages is the thioether linkage (Fig. 4). This linkage is among the most attractive for scale-up procedures because of its strength, in vivo stability, and reaction efficiency. In addition, the final coupling method of choice must retain the binding selectivity and affinity to target molecules. Ultimately, the choice of coupling reaction and ligand should be made based on the demands of specific drugs and target applications. With recent advances in understanding target molecule expression related to disease cells and tissue phenotypes, along with a systems approach to identify the degree of background expression and link(s) to disease development, active drug targeting with liposomes and lipid nanoparticles is now in sight.
OTHER APPLICATIONS
Multifunctional Liposomes and Lipid Nanoparticles
There has been a recent increase in focus on the development of liposomal delivery systems that combine multiple drugs and functions into a single particle. Through combined innovations in targeting designs, selection of therapeutic agents, and imaging capabilities, there is great potential for the production of highly specialized, safe, and effective therapeutic and diagnostic nanoparticles. This section focuses on liposomes and lipid nanoparticles that combine several targeting methods and different therapeutic or diagnostic modalities into a single nanoparticle to promote safety and efficacy.
The term “multifunctional” has gained popularity in recent years and is used here to describe a single nanoparticle that exhibits multiple functions. These functions may include multiple methods of targeting, targeting to multiple molecules, delivery of multiple therapeutic agents, or a diagnostic function with more than one biologic capability. A similar term with a more ambiguous definition is “multivalent”. Simply put, the “valence” of a molecule refers to its relative binding capacity. In the context of liposomes and lipid nanoparticles, “multivalency” refers to displaying many copies of one or more binding ligands capable of recognizing multiple target molecules on the same or different cells.163,164 Some researchers have used the term “hetero-multivalent” to signify liposomes expressing multiple and different binding peptides.165 The surface of a liposome or lipid nanoparticle 50–100 nm in diameter can carry significantly more than the two valencies available on an immunoglobulin G molecule. Thus, one can envision liposomes with multiple binding epitopes for enhancing biological functions. For example, the lipid membrane could be coated with a binding moiety to recognize prostate-specific antigen (PSA) on cancer cells along with another binding moiety that recognizes activated T or effector cell antigens such as CD64, CD8, and CD3. These lipid particles would bring together the cancer cell with activated T cells, mediating cancer cell lysis. In theory, such an approach with multivalency and high ligand density expressed on liposomal surfaces may provide higher affinity and avidity than the bifunctional antibody approach; many bi-functional antibodies are undergoing clinical evaluation such as blinatumomab for B cell acute lymphoblastic leukemia (ALL), which targets to CD19 on ALL B cells and effector CD3+ T cells.166,167 By bringing the effector T cells to leukemia B cells, blinatumomab (MT103) has progressed to Phase II studies.
If lipid particles are coated with a single targeting moiety, considerable off-target delivery is often detected in vivo because of the relatively high level of background expression in healthy, nontarget tissues. Folate receptor targeting is a good example because, although many cancer cells express high-affinity receptors and high receptor density, low-affinity receptors are also expressed in macrophages, the proximal tubule of the kidney, and in some normal epithelial cells.168 To reduce off-target delivery and improve selectivity in vivo, a number of modifications in the design of targeting ligand derivatives, binding structures, and environment-sensitive approaches have been explored. Perche and Torchilin169 recently reviewed various strategies to enhance multifunctional liposome targeting to cancer cells. Environmentally responsive PEGylated liposomes have been developed, which exhibit the prolonged blood circulation and reduced cellular uptake benefits of PEG conjugation. They can release their protective PEG coating at the target tissue to expose a previously hidden target recognition moiety. This environmentally responsive attribute is achieved through a pH-sensitive hydrazone link between the PEG chain and the PE lipid, which is cleaved at low pH, typical of inflamed or neoplastic tissues.170 Using this method, off-site targeting could be decreased by exposing secondary targeting proteins only in certain environmental conditions. Other environment-specific factors can be similarly exploited to aid targeting specificity, such as a matrix metalloproteinase 2 (MMP2)-cleavable linker that relies on the high local MMP2 concentration in tumor tissues.171 Similarly, the enzymatic activity of PSA could be utilized to target prostate cancer. Folate-expressing liposomes carrying antitumor siRNA are coated with cell-penetrating peptides, but these peptides are shielded by PSA-sensitive linkages. Upon binding via a folate receptor to cells expressing prostate-specific membrane antigen (PSMA), PSA cleaves the protective attachment and the cell-penetrating peptides become exposed, allowing liposomal entry and delivery of siRNA.172 By loading a therapeutic agent such as an anticancer drug into multifunctional environmentally responsive liposomes, targeting efficiency can be improved to increase drug delivery to the region or cells of interest and decrease the risk of negative side effects.
The concept of an outside trigger, or “remote-controlled targeting,” has also been explored with multifunctional liposomes. Some methods include low-wavelength UV-light application to disrupt liposome membranes, application of laser light to induce photosensitive release, and induction of local hyper-thermia by high-intensity focused ultrasound (HIFU) to trigger thermosensitive or pressure-sensitive release.173 These can also be used in combination, such as the use of temperature-sensitive liposomes in conjunction with the ultrasound-induced cavitation of coadministered microbubbles to enhance cellular permeability. Mild local hyperthermia to induce drug release combined with HIFU to enhance cellular permeability allows targeted intracellular accumulation of the encapsulated drug.174 Remotely triggered drug release could provide another layer of highly controllable targeting to improve the efficacy and safety profile of multifunctional lipid nanoparticles.
Finally, multifunctional liposomes may serve functions within multiple therapeutic or diagnostic modalities. For example, siRNA intended to silence the genes critical for cancer growth can be combined with the anticancer drug doxorubicin in PEGylated liposomes to reduce drug resistance via a combination of pharmaceutical and gene therapeutics.175 Therapeutic and diagnostic capabilities can even reside within the same particle. These “theranostic” liposomes could deliver drug while providing real-time imaging using one or more modalities. In many cases, a definitive diagnosis is needed prior to the initiation of therapy, and therefore it is not always practical to implement theranostic procedures. However, in some situations where both diagnostic imaging and therapeutic treatment coincide, such as a cancer patient whose tumor cells may become metastatic, theranostic liposomes could simulta neously provide quantitative information and treatment. Janib et al.176 have discussed the various classes of nanoparticles and contrast agents for multiple imaging modalities including magnetic resonance imaging (MRI), nuclear imaging such as positron emission tomography (PET) and single-positron emission computed tomography (CT), X-ray-based CT, ultrasound, and optical imaging with fluorophores. Without the use of contrast agents, ultrasound and X-ray-based CT are more convenient but provide lower resolution and detail compared with more costly MRI. The use of liposomes and lipid particles with associated contrast or PET agents has often been challenged by either dissociation of contrast from the lipid particles or rapid clearance via the liver, lung, and spleen. This issue has been recently addressed in lipid particles carrying pentachelate diethylene triamine pentaacetic acid (DTPA) that not only stably bind the MRI contrast agent gadolinium in vivo, but also produce contrast-enhanced resolution in the blood pool at low doses and high vascular resolution in the liver and kidney without significant distribution outside of blood vessels.177 Having successfully produced high-performance particles with outstanding MR contrast properties, it expands the potential for multi-valent target visualization. Lipid particles carrying gadolinium and/or PET agents (e.g., 111In) on the chelate DTPA expressed on their surface could be engineered with a target-seeking moiety such as PSMA or HER-2 to aid in the diagnosis and staging of cancer.
Vaccines
Although researchers focus on exploring liposomes to enhance weak antigenic or vaccine responses to one or more specific components of a pathogen (i.e., virus, bacterial, or microbes), liposomes and lipid membrane preparations may also be used as an adjuvant to boost the protective or therapeutic immune responses. As vaccine adjuvants, the primary role of liposomes is intended to induce antigen-dependent and specific humoral and cell-mediated immunity. Liposomal adjuvants are among the most promising candidates to replace the widely used alum-based adjuvants, which elicit a weak T helper cell (TH1) response and inadequate cell-mediated immunity. Although the antigen encapsulation or attachment strategy may vary, the antigen presented in liposomal or lipid nanoparticle form could potentially stimulate both humoral and cell-mediated immunity. Route of administration, antigen dose, and antigen nature—as in size and density—may also modulate the type and extent of immune response.
The mechanistic basis of how liposomes act as adjuvants and enhance antigen-specific immune responses is not yet fully understood. Currently, broad outlines of the adjuvanticity of liposomes have been described. Larger (~250–700 nm in diameter) and positively charged particles persist at subcutaneous or intramuscular injection sites and promote TH1 responses.178 In general, cationic liposome vaccines are more potent adjuvants and have superior immunogenicity than anionic or neutrally charged vaccines. MLVs, with usually 10 lamellae, may potentiate TH2 responses.179 Lipids that melt from a gel to fluid phase at higher temperatures (DSPC, 54°C; DPPC, 42°C; DMPC, 23°C; 1,2-dilauroyl-sn-glycero-3-phosphocholine, DOPC, –2°C; – 17°C) induce stronger antibody and T cell immune responses180; however, exceptions have been reported.181,182 As details of the human immune system become clearer, confounding factors such as immunologic variability among animal strains and species, and differences among rodent, primate, and human immunity and physiology must be carefully considered when designing studies and interpreting results.
The method of antigen attachment is essential to the immunogenicity of liposome vaccines. As discussed, a variety of antigens can be added to liposomes, including small molecule haptens, nucleic acids, carbohydrates, peptides, and proteins. Agonists for pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), NOD-like receptors, and C-type lectin receptors are essential immunomodulators. For instance, the TLR agonist monophosphoryl lipid A is a potent immunostimulant.183 Adding multiple PRR agonists to a single liposomal vaccine has been shown to be beneficial.184 Compared with antigens encapsulated inside liposomal vaccines, surface-associated antigens induce better antibody immune responses,185,186 likely as a result of increased exposure to B cell receptors. However, T cell responses are equivalent for encapsulated and surface-associated antigens.187,188 Processing and presentation of antigens on antigen-presenting cells (APCs) may occur either by MHC II-containing organelles or through endosomal escape and cytosolic delivery followed by loading onto MHC I molecules. Liposome fusion with other cellular or endosomal bilayer membranes and lipid transfer is known to promote immunogenicity by increasing T cell responses.189,190 Fusogenicity has also been widely used to improve the transfection efficiency in gene therapy. Cationic liposomes anneal with pDNA to form an electrostatic complex (lipoplex) and the pDNA serves as both an antigen and adjuvant.191 Following fusion, endosomal escape and cytosolic delivery may occur by cationic liposomes causing endosomal osmolysis192 or pH-sensitive liposomes inducing acidosis and a lamellar-to-hexagonal phase transition to disrupt endosomal compartments.193
Two marketed liposome-based vaccines are Inflexal ® V and Epaxal ®. The former is an influenza vaccine and the latter is a vaccine against hepatitis A. Both are virosomes, which contain functional influenza virus membranes (phospholipids, hemagglutinin, and neuraminidase) and are unilamellar (mono or bi-layer) vesicles approximately 150 nm in diameter. Liposomal vaccines Stimuvax (BLP-25), for nonsmall cell lung carcinoma, and RTS,S/AS01, for malaria, are currently undergoing Phase III/II clinical trials (Table 2) and are progressing toward marketing approval.
Gene Therapeutics
Liposomal vaccines and nonviral vector gene therapeutics share similar principles as they both use cationic liposomes to deliver cargo to the cytoplasm upon endosomal disruption caused by the proton sponge effect.194 Although cytosolic delivery is well studied, an insurmountable obstacle has been the challenge of nonviral vector gene therapy because pDNA is associated with low transgene expression and immunogenicity. For short-term expression of transgenes, gene therapeutics appear to work in the case of vaccines that require a few days of expression.195,196 However, gene therapeutics for chronic protein expression is a distance away from clinical translation. To date, even the most novel and sophisticated gene delivery vehicles have not been successful in overcoming these problems. An alternative approach is using mRNA in place of pDNA to translate gene products without the need to enter the nucleus.197 Recently, mRNA was chemically modified and encapsulated in liposomes for successful systemic delivery to tumor sites.198 The chemical modification involved first structurally enhancing mRNA transcripts and then substituting cytidine triphosphate and uridine triphosphate with 5-methylcytidine triphosphate and pseudouridine (ψ) triphosphate, respectively. These two nucleotide analogs reduced the activation of the innate immune response through the TLR pathway. mRNA was condensed with polycationic protamine into nanosized complexes to protect against nuclease degradation.
Antisense nucleic acid sequences—including RNA inhibitor or RNAi, siRNA, and miRNA—bind to target gene sequences and inhibit or modulate gene expression. Lipid nanoparticles are a leading delivery system for these gene therapeutics, but particle structures have been debated. Recently, stable nucleic acid lipid particles for delivering siRNA were shown to have an outer layer of PEG–lipid encapsulating immobilized siRNA bound to bilayer membrane surfaces that separate irregular water-filled cavities, which is counter to the view of a bilayer vesicle with freely tumbling siRNA in the inner aqueous compartment.199,200 About 10 years ago, siRNA and RNAi were regarded as highly innovative therapeutic platforms for multiple disease targets. However, the small fraction of siRNA found in target cells, the even smaller fractions found within target organelles or sites of action, and the challenge of rapid clearance (or excretion) from the body, have led to a lack of therapeutic efficacy and a broad divestment by biopharmaceutical development programs.
Oral Drug Delivery
Oral dosing of liposomal drug delivery systems has advantages over invasive routes because of the potential increase in patient compliance and ease of use. However, bile salts, pH, and pancreatic enzymes in the gut dismantle liposomal bilayers. To help resist these destabilizing factors, liposome formulations incorporate protective polymeric coatings or cholesterol and saturated phospholipids that increase membrane rigidity and decrease enzymatic degradation.201 Other ways to achieve gastrointestinal (GI) stability include liposomes with a carrageenan and collagen core202 or the addition of gangliosides GM1 and GM type III.203 However, disruption of the liposomal vesicles because of GI fluids, especially bile salts, remains a hindrance. In vitro lipolysis models are used to simulate human intestinal digestion and are useful for testing the GI stability of liposome formulations.204 Overall, successful oral liposomal delivery systems must be stable and move from the gut into the circulatory system prior to releasing their cargo in the blood or at specific target sites. Lipid-based excipients (e.g., liposomes, micellular nanoemulsions and microemulsions) have indeed improved the bioavailability of oral drugs by solubilizing poorly water-soluble drugs and increasing intestinal membrane permeability. For example, oral paclitaxel avoids P-glycoprotein efflux transporters when loaded into liposomes.205,206 Mechanisms of oral absorption of lipophilic drugs with lipid-based delivery systems have been thoroughly reviewed.207 Liposomal excipients also reduce GI side effects of nonsteroidal anti-inflammatory agents and eliminate the bitter taste of oral drugs.208 An oral PEG-coated liposomal vaccine with ovalbumin and diameters of 1.7–3.3 μm induced a mucosal immune response in mice209 and oral liposomal β-sitosterol was shown to upregulate the host defense of metastatic cancer cells and may enhance mucosal immunity.210 Stimulating mucosal immune responses may enhance delivery of antigen to APCs that actively take up particles in the GI tract. Generally, some benefits of using liposomes for such applications include biocompatibility, protection of antigen, flexibility in design, targeting of antigens to APCs, and improved stimulation of immune responses by oral delivery of soluble antigens.211 In some cases, a lipid mixture may be used as a solubilizing or suspension agent for highly insoluble or lipophilic drugs to be delivered as a microemulsion in softgel capsules for oral dosage. A drug and lipid microemulsion encased in softgel capsules was used to enhance reproducibility and bioavailability of cyclosporin A, originally formulated as a tablet.212
FUTURE DIRECTIONS
From a regulatory perspective, it is important to design a drug delivery strategy based on predefined desired attributes and to establish clinically meaningful specifications. In particular, a lingering issue is the lack of in vitro/in vivo correlation of liposome drug release profiles. As a result, current regulation relies heavily on time-consuming clinical studies to establish bioequivalence and set regulatory specifications. To evaluate the quality, safety, and efficacy of liposomal products, systematic understanding of the relationships between liposome physiochemical properties and its absorption, distribution, metabolism, and excretion (ADME) behaviors is critical. Physiologically based pharmacokinetic (PBPK) modeling could serve as a powerful tool to quantitatively describe and predict the biodistribution of liposomal vesicles and drug substances. Preclinical PBPK models can be readily extrapolated to humans by substituting related physiologic and pharmacokinetic parameters, making it possible to quantitatively predict the effects of changes in liposome physiochemical properties on ADME of liposomal drugs in humans.
Advances and continual growth in the identification of drug targets have provided ever-expanding drug candidates. Yet translating these compounds into therapeutic products continues to face challenges because of the off-target distribution and lack of efficacy in late-stage clinical trials. Many highly potent drugs often face limited solubility and target tissue exposure. Improved understanding of drug target distribution profiles within the body along with pharmacokinetics, drug disposition, and elimination, as they relate to therapeutic outcome, point to the need for consideration of drug delivery at a systems level. A systems approach not only considers the physiological context of target tissues and cells that link to disease symptom development, but also the ability and capacity of a drug delivery platform to penetrate and localize at sufficient levels necessary to modify therapeutic impacts. To make a significant therapeutic impact, drug delivery systems such as liposomes and lipid nanoparticles must serve as a drug carrier not only to improve drug stability and exposure, but also to enhance the accumulation of a significant amount of drug in the target tissue. Without significant drug exposure in target tissues, enhancing delivery of drug to cells or intracellular drug targets is unlikely. In this context, clinical insights in liposome and lipid nanoparticle disposition mechanisms have led to the design of small (~50 nm diameter) and sterically stabilized lipid particles to increase their blood residence time required for clinical applications. Advances in molecular design and chemistry for expression of ligand or receptor molecules on the liposome and lipid nanoparticle surface may further improve their interaction with target cells. Improved drug residence time (because of the reduced clearance of liposomes and lipid nanoparticles) will provide carrier-associated drugs the sufficient time they need to eventually reach their intended target sites. A first step for increasing the intracellular uptake of liposomal drugs (e.g., anticancer agents, antibiotics, and DNA) is to enhance their localization selectivity within the target tissue. As additional ligands with higher affinity and specificity continue to be developed, and progress is made in antibody and peptide engineering to mass-produce targeted lipid nanoparticle preparations, lipid–drug complexes with extended therapeutic indices are now within reach.
ACKNOWLDGMENTS
This study was supported in part by NIH grants AI077390, AI071971, RR00166, RR025014, and UL1TR000423. R.J.Y.H. was also supported in part by the Milo Gibaldi endowment.
Abbreviations Used
- APC
antigen-presenting cell
- DMPC
dimyristoylphosphatidylcholine
- DOPC
dioleoylphosphatidylcholine
- DPPC
dipalmitoylphosphatidylcholine
- DSPC
distearoylphosphatidylcholine
- DTPA
diethylene triamine pentaacetic acid
- EDC
ethyl(dimethylaminopropyl) carbodiimide
- NHS
N-hydroxysuccinimide
- EPR
enhanced permeability and retention
- FDA
US Food and Drug Administration
- HER-2
human epidermal growth factor receptor 2
- HIFU
high-intensity focused ultrasound
- MLV
multilamellar vesicle
- MMP
matrix metalloproteinase
- MPS
mononuclear phagocyte system
- MRI
magnetic resonance imaging
- MUC1
mucin 1
- MVL
multi-vesicular liposome
- NGPE
N-glutaryl phosphatidylethanolamine
- PC
phosphatidylcholine
- pDNA
plasmid DNA
- PE
phosphatidylethanolamine
- PEG
polyethylene glycol
- PET
positron emission tomography
- PG
phosphatidylglycerol
- PRR
pattern recognition receptor
- PSA
prostate-specific antigen
- PSMA
prostate-specific membrane antigen
- RGD
arginine–glycine–aspartate
- PS
phosphatidylserine
- SMPB
succinimidyl-4-(p-maleimidophenyl) butyrate
- SPH
sphingomyelin
- Tc
lipid-phase transition temperature
- TLR
Toll-like receptor
REFERENCES
- 1.Saunders L, Thomas IL. Diffusion studies with lysolecithin. J Chem Soc. 1958:483–485. [Google Scholar]
- 2.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, Anderson D, Morton WR, Hu SL, Ho RJ. Lipid–drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: A proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34(4):387–397. doi: 10.1097/00126334-200312010-00005. [DOI] [PubMed] [Google Scholar]
- 5.James JS. DOXIL approved for KS. AIDS treatment news (no. 236) 1995:6. [PubMed] [Google Scholar]
- 6.James JS. DOXIL approved by FDA. AIDS patient care (no. 9) 1995:306. [PubMed] [Google Scholar]
- 7.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11(8):1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 8.Northfelt DW. Stealth liposomal doxorubicin (SLD) delivers more doxorubicin (DOX) to AIDS-Kaposis sarcoma (AIDS-KS) lesions than to normal skin (abstract #5). Proc Am Soc Clin Oncol. 1994;13:51. [Google Scholar]
- 9.NDA . Doxil: New drug application. Center for Drug Evaluation & Research—FDA; 2004. [Google Scholar]
- 10.Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90(6):667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 11.Ramishetti S, Huang L. Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy. Ther Deliv. 2012;3(12):1429–1445. doi: 10.4155/tde.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregoriadis G. Liposome technology. 3rd ed. I–III. Informa Healthcare; New York: 2007. [Google Scholar]
- 13.Torchilin VP, Weissig V. Liposomes: A practical approach. 2nd ed. Oxford University Press; Oxford; New York: 2003. p. xxiii.p. 396. [Google Scholar]
- 14.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2012;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Mabrey S, Mateo PL, Sturtevant JM. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 16.Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam: A sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153–1176. doi: 10.2165/00003088-200645120-00002. [DOI] [PubMed] [Google Scholar]
- 17.Katre NV. Liposomes-based depot injection technologies. Am J Drug Deliv. 2004;2(4):213–227. [Google Scholar]
- 18.Cullis PR, de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 19.Edidin M. Lipids on the frontier: A century of cell-membrane bilayers. Nat Rev Mol Cell Biol. 2003;4(5):414–418. doi: 10.1038/nrm1102. [DOI] [PubMed] [Google Scholar]
- 20.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 21.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233(2):134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 22.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269(5):3198–3204. [PubMed] [Google Scholar]
- 23.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743. [PubMed] [Google Scholar]
- 24.Plank C, Mechtler K, Szoka FC, Jr., Wagner E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7(12):1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 25.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 26.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146(12):4234–4241. [PubMed] [Google Scholar]
- 27.Szebeni J, Bedocs P, Rozsnyay Z, Weiszhar Z, Urbanics R, Rosivall L, Cohen R, Garbuzenko O, Bathori G, Toth M, Bunger R, Barenholz Y. Liposome-induced complement activation and related cardiopulmonary distress in pigs: Factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine. 2012;8(2):176–184. doi: 10.1016/j.nano.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipidmethoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20(14):2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 29.Sou K, Tsuchida E. Electrostatic interactions and complement activation on the surface of phospholipid vesicle containing acidic lipids: Effect of the structure of acidic groups. Biochim Biophys Acta. 2008;1778(4):1035–1041. doi: 10.1016/j.bbamem.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Risbo J, Jorgensen K, Sperotto MM, Mouritsen OG. Phase behavior and permeability properties of phospholipid bilayers containing a short-chain phospholipid permeability enhancer. Biochim Biophys Acta. 1997;1329(1):85–96. doi: 10.1016/s0005-2736(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 31.McMullen TP, Lewis RN, McElhaney RN. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Szoka FC., Jr Sterol-modified phospholipids: cholesterol and phospholipid chimeras with improved biomembrane properties. J Am Chem Soc. 2008;130(46):15702–15712. doi: 10.1021/ja8065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen K, Ipsen JH, Mouritsen OG, Zuckermann MJ. The effect of anaesthetics on the dynamic heterogeneity of lipid membranes. Chem Phys Lipids. 1993;65(3):205–216. doi: 10.1016/0009-3084(93)90018-x. [DOI] [PubMed] [Google Scholar]
- 34.Krill SL, Lau KY, Plachy WZ, Rehfeld SJ. Penetration of dimyristoylphosphatidylcholine monolayers and bilayers by model beta-blocker agents of varying lipophilicity. J Pharm Sci. 1998;87(6):751–756. doi: 10.1021/js970374z. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan SM, Huang L. Enhanced delivery to target cells by heat-sensitive immunoliposomes. Proc Natl Acad Sci USA. 1986;83(16):6117–6121. doi: 10.1073/pnas.83.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanez AM, Wallace M, Ho R, Shen D, Yaksh TL. Touch-evoked agitation produced by spinally administered phospholipid emulsion and liposomes in rats. Structure–activity relation. Anesthesiology. 1995;82(5):1189–1198. doi: 10.1097/00000542-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Beauchamp C, Daddona PE, Menapace DP. Properties of a novel PEG derivative of calf adenosine deaminase. Adv Exp Med Biol. 1984;165(Pt A):47–52. doi: 10.1007/978-1-4684-4553-4_9. [DOI] [PubMed] [Google Scholar]
- 38.Zhao XB, Muthusamy N, Byrd JC, Lee RJ. Cholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomes. J Pharm Sci. 2007;96(9):2424–2435. doi: 10.1002/jps.20885. [DOI] [PubMed] [Google Scholar]
- 39.Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama K, Yuda T, Okamoto A, Kojima S, Suginaka A, Iwatsuru M. Prolonged circulation time in vivo of large unilamelar liposomes composed of distearoyl phosphatidylcholine and cholesterol containing amphipathic poly(ethylene glycol). Biochim Biophys Acta. 1992;1128(1):44–49. doi: 10.1016/0005-2760(92)90255-t. [DOI] [PubMed] [Google Scholar]
- 41.Woodle MC, Matthay KK, Newman MS, Hidayat JE, Collins LR, Redemann C, Martin FJ, Papahadjopoulos D. Versatility in lipid compositions showing prolonged circulation with sterically stabilized liposomes. Biochim Biophys Acta. 1992;1105(2):193–200. doi: 10.1016/0005-2736(92)90194-q. [DOI] [PubMed] [Google Scholar]
- 42.Huang SK, Lee KD, Hong K, Friend DS, Papahadjopoulos D. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res. 1992;52(19):5135–5143. [PubMed] [Google Scholar]
- 43.Wu NZ, Da D, Rudoll TL, Needham D, Whorton AR, Dewhirst MW. Increased microvascular permeability contributes to preferential accumulation of Stealth liposomes in tumor tissue. Cancer Res. 1993;53(16):3765–3770. [PubMed] [Google Scholar]
- 44.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–992. [PubMed] [Google Scholar]
- 45.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 46.Metselaar JM, Bruin P, de Boer LW, de Vringer T, Snel C, Oussoren C, Wauben MH, Crommelin DJ, Storm G, Hennink WE. A novel family of L-amino acid-based biodegradable polymer-lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjug Chem. 2003;14(6):1156–1164. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release. 2001;75(1–2):83–91. doi: 10.1016/s0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP, Levchenko TS, Whiteman KR, Yaroslavov AA, Tsatsakis AM, Rizos AK, Michailova EV, Shtilman MI. Amphiphilic poly-N-vinylpyrrolidones: Synthesis, properties and liposome surface modification. Biomaterials. 2001;22(22):3035–3044. doi: 10.1016/s0142-9612(01)00050-3. [DOI] [PubMed] [Google Scholar]
- 49.Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticuate drug carriers long-circulating? Adv Drug Deliv Rev. 1995;16:141–155. [Google Scholar]
- 50.Whiteman KR, Subr V, Ulbrich K, Torchilin VP. Poly(Hpma)coated liposomes demonstrate prolonged circulation in mice. J Lipo-some Res. 2001;11(2–3):153–164. doi: 10.1081/LPR-100108459. [DOI] [PubMed] [Google Scholar]
- 51.Woodle MC. Controlling liposome blood clearance by surface-grafted polymers. Adv Drug Deliv Rev. 1998;32(1–2):139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 52.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54(4):459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 53.Pasut G, Veronese FM. Polymer–drug conjugation, recent achievements and general strategies. Progr Polym Sci. 2007;32:933–961. [Google Scholar]
- 54.Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin on Drug Deliv. 2012;9(11):1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 55.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122(3):349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylene glycol: Facts and fiction. Pharm Res. 2013;30(7):1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 58.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 59.Bertrand N, Leroux JC. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J Control Release. 2012;161(2):152–163. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 60.Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A. 2011;108(16):6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation. 1990;82(1):1–7. doi: 10.1161/01.cir.82.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Townsley MI, Parker JC, Longenecker GL, Perry ML, Pitt RM, Taylor AE. Pulmonary embolism: Analysis of endothelial pore sizes in canine lung. Am J Physiol. 1988;255(5 Pt 2):H1075–1083. doi: 10.1152/ajpheart.1988.255.5.H1075. [DOI] [PubMed] [Google Scholar]
- 64.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15(17):1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 65.Luft FC, Aronoff GR, Evan AP, Connors BA, Blase DK, Gattone VH. Effects of moxalactam and cefotaxime on rabbit renal tissue. Antimicrobial Agents Chemother. 1982;21(5):830–835. doi: 10.1128/aac.21.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogawa S, Ota Z, Shikata K, Hironaka K, Hayashi Y, Ota K, Kushiro M, Miyatake N, Kishimoto N, Makino H. High-resolution ultra-structural comparison of renal glomerular and tubular basement membranes. Am J Nephrol. 1999;19(6):686–693. doi: 10.1159/000013543. [DOI] [PubMed] [Google Scholar]
- 67.Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestila M, Koskimies O, Jalanko H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004;15(10):2611–2618. doi: 10.1097/01.ASN.0000139478.03463.D9. [DOI] [PubMed] [Google Scholar]
- 68.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 69.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am J Physiol. 2005;288(4):F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 73.Beaumier PL, Hwang KJ. Effects of liposome size on the degradation of bovine brain sphingomyelin/cholesterol liposomes in the mouse liver. Biochim Biophys Acta. 1983;731(1):23–30. doi: 10.1016/0005-2736(83)90393-0. [DOI] [PubMed] [Google Scholar]
- 74.Proffitt RT, Williams LE, Presant CA, Tin GW, Uliana JA, Gamble RC, Baldeschwieler JD. Tumor-imaging potential of liposomes loaded with In-111-NTA: Biodistribution in mice. J Nucl Med. 1983;24(1):45–51. [PubMed] [Google Scholar]
- 75.Gabizon AA, Barenholz Y, Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: Pharmacokinetic studies in rodents and dogs. Pharm Res. 1993;10(5):703–708. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 76.Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barenholz Y, Amselem S. Quality control assays in the development and clinical use of liposome-based formulations. In: Gregoriadis G, editor. Liposome technology. 2nd ed. CRC Press; Boca Raton: 1993. pp. 527–616. [Google Scholar]
- 78.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 79.Asmal M, Whitney JB, Luedemann C, Carville A, Steen R, Letvin NL, Geiben-Lynn R. In vivo anti-HIV activity of the heparin-activated serine protease inhibitor antithrombin III encapsulated in lymph-targeting immunoliposomes. PLoS One. 2012;7(11):e48234. doi: 10.1371/journal.pone.0048234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 81.Tejera-Garcia R, Ranjan S, Zamotin V, Sood R, Kinnunen PK. Making unilamellar liposomes using focused ultrasound. Langmuir. 2011;27(16):10088–10097. doi: 10.1021/la201708x. [DOI] [PubMed] [Google Scholar]
- 82.Mayhew E, Lazo R, Vail WJ, King J, Green AM. Characterization of liposomes prepared using a microemulsifier. Biochim Biophys Acta. 1984;775(2):169–174. doi: 10.1016/0005-2736(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 83.Barnadas-Rodriguez R, Sabes M. Factors involved in the production of liposomes with a high-pressure homogenizer. Int J Pharm. 2001;213(1–2):175–186. doi: 10.1016/s0378-5173(00)00661-x. [DOI] [PubMed] [Google Scholar]
- 84.Dennison SM, Sutherland LL, Jaeger FH, Anasti KM, Parks R, Stewart S, Bowman C, Xia SM, Zhang R, Shen X, Scearce RM, Ofek G, Yang Y, Kwong PD, Santra S, Liao HX, Tomaras G, Letvin NL, Chen B, Alam SM, Haynes BF. Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41. PLoS One. 2011;6(11):e27824. doi: 10.1371/journal.pone.0027824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amselem S, Gabizon A, Barenholz Y. A large-scale method for the preparation of sterile and nonpyrogenic liposomal formulations of defined size distributions for clinical use. In: Gregoriadis G, editor. Liposome technology. CRC Press; Boca Raton: 1993. pp. 501–525. [Google Scholar]
- 86.Rameez S, Bamba I, Palmer AF. Large scale production of vesicles by hollow fiber extrusion: A novel method for generating polymersome encapsulated hemoglobin dispersions. Langmuir. 2010;26(7):5279–5285. doi: 10.1021/la9036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szoka F, Jr., Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA. 1978;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maitani Y, Soeda H, Junping W, Takayama K. Modified ethanol injection method for liposomes containing beta-sitosterol beta-D-glucoside. J Liposome Res. 2001;11(1):115–125. doi: 10.1081/LPR-100103174. [DOI] [PubMed] [Google Scholar]
- 89.Gentine P, Bourel-Bonnet L, Frisch B. Modified and derived ethanol injection toward liposomes: Development of the process. J Liposome Res. 2012;23(1):11–19. doi: 10.3109/08982104.2012.717298. [DOI] [PubMed] [Google Scholar]
- 90.Jaafar-Maalej C, Charcosset C, Fessi H. A new method for liposome preparation using a membrane contactor. J Liposome Res. 2011;21(3):213–220. doi: 10.3109/08982104.2010.517537. [DOI] [PubMed] [Google Scholar]
- 91.Wagner A, Platzgummer M, Kreismayr G, Quendler H, Stiegler G, Ferko B, Vecera G, Vorauer-Uhl K, Katinger H. GMP production of liposomes—A new industrial approach. J Liposome Res. 2006;16(3):311–319. doi: 10.1080/08982100600851086. [DOI] [PubMed] [Google Scholar]
- 92.Meure LA, Foster NR, Dehghani F. Conventional and dense gas techniques for the production of liposomes: a review. AAPS Pharm Sci Tech. 2008;9(3):798–809. doi: 10.1208/s12249-008-9097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lasch J, Weissig V, Brandl M. Preparation of liposomes. In: Torchilin V, Weissig V, editors. Liposomes: A practical approach. Oxford University Press; Oxford/New York: 2003. pp. 3–29. [Google Scholar]
- 94.Schwendener RA. The preparation of large volumes of sterile liposomes for clinical applications. In: Gregoriadis G, editor. Liposome Technology. 2 ed. CRC Press; Boca Raton: 1993. pp. 487–500. [Google Scholar]
- 95.Frederiksen L, Anton K, van Hoogevest P, Keller HR, Leuenberger H. Preparation of liposomes encapsulating water-soluble compounds using supercritical carbon dioxide. J Pharm Sci. 1997;86(8):921–928. doi: 10.1021/js960403q. [DOI] [PubMed] [Google Scholar]
- 96.Spilimbergo S, Bertucco A. Non-thermal bacterial inactivation with dense CO(2). Biotechnol Bioeng. 2003;84(6):627–638. doi: 10.1002/bit.10783. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M. Sterilization using high-pressure carbon dioxide. J Supercritical Fluids. 2006;38(3):354–372. [Google Scholar]
- 98.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HR, Andrieux K, Delomenie C, Chacun H, Appel M, Desmaele D, Taran F, Georgin D, Couvreur P, Taverna M. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip system. Electrophoresis. 2007;28(13):2252–2261. doi: 10.1002/elps.200600694. [DOI] [PubMed] [Google Scholar]
- 100.Goppert TM, Muller RH. Protein adsorption patterns on poloxamer- and poloxamine-stabilized solid lipid nanoparticles (SLN). Eur J Pharm Biopharm. 2005;60(3):361–372. doi: 10.1016/j.ejpb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, Linse S, Dawson KA. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl. 2007;46(30):5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 102.Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci. 2007;134–135:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Scherphof G, Roerdink F, Waite M, Parks J. Disintegration of phosphatidylcholine liposomes in plasma as a result of interaction with high-density lipoproteins. Biochim Biophys Acta. 1978;542(2):296–307. doi: 10.1016/0304-4165(78)90025-9. [DOI] [PubMed] [Google Scholar]
- 104.Tall AR, Small DM. Solubilisation of phospholipid membranes by human plasma high density lipoproteins. Nature. 1977;265(5590):163–164. doi: 10.1038/265163a0. [DOI] [PubMed] [Google Scholar]
- 105.Mendez AJ, He JL, Huang HS, Wen SR, Hsia SL. Interaction of rabbit lipoproteins and red blood cells with liposomes of egg yolk phospholipids. Lipids. 1988;23(10):961–967. doi: 10.1007/BF02536344. [DOI] [PubMed] [Google Scholar]
- 106.Papahadjopoulos D, Moscarello M, Eylar EH, Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- 107.Devine DV, Wong K, Serrano K, Chonn A, Cullis PR. Liposome–complement interactions in rat serum: Implications for liposome survival studies. Biochim Biophys Acta. 1994;1191(1):43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 108.Yan X, Scherphof GL, Kamps JA. Liposome opsonization. J Liposome Res. 2005;15(1–2):109–139. doi: 10.1081/lpr-64971. [DOI] [PubMed] [Google Scholar]
- 109.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macro-molecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 110.Bui T, Faltynek C, Ho RJ. Differential disposition of soluble and liposome-formulated human recombinant interleukin-7: Effects on blood lymphocyte population in guinea pigs. Pharm Res. 1994;11(5):633–641. doi: 10.1023/a:1018955708443. [DOI] [PubMed] [Google Scholar]
- 111.Patel HM. Fate of liposomes in the lymphatics. In: Gregoriadis G, editor. Liposomes as drug carriers. John Wiley & Sons; New York: 1988. p. 51. [Google Scholar]
- 112.Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–156. doi: 10.1016/s0169-409x(01)00154-5. [DOI] [PubMed] [Google Scholar]
- 113.Higuchi M, Fokin A, Masters TN, Robicsek F, Schmid-Schonbein GW. Transport of colloidal particles in lymphatics and vasculature after subcutaneous injection. J Appl Physiol. 1999;86(4):1381–1387. doi: 10.1152/jappl.1999.86.4.1381. [DOI] [PubMed] [Google Scholar]
- 114.Ikomi F, Hanna GK, Schmid-Schonbein GW. Size- and surface-dependent uptake of colloid particles into the lymphatic system. Lymphology. 1999;32(3):90–102. [PubMed] [Google Scholar]
- 115.Oussoren C, Velinova M, Scherphof G, van der Want JJ, van Rooijen N, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. IV. Fate of liposomes in regional lymph nodes. Biochim Biophys Acta. 1998;1370(2):259–272. doi: 10.1016/s0005-2736(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 116.Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim Biophys Acta. 1997;1328(2):261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 117.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192(10):1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fidler IJ, Sone S, Fogler WE, Barnes ZL. Eradication of spontaneous metastases and activation of alveolar macrophages by intravenous injection of liposomes containing muramyl dipeptide. Proc Natl Acad Sci USA. 1981;78(3):1680–1684. doi: 10.1073/pnas.78.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S, Goins B, Hrycushko BA, Phillips WT, Bao A. Feasibility of eradication of breast cancer cells remaining in postlumpectomy cavity and draining lymph nodes following intracavitary injection of radioactive immunoliposomes. Mol Pharm. 2012;9(9):2513–2522. doi: 10.1021/mp300132f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 121.Wong JK, Gunthard HF, Havlir DV, Zhang ZQ, Haase AT, Ignacio CC, Kwok S, Emini E, Richman DD. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci USA. 1997;94(23):12574–12579. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruiz L, van Lunzen J, Arno A, Stellbrink HJ, Schneider C, Rull M, Castella E, Ojanguren I, Richman DD, Clotet B, Tenner-Racz K, Racz P. Protease inhibitor-containing regimens compared with nucleoside analogues alone in the suppression of persistent HIV-1 replication in lymphoid tissue. AIDS. 1999;13(1):F1–F8. doi: 10.1097/00002030-199901140-00001. [DOI] [PubMed] [Google Scholar]
- 123.Hockett RD, Kilby JM, Derdeyn CA, Saag MS, Sillers M, Squires K, Chiz S, Nowak MA, Shaw GM, Bucy RP. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med. 1999;189(10):1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kinman L, Bui T, Larsen K, Tsai CC, Anderson D, Morton WR, Hu SL, Ho RJ. Optimization of lipid–indinavir complexes for localization in lymphoid tissues of HIV-infected macaques. J Acquir Immune Defic Syndr. 2006;42(2):155–161. doi: 10.1097/01.qai.0000214822.33905.87. [DOI] [PubMed] [Google Scholar]
- 125.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 126.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 127.Northfelt DW, Kaplan L, Russell J. Pharmacokinetics and tumor localization of DOX-SL (Stealth liposomal doxorubicin) by comparison with Adriamycin in patients with AIDS and Kaposi's sarcoma. In: Lasic DD, Martin FJ, editors. Stealth liposomes. CRC Press; Boca Raton, Florida: 1995. pp. 257–266. [Google Scholar]
- 128.Forssen EA, Coulter DM, Proffitt RT. Selective in vivo localization of daunorubicin small unilamellar vesicles in solid tumors. Cancer Res. 1992;52(12):3255–3261. [PubMed] [Google Scholar]
- 129.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev. 2009;2(1):99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 130.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Treating metastatic cancer with nanotechnology. Nat Rev. 2012;12(1):39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 131.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev. 2008;8(6):473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 133.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci USA. 2010;107(42):17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heath TD, Fraley RT, Papahdjopoulos D. Antibody targeting of liposomes: Cell specificity obtained by conjugation of F(ab’)2 to vesicle surface. Science. 1980;210(4469):539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 135.Leserman LD, Barbet J, Kourilsky F, Weinstein JN. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature. 1980;288(5791):602–604. doi: 10.1038/288602a0. [DOI] [PubMed] [Google Scholar]
- 136.Endsley AN, Ho RJ. Enhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticles. J Acquir Immune Defici Syndromes. 2012;61(4):417–424. doi: 10.1097/QAI.0b013e3182653c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9(1):29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 143.Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264(5167):1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 144.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394(6695):798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 145.Schnitzer JE. Caveolae: From basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49(3):265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 146.Lv Q, Li LM, Han M, Tang XJ, Yao JN, Ying XY, Li FZ, Gao JQ. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int J Pharm. 2013;444(1–2):1–9. doi: 10.1016/j.ijpharm.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 147.Miller K, Shipman M, Trowbridge IS, Hopkins CR. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65(4):621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- 148.Altschuler Y, Kinlough CL, Poland PA, Bruns JB, Apodaca G, Weisz OA, Hughey RP. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell. 2000;11(3):819–831. doi: 10.1091/mbc.11.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang CY, Huang L. Plasmid DNA adsorbed to pH-sensitive liposomes efficiently transforms the target cells. Biochem Biophys Res Commun. 1987;147(3):980–985. doi: 10.1016/s0006-291x(87)80166-3. [DOI] [PubMed] [Google Scholar]
- 150.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8(4):335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 151.Ho RJ, Chien JY. Trends in translational medicine and drug targeting and delivery: New insights on an old concept—Targeted drug delivery with antibody–drug conjugates for cancers. J Pharm Sci. 2013 doi: 10.1002/jps.23761. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handbook Exp Pharm. 2010;(197):55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 153.Torchilin VP. Passive and active drug targeting: Drug delivery to tumors as an example. Handbook Exp Pharma. 2010;(197):3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 154.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 155.Bhattacharyya S, Bhattacharya R, Curley S, McNiven MA, Mukherjee P. Nanoconjugation modulates the trafficking and mechanism of antibody induced receptor endocytosis. Proc Natl Acad Sci USA. 2010;107(33):14541–14546. doi: 10.1073/pnas.1006507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mann AP, Bhavane RC, Somasunderam A, Liz Montalvo-Ortiz B, Ghaghada KB, Volk D, Nieves-Alicea R, Suh KS, Ferrari M, Annapragada A, Gorenstein DG, Tanaka T. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget. 2011;2(4):298–304. doi: 10.18632/oncotarget.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Willis MC, Collins BD, Zhang T, Green LS, Sebesta DP, Bell C, Kellogg E, Gill SC, Magallanez A, Knauer S, Bendele RA, Gill PS, Janjic N. Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug Chem. 1998;9(5):573–582. doi: 10.1021/bc980002x. [DOI] [PubMed] [Google Scholar]
- 158.Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, Lu Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed Engl. 2009;48(35):6494–6498. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 159.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16(1):163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakagawa O, Ming X, Huang L, Juliano RL. Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. J Am Chem Soc. 2010;132(26):8848–8849. doi: 10.1021/ja102635c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reddy BS, Banerjee R. 17Beta-estradiol-associated stealthliposomal delivery of anticancer gene to breast cancer cells. Angew Chem Int Ed Engl. 2005;44(41):6723–6727. doi: 10.1002/anie.200501793. [DOI] [PubMed] [Google Scholar]
- 162.Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113(3):1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- 163.Gray BP, Li S, Brown KC. From phage display to nanoparticle delivery: Functionalizing liposomes with multivalent peptides improves targeting to a cancer biomarker. Bioconjug Chem. 2013;24(1):85–96. doi: 10.1021/bc300498d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Humphries HE, Williams JN, Blackstone R, Jolley KA, Yuen HM, Christodoulides M, Heckels JE. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine. 2006;24(1):36–44. doi: 10.1016/j.vaccine.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 165.Ravikumar M, Modery CL, Wong TL, Gupta AS. Peptide-decorated liposomes promote arrest and aggregation of activated platelets under flow on vascular injury relevant protein surfaces in vitro. Biomacromolecules. 2012;13(5):1495–1502. doi: 10.1021/bm300192t. [DOI] [PubMed] [Google Scholar]
- 166.Portell CA, Advani AS. Novel targeted therapies in acute lymphoblastic leukemia. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.823493. [DOI] [PubMed] [Google Scholar]
- 167.Portell CA, Wenzell CM, Advani AS. Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin Pharmacol. 2013;5(Suppl 1):5–11. doi: 10.2147/CPAA.S42689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 169.Perche F, Torchilin VP. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Deliv. 2013;2013:705265. doi: 10.1155/2013/705265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: Double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem. 2006;17(4):943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6(4):3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xiang B, Dong DW, Shi NQ, Gao W, Yang ZZ, Cui Y, Cao DY, Qi XR. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials. 2013;34(28):6976–6991. doi: 10.1016/j.biomaterials.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 173.Oude Blenke E, Mastrobattista E, Schiffelers RM. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin Drug Deliv. 2013 doi: 10.1517/17425247.2013.805742. [DOI] [PubMed] [Google Scholar]
- 174.Yudina A, de Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, Bouchaud V, Voisin P, Grull H, Moonen CT. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J Control Release. 2011;155(3):442–448. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 175.Gomes-da-Silva LC, Fonseca NA, Moura V, Pedroso de Lima MC, Simoes S, Moreira JN. Lipid-based nanoparticles for siRNA delivery in cancer therapy: Paradigms and challenges. Acc Chem Res. 2012;45(7):1163–1171. doi: 10.1021/ar300048p. [DOI] [PubMed] [Google Scholar]
- 176.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bui T, Stevenson J, Hoekman J, Zhang S, Maravilla K, Ho RJ. Novel Gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mann JF, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 179.Bhowmick S, Mazumdar T, Sinha R, Ali N. Comparison of liposome based antigen delivery systems for protection against Leish-mania donovani. J Control Release. 2010;141(2):199–207. doi: 10.1016/j.jconrel.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 180.Badiee A, Jaafari MR, Khamesipour A, Samiei A, Soroush D, Kheiri MT, Barkhordari F, McMaster WR, Mahboudi F. Enhancement of immune response and protection in BALB/c mice immunized with liposomal recombinant major surface glycoprotein of Leishmania (rgp63): The role of bilayer composition. Colloids Surf. 2009;74(1):37–44. doi: 10.1016/j.colsurfb.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 181.Davis D, Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: Influence of liposomal characteristics. Immunology. 1987;61(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- 182.Gregoriadis G, Davis D, Davies A. Liposomes as immunological adjuvants: Antigen incorporation studies. Vaccine. 1987;5(2):145–151. doi: 10.1016/0264-410x(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 183.Alving CR, Rao M. Lipid A and liposomes containing lipid A as antigens and adjuvants. Vaccine. 2008;26(24):3036–3045. doi: 10.1016/j.vaccine.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 184.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shahum E, Therien HM. Correlation between in vitro and in vivo behaviour of liposomal antigens. Vaccine. 1994;12(12):1125–1131. doi: 10.1016/0264-410x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 186.Therien HM, Lair D, Shahum E. Liposomal vaccine: Influence of antigen association on the kinetics of the humoral response. Vaccine. 1990;8(6):558–562. doi: 10.1016/0264-410x(90)90008-a. [DOI] [PubMed] [Google Scholar]
- 187.Guan HH, Budzynski W, Koganty RR, Krantz MJ, Reddish MA, Rogers JA, Longenecker BM, Samuel J. Liposomal formulations of synthetic MUC1 peptides: Effects of encapsulation versus surface display of peptides on immune responses. Bioconjug Chem. 1998;9(4):451–458. doi: 10.1021/bc970183n. [DOI] [PubMed] [Google Scholar]
- 188.Engler OB, Schwendener RA, Dai WJ, Wolk B, Pichler W, Moradpour D, Brunner T, Cerny A. A liposomal peptide vaccine inducing CD8+ T cells in HLA-A2.1 transgenic mice, which recognise human cells encoding hepatitis C virus (HCV) proteins. Vaccine. 2004;23(1):58–68. doi: 10.1016/j.vaccine.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 189.Nair S, Zhou F, Reddy R, Huang L, Rouse BT. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992;175(2):609–612. doi: 10.1084/jem.175.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Perrie Y, Frederik PM, Gregoriadis G. Liposome-mediated DNA vaccination: The effect of vesicle composition. Vaccine. 2001;19(23–24):3301–3310. doi: 10.1016/s0264-410x(00)00432-1. [DOI] [PubMed] [Google Scholar]
- 191.Chen WC, Huang L. Non-viral vector as vaccine carrier. Adv Genet. 2005;54:315–337. doi: 10.1016/S0065-2660(05)54013-6. [DOI] [PubMed] [Google Scholar]
- 192.Sonawane ND, Szoka FC, Jr., Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine–DNA polyplexes. J Biol Chem. 2003;278(45):44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 193.Legendre JY, Szoka FC., Jr. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: Comparison with cationic liposomes. Pharm Res. 1992;9(10):1235–1242. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- 194.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 195.Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: A versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010;7(12):1433–1446. doi: 10.1517/17425247.2010.538047. [DOI] [PubMed] [Google Scholar]
- 196.Chowdhery R, Gonzalez R. Immunologic therapy targeting metastatic melanoma: Allovectin-7. Immunotherapy. 2011;3(1):17–21. doi: 10.2217/imt.10.89. [DOI] [PubMed] [Google Scholar]
- 197.Van Driessche A, Ponsaerts P, Van Bockstaele DR, Van Tendeloo VF, Berneman ZN. Messenger RNA electroporation: An efficient tool in immunotherapy and stem cell research. Folia Histochem Cytobiol. 2005;43(4):213–216. [PubMed] [Google Scholar]
- 198.Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P, Huang L. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther. 2013;21(2):358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leung AK, Hafez IM, Baoukina S, Belliveau NM, Zhigaltsev IV, Afshinmanesh E, Tieleman DP, Hansen CL, Hope MJ, Cullis PR. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core. J Phys Chem C. 2012;116(34):18440–18450. doi: 10.1021/jp303267y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 201.Kokkona M, Kallinteri P, Fatouros D, Antimisiaris SG. Stability of SUV liposomes in the presence of cholate salts and pancreatic lipases: Effect of lipid composition. Eur J Pharm Sci. 2000;9(3):245–252. doi: 10.1016/s0928-0987(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 202.Moutardier V, Tosini F, Vlieghe P, Cara L, Delpero JR, Clerc T. Colloidal anticancer drugs bioavailabilities in oral administration models. Int J Pharm. 2003;260(1):23–38. doi: 10.1016/s0378-5173(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 203.Taira MC, Chiaramoni NS, Pecuch KM, Alonso-Romanowski S. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 2004;11(2):123–128. doi: 10.1080/10717540490280769. [DOI] [PubMed] [Google Scholar]
- 204.Parmentier J, Thomas N, Mullertz A, Fricker G, Rades T. Exploring the fate of liposomes in the intestine by dynamic in vitro lipolysis. Int J Pharm. 2012;437(1–2):253–263. doi: 10.1016/j.ijpharm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 205.Peltier S, Oger JM, Lagarce F, Couet W, Benoit JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006;23(6):1243–1250. doi: 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- 206.Yang S, Gursoy RN, Lambert G, Benita S. Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm Res. 2004;21(2):261–270. doi: 10.1023/b:pham.0000016238.44452.f1. [DOI] [PubMed] [Google Scholar]
- 207.Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 208.Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert RO, Martin F, Muller-Goymann C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm Res. 2010;27(8):1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 209.Minato S, Iwanaga K, Kakemi M, Yamashita S, Oku N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: Effect of lipid dose on systemic and mucosal immunity. J Control Release. 2003;89(2):189–197. doi: 10.1016/s0168-3659(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 210.Imanaka H, Koide H, Shimizu K, Asai T, Kinouchi Shimizu N, Ishikado A, Makino T, Oku N. Chemoprevention of tumor metastasis by liposomal beta-sitosterol intake. Biol Pharm Bull. 2008;31(3):400–404. doi: 10.1248/bpb.31.400. [DOI] [PubMed] [Google Scholar]
- 211.Rogers JA, Anderson KE. The potential of liposomes in oral drug delivery. Crit Rev Ther Drug Carrier Syst. 1998;15(5):421–480. [PubMed] [Google Scholar]
- 212.White M, Pelletier GB, Tan A, Jesina C, Carrier M. Pharmacokinetic, hemodynamic, and metabolic effects of cyclosporine sandimmune versus the microemulsion neoral in heart transplant recipients. J Heart Lung Transplant. 1997;16(8):787–794. [PubMed] [Google Scholar]