Abstract
Background
Plasma 25 hydroxycholecalciferol (vit D) deficiency has been associated with adverse cardiovascular outcomes in epidemiological studies. Chronic kidney disease is associated with loss of 1-α-hydroxylase and consequently vit D deficiency. We hypothesized that vitamin D deficiency was associated with increased mortality and increased vascular access failure in patients undergoing permanent vascular access for end stage renal disease.
Methods
This retrospective cohort study analyzed 128 patients undergoing permanent vascular access surgery between 2003 and 2012 who also had concurrent plasma vit D levels. Levels were considered deficient at <20 ng/mL. Multivariable analysis was used to determine the association between vit D and mortality and vascular access outcomes.
Results
The mean age was 66.7; 96.8% were male and 32.0% African American; 60.9% had diabetes mellitus. In the entire cohort 55.5% were vitamin D (vit D) deficient, despite similar rates of repletion among the vit D deficient and non-deficient groups. During median follow up of 2.73 years there were 40 (31%) deaths. Vit D deficient patients tended to be younger (P=.01), have higher total cholesterol (P=.001), lower albumin levels (P=.017), and lower calcium levels (P=.007). Despite their younger age, mortality was significantly higher (P=.026) and vascular access failure was increased (P=.008) in the vit D deficient group. In multivariate logistic regression analysis vit D deficiency OR=3.64; (CI 1.12-11.79) P=.031, hemodialysis via central catheter OR=3.08; (CI 1.04-9.12) P=.042, coronary artery disease OR=3.08; (CI 1.06-8.94) P=.039, increased age OR=1.09; (CI 1.03-1.15) P=.001 and albumin OR=0.27 (CI 0.09-0.83) P=.023 remained independent predictors of mortality. Vit D deficiency HR=2.34 (CI 1.17-4.71) P=.02, synthetic graft HR=3.50 (CI 1.38-8.89) P=.009, , and hyperlipidemia HR=0.42 (CI .22-.81) P=.01 were independent predictors of vascular access failure in a Cox proportional harzardmodel.
Conclusion
Vit D deficiency is highly prevalent in patients undergoing vascular access procedures. Patients who are deficient have worse survival and worse vascular access outcomes. Further study is warranted to assess whether aggressive vitamin D repletion will improve outcomes in this population.
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem, affecting approximately 8 million individuals in the United States alone.1 Of these patients, nearly 400,000 are currently on chronic hemodialysis (HD). CKD has been associated with higher all-cause mortality rates, which increase sharply as kidney function declines. Patients with stage 3 CKD have a 17% increase in mortality; whereas, those with stage 5 CKD have a 600% increase in mortality compared to an age matched population with normal renal function.2 Even once hemodialysis is initiated; mortality in the dialysis population remains ten times greater than among Medicare patients of similar age without kidney disease.3 Dialysis patients aged 40 to 44 have an expected remaining life span of approximately 8 years, and approximately 4.5 years for those 60 to 64 years of age. These values in older patients are only slightly better than those in patients with lung cancer and are much worse than the general population (which is 30 to 40 years for those aged 40 to 44, and 17 to 22 years for individuals aged 60 to 64).3
Performance of a successful hemodialysis procedure requires a functional vascular access that can be cannulated reproducibly with two needles and deliver adequate dialysis blood flow. However, vascular access dysfunction is one of the leading causes of morbidity in the hemodialysis population4. Medicare data has estimated that vascular access dysfunction is responsible for 20% of all hospitalizations in the hemodialysis population.5,6 The 2007 United States Renal Data System report found that only 39.4% of prevalent end-stage renal disease patients are able to maintain a functioning fistula7. Autogenous fistulas have a 40-70% rate of primary non-maturation. This is a major obstacle to increasing fistula use in the US dialysis population 8. Currently there are no existing medical therapies aimed at the underlying pathophysiology of vascular access failure, either to improve initial maturation rates of fistulas or prolong fistula or graft patency.
Nutritional vitamin D deficiency in otherwise healthy individuals has been associated with a higher all-cause mortality. Beyond the well-known mineral metabolism effects, Vitamin D deficiency has been associated with hypertension, insulin resistance, immune abnormalities that enhance the risk of viral and bacterial infections, autoimmune disorders, cancer, and multiple organ damage due to excessive systemic inflammation causing atherosclerosis, and vascular dysfunction.9 The frequency and severity of all of these disorders markedly increase in CKD10. The multiple immune-modulatory and anti-inflammatory effects of vitamin D may have particular relevance in the CKD population, given that the uremic state in humans results in a perturbed biochemical state characterized by inflammatory and oxidative stress as well as vitamin D deficiency11. Given the known effects of vitamin D in the vasculature 12,13, we hypothesized that vitamin D deficiency in this population would be associated with increased mortality and poorer vascular access outcomes.
Methods
Study design and inclusion criteria
With the approval of the UCSF Institutional Review Board, a cohort of 128 patients from the San Francisco Veteran's Administration Medical Center was identified who had undergone surgical placement of permanent vascular access for hemodialysis between 2003 and 2012 and who also had a concurrent vitamin D level documented (plasma 25-hydroxycholecalciferol) as part of routine clinical care. Patients were excluded if their vascular access was performed using a bovine graft, if they did not have a vitamin D level documented or if there was no documented post-operative follow up. Each subject in the cohort was unique. For patients who had multiple fistulas placed, only the procedure closest to the date of vitamin D measurement was included in the dataset; all others were excluded. Patients were considered vitamin D deficient if their level was ≤ 20ng/mL15. Plasma 25-hydroxycholecalciferol was used as the metric of vitamin D status. The immunoassay was run on the Abott Architect i1000, with a coefficient of variance less than 7%, and a limit of detection of 3.1ng/mL. Given its relative ease of measurement as compared to 1,25-dihydroxycholecalciferol (the active form of vit D), it is the most widely accepted indicator of vitamin D status in clinical laboratories. Electronic medical records were used to compile patient's baseline demographics, comorbidities, medications, and laboratory data. This was performed via the Veterans Health Information Systems and Technology Architecture (VistA), an enterprise-wide information system built around an electronic health record, used throughout the United States Department of Veterans Affairs (VA) medical system.
Procedure
The type of vascular access placed was at the discretion of the operating surgeon. However it is the preference at our institution to only use arteries > 2 mm and veins > 3 mm for autogenous access. If these size criteria are met, we prefer to create radio-cephalic fistulas first, followed by brachio-cephalic fistulas at the elbow, then finally a two-stage brachio-basilic. If one of these conditions is not met, an AV graft is placed. Venous segments are meticulously dissected, ligating all side braches. Veins are mobilized sufficiently to create a tension-free end-to-side arterio-venous anastomosis using a running 6-0 Prolene suture. Vessels are flushed with heparinized saline prior to completion of the anastomosis. Patients do not routinely receive systemic heparin.
Endpoint definitions
Clinical endpoints included all-cause mortality and vascular access failure. Access related events were recorded for each patient, including interventions designed to maintain or reestablish patency, access thrombosis, access abandonment or successful use of the access for hemodialysis14. Failure was defined as any thrombosis or abandonment for non-maturation or patency reasons. Primary, primary-assisted, and secondary patency were recorded; however, failure, as defined here, equates to loss of primary assisted patency and is the focus of this manuscript. Primary and secondary patency were not significantly different between the two groups as measured by log-rank test. AVF ligation for steal, infection or transplant were not considered failures. All of these AVFs were mature, successful fistulas; however, ligation is a competing event, and these AVFs were censored as open at the time of ligation. Fistula maturity was clinically defined as one that was able to sustain adequate blood flows for hemodialysis use. Routine surveillance of vascular access is not performed at our institution. Patients are seen within 1 month post-operatively and then on an as needed basis thereafter.
Statistical methods
Normally distributed data are represented with means and standard deviations. Non-normally distributed data are represented as medians with interquartile ranges. Distributions of data were determined using normal probability plots. Student's t-tests and Chi-squared tests were performed where appropriate to assess for any differences between the vitamin D deficient and non-deficient groups. Univariate and multivariate logistic regression models were used to assess for independent predictors of survival. A cox proportional hazards model and Kaplan-Meier curve were used to analyze vascular access failure. In each model all variables with p-value of 0.1 or less in the univariate analysis were then included in the multivariate analysis. Analyses were performed both with and without the 16 grafts; however, results were not substantially different and conclusions were unchanged. Therefore, only the analysis of the full cohort of 128 patients is presented here. All statistical analysis was performed using STATA version 12.1 (StataCorp LP, College Station, TX).
Results
Patient Demographics and Comorbidities
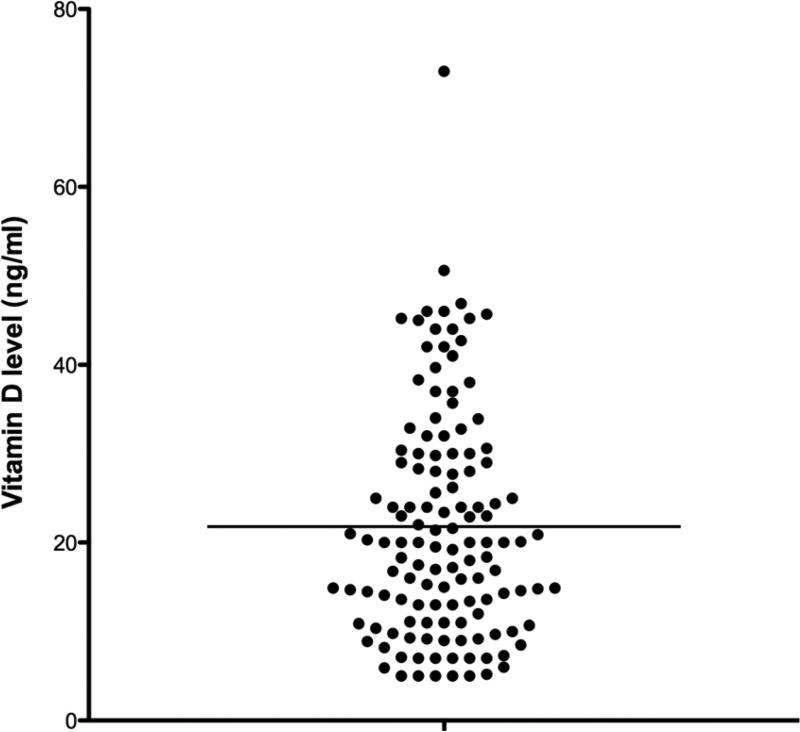
There were 339 hemodialysis accesses created at our institution between 2003 and 2012. 119 were excluded due to a lack of documented vit D level, 88 were excluded as they were performed in patients already included in the cohort, and 4 were excluded for use of bovine conduit. The remaining cohort included 128 patients with a median follow-up of 2.73 years (IQR 1.00-4.22) and a mean age of 66.8 years (SD 11.7), 96.9% were male and 32.0% African American. Diabetics comprised 60.9% of the cohort, and 18.8% were current smokers (Table I). Vit D deficiency was highly prevalent with 71 of the 128 patients (55.5%) having levels ≤20 ng/mL (Figure 1). Vit D levels were measured a median of 9.5 days from the index procedure, which was considered time 0. Patient demographics were similar between the Vitamin D deficient group and the non-deficient group. This included rates of vitamin D repletion: 39.4% of the vitamin D deficient group versus 50.9% of the non-deficient group were taking some form of vitamin D replacement (P=.2). Notable differences between the groups included that Vitamin D deficient patients tended to be younger (64.4yr vs 69.7yr, P=.01), were more likely to have a pre-existing history of stroke (21.1% vs 7.0%, P=.02), had lower albumin levels (3.3 gm/dL vs 3.6 gm/dL, P=.02), lower calcium levels (8.7 mg/dL vs 9.0 mg/dL, P=.007), and higher total cholesterol (158 mg/dL vs 129 mg/dL, P=.001), see Table I.
Table I.
Patient Demographics
| Overall (n=128) | Vit D ≤ 20 (n=71) | Vit D >20 (n=57) | |||||
|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | p-value | |
| Age | 66.8 | 11.7 | 64.4 | 12.1 | 69.7 | 10.4 | .01 |
| Male | 124 | 96.9 | 68 | 95.8 | 56 | 98.3 | .4 |
| African American | 41 | 32.0 | 24 | 33.8 | 17 | 29.8 | .6 |
| DM | 78 | 60.9 | 42 | 59.2 | 36 | 63.2 | .6 |
| HTN | 125 | 97.7 | 68 | 95.8 | 57 | 100.0 | .1 |
| CAD | 64 | 50.0 | 34 | 47.9 | 30 | 52.6 | .6 |
| Stroke | 19 | 14.8 | 15 | 21.1 | 4 | 7.0 | .02 |
| HLD | 94 | 73.4 | 48 | 67.6 | 46 | 80.7 | .10 |
| Cr | 5.0 | 2.4 | 5.3 | 2.5 | 4.7 | 2.2 | .2 |
| eGFR | 15.5 | 6.8 | 15.7 | 7.5 | 15.3 | 6.2 | .8 |
| Albumin | 3.4 | 0.7 | 3.3 | 0.5 | 3.6 | 0.9 | .02 |
| Current Smoker | 24 | 18.8 | 15 | 21.1 | 9 | 15.8 | .7 |
| ASA | 69 | 53.9 | 39 | 54.9 | 30 | 52.6 | .8 |
| Statin | 86 | 67.2 | 45 | 63.4 | 41 | 71.9 | .3 |
| Total Cholesterol | 145.3 | 46.4 | 158 | 49.5 | 128.6 | 36.3 | .001 |
| Triglycerides | 137.0 | 112.8 | 139.8 | 116.9 | 133.1 | 108.3 | .8 |
| HgbA1C | 6.3 | 1.3 | 6.1 | 1.1 | 6.5 | 1.4 | .1 |
| Calcium | 8.8 | 0.7 | 8.7 | 0.6 | 9.0 | 0.7 | .007 |
| Phosphorus | 4.5 | 1.2 | 4.7 | 1.4 | 4.3 | 1.0 | .06 |
| PTH | 237.8 | 175.7 | 259.1 | 190.1 | 210.1 | 152.1 | .1 |
| Hematocrit | 34.8 | 5.2 | 34.7 | 4.8 | 34.9 | 5.7 | .8 |
| Vit D replacement | 57 | 44.5 | 28 | 39.4 | 29 | 50.9 | .2 |
Figure 1.
Distribution of Vitamin D levels within cohort (ng/mL)
Survival
This was a highly morbid cohort with 40 deaths (31%) observed during follow up. Causes of death were as follows: 8 infection/sepsis, 7 end stage renal disease after stopping HD, 6 cardiac arrest, 4 cancer, 1 GI bleed, and 14 unknown. There were 28 (39%) deaths in the vitamin D deficient group versus 12 (21%) deaths in the non-deficient group, P=.03, see Figure 2. Predictors of mortality found to be significant (p-value ≤.1) in our univariate logistic model included: vitamin D deficiency, age, initiation of HD through a catheter, coronary artery disease (CAD), albumin and hemoglobin A1C. These predictors were then entered into a multivariate logistic regression model, See Table II. After adjustment, vitamin D deficiency was the strongest predictor of mortality with an odds ratio of 3.64 (95%CI; 1.13-11.79; P=.03). Receiving HD through a catheter conferred a 3.08 greater odds of death (95%CI 1.04-9.11; P=.04), pre-existing diagnosis of CAD had 3.07 greater odds (95%CI 1.06-8.94; P=.04), and each year increase in age lead to 1.09 increase in odds of death (95%CI 1.03-1.15; P=.001). Finally albumin was protective, with each unit increase translating into a 0.27 odds of mortality (95%CI 0.09-.83; P=.02).
Figure 2.
Difference in Mortality and Vascular Access Failure between Vitamin D Deficient and Non-deficient.
Table II.
Predictors of Mortality
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | p-value | OR | 95% CI | p-value |
| Vit D deficiency | .03 | 3.64 | 1.13-11.79 | .03 |
| Age | .003 | 1.09 | 1.03-1.15 | .001 |
| AVF vs Graft | 1 | |||
| HD via Central Cath | .02 | 3.08 | 1.04-9.11 | .04 |
| Previous AVF/AVG | .7 | |||
| Sroke/TIA | .9 | |||
| Heart Failure | .3 | |||
| CAD | .001 | 3.08 | 1.06-8.94 | .04 |
| HTN | .2 | |||
| HLD | .9 | |||
| DM | .5 | |||
| Tobacco Use | .8 | |||
| ASA | .6 | |||
| Statin | .9 | |||
| eGFR | .9 | |||
| Cr | .5 | |||
| Albumin | .05 | 0.27 | .09-.83 | .02 |
| Total Chol | .5 | |||
| Trig | .1 | |||
| HgbA1C | .07 | 0.71 | .40-1.28 | .3 |
| Calcium | .7 | |||
| Phos | .2 | |||
| PTH | .3 | |||
| Hematocrit | .3 | |||
Fistula Types and Interventions
Of the procedures performed there were 16 arteriovenous grafts placed and 112 autogenous fistulas: 58 radio-cephalic fistulas, 32 brachio-cephalic, and 22 brachio-basilic. Sixty-five (50.8%) of the fistulas matured to a usable state. Another 80 (63%) underwent additional interventions to aid maturation and maintain or regain patency. Procedures performed in order to maintain patency were as follows: 41 (32%) patients required balloon angioplasty and 5 (4%) required anastomotic revisions. Another 17 (13%) patients underwent thrombectomy in order to regain lost patency. Other ancillary interventions performed unrelated to patency included 23 second stage superficializations, 14 venous side branch ligations, and 3 pseudoaneurysm revisions (See Table III). Forty (31%) of the fistulas were abandoned due to loss of patency. However, many of the fistulas underwent ligation for reasons unrelated to patency: 5 for steal syndrome, 10 for transplant/non-use, 2 for pseudoaneurysm, and 2 for infection, see table III.
Table III.
Procedure Characteristics.
| Overall (n=128) | Vit D ≤ 20 (n=71) | Vit D >20 (n=57) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| Fistula Type: | |||||||
| Cimino | 58 | 45.3 | 37 | 52.1 | 21 | 36.8 | .05 |
| Brachio-cephalic | 32 | 25.0 | 17 | 23.9 | 15 | 26.3 | .4 |
| Brachio-basilic | 22 | 17.2 | 8 | 11.3 | 14 | 24.6 | .04 |
| AV Graft | 16 | 12.5 | 9 | 12.7 | 7 | 12.3 | .6 |
| Interventions: | |||||||
| Balloon angioplasty | 41 | 32.0 | 21 | 29.6 | 20 | 35.1 | .6 |
| Thrombectomy | 17 | 13.3 | 14 | 19.7 | 3 | 5.3 | .02 |
| Branch ligation | 14 | 10.9 | 9 | 12.7 | 5 | 8.8 | .6 |
| Pseudoaneurysm revision | 3 | 2.3 | 3 | 4.2 | 0 | -- | .3 |
| Anastomotic revision | 5 | 3.9 | 2 | 2.8 | 3 | 5.3 | .7 |
| 2nd stage superficialization | 23 | 18.0 | 10 | 14.1 | 13 | 22.8 | .2 |
| Outcomes: | |||||||
| Mature | 65 | 50.8 | 36 | 50.1 | 29 | 50.9 | .4 |
| Thrombosed | 42 | 32.8 | 27 | 38 | 15 | 26.3 | .1 |
| Ligated: | |||||||
| Steal | 5 | 3.9 | 2 | 2.8 | 3 | 5.3 | .7 |
| Transplant/non-use | 10 | 7.8 | 8 | 11.3 | 2 | 3.5 | .2 |
| Pseudoaneurysm | 2 | 1.6 | 1 | 1.4 | 1 | 1.8 | 1.0 |
| Infection | 2 | 1.6 | 2 | 2.8 | 0 | -- | .5 |
Failure
We found that there were significantly higher incidence of vascular access failure in the vitamin D deficient group, i.e. this group was much more likely to have interrupted patency (34 events (48%) versus 15(26%); P=.008), see Figure 2. In addition, Kaplan-Meier curves showed a significant difference in the rates of failure between the two groups, log-rank test = .037, see Figure 3. Univariate predictors of access failure in our Cox proportional hazards included vitamin D deficiency, initiation of HD through a catheter, previous failed permanent vascular access, synthetic graft, hypertension, hyperlipidemia, aspirin use, creatinine level, and hematocrit, see Table IV. Vitamin D deficiency, synthetic graft and hyperlipidemia remained statistically significant in the multivariate model. After adjustment synthetic graft was the strongest predictor of failure, HR=3.5 (95% CI 1.38-8.89) P=.009. However, vitamin D deficiency remained a strong predictor with a HR of 2.34 (95% CI 1.17-4.71) P=.02. Finally, history of hyperlipidemia was protective with HR of 0.42 (95%CI .22-.81) P=.01. Location of fistula creation (wrist, elbow), was not predictive of failure.
Figure 3.
Kaplan-Meier Curve of Access Failure by Vitamin D Deficient and Non-deficient.
Table IV.
Cox Proportional Hazard Model of Predictors of Failure
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | p-value | HR | 95% CI | p-value |
| Vit D deficiency | .04 | 2.34 | 1.17-4.71 | .02 |
| Age | .8 | |||
| Gender | .9 | |||
| Race | .5 | |||
| HD via Central Cath | .08 | 1.50 | .74-3.06 | .3 |
| Previous failed AVF/AVG | .0002 | 1.50 | .56-3.85 | .4 |
| AVG v AVF | .0003 | 3.50 | 1.38-8.89 | .009 |
| Sroke/TIA | .4 | |||
| Heart Failure | .1 | |||
| CAD/MI | .8 | |||
| HTN | .002 | .20 | .04-1.01 | .051 |
| HLD | .02 | .42 | .22- .81 | .01 |
| DM | 0.2 | |||
| Tobacco Use | 0.4 | |||
| ASA | 0.09 | .67 | .37-1.23 | .2 |
| Statin | 0.2 | |||
| eGFR | 0.9 | |||
| Cr | 0.07 | .96 | .85-1.08 | .5 |
| Albumin | 0.4 | |||
| Trig | 0.4 | |||
| HDL | 0.7 | |||
| LDL | 0.8 | |||
| HgbA1C | 0.7 | |||
| Calcium | 0.4 | |||
| Phos | 0.7 | |||
| PTH | 0.6 | |||
| Hematocrit | 0.03 | 1.07 | .999-1.15 | .052 |
Discussion
The purpose of this study was to investigate the hypothesis that the potentially modifiable risk factor of vitamin D deficiency is associated with mortality and access failure. We found that vitamin D deficiency was highly prevalent in this cohort with 55.5% of the subjects having levels ≤20ng/mL; the commonly used cutoff for severe deficiency 15. This was despite similar rates of vitamin D replacement therapy. The end stage renal disease population is at high risk for vitamin D deficiency. The reasons for this are multifactorial, but are at least in part due to the progressive decline in production of 1α hydroxylase from the kidney 16. Therefore, these patients are not able to adequately regulate the active form of the hormone, 1,25-dihydroxyvitamin D. Beyond its traditionally known mineral metabolism effects, vitamin D is now recognized to have multiple effects in a wide range of tissue types including the vasculature. Vitamin D deficiency is associated with increased arterial stiffness as assessed by pulse wave velocity (PWV) and decreased endothelial function as assessed by flow mediated vasodilation (FMD) in patients with ESRD.17 Partial reversibility of vascular changes is evidenced by the fact that vitamin D replacement reduces left ventricular mass index by 9-10% in vitamin D deficient patients,12 reduces PWV,18 and improves elastic properties of arteries.19 The vascular effects of vitamin D may be mediated through vitamin D receptors and peripheral 1α-hydroxylase, both present in endothelial and smooth muscle cells of the vascular wall.20
Our analysis found a dramatic association with severe vitamin D deficiency and mortality in this population. This was a particularly notable finding given the fact that the vitamin D deficient group tended to be younger and had similar rates of vitamin D supplementation. Our findings reinforce the link between vitamin D deficiency and decreased survival, and add to a burgeoning body of literature in various patient populations linking vitamin D deficiency to mortality as well as adverse cardiovascular outcomes13, 21-30. Observational studies suggest that vitamin D deficiency is associated with increased risk of cardiovascular events31, hypertension, and congestive heart failure32 and supplementation of vitamin D improves survival.33
However, this study is the first that we know of to show an independent association between vitamin D deficiency and vascular access failure. This finding is of particular relevance in this population who is at high risk for vitamin D deficiency, and in whom vascular access is so critical, yet so frequently fails. In addition, we found that 62.5% of patients initiated HD with a catheter. This was concerning, given that our analysis found that initiating HD through a catheter to be a dramatic independent predictor of mortality. There was no association with vitamin D deficiency and primary or secondary patency; however, the reasons for loss of primary patency were heterogeneous e.g., balloon angioplasty for non-maturation or outflow obstruction, intrinsic venous disease or stenosis developing within a functional graft or fistula. Some of these reasons are related to vessel wall biology, while others are not. Loss of primary-assisted patency, as we have focused on here, does reflect a loss of functionality that can be most closely related to vessel wall biology. It is most commonly due to intimal hyperplasia and thrombosis. It is the most clinically relevant endpoint in this context and thus the focus of this manuscript.
The proposed mechanisms of these associations with vitamin D deficiency and adverse cardiovascular measures and outcomes, include the multiple known immune-modulatory and anti-inflammatory effects of this steroid hormone. Vitamin D deficiency is associated with elevated circulating markers of inflammation and oxidative stress including IL-6, soluble vascular cell adhesion molecule (sVCAM), malondialdehyde (MDA) and myeloperoxidase.34 This is particularly problematic in uremic patients who are already in an inflammatory state. Histologic analysis of uremic human vein specimens has shown increased activation of the innate immune system with increased expression of IL-6, transforming growth factor β (TGF-β), and tumor necrosis factor α (TNF-α) accompanied by markers of oxidative damage.35 Correction of vitamin D deficiency through supplementation reduces IL-1β, IL-6 and CRP and raises serum albumin in renally impaired patients.11, 12, 36 In addition, ESRD patients have marked endothelial dysfunction manifested by impaired endothelium-dependent, flow mediated vasodilation (FMD).17, 37-39 This inflammatory state characterized by vitamin D deficiency may contribute to the endothelial dysfunction and development of vascular disease, and thus poorer vascular access outcomes.
Our study has several limitations. First it is a single center, observational study, with a relatively small number of patients. The design of this study does not allow us to make any inferences about the causal nature of vitamin D deficiency. We can only state the observed associations. Additionally, the small sample size of the study resulted in very wide confidence intervals. So while we can say that there are associations between our predictors and outcomes, it is difficult to say how strong these associations are. Additionally, we do not know if this population of patients is different than the ESRD patients who did not have vitamin D levels measured.
The active form of vitamin D, plasma 1,25-dihydroxyvitamin D was not used as a metric in this study. We were limited by the retrospective nature of this study, and the active form of vitamin D is rarely measured as part of clinical routine. Its pro-hormone, 25-hydroxyvitamin D, is bound to circulating vitamin D binding protein (VBP), making it much more stable with concentrations up to 1000 fold higher than 1,25-dihydroxyvitamin D, and a significantly longer half-life, on the order of weeks as opposed to hours 40. Measuring 1,25-dihydroxyvitamin D requires complicated and labor intensive methods. For these reasons serum concentrations of 25-hydroxyvitamin D are the most widely used clinical measure of vitamin D status.
Given the retrospective nature of the study several factors were unavailable to us for analysis. For instance, the difference in serum albumin between the two groups raises the possibility that vitamin D level was a surrogate for overall nutrition in this cohort. However, prealbumin was not available as a measure to further probe this question. Nor were there serial measurements of vit D, which may have been informative to see how levels changed over time, given replacement therapy or not, in relation to our outcomes of interest.
Finally, time to death, or survival analyses were not performed given the lack of routine surveillance and inability to ascertain exact dates of death in this retrospective study. Nevertheless, access failure as defined herein is the most clinically meaningful access-related event often necessitating creation of new access and placement of a temporary or permanent central venous catheter.
In conclusion, this study found that vitamin D deficiency is a highly prevalent condition with serious implications in this patient population. Given the potential ramifications of increased mortality and vascular access failure, further study is warranted to assess if increased scrutiny of vitamin D levels or aggressive vitamin D repletion will improve survival or vascular access outcomes in this population.
Acknowledgements
Vascular Integrated Physiology and Experimental Therapeutics (VIPERx) Lab, San Francisco VA Medical Center
Support:
K23 NIH HL-92163 Grant (Dr. Christopher Owens)
National Center for Advancing Translational Sciences, NIH, through UCSF Clinical and Translational Science Institute grants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previously presented: Society for Vascular Surgery Annual Meeting San Francisco, CA June 1, 2013 Rapid paced paper session
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2009;55:S1–420, A6-7. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins AJ, Roberts TL, St Peter WL, Chen SC, Ebben J, Constantini E. United States Renal Data System assessment of the impact of the National Kidney Foundation-Dialysis Outcomes Quality Initiative guidelines. Am J Kidney Dis. 2002;39:784–95. doi: 10.1053/ajkd.2002.31999. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–22. doi: 10.1053/ajkd.2002.34924. [DOI] [PubMed] [Google Scholar]
- 6.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Understanding the pathophysiology of hemodialysis access problems as a prelude to developing innovative therapies. Nat Clin Pract Nephrol. 2008;4:628–38. doi: 10.1038/ncpneph0947. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–8. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109–24. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 9.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, et al. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 10.Dusso AS. Update on The Biologic Role of The Vitamin D Endocrine System. Curr Vasc Pharmacol. 2013 doi: 10.2174/15701611113119990026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Bucharles S, Barberato SH, Stinghen AE, Gruber B, Piekala L, Dambiski AC, et al. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22:284–91. doi: 10.1053/j.jrn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–11. doi: 10.2215/CJN.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer LC, Michos ED, Reis JP. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr Drug Targets. 2011;12:54–60. doi: 10.2174/138945011793591617. [DOI] [PubMed] [Google Scholar]
- 14.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Jr, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–10. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.Doorenbos CR, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009;5:691–700. doi: 10.1038/nrneph.2009.185. [DOI] [PubMed] [Google Scholar]
- 17.London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65:700–4. doi: 10.1111/j.1523-1755.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–91. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 19.Braam LA, Hoeks AP, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost. 2004;91:373–80. doi: 10.1160/TH03-07-0423. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol. 2010;118:135–41. doi: 10.1016/j.jsbmb.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–72. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- 22.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54:1103–13. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 23.Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, Marz W. Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr Drug Targets. 2011;12:88–96. doi: 10.2174/138945011793591563. [DOI] [PubMed] [Google Scholar]
- 24.Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603–9. doi: 10.1093/ndt/gfr076. [DOI] [PubMed] [Google Scholar]
- 25.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–36. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–33. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–9. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 30.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–61. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631–8. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 32.Wolf M, Thadhani R. Beyond minerals and parathyroid hormone: role of active vitamin D in end-stage renal disease. Semin Dial. 2005;18:302–6. doi: 10.1111/j.1525-139X.2005.18406.x. [DOI] [PubMed] [Google Scholar]
- 33.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 34.Codoner-Franch P, Tavarez-Alonso S, Simo-Jorda R, Laporta-Martin P, Carratala-Calvo A, Alonso-Iglesias E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161:848–54. doi: 10.1016/j.jpeds.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 35.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access. 2012;13:168–74. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panichi V, Andreini B, De Pietro S, Migliori M, Taccola D, Giovannini L, et al. Calcitriol oral therapy for the prevention of secondary hyperparathyroidism in patients with predialytic renal failure. Clin Nephrol. 1998;49:245–50. [PubMed] [Google Scholar]
- 37.Vita JA, Holbrook M, Palmisano J, Shenouda SM, Chung WB, Hamburg NM, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117:3126–33. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annuk M, Lind L, Linde T, Fellstrom B. Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant. 2001;16:302–6. doi: 10.1093/ndt/16.2.302. [DOI] [PubMed] [Google Scholar]
- 39.Blackwell S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann Clin Biochem. 2010;47:17–28. doi: 10.1258/acb.2009.009196. [DOI] [PubMed] [Google Scholar]
- 40.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]