Abstract
Objective
Statins reduce atherosclerosis and cardiovascular morbidity in the general population, but their efficacy and safety in children and adolescents with systemic lupus erythematosus (SLE) are unknown. This study was undertaken to determine the 3-year efficacy and safety of atorvastatin in preventing subclinical atherosclerosis progression in pediatric-onset SLE.
Methods
A total of 221 participants with pediatric SLE (ages 10–21 years) from 21 North American sites were enrolled in the Atherosclerosis Prevention in Pediatric Lupus Erythematosus study, a randomized double-blind, placebo-controlled clinical trial, between August 2003 and November 2006 with 36-month followup. Participants were randomized to receive atorvastatin (n = 113) or placebo (n = 108) at 10 or 20 mg/day depending on weight, in addition to usual care. The primary end point was progression of mean-mean common carotid intima-media thickening (CIMT) measured by ultrasound. Secondary end points included other segment/wall-specific CIMT measures, lipid profile, high-sensitivity C-reactive protein (hsCRP) level, and SLE disease activity and damage outcomes.
Results
Progression of mean-mean common CIMT did not differ significantly between treatment groups (0.0010 mm/year for atorvastatin versus 0.0024 mm/year for placebo; P = 0.24). The atorvastatin group achieved lower hsCRP (P = 0.04), total cholesterol (P < 0.001), and low-density lipoprotein (P < 0.001) levels compared with placebo. In the placebo group, CIMT progressed significantly across all CIMT outcomes (0.0023–0.0144 mm/year; P < 0.05). Serious adverse events and critical safety measures did not differ between groups.
Conclusion
Our results indicate that routine statin use over 3 years has no significant effect on subclinical atherosclerosis progression in young SLE patients; however, further analyses may suggest subgroups that would benefit from targeted statin therapy. Atorvastatin was well tolerated without safety concerns.
Improvements in the diagnosis and management of systemic lupus erythematosus (SLE) have significantly improved short-term prognosis. As a result, detection and prevention of long-term SLE complications has become a focus of clinical and research attention. One long-term complication, accelerated atherosclerosis, has emerged as a major cause of morbidity and mortality in patients with SLE and is not completely explained by traditional risk factors (1–5). Compared with adults, children with SLE have more severe organ disease and longer exposure to illness and potentially toxic treatments (6,7). Although the prevalence of atherosclerosis in pediatric SLE is unknown, increased rates of subclinical atherosclerosis (measured by carotid intima-media thickening [CIMT], flow-mediated brachial artery dilation, and myocardial perfusion) have been reported (8–13).
Statins reduce mortality and morbidity from atherosclerosis in adults (14). In addition to lipid-modifying effects, statins have intrinsic antiinflammatory and immunomodulatory properties (15,16), which may convey particular benefit in the prevention and treatment of atherosclerosis in SLE. Clinical trials in familial hypercholesterolemia have shown statins to be safe and effective in children, but statins have not been studied in pediatric SLE (17). The Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial was designed to assess whether 36 months of atorvastatin therapy in children and adolescents with SLE is effective and safe in reducing atherosclerosis progression as measured by CIMT.
PATIENTS AND METHODS
Study oversight
APPLE was a multicenter, randomized double-blind, placebo-controlled clinical trial conducted at 21 Childhood Arthritis and Rheumatology Research Alliance (CARRA) sites in North America. The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the trial. Pfizer provided atorvastatin and matching placebo but had no role in the study design, data collection or analysis, or manuscript preparation. The Duke Clinical Research Institute (Durham, NC) served as the data-coordinating center and provided oversight of all aspects of the study’s conduct, management, and statistical analysis. APPLE investigators who participated in the study (in addition to the authors) are listed in Appendix A. Investigators were blinded with regard to all centrally measured laboratory results, including lipid and high-sensitivity C-reactive protein (hsCRP) levels, as well as results of CIMT measurement and magnetic resonance imaging (MRI). Local institutional review board approval was obtained, and all patients or their guardians gave informed consent and assent following local guidelines. The APPLE Data and Safety Monitoring Board (DSMB) approved the protocol and amendments and reviewed study progress, serious adverse events, and safety parameters.
Patient population
Children and adolescents ages 10–21 years at enrollment who met the American College of Rheumatology (ACR) revised diagnostic criteria for SLE (18) were eligible. Additional inclusion criteria included weight ≥25 kg, ability to complete questionnaires in English or Spanish, and willingness to comply with American Heart Association Therapeutic Lifestyle Changes diet and approved birth control methods. In addition, patients who had active nephrotic syndrome, myositis, liver disease, renal insufficiency, or hypercholesterolemia (total cholesterol >350 mg/dl) or were being treated with cyclosporine or tacrolimus were excluded from the study. Participants in whom CIMT scans of adequate quality could not be obtained were excluded before randomization.
Study design
Participants were assigned via site-based block randomization (with random blocks of sizes 2, 4, and 6) in a 1:1 ratio to receive daily atorvastatin or placebo in a double-dummy, double-blind manner. All participants were started on atorvastatin or placebo 10 mg daily. The dosage was increased to 20 mg/day at the 1-month visit for children weighing >50 kg. Dosages were adjusted for weight during the trial. Participants received medical treatment for SLE at the discretion of the treating pediatric rheumatologist. Hydroxychloroquine, low-dose aspirin, and a multivitamin containing folate (400 µg) were recommended. Participants received cardiovascular risk and dietary counseling (American Heart Association Therapeutic Lifestyle Changes diet) at enrollment and every 12 months. Participants were seen for study visits at 1 and 3 months after randomization and every 3 months thereafter for a total of 36 months. There were 7 CIMT examinations over the course of the study, with 2 at enrollment and 2 at study end. Additional CIMT measurements were scheduled at 6, 12, and 24 months.
B-mode ultrasonography of carotid arteries
Standardized measurements of CIMT were obtained using an ultrasound protocol adapted from several previous clinical trials (19–21). Portable Siemens Cypress systems were shared between clinical centers. All scanners were equipped with 7L3 transducers, a cardiology package with electrocardiogram (EKG) tracing, and specific APPLE presets in order to reduce variability across centers. Standardized longitudinal B-mode images were collected for 3 arterial segments defined relative to the tip of the flow divider (TFD) as the common carotid artery (10–20 mm proximal to the TFD), the carotid bifurcation (from the TFD to 10 mm proximal to the TFD), and the proximal 10 mm of the internal carotid artery. Near and far walls were imaged simultaneously in the common carotid artery, but separately in the carotid bifurcation and internal carotid artery to improve the ability to align each wall horizontally in these segments. For each arterial segment, Meijer’s Arc was used to collect images at 90°, 120°, 150°, and 180° on the right side and at 270°, 240°, 210°, and 180° on the left side.
Image selections were saved as 5-second digital clips and written to 640-megabyte magnetic optical disks for transfer to a central reading center (Ward A. Riley Ultrasound Center, Wake Forest University School of Medicine, Winston-Salem, NC). All ultrasound scans were read using Image Pro software (Media Cybernetics) by a single reader with more than 20 years of experience reading CIMT research studies. For each image sequence, the reader selected 1 frame for measurement when the heart was in systole (EKG tracing on QRS complex). Leading (far wall) and trailing (near wall) edges of visualized blood–intima and media–adventitia boundaries were traced with a computer mouse within a region of interest specified by the reader. Quality assurance procedures included central training and certification of all sonographers and the reader as well as regular site visits and performance reviews. For a set of 68 studies reread to evaluate intrareader reliability, the intraclass correlation coefficient was 0.74 (95% confidence interval [95% CI] 0.61, 0.83) for mean-mean common and 0.71 (95% CI 0.56, 0.81) for mean-max CIMT measurements.
The combination of 3 arterial segments, 2 walls, and 2 sides of the neck provides a set of 12 CIMT measurement sites, each imaged from 4 angles. For each measurement site, a maximum CIMT value was defined as the largest of the 4 angle-specific maximum CIMTs. The 12 maximum CIMT values were then averaged to determine the mean-max CIMT over near and far walls of the right and left common carotid artery, carotid bifurcation, and internal carotid artery. For each of the 4 measurement sites in the common carotid artery, a mean CIMT value defined as the average of the 4 angle-specific mean CIMTs was also calculated. The 4 mean CIMT values were then averaged to determine the mean-mean common CIMT. Overall mean-mean and other segment/wall-specific mean-max or mean-mean CIMT measures were computed accordingly.
Magnetic resonance imaging studies
Due to concerns that statins may impair central nervous system white matter development (22), serial brain MRI was performed at randomization, 9 months, and 36 months in a subset of participants. Forty-four (63%) of the 70 participants at the 5 sites taking part in the MRI substudy were enrolled. Both conventional MRI and diffusion tensor imaging were performed using local 1.5T or 3.0T MRI magnets with prespecified imaging protocols. Change in white matter integrity was assessed by the rate of decrease in fractional anisotropy and development of new hyperintense white matter lesions on T2-weighted MRI. All MRIs were read by a neuroradiologist (JP) who was blinded with regard to treatment group (Duke University School of Medicine, Durham, NC).
Other assessments
Lipid profiles from serum samples obtained after 12-hour or 4-hour fasts at randomization and 3, 6, 12, 18, 24, and 36 months were analyzed at a central commercial laboratory (PPD Global Central Laboratories, Highland Heights, KY), along with homocysteine, lipoprotein A and B, and hsCRP levels. Other blood and urine tests were performed at local laboratories every 3 months, including chemistries, complete blood counts, urine protein-to-creatinine ratio, and SLE disease activity measures.
SLE disease activity was assessed using the modified Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE Disease Activity Index (SLEDAI) (23) at randomization and every 3 months during the study period. Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI) (24), health-related quality of life (PedsQL 4.0) (25), and Tanner staging were measured at randomization and every 12 months thereafter. Physical assessment, including manual muscle testing, was performed every 3 months, and adverse events were recorded according to National Cancer Institute Common Toxicity Criteria Scale, version 2.0 standards (26). Study drug adherence was defined as taking ≥75% of prescribed drug and was determined by pill counts at each study visit.
Study outcome measures
Two CIMT summary measures, the mean-mean common and the mean-max CIMT averaged over the common, bifurcation, and internal carotid segments, have been widely used as primary outcomes in clinical trials and are generally recognized as having equivalent clinical significance and similar expected progression rates (19–24,27). The original APPLE protocol specified progression of mean-max CIMT over 12 arterial sites as the primary outcome, with a sample size of 280 participants expected to provide >90% power to detect a difference of 0.0045 mm/year in progression rates between treatment groups. However, recruitment was slower than expected, and at the second planned interim analysis in August 2006, only 179 subjects had been enrolled despite implementation of aggressive recruitment strategies. Recognizing the recruitment challenges inherent to a prevention trial for a rare and complex chronic disease (27), the investigators considered several approaches to decreasing the sample size at that time, including reevaluating the CIMT segments used for measuring the primary outcome. Blinded review of baseline mean-mean common and meanmax CIMT values revealed increased precision in the meanmean common CIMT measurement (SD 0.043) compared with the mean-max CIMT measurement (SD 0.054). Therefore, the primary outcome was changed to the mean-mean common CIMT, allowing the sample size to be reduced to 220 participants while preserving >90% power to detect the same prespecified clinically relevant difference between groups (0.0045 mm/year). This change was approved by the DSMB on August 25, 2006.
Secondary outcomes included the mean-max CIMT of 12 segments and the mean-max and mean-mean CIMT of the different carotid artery segments, SLE disease activity, organ system damage, health-related quality of life, and laboratory measures, including hsCRP and serum lipid levels.
Statistical analysis
We defined clinically significant change in the primary end point as a 0.0045 mm/year difference in mean CIMT progression rates between the atorvastatin and placebo groups. Informing this definition of clinical significance were large epidemiologic studies in adults (28), which demonstrated a 41–47% increase in risk of cardiovascular events for every 0.16–0.20 mm increase in CIMT (28–31). For a 15-year-old SLE patient to achieve 40% reduction of risk by age 50, a decrease in the CIMT progression rate of at least 0.0045 mm/year over 35 years (0.0045 mm/year × 35 years = 0.16 mm) would be required. As described above, sample size calculations were revised based on blinded results from the second planned interim analysis using observed estimates of the SD of mean-mean common CIMT (0.043 mm [95% CI 0.038, 0.047]), the dropout rate (11.6% [95% CI 1.1, 23.2%]), the study drug adherence rate (77.6% [95% CI 71.1, 84.1%]), and estimates of visit-to-visit correlations between repeated measures of CIMT (~0.60). Using point estimates for these parameters, a total sample size of 220 provided 94% power to detect a 0.0045 mm/year difference in mean-mean common CIMT progression rates between the 2 groups, assuming an overall 0.05 type I error accounting for interim analyses and a 2-sided significance test. In sensitivity analyses based on the use of 95% confidence limits for these parameters, estimated power ranged from 82% to 98%.
The primary efficacy analysis compared mean rates of mean-mean common CIMT progression between treatment groups based on a test of interaction between treatment group and time in a longitudinal linear mixed-effects model under data-missing-at-random assumptions. In subsequent exploratory analyses prespecified in the statistical analysis plan, additional covariates were considered to adjust for possible confounders (variables that were imbalanced at baseline), and to improve precision of the estimates (variables that were found to associate with CIMT) (10). A stepwise process was used to derive the adjusted final model. Similar mixed-effects models were used for analyzing continuous secondary longitudinal end points or changes from baseline over time for lipid data. Log transformation was used for hsCRP to achieve normality. Generalized estimating equations were used for binary longitudinal outcomes. Baseline characteristics of the APPLE cohort were summarized using descriptive statistics with categorical data presented as percentages and continuous data presented as the mean ± SD and median. Differences between treatment groups were assessed using the chi-square test, Fisher’s exact test, or Wilcoxon’s test. Data were analyzed according to the intent-to-treat principle. Efficacy boundaries based on the Lan-DeMets alpha spending function were used for sequential monitoring by the DSMB. A total of 2 planned interim analyses were carried out. With adjustment for these 2 interim analyses, P values less than or equal to 0.049 were considered significant for the final primary analysis. The level of significance for other efficacy and safety analyses was 0.05, and all analyses were 2 sided. Analyses were performed with SAS software, version 8.2.
RESULTS
Disposition of the patients
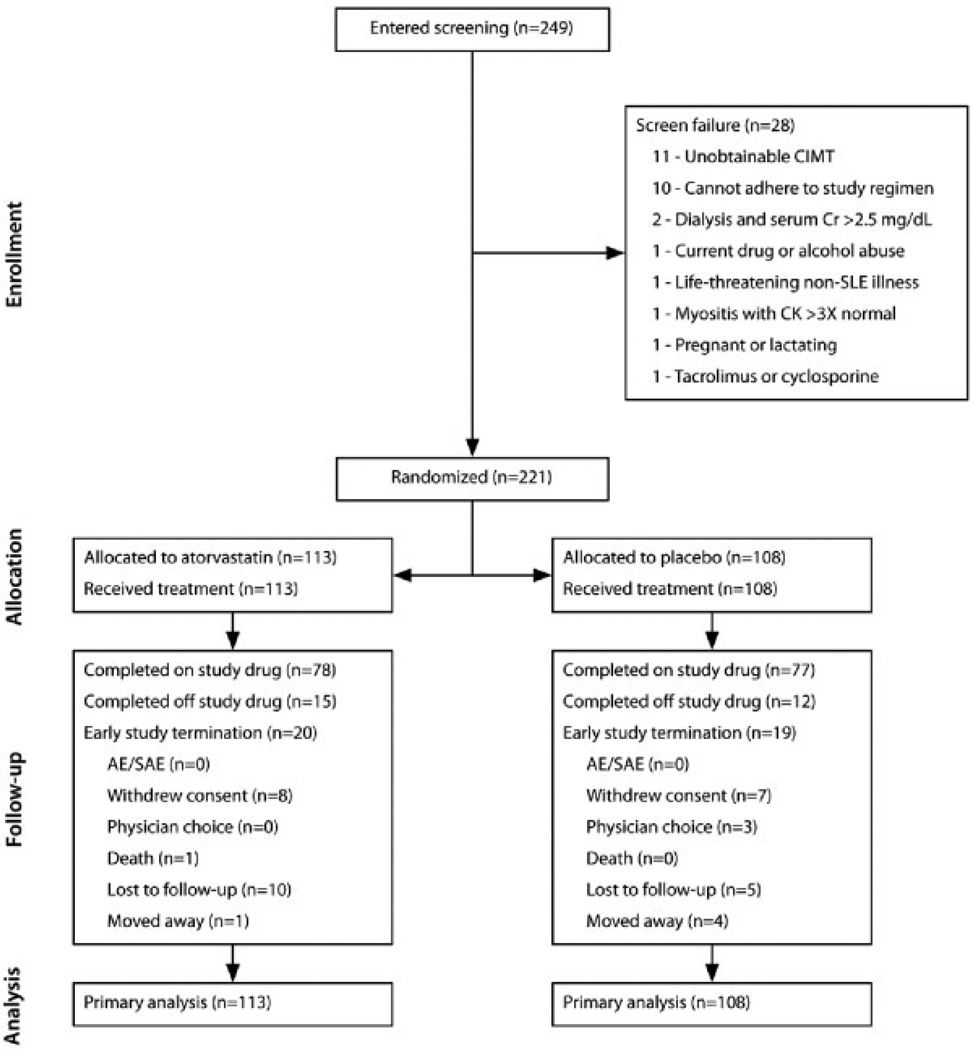
Enrollment occurred between August 2003 and November 2006. Participants were followed up for 36 months, with the last participant completing in December 2009. As shown in Figure 1, a total of 221 participants were randomized, with 113 patients in the atorvastatin group and 108 patients in the placebo group. A total of 182 (82.3%) of the participants completed the trial. Dropout rates were similar between treatment groups (17.7% in the atorvastatin group versus 17.6% in the placebo group). The estimated drug adherence rates were similar between groups (59.3% in the atorvastatin group versus 65.0% in the placebo group, P = 0.39). Table 1 shows that the atorvastatin- and placebo-treated groups were well matched with regard to most baseline characteristics, except for mean creatinine clearance, hsCRP level, body mass index (BMI), and low-density lipoprotein (LDL) level.
Figure 1.
Consolidated Standards of Reporting Trials diagram for the Atherosclerosis Prevention in Pediatric Lupus Erythematosus study. CIMT = carotid intima-media thickening; Cr = creatinine; SLE = systemic lupus erythematosus; CK = creatine kinase; AE = adverse event; SAE = serious adverse event.
Table 1.
Baseline characteristics of the APPLE study participants*
| Atorvastatin (n = 113) |
Placebo (n = 108) |
P | |
|---|---|---|---|
| Sex, no. (%) female | 95 (84.1) | 89 (82.4) | 0.741 |
| Age, mean ± SD years | 15.7 ± 2.8 | 15.8 ± 2.5 | 0.856 |
| No. (%) white | 55 (48.7) | 59 (54.6) | 0.376 |
| No. (%) Hispanic or Latino | 29 (25.7) | 25 (23.1) | 0.664 |
| History of smoking, no. (%) | 3 (2.7) | 4 (3.7) | 0.717 |
| Family history of angina/MI/atherosclerosis, no. (%)† | 38 (35.5) | 41 (39.1) | 0.595 |
| Family history of hyperlipidemia, no. (%)† | 35 (32.7) | 45 (43.3) | 0.114 |
| Annual household income, no. (%)‡ | 0.984 | ||
| <$25,000 | 30 (29.4) | 34 (33.0) | |
| $25,000–49,999 | 29 (28.4) | 26 (25.2) | |
| $50,000–74,999 | 16 (15.7) | 17 (16.5) | |
| $75,000–99,999 | 12 (11.8) | 13 (12.6) | |
| $100,000–150,000 | 10 (9.8) | 8 (7.8) | |
| >$150,000 | 5 (4.9) | 5 (4.9) | |
| Body mass index, mean ± SD kg/m2 | 25.0 ± 5.2 | 23.8 ± 5.4 | 0.045 |
| Duration of lupus, mean ± SD months | 32.5 ± 29.1 | 29.4 ± 27.8 | 0.448 |
| SDI score of 0, no. (%) | 79 (69.9) | 83 (76.9) | 0.244 |
| SLEDAI, mean ± SD§ | 4.92 ± 4.46 | 4.57 ± 4.07 | 0.726 |
| History of hypertension, no. (%)¶ | 38 (34.2) | 35 (34) | 0.969 |
| History of nephritis/nephrotic syndrome, no. (%)¶ | 46 (40.7) | 45 (42.1) | 0.839 |
| Creatinine clearance, mean ± SD ml/minute/m2 | 145.2 ± 36.9 | 133.2 ± 26.6 | 0.021 |
| Proteinuria, no. (%)# | 25 (22.1) | 31 (29.0) | 0.244 |
| Lupus anticoagulant positive, no. (%)** | 45 (42.1) | 35 (34.3) | 0.250 |
| Anticardiolipin IgG or IgM positive, no. (%)** | 46 (41.8) | 54 (50.9) | 0.179 |
| dsDNA antibody positive, no. (%) | 94 (83.2) | 87 (80.6) | 0.612 |
| C3, mean ± SD mg/dl | 102.0 ± 30.1 | 99.3 ± 27.8 | 0.338 |
| C4, mean ± SD mg/dl | 15.8 ± 11.0 | 15.2 ± 8.0 | 0.953 |
| Medications (past 30 days)†† | |||
| Aspirin, no. (%) | 71 (62.8) | 76 (70.4) | 0.235 |
| Hydroxychloroquine, no. (%) | 110 (97.3) | 103 (95.4) | 0.491 |
| Multivitamin, no. (%) | 80 (70.8) | 76 (70.4) | 0.945 |
| Corticosteroids, no. (%) | 91 (81.3) | 89 (82.4) | 0.824 |
| Weight-adjusted prednisone dosage, mean ± SD mg/kg/day | 0.170 ± 0.166 | 0.204 ± 0.205 | 0.307 |
| Cyclophosphamide, no. (%) | 13 (11.6) | 13 (12.0) | 0.921 |
| Mycophenolate mofetil, no. (%) | 29 (25.9) | 24 (22.2) | 0.524 |
| Azathioprine, no. (%) | 12 (10.7) | 18 (16.7) | 0.198 |
| Methotrexate, no. (%) | 16 (14.3) | 13 (12.0) | 0.622 |
| Rituximab, no. (%) | 0 (0.0) | 1 (1.0) | 0.495 |
| NSAIDs, no. (%) | 35 (31.3) | 33 (30.6) | 0.911 |
| ACE inhibitor, no. (%) | 25 (22.3) | 29 (26.9) | 0.435 |
| hsCRP, mean ± SD mg/liter‡‡ | 4.4 ± 18.0 | 2.6 ± 7.4 | 0.044 |
| Homocysteine, mean ± SD µmoles/liter | 7.3 ± 2.7 | 7.7 ± 3.4 | 0.668 |
| Lipid levels, mean ± SD mg/dl | |||
| Total cholesterol | 159.6 ± 41.1 | 150.6 ± 34.0 | 0.110 |
| HDL cholesterol | 46.7 ± 12.9 | 46.0 ± 12.7 | 0.716 |
| LDL cholesterol | 91.8 ± 33.0 | 80.7 ± 28.7 | 0.019 |
| Triglycerides | 105.5 ± 52.8 | 122.9 ± 77.3 | 0.083 |
| Lipoprotein A | 25.9 ± 28.4 | 20.3 ± 24.9 | 0.104 |
| CIMT measurements§§ | |||
| Mean-mean ± SD common CIMT, mm | 0.465 ± 0.0439 | 0.471 ± 0.0409 | 0.578 |
| Mean-max ± SD CIMT, mm | 0.579 ± 0.059 | 0.587 ± 0.053 | 0.128 |
APPLE = Atherosclerosis Prevention in Pediatric Lupus Erythematosus; SDI = Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; dsDNA = double-stranded DNA; hsCRP = high-sensitivity C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; CIMT = carotid intima-media thickening.
Data on family history of angina/myocardial infarction (MI)/atherosclerosis were available for 107 patients in the atorvastatin group and 105 patients in the placebo group. Data on family history of hyperlipidemia were available for 107 patients in the atorvastatin group and 104 patients in the placebo group.
Data on annual household income were available for 102 patients in the atorvastatin group and 103 patients in the placebo group.
The median score was 4.0 in both groups.
Data on history of hypertension were available for 111 patients in the atorvastatin group and 103 patients in the placebo group. Data on history of nephritis/nephrotic syndrome were available for 107 patients in the placebo group.
Data on proteinuria (defined as >500 mg protein in a 24-hour urine collection or a random urine protein-to-creatinine ratio of >0.5) were available for 107 patients in the placebo group.
Data on lupus anticoagulant status were available for 107 patients in the atorvastatin group and 102 patients in the placebo group. Data on anticardiolipin IgG or IgM status were available for 110 patients in the atorvastatin group and 106 patients in the placebo group.
Corticosteroids were given by oral or intravenous administration. The dosage is presented as the prednisone equivalent. If alternate-day dosing of corticosteroids was used, the dose was averaged to obtain a daily dose. Data on corticosteroid use were available for 112 patients in the atorvastatin group. Data on cyclophosphamide, mycophenolate mofetil, azathioprine, methotrexate, nonsteroidal antiinflammatory drug (NSAID), and angiotensin-converting enzyme (ACE) inhibitor use were available for 112 patients in the atorvastatin group. Data on rituximab use were available for 106 patients in the atorvastatin group and 104 patients in the placebo group.
The normal range according to PPD Global Central Laboratories is 0–8.4 mg/liter. These normal values are for men and women older than 18 years; no normal values are available for younger populations.
Second baseline measurements.
Primary end point and CIMT secondary end points
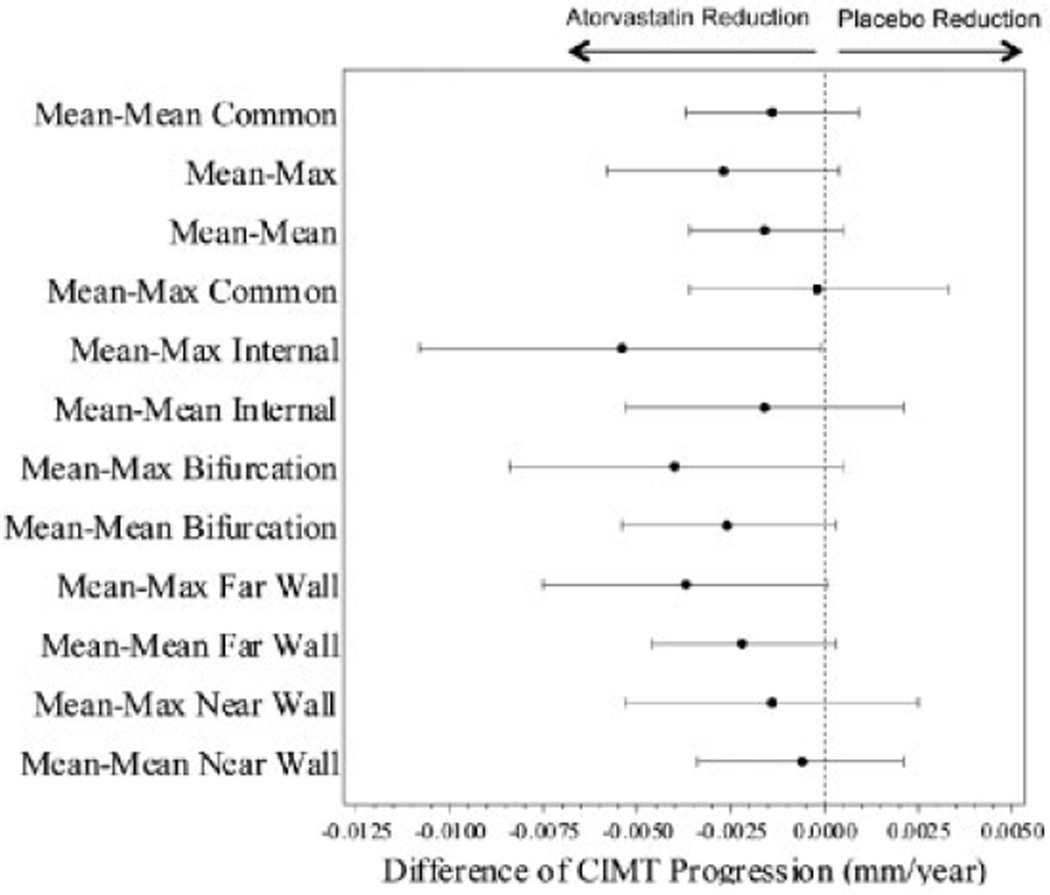
As shown in Figure 2, CIMT progression was not significantly different between the atorvastatin- and placebo-treated groups for either the primary outcome of mean-mean common CIMT (progression rate 0.0010 mm/year in the atorvastatin group versus 0.0024 mm/year in the placebo group [difference −0.0014 mm/year], P = 0.24) or the important secondary outcome mean-max CIMT (progression rate 0.0037 mm/year in the atorvastatin group versus 0.0064 mm/year in the placebo group [difference −0.0027 mm/year], P = 0.083). Diagnostics revealed no significant quadratic effects of time for either outcome. In prespecified exploratory models adjusting for baseline confounders or covariates known to impact CIMT, the adjusted difference in mean-mean common CIMT progression rate remained nonsignificant, while the adjusted difference in mean-max CIMT progression rate was −0.0042 mm/year (P = 0.006). The final adjusted models included additional fixed main and interaction effects for covariates of age, sex, baseline measurements of weight-adjusted prednisone dose, BMI, log hsCRP, LDL cholesterol, and baseline mean-mean common or mean-max CIMT.
Figure 2.
Difference in progression rates of carotid intima-media thickening (CIMT) in the atorvastatin and placebo treatment groups. Forest plots of the progression rate differences between the atorvastatin and placebo groups are shown for all CIMT outcomes. Whiskers indicate the 95% confidence interval.
Results of other CIMT secondary end points demonstrated lower CIMT progression rates in the atorvastatin group compared with the placebo group. However, none of the observed group differences were statistically significant except for mean-max internal CIMT (difference in progression rate −0.0054 mm/year, P = 0.047) (Table 2). This was the only CIMT outcome where the estimated treatment effect met or exceeded the prespecified definition of clinical significance (0.0045 mm/year). All CIMT outcomes demonstrated significant progression in the placebo group (ranged from 0.0023 to 0.0144 mm/year, P ≤ 0.05 for testing zero progression rate) except for mean-max common CIMT.
Table 2.
Progression of CIMT thickening over 36 months in the atorvastatin and placebo treatment groups*
| CIMT outcome | Baseline, mean ± SD |
Progression rate in atorvastatin-treated patients, estimate (95% CI) |
Progression rate in placebo-treated patients, estimate (95% CI) |
Difference between atorvastatin and placebo groups, estimate (95% CI) |
|---|---|---|---|---|
| Primary end point | ||||
| Mean-mean common CIMT | 0.4680 ± 0.0425 | 0.0010 (−0.0006, 0.0026) | 0.0024 (0.0007, 0.0040) | −0.0014 (−0.0037, 0.0009) |
| Secondary end points | ||||
| Mean-max CIMT† | 0.5830 ± 0.0562 | 0.0037 (0.0015, 0.0058) | 0.0064 (0.0042, 0.0086) | −0.0027 (−0.0058, 0.0004) |
| Mean-mean CIMT‡ | 0.4600 ± 0.0395 | 0.0033 (0.0018, 0.0047) | 0.0049 (0.0034, 0.0063) | −0.0016 (−0.0036, 0.0005) |
| Mean-max common CIMT | 0.5933 ± 0.0592 | 0.0006 (−0.0018, 0.0031) | 0.0008 (−0.0017, 0.0033) | −0.0002 (−0.0036, 0.0033) |
| Mean-max internal CIMT | 0.5070 ± 0.0835 | 0.0090 (0.0052, 0.0128) | 0.0144 (0.0106, 0.0182) | −0.0054 (−0.0108, −0.0001)§ |
| Mean-mean internal CIMT | 0.4113 ± 0.0533 | 0.0067 (0.0041, 0.0093) | 0.0082 (0.0056, 0.0109) | −0.0016 (−0.0053, 0.0021) |
| Mean-max bifurcation CIMT | 0.6315 ± 0.0688 | 0.0033 (0.0001, 0.0064) | 0.0072 (0.0040, 0.0104) | −0.0040 (−0.0084, 0.0005) |
| Mean-mean bifurcation CIMT | 0.4910 ± 0.0473 | 0.0030 (0.0009, 0.0050) | 0.0055 (0.0035, 0.0076) | −0.0026 (−0.0054, 0.0003) |
| Mean-max far wall CIMT | 0.5866 ± 0.0658 | 0.0045 (0.0019, 0.0072) | 0.0082 (0.0055, 0.0110) | −0.0037 (−0.0075, 0.0001) |
| Mean-mean far wall CIMT | 0.4564 ± 0.0447 | 0.0042 (0.0025, 0.0059) | 0.0064 (0.0047, 0.0081) | −0.0022 (−0.0046, 0.0003) |
| Mean-max near wall CIMT | 0.5795 ± 0.0624 | 0.0024 (−0.0003, 0.0052) | 0.0038 (0.0011, 0.0066) | −0.0014 (−0.0053, 0.0025) |
| Mean-mean near wall CIMT | 0.4646 ± 0.0460 | 0.0022 (0.0002, 0.0041) | 0.0028 (0.0008, 0.0047) | −0.0006 (−0.0034, 0.0021) |
Missing data were assumed to be missing at random, with a rate of missing data for carotid intima-media thickening (CIMT) of 11.6%. 95% CI = 95% confidence interval.
Summary measure of 12 segments.
Summary measure of 3 segments.
Differed significantly between the 2 treatment groups (P = 0.047).
Non-CIMT secondary end points
Table 3 shows that the atorvastatin group achieved significant reductions from baseline in total cholesterol, LDL, and hsCRP levels and that these reductions were maintained over time. Changes from baseline in SLEDAI, SDI, and PedsQL did not differ significantly between groups.
Table 3.
Non-CIMT cardiovascular secondary end points in the APPLE study, by treatment group*
| Lipid outcome | Baseline, mean ± SD |
Change in atorvastatin group (95% CI) |
Change in placebo group (95% CI) |
Difference between atorvastatin and placebo groups, estimate (95% CI)† |
P |
|---|---|---|---|---|---|
| Log of hsCRP | −0.283 ± 1.523 | −0.13 (−0.48, 0.22) | 0.27 (−0.08, 0.63) | −0.41 (−0.79, −0.02) | 0.037 |
| Total cholesterol, mg/dl | 155.1 ± 38.0 | −30.30 (−37.06, −23.53) | −0.72 (−7.57, 6.12) | −29.57 (−38.63, −20.52) | <0.001 |
| HDL cholesterol, mg/dl | 46.3 ± 12.8 | −0.43 (−2.23, 1.37) | 0.89 (−0.93, 2.71) | −1.32 (−3.71, 1.07) | 0.277 |
| LDL cholesterol, mg/dl | 86.4 ± 31.4 | −27.63 (−32.79, −22.47) | −1.48 (−6.72, 3.76) | −26.15 (−32.98, −19.31) | <0.001 |
| Triglycerides, mg/dl | 114.0 ± 66.4 | −11.04 (−23.24, 1.17) | −5.62 (−17.99, 6.75) | −5.42 (−22.00, 11.17) | 0.522 |
| Lipoprotein A, mg/dl | 23.17 ± 26.85 | 2.00 (−2.17, 6.17)† | 6.34 (2.09, 10.59)† | −4.34 (−10.30, 1.61) | 0.152 |
| Homocysteine, µmole/liter | 7.49 ± 3.07 | 1.84 (1.08, 2.60)† | 1.76 (0.98, 2.53)† | −0.08 (−1.00, 1.17) | 0.880 |
CIMT = carotid intima-media thickening; APPLE = Atherosclerosis Prevention in Pediatric Lupus Erythematosus; 95% CI = 95% confidence interval; hsCRP = high-sensitivity C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Mean changes from baseline at 36 months.
Safety
The occurrence of serious adverse events and predefined safety events (muscle, liver, and neurotoxicity) did not differ between the treatment groups (Table 4). One death due to pneumococcal sepsis occurred in the atorvastatin group. Twenty-four atorvastatin-treated and 20 placebo-treated patients at 5 sites underwent serial brain MRI. Central nervous system hyperintense lesions developed in 3 subjects in the atorvastatin group and 2 subjects in the placebo group (P > 0.999); significant fractional anisotropy changes occurred in ≥3 brain locations in 2 atorvastatin-treated and 4 placebo-treated subjects at 36 months (P = 0.387).
Table 4.
Serious adverse events and critical safety end points in the APPLE trial*
| Atorvastatin (n = 113) |
Placebo (n = 108) |
|
|---|---|---|
| Death | 1 (0.9) | 0 (0.0) |
| Serious adverse events | ||
| All events | 34 (30.1) | 40 (37.0) |
| Infections | 15 (13.3) | 14 (13.0) |
| Musculoskeletal and connective tissue disorders | 11 (9.7) | 9 (8.3) |
| Psychiatric disorders | 4 (3.5) | 5 (4.6) |
| Nervous system disorders | 1 (0.9) | 6 (5.6) |
| Renal and urinary disorders | 3 (2.7) | 3 (2.8) |
| Gastrointestinal disorders | 3 (2.7) | 2 (1.9) |
| Skin and subcutaneous tissue disorders | 2 (1.8) | 3 (2.8) |
| Blood and lymphatic system disorders | 2 (1.8) | 2 (1.9) |
| General disorders and administration site conditions | 1 (0.9) | 2 (1.9) |
| Metabolism and nutrition disorders | 1 (0.9) | 2 (1.9) |
| Pregnancy, puerperium, and perinatal complications | 3 (2.7) | 0 (0.0) |
| Vascular disorders | 1 (0.9) | 2 (1.9) |
| Cardiac disorders | 1 (0.9) | 1 (0.9) |
| Congenital, familial, and genetic disorders | 1 (0.9) | 1 (0.9) |
| Hepatobiliary disorders | 1 (0.9) | 1 (0.9) |
| Immune system disorders | 0 (0.0) | 2 (1.9) |
| Neoplasms (benign, malignant, and unspecified, including cysts and polyps) | 0 (0.0) | 2 (1.9) |
| Respiratory, thoracic, and mediastinal disorders | 2 (1.8) | 0 (0.0) |
| CK elevation requiring investigation | 1 (0.9) | 0 (0.0) |
| Critical safety end points† | ||
| Muscle toxicity | 29 (26.1) | 33 (30.6) |
| Myositis | 14 (12.5) | 9 (8.3) |
| Rhabdomyolysis | 0 (0.0) | 0 (0.0) |
| CK >3 times the upper limit of normal | 5 (4.5) | 7 (6.5) |
| Myalgia | 16 (14.3) | 20 (18.5) |
| Muscle weakness | 6 (5.4) | 4 (3.7) |
| Liver toxicity | 27 (24.1) | 20 (18.7) |
| Neurotoxicity | 27 (24.1) | 32 (29.6) |
There were no significant differences between the 2 groups. APPLE = Atherosclerosis Prevention in Pediatric Lupus Erythematosus.
Data on muscle toxicity were available for 111 patients in the atorvastatin group. Data on myositis, rhabdomyolysis, creatine kinase (CK) level, myalgia, muscle weakness, liver toxicity, and neurotoxicity were available for 112 patients in the atorvastatin group. Data on liver toxicity were available for 107 patients in the placebo group.
Seventeen pregnancies occurred in 15 patients during the trial (9.5% of the female patients in the atorvastatin group versus 7% of the female patients in the placebo group; P = 0.5) with 7 live births (4 in the atorvastatin group, 1 of which had fetal anomalies consistent with methotrexate exposure and died 2 hours after birth), 5 spontaneous abortions (3 in the atorvastatin group), 4 elective abortions (3 in the atorvastatin group), and 1 lost to followup.
DISCUSSION
The APPLE trial was designed to determine whether 36 months of atorvastatin treatment afforded children and adolescents with pediatric-onset SLE a clinically important reduction in CIMT progression rate. The treatment group difference in the primary end point, progression of mean-mean common CIMT, was not significant, and the 95% CI (−0.0037, 0.0009 mm/year) did not include the prespecified clinically relevant reduction of −0.0045 mm/year. The important secondary end point, mean-max CIMT over 12 arterial sites, approached statistical significance (P = 0.08) in the primary analysis and became significant (P = 0.006) in exploratory analyses after controlling for covariates imbalanced at baseline or related to baseline CIMT. However, due to limitations inherent in secondary and exploratory analyses, this result cannot be interpreted as definitive confirmation of treatment benefit. Moreover, among 10 additional secondary CIMT end points, only the mean-max internal CIMT exceeded the clinically relevant threshold of −0.0045 mm/year, attaining nominal statistical significance. Collectively, these results suggest that the potential benefits of statin therapy for slowing CIMT progression are not large enough to warrant routine use in children with SLE.
Other studies have evaluated the use of statins in adult SLE. The Lupus Atherosclerosis Prevention Study was a 24-month double-blind, placebo-controlled trial testing the efficacy of higher-dose atorvastatin (40 mg) in reducing coronary artery calcium in 200 adult lupus patients (32). Results showed no difference in either the primary outcome (coronary artery calcium) or the secondary outcome (mean common CIMT) between treatment groups. In addition, there was no difference in hsCRP level. In contrast, post hoc subgroup analysis of the Assessment of Lescol in Renal Transplantation study showed that SLE patients treated with statins experienced a reduction in major cardiac events (33). Other smaller, shorter-term clinical trials have demonstrated that statins are effective in reducing total cholesterol and LDL and in improving endothelial function (as measured by brachial artery flow-mediated dilation) in adult SLE patients (34–36).
In the APPLE study, slower than expected recruitment necessitated a change in the primary outcome, allowing sample size reduction and study completion without compromising power or changing the definition of clinical significance. Mean-max and mean-mean common CIMT have been used interchangeably in both cohort studies and clinical trials for years. Trials supporting the use of CIMT as a surrogate outcome for lipid-lowering interventions in adults included studies using both measurements with similar results (37). Furthermore, a recent meta-analysis of population-based studies relating CIMT measurements to incident cardiovascular events demonstrated that multiple sources of heterogeneity between studies, including differences in the definition and inclusion of various carotid artery segments, did not significantly impact the resulting hazard estimates (38). Recent analyses of CIMT intervention studies suggest that treatment differences for lipid-lowering interventions are, on average, similar between the 2 CIMT measurements, with the mean-mean common CIMT affording more precision and reproducibility (29,39).
The observed variability in carotid segment progression rates may indicate differences in the pathogenesis of premature atherosclerosis in SLE and other causes of atherosclerosis. Similar variability has been shown in adult SLE studies that described increased carotid plaque in SLE patients compared with controls, while CIMT was less affected (3,40). However, carotid plaque is a later effect of early atherosclerosis and is not present in children and adolescents. In this trial, observed correlations between mean-mean common and mean-max CIMT measures at different time points within each group ranged from 0.6 to 0.8 (data not shown). Models adjusted for baseline covariates, such as age, sex, BMI, weight-adjusted prednisone dose, log hsCRP, and LDL cholesterol, have varying strengths of association with mean-mean common and mean-max CIMT measures. The impact of baseline body size on CIMT measurement was of particular interest given the age of the study participants; however, inclusion of baseline height in the model did not alter the adjusted results. Further research is needed to better define the biologic relevance of different CIMT measures in SLE patients compared with the general population.
Although the primary efficacy end point was not achieved, this study included many clinically significant results. Importantly, the APPLE study found CIMT progression rates in all but 1 carotid segment to be higher in the placebo group (0.0023 to 0.0144 mm/year) than previously reported for both a general pediatric population (<0.002 mm/year between 10 and 18 years of age) (41,42), and children with familial hypercholesterolemia (17). These results underscore that subclinical atherosclerosis does indeed begin early in pediatric SLE, with CIMT progression rates comparable with those in children with familial hypercholesterolemia, a disease that is clearly associated with premature atherosclerosis and cardiovascular morbidity and mortality (43).
Atorvastatin effectively reduced LDL (30% decline) and triglyceride (10% decline) levels in this pediatric SLE cohort. The magnitude of reduction was comparable with that observed in a clinical trial of pravastatin-treated children with familial hypercholesterolemia (17), despite the fact that familial hypercholesterolemia patients had significantly higher baseline LDL levels (>300 mg/dl) compared with APPLE participants (LDL <100 mg/dl). Importantly, the APPLE trial also showed that 3 years of statin therapy is safe in pediatric SLE, a complex disease in which the involvement of multiple organ systems and the use of numerous medications is typical.
Actual adherence rates were lower than those assumed for the power calculations. With the observed 17.2% dropout rate and 62% adherence rate, the post hoc unconditional power was 90% with an alpha value of 0.05 to detect a 0.0045 mm/year difference in progression rates of mean-mean common CIMT between the 2 groups. While many clinical trials do not report adherence rates, those that do often show results comparable with APPLE. Adherence in multiyear trials is challenging across all disease groups and ages, ranging from 43% to 75% (44). Additional analysis of APPLE participants showed that CIMT progression was slower in adherent participants compared with nonadherent participants in both treatment groups, but there were no treatment group differences (P ≥ 0.087). Since adherence with the study drug is likely to reflect the overall adherence with medical therapy, this observation may indicate that better control of lupus activity may result in decreased cardiovascular risk, as previously suggested by others (3). Despite suboptimal adherence rates, the sustained reductions in LDL levels observed in this trial suggest that atorvastatin achieved the expected lipid-lowering effects known to impact atherosclerosis risk and CIMT progression in other populations (45).
Due to safety concerns, the APPLE trial did not include pediatric SLE patients with severe hypercholesterolemia, renal insufficiency, or currently active nephrotic syndrome, which are all known independent risk factors for cardiovascular events (46). Exclusion of these patients may have omitted participants more likely to benefit from long-term statin therapy. In addition, the APPLE cohort had mild to moderate SLE disease activity (mean SLEDAI <5) and a low burden of disease-related damage (73% with SDI score of 0), raising the possibility that children and adolescents with more active disease and higher damage indices might show more benefit from statin therapy. While many studies, including analysis of the baseline APPLE cohort (10), have not shown a significant relationship between measures of SLE disease activity and subclinical atherosclerosis measured by carotid ultrasound (9,47,48), Roman et al demonstrated an association between cumulative disease damage and increased carotid plaque in an adult SLE cohort (3). In addition, more aggressive immunosuppressive treatment of SLE was associated with less progression of carotid plaque (3,49). Finally, APPLE enrolled children and adolescents ages 10–21 years, spanning the full spectrum of pubertal development. A study focused on a postpubertal and young adult population with SLE may have demonstrated significant response to statin therapy, as this is when normal age-related increases in CIMT are first observed (41,50). Post hoc subgroup analyses are currently under way to address this question.
Despite the fact that pediatric SLE is a rare and complex disease with notable health disparities, successful multisite recruitment was achieved for this prevention trial due to the collaborative efforts of the CARRA network. Networks such as CARRA can overcome barriers that have traditionally hampered pediatric rheumatology research, particularly in pediatric SLE. Challenges include rare and heterogeneous disease processes, site and investigator inexperience, limited funding, and a shortage of pediatric rheumatology providers.
In summary, the APPLE trial found no significant effect of atorvastatin on the primary end point, and the results do not support a recommendation for the routine use of statins in all pediatric patients with SLE. However, there was a nonsignificant trend toward reduced CIMT progression in the atorvastatin-treated group. Future post hoc analyses may suggest subgroups that could benefit from early statin therapy. APPLE study results showed that 3 years of statin therapy is safe and effectively lowers total cholesterol, LDL, and hsCRP levels in pediatric SLE. Additionally, there was significant progression in all CIMT outcomes in the placebo group, showing that subclinical atherosclerosis in SLE begins in the pediatric age group and underscoring the pressing need for pediatric rheumatologists to address modifiable cardiovascular risk factors in daily practice, including identification and management of dyslipidemia, hypertension, obesity, smoking, and low physical activity (51–53). At this time, a clinically useful measure of subclinical atherosclerosis in children and adolescents is not available, and routine CIMT measurement is not recommended in clinical practice. Additional research is needed to further clarify effective cardiovascular prevention in this population.
ACKNOWLEDGMENTS
We thank the APPLE Data and Safety Monitoring Board (Susan Manzi, MD [Chair], Deborah Friedman, MD, Janet Holbrook, PhD, Peter Horn, PhD, Ilona Szer, MD, Lori Tucker, MD, and Richard Vehe, MD) and its National Institute of Arthritis and Musculoskeletal and Skin Diseases representatives (Susana Serrate-Sztein, MD, Madeline Turkeltaub, PhD [deceased], Anna Nicholson, and Shahnaz Kahn) for their dedication and oversight. We thank R. Califf and R. Harrington (Duke University Medical Center) for reviewing the manuscript.
ROLE OF THE STUDY SPONSOR
Pfizer had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Pfizer.
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases contract N01-AR-2-2265), the Edna and Fred L. Mandel Jr. Center for Hypertension and Atherosclerosis, and Pfizer, which provided atorvastatin and matching placebo. Dr. Singer’s work was supported by the NIH (Clinical and Translational Science Award program grant UL1-RR-024989).
Dr. Schanberg has received consulting fees from Pfizer (less than $10,000). Dr. Ardoin has received honoraria for serving on the Johnson & Johnson Data Safety Monitoring Board (less than $10,000). Mr. Evans has received consulting fees from ImagePace and Astra-Zeneca (less than $10,000 each) and has received honoraria from Merck/Schering-Plough (less than $10,000). Dr. Kimura has received consulting fees, speaking fees, and/or honoraria from Genentech (more than $10,000). Dr. Singer has received consulting fees, speaking fees, and/or honoraria from Pfizer, UCB, and NovelMed Therapeutics (less than $10,000 each) and has received honoraria for service on the Pfizer Drug Safety Monitoring Board (less than $10,000). Dr. McCurdy owns stock or stock options in Amgen. Drs. Klein-Gitelman and Jung have received consulting fees, speaking fees, and/or honoraria from Genentech (less than $10,000 each). Dr. Wallace has received a grant from Pfizer for the Childhood Arthritis and Rheumatology Research Alliance Enhanced Drug Safety Surveillance Project in Juvenile Idiopathic Arthritis. Dr. Provenzale has received consulting fees, speaking fees, and/or honoraria from Bayer, Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceuticals, and Theradex (less than $10,000 each).
APPENDIX A
ATHEROSCLEROSIS PREVENTION IN PEDIATRIC LUPUS ERYTHEMATOSUS INVESTIGATORS
APPLE investigators (in addition to the authors) from the following institutions participated in this study. The clinical centers were Duke University Medical Center (Durham, NC; E. Morgan Dewitt, C. E. Rabinovich, J. Ellis, and J. Wootton), Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center (Hackensack, NJ; K. A. Haines, S. C. Li, J. E. Weiss, M. E. Riordan, and B. Vaidya), Columbia University Medical Center (New York, NY; A. Eichenfield, D. M. Levy, P. Kahn, M. Carson, C. Batres, and D. Cabral), Cohen Children’s Medical Center of New York (New Hyde Park, NY; B. Gottlieb, P. Irigoyen, J. Luftig, Shaz Siddiqi, Z. Ni, M. Orlando, and E. Pagano), Stanford University School of Medicine (Palo Alto, CA; P. Chira, J. Hsu, T. Lee, and J. Perea), University of California at San Francisco Medical Center (San Francisco, CA; M. Mietus-Snyder), Hospital for Sick Children (Toronto, Ontario, Canada; Lawrence Ng), Indiana University School of Medicine (Indianapolis, IN; S. Ballinger, T. Klausmeier, and D. Hinchman), Texas Scottish Rite Hospital for Children (Dallas, TX; A. Hudgins, S. Henry, and S. Zhang), University Hospitals/Case Medical Center (Cleveland, OH; E. B. Brooks, S. Miner, J. Ortiz, L. Scalzi, and N. Szabo), Children’s Hospital of Philadelphia (Philadelphia, PA; L. Dorfeld and S. Wilson), University of California Los Angeles Medical Center (Los Angeles, CA; T. Hernandez and J. Vitale), Children’s Memorial Hospital (Chicago, IL; A. Kress, N. Lowe, and F. Patel), Seattle Children’s Hospital and Regional Medical Center (Seattle, WA; S. Hamilton), Medical University of South Carolina (Charleston, SC; K. Caldwell and D. Kamen), University of Chicago (Chicago, IL; R. Puplava and A. Lonchev), Nationwide Children’s Hospital (Columbus, OH; M. Bacani), Cincinnati Children’s Hospital Medical Center (Cincinnati, OH; C. Rutherford and J. Meyers-Eaton), Creighton University Medical Center (Omaha, NE; T. Conway, L. Frank, and L. Kuss), University of Colorado (Denver, CO; H. Senz), and Mayo Clinic (Rochester, MN; T. Mason, J. Jaquith, and D. E. Paepke-Tollefsrud). The core labs were the Ward A. Riley Ultrasound Center, Wake Forest University School of Medicine (Winston-Salem, NC; W. A. Riley) for CIMT, Duke University Medical Center (Durham, NC; S. Chen and J. Isaacson) for radiology, and Cincinnati Children’s Hospital Medical Center (Cincinnati, OH; Susan Thompson) for tissue repository. The data coordinating center was Duke Clinical Research Institute (Durham, NC; M. Creed, L. Lytle, A. McDaniel, C. McLendon, P. Monds, D. Roth, J. Winsor, and H. Wood).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Schanberg had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Schanberg, Sandborg, Barnhart, Evans, Ilowite, Punaro, Provenzale.
Acquisition of data. Schanberg, Sandborg, Yow, Evans, Mieszkalski, Ilowite, Eberhard, Imundo, Kimura, von Scheven, Silverman, Bowyer, Punaro, Singer, Sherry, McCurdy, Klein-Gitelman, Wallace, Silver, Wagner-Weiner, Higgins, Brunner, Jung, Soep, Reed, Provenzale, Thompson.
Analysis and interpretation of data. Schanberg, Sandborg, Barnhart, Ardoin, Yow, Evans, Mieszkalski, Ilowite, von Scheven, Silver, Provenzale.
REFERENCES
- 1.Gladman DD, Urowitz MB. Morbidity in systemic lupus erythematosus. J Rheumatol Suppl. 1987;14(Suppl 13):223–226. [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the Nurses’ Health Stud. Arthritis Rheum. 2009;61:1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urowitz MB, Gladman D, Ibanez D, Bae SC, Sanchez-Guerrero J, Gordon C, et al. for the Systemic Lupus International Collaborating Clinics. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:881–887. doi: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58:556–562. doi: 10.1002/art.23204. [DOI] [PubMed] [Google Scholar]
- 7.Tucker LB, Uribe AG, Fernandez M, Vila LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII) Lupus. 2008;17:314–322. doi: 10.1177/0961203307087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascif AK, Hilario MO, Terreri MT, Ajzen SA, D’Almeida V, Plavnik FL, et al. Endothelial function analysis and atherosclerotic risk factors in adolescents with systemic lupus erythematosus. Int J Adolesc Med Health. 2007;19:497–505. [PubMed] [Google Scholar]
- 9.Falaschi F, Ravelli A, Martignoni A, Migliavacca D, Sartori M, Pistorio A, et al. Nephrotic-range proteinuria, the major risk factor for early atherosclerosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2000;43:1405–1409. doi: 10.1002/1529-0131(200006)43:6<1405::AID-ANR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, et al. for the Atherosclerosis Prevention in Pediatric Lupus Erythematosus Investigators. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the Atherosclerosis Prevention in Pediatric Lupus Erythematosus cohort. Arthritis Rheum. 2009;60:1496–1507. doi: 10.1002/art.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soep JB, Mietus-Snyder M, Malloy MJ, Witztum JL, von Scheven E. Assessment of atherosclerotic risk factors and endothelial function in children and young adults with pediatric-onset systemic lupus erythematosus. Arthritis Rheum. 2004;51:451–457. doi: 10.1002/art.20392. [DOI] [PubMed] [Google Scholar]
- 12.Chow PC, Ho MH, Lee TL, Lau YL, Cheung YF. Relation of arterial stiffness to left ventricular structure and function in adolescents and young adults with pediatric-onset systemic lupus erythematosus. J Rheumatol. 2007;34:1345–1352. [PubMed] [Google Scholar]
- 13.Gazarian M, Feldman BM, Benson LN, Gilday DL, Laxer RM, Silverman ED. Assessment of myocardial perfusion and function in childhood systemic lupus erythematosus. J Pediatr. 1998;132:109–116. doi: 10.1016/s0022-3476(98)70494-9. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 15.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–330. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331–337. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 19.Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima-media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. Eur Heart J. 2006;27:746–755. doi: 10.1093/eurheartj/ehi695. [DOI] [PubMed] [Google Scholar]
- 20.Crouse JR, III, Grobbee DE, O’Leary DH, Bots ML, Evans GW, Palmer MK, et al. Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther. 2004;18:231–238. doi: 10.1023/B:CARD.0000033645.55138.3d. [DOI] [PubMed] [Google Scholar]
- 21.Kastelein JJ, van Leuven SI, Evans GW, Riley WA, Revkin JH, Shear CL, et al. Designs of RADIANCE 1 and 2: carotid ultrasound studies comparing the effects of torcetrapib/atorvastatin with atorvastatin alone on atherosclerosis. Curr Med Res Opin. 2007;23:885–894. doi: 10.1185/030079907x182121. [DOI] [PubMed] [Google Scholar]
- 22.Pavlov OV, Bobryshev Y, Balabanov Y, Ashwell K. An in vitro study of the effects of lovastatin on human fetal brain cells. Neurotoxicol Teratol. 1995;17:31–39. doi: 10.1016/0892-0362(95)91641-w. [DOI] [PubMed] [Google Scholar]
- 23.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 24.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common toxicity criteria version 2.0. URL: http://ctep.cancer.gov/forms. [PubMed]
- 27.Costenbader KH, Karlson EW, Gall V, de Pablo P, Finckh A, Lynch M, et al. Barriers to a trial of atherosclerosis prevention in systemic lupus erythematosus. Arthritis Rheum. 2005;53:718–723. doi: 10.1002/art.21441. [DOI] [PubMed] [Google Scholar]
- 28.Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 29.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 30.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr for the Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 32.Petri M, Kiani AN, Post W, Magder L. Lupus Atherosclerosis Prevention Study (LAPS): randomized double blind placebo controlled trial of atorvastatin versus placebo [abstract] Arthritis Rheum. 2006;54(Suppl):S520. [Google Scholar]
- 33.Norby GE, Holme I, Fellstrom B, Jardine A, Cole E, Abedini S, et al. on behalf of the Assessment of Lescol in Renal Transplantation Study Group. Effect of fluvastatin on cardiac outcomes in kidney transplant patients with systemic lupus erythematosus: a randomized placebo-controlled study. Arthritis Rheum. 2009;60:1060–1064. doi: 10.1002/art.24379. [DOI] [PubMed] [Google Scholar]
- 34.De Kruif MD, Limper M, Hansen HR, de Ruiter J, Spek CA, van Gorp EC, et al. Effects of a 3-month course of rosuvastatin in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:1654. doi: 10.1136/ard.2009.109405. [DOI] [PubMed] [Google Scholar]
- 35.Kotyla PJ. Comment on: atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 week controlled trial [letter] Rheumatology (Oxford) 2008;47:381–382. doi: 10.1093/rheumatology/kem381. [DOI] [PubMed] [Google Scholar]
- 36.Costenbader KH, Liang MH, Chibnik LB, Aizer J, Kwon H, Gall V, et al. A pravastatin dose-escalation study in systemic lupus erythematosus. Rheumatol Int. 2007;27:1071–1077. doi: 10.1007/s00296-007-0341-6. [DOI] [PubMed] [Google Scholar]
- 37.Espeland MA, O’Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi: 10.1186/1468-6708-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 39.Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, et al. on behalf of the RADIANCE 1 and RADIANCE 2 Study Groups. Ultrasound protocols to measure carotid intima-media thickness in trials; comparison of reproducibility, rate of progression, and effect of intervention in subjects with familial hypercholesterolemia and subjects with mixed dyslipidemia. Ann Med. 2010;42:447–464. doi: 10.3109/07853890.2010.499132. [DOI] [PubMed] [Google Scholar]
- 40.Thompson T, Sutton-Tyrrell K, Wildman RP, Kao A, Fitzgerald SG, Shook B, et al. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58:835–842. doi: 10.1002/art.23196. [DOI] [PubMed] [Google Scholar]
- 41.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age sex, anthropometry and blood pressure. J Hypertens. 1998;16:1593–1602. doi: 10.1097/00004872-199816110-00005. [DOI] [PubMed] [Google Scholar]
- 42.Jourdan C, Wuhl E, Litwin M, Fahr K, Trelewicz J, Jobs K, et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23:1707–1715. doi: 10.1097/01.hjh.0000178834.26353.d5. [DOI] [PubMed] [Google Scholar]
- 43.Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 44.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 45.Crouse JR, III, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’Leary DH, et al. for the METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 46.Shurraw S, Tonelli M. Statins for treatment of dyslipidemia in chronic kidney disease. Perit Dial Int. 2006;26:523–539. [PubMed] [Google Scholar]
- 47.Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosu. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Maksimowicz-McKinnon K, Magder LS, Petri M. Predictors of carotid atherosclerosis in systemic lupus erythematosus. J Rheumatol. 2006;33:2458–2463. [PubMed] [Google Scholar]
- 49.Roman MJ, Crow MK, Lockshin MD, Devereux RB, Paget SA, Sammaritano L, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412–3419. doi: 10.1002/art.22924. [DOI] [PubMed] [Google Scholar]
- 50.Bohm B, Hartmann K, Buck M, Oberhoffer R. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis. 2009;206:458–463. doi: 10.1016/j.atherosclerosis.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Ardoin SP, Schanberg LE. The management of pediatric systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2005;1:82–92. doi: 10.1038/ncprheum0046. [DOI] [PubMed] [Google Scholar]
- 52.Sandborg C, Ardoin SP, Schanberg L. Therapy insight: cardiovascular disease in pediatric systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4:258–265. doi: 10.1038/ncprheum0789. [DOI] [PubMed] [Google Scholar]
- 53.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]