Abstract
A number of prior fMRI studies have focused on the ways in which the midbrain dopaminergic reward system co-activates with hippocampus to potentiate memory for valuable items. However, another means by which people could selectively remember more valuable to-be-remembered items is to be selective in their use of effective but effortful encoding strategies. To broadly examine the neural mechanisms of value on subsequent memory, we used fMRI to examine how differences in brain activity at encoding as a function of value relate to subsequent free recall for words. Each word was preceded by an arbitrarily assigned point value, and participants went through multiple study-test cycles with feedback on their point total at the end of each list, allowing for sculpting of cognitive strategies. We examined the correlation between value-related modulation of brain activity and participants’ selectivity index, a measure of how close participants were to their optimal point total given the number of items recalled. Greater selectivity scores were associated with greater differences in activation of semantic processing regions, including left inferior frontal gyrus and left posterior lateral temporal cortex, during encoding of high-value words relative to low-value words. Although we also observed value-related modulation within midbrain and ventral striatal reward regions, our fronto-temporal findings suggest that strategic engagement of deep semantic processing may be an important mechanism for selectively encoding valuable items.
Keywords: value, memory, selective encoding, reward, metacognitive control, functional magnetic resonance imaging (fMRI)
It is generally true that some of what a person encounters is important to remember, while other things are less important. One critical operation is to selectively remember important information, often at the expense of less important information. For instance, when studying for an exam, some students might maximize efficiency, focusing exclusively on the most important material. Other students might not be as selective; even though they know that some items are more important than others, they may still try to remember as much as possible, a strategy that often leads to poorer results. The present work uses fMRI to better understand what people do differently, on both a cognitive and neural level, when remembering items deemed important.
In order to address these questions, we used a variant of the value-directed remembering (VDR) paradigm (Castel, Benjamin, Craik, & Watkins, 2002; Castel, 2008). The VDR paradigm involves having participants study a list of words paired with point values, with the participants’ goal being to maximize the total score, which is the sum of the values associated with recalled words. A number of behavioral studies (e.g., Ariel & Castel, 2014; Castel et al., 2002; Castel, Farb, & Craik, 2007; Castel, Murayama, Friedman, McGillivray, & Link, 2013; Hanten et al., 2007; Loftus & Wickens, 1970; Soderstrom & McCabe, 2011; Watkins & Bloom, 1999) have shown that words that are arbitrarily determined to be valuable (via high point values) tend to be recalled better than words that are arbitrarily assigned lower values. However, prior studies with this paradigm have been limited in fully explaining the effect on a mechanistic level, with explanations ranging from differential forms of rehearsal, use of imagery, and strategic encoding and retrieval operations.
There is reason to believe that people are making an explicit effort to prioritize encoding of high-value items in the VDR paradigm. Specifically, the degree to which people optimize their point score, as measured by the selectivity index (Castel et al., 2002), increases from earlier lists to later lists (Castel, 2008; Castel et al., 2011a). The VDR paradigm is structured such that people learn multiple distinct word lists, with a free recall test after each list and immediate feedback on the number of points earned after each test. The improvement in selectivity across lists suggests that people may be learning about how many words they can remember and about which encoding strategies will lead to the highest point total. This pattern of results would be consistent with the use of explicit cognitive strategies to enhance encoding of high-value items.
A number of functional neuroimaging studies have examined the brain mechanisms that might mediate the enhancement of memory for high-value items. Adcock, Thangavel, Whitfield-Gabrieli, Knutson, and Gabrieli (2006) were the first to do so in the context of an intentional encoding paradigm. They found that increased activity in regions of the dopaminergic reward system, specifically the ventral tegmental area (VTA) in the midbrain and nucleus accumbens (NAcc) in the ventral striatum, elicited in response to a value cue that preceded presentation of the actual stimulus, predicted successful encoding of high-value items. A similar pattern was observed in the hippocampus, and moreover the functional connectivity between VTA and hippocampus was strongest during cues preceding high-value items that were subsequently remembered. These findings suggest that input from the midbrain reward system might serve to prepare the hippocampus to better encode the important information that is about to be encountered, in this case a photograph of a landscape scene. Such connections between dopaminergic midbrain systems and the hippocampus had previously been shown to be important in rodents (Huang & Kandel, 1995; Jay, 2003; Lisman & Grace, 2005), but this was the first direct evidence for such a mechanism in humans. While the study by Adcock et al. (2006), and subsequent work by others (e.g., Murty, Labar, and Adcock, 2012; Wolosin, Zeithamova, and Preston, 2012) have contributed valuable insights about the neural mechanisms that can underlie reward-based learning, there are likely to be additional mechanisms whereby people strategically process high value items differentially to optimize limited memory. We focus primarily on those mechanisms in the present paper.
One difference between selective strategic enhancement of memory for valuable items and midbrain reward-motivated learning mechanisms is the time course of these effects. For example, Adcock et al. (2006) tested memory at a delay of 24 hours, following evidence from rodent work (e.g., O’Carroll, Martin, Sandin, Frenguelli, & Morris, 2006; Frey, Schroeder, & Matthies, 1990; Frey, Matthies, Reymann, & Matthies, 1991) suggesting that enhancement of encoding for valuable items via dopamine-driven increases in hippocampal plasticity is likely to emerge only after a delay. Although Adcock et al.’s study did not include an immediate memory test for comparison, Spaniol, Schain, and Bowen (2013) tested young and older adults on a very similar task and found that on an immediate test, value did not reliably enhance memory in either age group. With a test given 24 hours after encoding, however, they replicated the finding of a significant enhancement of memory for valuable items. Similarly, Murayama and Kuhbandner (2011) found that after a 1-week delay, monetary rewards increased memory for trivia questions that were not inherently interesting, an effect believed to be dopamine-driven. No effect of reward on memory was observed on an immediate test, however, again suggesting that effects of the putative dopaminergic reward-motivated learning mechanism that Adcock et al. and others have examined only emerge after a delay. Reward-related activity in the VTA-hippocampal circuit thus appears to engage a consolidation process that makes memory for valuable items less vulnerable to forgetting after a delay, but this process does not seem to affect retrievability in the shorter term. However, under different circumstances, people can improve their memory for valuable items in a way that is apparent in tests administered immediately following learning (e.g., Castel et al., 2002; Castel, 2008). It thus seems likely that there is an additional mechanism capable of enhancing the encoding of valuable items that is engaged by the VDR paradigm, and most likely by certain real-world situations as well.
As noted above, there is reason to believe that participants in the VDR paradigm gradually learn to employ effective mnemonic strategies that allow them to strengthen encoding of high-value items; this is apparent both from the pattern of recall data across lists and from post-experiment self-reports (e.g., Castel, 2008; Castel, McGillivray, & Friedman, 2012). In contrast, participants in most studies of reward-motivated learning (e.g., Adcock et al., 2006) are presented with a long list of stimuli, and memory is only tested after all encoding is complete with no opportunity to modify encoding strategy based on feedback. Additionally, performance in the VDR paradigm is typically assessed via free recall, whereas memory in reward-based learning tasks is usually assessed by a yes/no recognition task. Thus, the VDR task is more likely to tap into strategic enhancement of encoding for high-value items than are paradigms that are typically used to assess reward-based learning. Additional neural mechanisms may be engaged during encoding of high-value items in the VDR paradigm that may reflect real-life situations in which people are able to preferentially remember valuable information.
To our knowledge, no prior neuroimaging studies have examined effects of value on neural mechanisms of strategy use during encoding of items with different values. However, a number of studies have examined which brain areas are preferentially recruited when people engage in deep encoding of study materials versus shallower encoding. One of the first such studies (Kapur et al., 1994) examined how tasks structured to promote different levels of processing (Craik & Lockhart, 1972; Craik & Tulving, 1975) differentially affected cerebral blood flow. They found that a task that engaged deep encoding by evoking semantic representation of words was associated with greater activity in the left inferior prefrontal cortex (PFC), relative to a task that required only surface-level encoding. Thompson-Schill, D’Esposito, Aguirre, and Farah (1997) provided a more precise account of left inferior PFC function, suggesting that the role of this region is specifically in the selection of the most relevant semantic representation(s) for a given task, rather than in the retrieval of semantic knowledge more generally. Subsequent studies (e.g., Wagner et al., 2001; Badre et al., 2005) have further clarified how left inferior PFC contributes to controlled semantic processing; see also reviews by Bookheimer (2002), Costafreda et al. (2006), and Badre & Wagner (2007).
Other work has more directly implicated left PFC in the use of verbal or semantic strategies at encoding. When participants are instructed to use a semantic clustering strategy, they tend to show increased activity in areas including left dorsolateral and left ventrolateral PFC at encoding, relative to earlier blocks when such a strategy is possible but has not been explicitly instructed (Savage et al., 2001; Miotto et al., 2006). Similarly, Kirchhoff and Buckner (2006) showed that individual differences in encoding-related activity in left inferior PFC are associated with the degree to which people report having used a verbal elaboration strategy during encoding. Use of these elaborative strategies was associated with better memory performance, suggesting that the often-observed association between left ventrolateral prefrontal activity at encoding and successful subsequent memory (e.g., Wagner et al., 1998; Kim, 2011) is mediated by increased use of semantic strategies at encoding. One possible neural mechanism underlying enhanced memory for high-value items in the VDR paradigm may be the differential engagement of regions associated with the use of semantic strategies at encoding. Such a finding would be particularly interesting given that prior work has largely ignored the ways in which intentional strategic processing can mediate the effects of value on memory.
Method
Participants
Twenty-two young adults participated in the study. Data from two participants were excluded, one for being a non-native English speaker, and a second who was only able to complete 3 lists due to discomfort in the scanner. The remaining twenty participants (mean age = 21.65, SD = 3.66, age range = 18–30; 11 female, 9 male) were all right-handed, native English speakers who reported no current psychoactive medications or severe psychiatric or neurological disorders. All participants either had normal or corrected-to-normal vision. Written consent was obtained from each participant, and all procedures were approved by UCLA’s Medical Institutional Review Board. Participants were recruited via flyers posted on the UCLA campus, and were paid $10/hour, plus additional earnings from the Monetary Incentive Delay (MID) task (typically $10-$12), and also had the chance to win up to an additional $25 in a delay-discounting task (Kirby, Petry, & Bickel, 1999) that we ran after the scan. For one participant, we were unable to finish data collection on one run of the VDR task due to discomfort, but the remaining four VDR runs for that participant are included in our analyses.
Task Stimuli and Behavioral Procedures
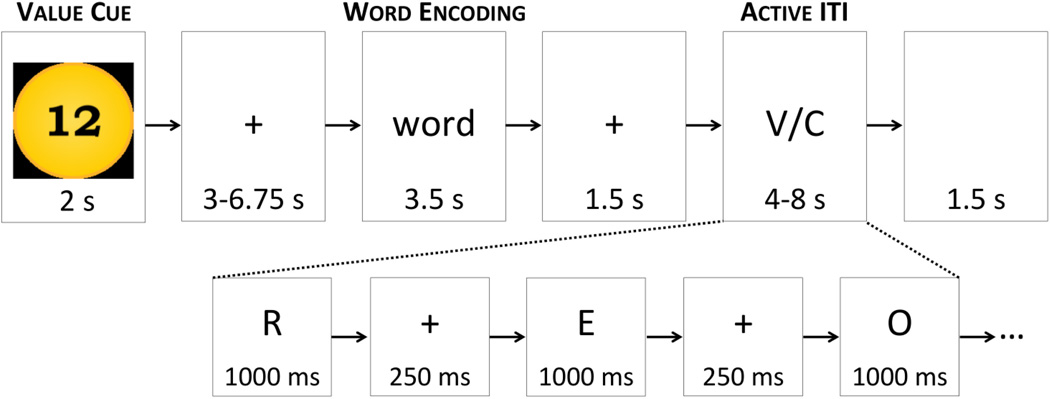
Our VDR task paradigm was based on that used by Castel et al. (2002), but was altered to make it more amenable to neuroimaging (see Figure 1). Each trial of our task began with a cue for point value, either high-value (10, 11, or 12 points) or low-value (1, 2, or 3 points), presented as a number inside of a gold “coin” on the screen for 2 s. This was followed by a fixation cross of jittered duration (equal proportions 3 s, 4.25 s, 5.5 s, and 6.75 s). Next, a word was presented for 3.5 s, followed by 1.5 s of fixation and then an active baseline task (Stark & Squire, 2001) of jittered duration (50% 4 s, 25% 6.5 s, 25% 8 s). The vowel-consonant baseline task involved the presentation of a pseudorandom series of letters, with an approximately equal ratio of vowels and consonants. Each letter was presented for 1 s, with a 0.25 s fixation between letters, and a 1.5 s blank screen at the end of the trial. Participants were instructed to respond to each letter while it was still on the screen. Button mappings were fixed across subjects, such that all individuals used their index finger if the letter was a consonant and their middle finger if the letter was a vowel. Letters in the vowel-consonant task were arranged such that they did not spell any words. We used a vowel-consonant task in order to continually engage verbal processing resources throughout the inter-trial intervals, thereby reducing our participants’ ability to simultaneously engage in verbal rehearsal of the words during this time.
Figure 1.
Value-directed remembering task design. On each trial, participants are first presented with the value cue, then with a to-be-remembered word, and finally with a 2–6 trials of an active baseline task (vowel/consonant judgment) to be performed during the inter-trial interval (ITI).
Each list included 24 different words, of which 12 were arbitrarily defined as high-value and 12 arbitrarily defined as low-value (with 4 words at each specific value level). Five lists of the VDR task were presented in the scanner. Items were drawn from clusters 6 and 7 of the Toglia and Battig (1978/2009) “Colorado” word norms. All stimuli were 4–8 letter, 1–2 syllable nouns, rated as highly familiar (range 5.5–7 on a 1–7 scale), moderate to high on concreteness and imagery (range 4–6.5 on a 1–7 scale), and moderate in pleasantness (range 2.5–5.5 on a 1–7 scale). Values were pseudorandomly assigned to words, with the assignment of particular words to value group (high or low) counterbalanced across subjects. The order in which the different lists were presented in the scanner was also counterbalanced. Each list began with 12.5 s of fixation and ended with an extra 15 s of the vowel-consonant task. Within about 10– 20 s after the end of each scan, the recall test began, and the participant was given 90 s to recall as many words as possible from the preceding list. Immediately after recall was complete, the experimenter scored the test, and gave feedback on the point score earned for that list.
Prior to scanning, participants were given detailed instructions about the VDR task, and then completed six practice items, followed by two full practice lists. Each of the two full practice lists included recall tests with feedback. Prior work has shown that selectivity is typically stronger on the third and subsequent lists than on the first two lists (Ariel & Castel, 2014; Castel, 2008; McGillivray & Castel, 2011). Thus, we assumed that by presenting two full lists prior to scanning, strategy use would be relatively well established and consistent in the scanner.
After completion of the VDR task, participants remained in the scanner to perform one run of the MID task (Knutson, Adams, Fong, and Hommer, 2001), which was intended to serve as a functional localizer task for the VTA and NAcc. This task included a total of 48 trials, equally divided into high-reward (+$1.00), low-reward (+$0.10), and no-reward (+$0.00). Loss/punishment trials were not included, as these were not relevant for our purposes. In addition, our version of the task includes feedback in word form, unlike the symbolic cues used in the classic MID paradigm, but consistent with the version used by Samanez-Larkin et al. (2007). This version is intended to be more amenable for use with older adult participants, who we expect to test in follow-up studies. While the number of trials of each type may appear low, a recent study by Wu, Samanez-Larkin, Katovich, & Knutson (2014) used a similar number of trials of each type and reported robust and consistent changes in BOLD signal as a function of value.
Each trial began with a text cue indicating the potential value of that trial (e.g., “Win $1.00”). To earn this reward, the participant was required to make a button-press during the brief window of time that a square stimulus appeared on the screen. As in prior studies with this paradigm, we used an adaptive algorithm, which adjusted the response period to keep the overall win percentage at approximately 66%. The initial response period for the practice run outside the scanner was 300 ms, and the initial response period in the scanner was determined based on the average response time for successful responses during practice. If the participant’s win percentage exceeded 66%, the response period would tend to be made shorter (i.e., more difficult) on the next trial. If the participant’s win percentage was less than 66%, the response period would tend to be made longer (i.e., easier) on the next trial, down to a minimum possible response period of 140 ms. Overall, mean accuracy across the 18 participants for whom we have behavioral data, was 60.4% (SD = 8.3%) for $0.00 trials, 60.4% (SD = 7.1%) for $0.10 trials, and 60.1% (SD = 6.5%) for $1.00 trials. Mean RTs for correct trials were 195.8 ms (SD = 27.3 ms) for $0.00 trials, 178.6 ms (SD = 29.3 ms) for $0.10 trials, and 169.9 ms (SD = 26.9 ms) for $1.00 trials.
The experimental session also included several supplementary behavioral measures before and after scanning. Prior to scanning, we ran reading span and counting span tests (Kane et al., 2004) to measure working memory capacity. We used a partial-credit load-weighted scoring procedure such that each unit that was correctly recalled, in the correct serial position, was scored as 1 point (Conway et al., 2005). Following guidance by Conway et al. (2005), we generated a composite measure of working memory from scores on these two tests. Because we did not have enough data to do a true latent variable analysis, we computed z scores for each measure, and averaged the z scores to yield a composite measure of working memory.
At the end of the session, we administered a debriefing questionnaire that included questions about what strategies participants had used to encode the words, what (if anything) they had done differently during encoding of the high-value words, and questions about what (if anything) they were rehearsing during the fixation and vowel-consonant periods. Self-reported encoding strategies were categorized as either relying upon semantic aspects of the words, or as relying more on surface features of the words. We also classified each participant into one of 3 categories: only attempting to encode high-value items (ignoring low-value items), trying harder on high-value items, or ignoring value entirely. We also categorized self-reported encoding strategies as either relying upon semantic aspects of the words, or as relying more on surface features of the words.
Scanning Procedure
T2*-weighted echoplanar (EPI) images sensitive to blood oxygenation level dependent (BOLD) contrast were collected using a 3 T Siemens Tim Trio MRI scanner at the UCLA Staglin IMHRO Center for Cognitive Neuroscience. For the VDR task, each 179-volume functional run lasted approximately 7.5 min; five such runs were acquired for each participant. Each functional volume consisted of 45 interleaved axial slices, TR = 2500 ms, TE = 25 ms, flip angle = 75°, slice thickness = 3.0 mm, in-plane resolution = 3.0 × 3.0 mm, matrix = 64 × 64, FOV = 192 mm, and no gap between slices. For the MID task, similar scan parameters were used, except that the TR was shortened to 2 s, only 36 slices were acquired per volume, and only one 246-volume run was collected. In addition, we collected matched-bandwidth T2-weighted coplanar structural scans to use as an intermediate step in spatial registration. We also collected a high-resolution structural scan (MPRAGE), using the following parameters: TR = 1900 ms, TE = 3.26 ms, flip angle = 9°, 176 slices, 1 mm3 voxels, 18.2% slice oversampling, FOV = 250 mm, with GRAPPA acceleration. To minimize head movement during scanning, we placed extra cushions between the subject’s head and the coil. Stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA), and images were shown via either a custom-built MR-compatible rear projection system, or via MR-compatible goggles (Resonance Technology, Inc.).
fMRI Data Analysis
Preprocessing
Analyses of EPI data were carried out using FEAT v5.98 (fMRI Expert Analysis Tool), as implemented in FSL v4.1.9 (www.fmrib.ox.ac.uk/fsl). We corrected for head motion using MCFLIRT (FMRIB's motion correction linear image registration tool; Jenkinson et al., 2002), and also used the fsl_motion_outliers script to detect and censor any volumes with excessive head motion. We then removed non-brain tissue using BET (Brain Extraction Tool; Smith, 2002). Grand-mean intensity normalization was applied to the 4D dataset from each run based on a multiplicative scaling factor. We applied a Gaussian kernel of 5 mm FWHM for spatial smoothing, and for temporal filtering, a high-pass filter to remove low-frequency noise using a Gaussian-weighted least-squares straight-line fitting with a sigma of 100 s. Temporal autocorrelation was corrected for using prewhitening as implemented by FILM (FMRIB’s improved linear model; Woolrich et al., 2001). Functional images were registered to a coplanar structural scan and then to a high-resolution structural scan using FLIRT (FMRIB’s Linear Image Registration Tool) linear registration. Registration from the high-resolution structural scan to standard Montreal Neurological Institute (MNI) space was further refined using FNIRT (FMRIB’s Non-linear Image Registration Tool).
Analysis of Value-Directed Remembering Task
We included four different event types in the statistical model: high-value cue period, high-value word-encoding period, low-value cue period, and low-value word-encoding period. The cue period was defined based on the time period in which each value cue was on-screen, 2 s in duration, convolved with a double-gamma hemodynamic response function (HRF). The word-encoding period was defined as a separate event, based on the time period in which the to-be-learned word was on-screen, 3.5 s in duration, convolved with a double-gamma HRF. Temporal derivatives were included in the model for all four event types. Motion regressors generated by MCFLIRT and regressors coding for any motion outlier TRs were also included in the model as covariates of no interest.
First-level general linear model (GLM) analysis was carried out separately for each run. Then, in a second-level fixed-effects analysis, we combined the parameter estimates across all five runs of the VDR task, and created a set of linear contrasts. Our primary contrasts of interest compared the BOLD signal during high-value vs. low-value items, looking separately at the cue period data and the word-encoding period data. For whole-brain analyses across subjects, we used the FLAME stages 1 and 2 mixed effects model in FSL, with automatic outlier detection. Clusters were determined using a voxel-level threshold of Z > 2.3, with a cluster-corrected significance level of p < .05.1 Cortical surface renderings were created using Caret v5.65 (http://brainvis.wustl.edu; Van Essen et al., 2001) on the inflated Conte69 atlas in FNIRT space (Van Essen, Glasser, Dierker, Harwell, and Coalson, 2012), with FSL activation maps transformed from volume to surface space using Caret’s interpolated voxel algorithm. Activation peaks noted in the tables were a subset of the local maxima generated for each contrast by FSL’s “cluster” command, with a minimum distance of 10 mm between peaks. Labels were determined using the Harvard-Oxford structural atlas and other relevant brain maps (e.g., Talairach & Tournoux, 1988; Brodmann, 1909), and redundant peaks were eliminated.
We computed each participant’s selectivity index for each list using the formula [(actual score – chance score) / (ideal score – chance score)], as described in prior literature (Castel et al., 2002; Watkins & Bloom, 1999). We then averaged the selectivity indices across all scanned lists to yield a single score. To search the whole brain for correlations between behavioral measures (e.g., selectivity index) and changes in BOLD signal, we included the behavioral measure as an EV in an FSL group-level model, in addition to the group mean. For region of interest (ROI) analyses, we computed Pearson correlation coefficients across participants using each individual’s mean selectivity index and the mean parameter estimates for a given contrast in a given ROI for each participant. We applied a Bonferroni-Holm correction (Holm, 1979) to correct for multiple comparisons across each set of related ROIs; unless otherwise indicated, all effects survived this correction for the particular cohort of ROIs tested.
Analysis of Monetary Incentive Delay Task
The analysis workflow applied to MID task data was generally similar to that described for the VDR task. We modeled the cue period and the feedback period as separate event types, each convolved with a double-gamma HRF. The cue period was defined as an event of 2 s duration during which the value cue was on-screen. The feedback period was defined as an event of 1.92 s duration during which feedback (i.e., whether or not the participant had “won” on a given trial) was on-screen. High-value, low-value, and no-reward trials were defined as separate event types. Our primary analysis of interest compared activity during the cue period on high-value trials with activity during the cue period on no-reward trials. Group level analyses followed the same procedure as described for the VDR task.
Results
Behavioral data
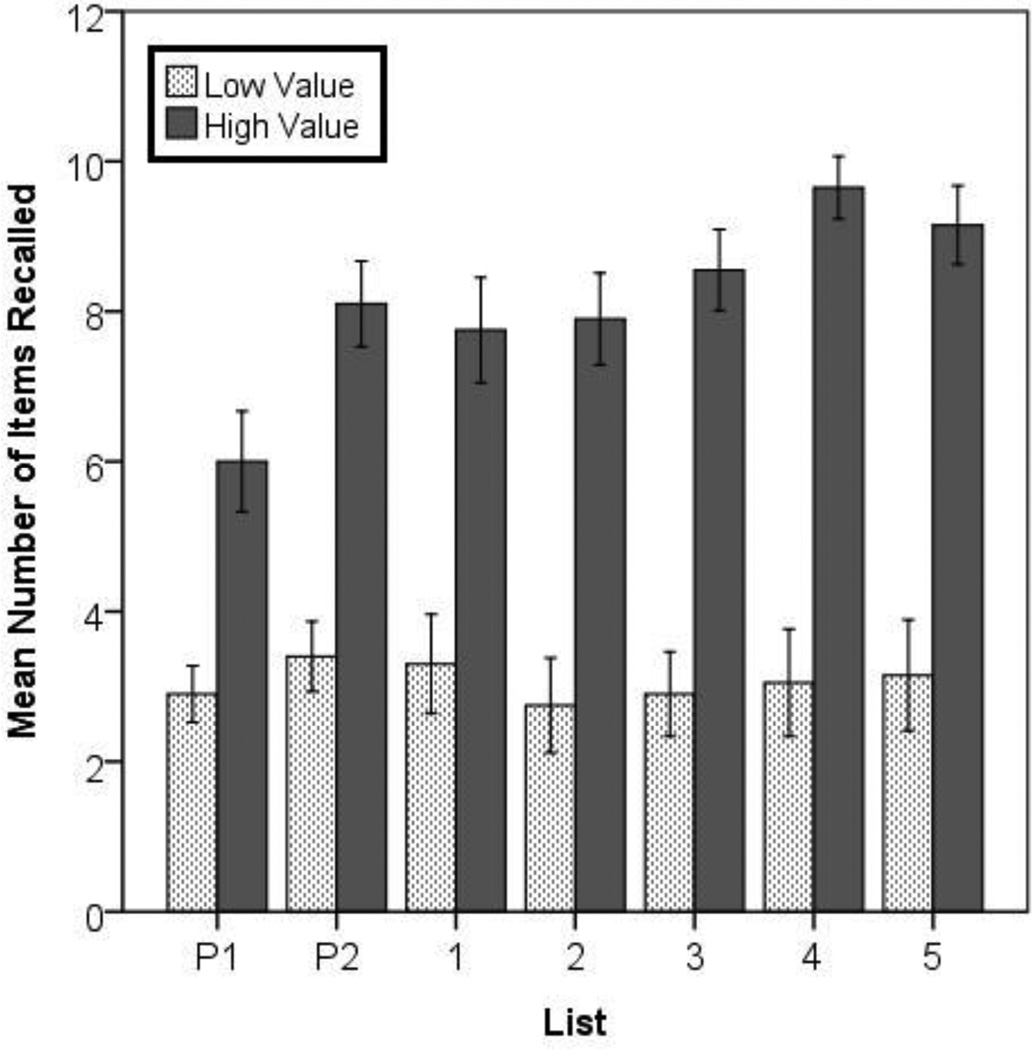
We first examined the behavioral data to confirm that high-value words were consistently recalled better than low-value words (Figure 2). Using paired-samples t-tests (two-tailed), we found that high-value words were remembered better than low-value words even on the first practice list, t(19) = 4.13, p = .001, and on the second practice list, t(19) = 7.02, p < .001. For the five lists presented in the scanner, a paired-samples t-test (performed on the data from the 19 subjects who completed all five lists) confirmed that the mean number of items recalled across all five scanned lists was significantly greater for high-value words, t(19) = 9.58, p < .001. A 2 × 5 (value-group x list) repeated-measures ANOVA on the proportion of items recalled additionally showed an interaction between list and value-group, F(4, 72) = 3.15, MSE = 1.96, p = .019, ηp2 = .149, but no main effect of list, F(4, 72) = 1.79, ηp2 = .090. The significant interaction suggests that point values had a reliably stronger effect on recall on later lists. Separate paired-samples t-tests for each list confirmed that there was still a highly reliable effect of value on all five scanned lists, with all ts > 6.00, and all ps < .001.
Figure 2.
Mean number of high-value and low-value items recalled on each list (including on the two practice lists shown prior to scanning). Significantly more high-value items were recalled than low-value items on all lists. Error bars represent +/− 1 SE.
In addition, we examined data for the individual value levels, in part to confirm that the binarization into high vs. low value that we use throughout the remainder of the paper is justified by the data (Table 1). For low-value items, a one-way within-subjects ANOVA did not find a difference in the number of items recalled across the three low-value conditions, F(2, 38) < 1, ηp2 = .004. Within high-value conditions, a one-way repeated measures ANOVA showed a trend towards an effect of point value, F(2, 38) = 2.97, MSE = 1.16, p = .063, ηp2 = .135. Significantly more 12-point items (M = 9.17) were recalled than 11-point items (M = 8.50), t(19) = 2.83, p = .028, but the difference between 11-point and 10-point items (M = 8.42) was not significant, t(19) < 1.
Table 1.
Mean (SE) number of items recalled across the 5 scanned lists by specific point value.
| High Value | Low Value | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 10 | 11 | 12 |
| 2.97 (0.60) |
3.09 (0.66) |
3.04 (0.65) |
8.42 (0.51) |
8.50 (0.45) |
9.17 (0.40) |
Main effects of value
Cue Period
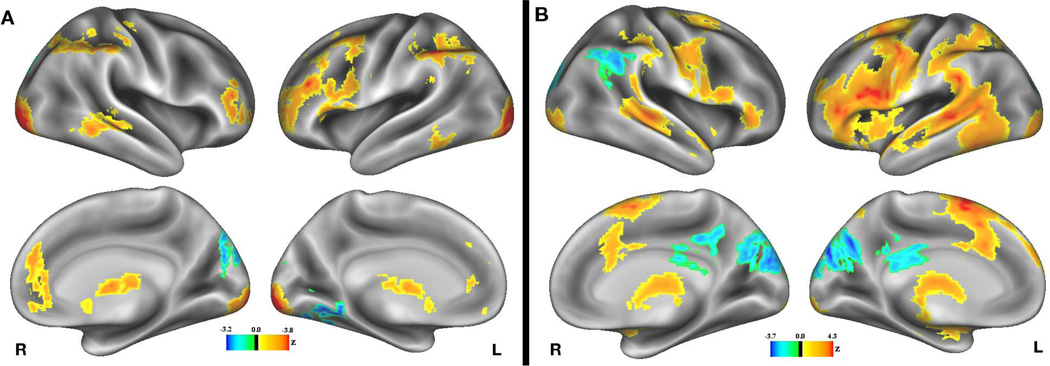
We first examine how brain activity differs during high-value trials as compared to low-value trials across individuals, during the cue period. A whole-brain analysis revealed several frontoparietal regions that showed greater BOLD signal in response to high value compared to low value cues (Figure 3A; Supplemental Table 1). In addition, as predicted, we observed significant effects in mesolimbic reward structures, including clusters in left nucleus accumbens (NAcc; peak voxel MNI coordinates: −6, 8, −4), and in right NAcc (peak voxel: 8, 10, −6). The whole-brain analysis also revealed a cluster in right pregenual cingulate cortex (peak voxel: 4, 44, 24), an area that has been associated with reward processing in a recent meta-analysis (Liu, Hairston, Schrier, & Fan, 2011). This cluster is immediately dorsal to the ventromedial prefrontal cortex, which is widely considered to be important in reward processing (e.g., O’Doherty, 2013).
Figure 3.
Group activation contrast showing main effects of value on BOLD signal (A) during the cue period, and (B) during the word encoding period. Warm colors indicate regions showing greater activity on high value trials, and cool colors indicate regions showing greater activity on low value trials. Note that scales were chosen separately for each contrast, and for positive and negative activations within each contrast, to maximize dynamic range, but the actual thresholds were constant across this and all other figures.
In addition, we conducted ROI analyses to probe for differential levels of activity in those specific reward-sensitive regions for which we had a priori hypotheses, specifically, VTA/midbrain and nucleus accumbens (NAcc)/ventral striatum. Our primary method of localizing these reward-sensitive regions was to locate the peak coordinates in midbrain and ventral striatum from a group-level analysis of the MID task. To localize the reward-sensitive midbrain, we placed a sphere of radius 4 mm around the peak midbrain coordinate obtained from the MID functional localizer task (L hemisphere: −6, −24, −6; R hemisphere: 6, −26, −6). We also functionally defined a NAcc/ventral striatal reward-sensitive region by placing a sphere of radius 4 mm around the peak coordinates in the vicinity of NAcc from the MID task (L hemisphere: −8, 12, −2; R hemisphere: 8, 10, −2). Because effects of value were reliably correlated across corresponding regions in the two hemispheres, and in order to increase statistical power, we combined L and R hemisphere spheres to create bilateral functionally-defined ROI for the NAcc and the midbrain. We found a significant effect of value in the bilateral NAcc/ventral striatum, t(19) = 3.73, p = .001. We also found greater activity during high-value cues in the reward-sensitive midbrain, t(19) = 2.48, p = .022.
Because our functionally-defined midbrain ROI is somewhat lateral, posterior, and superior to the typical anatomical definition of the VTA, possibly due to imperfect registration of midbrain BOLD signal to the anatomical template brain (e.g., Limbrick-Oldfield et al., 2012), we elected to also interrogate our data using an alternative VTA ROI, defined based on a probabilistic anatomical MRI atlas (Murty & Adcock, 2013; Shermohammed et al., 2012); we included all voxels that had non-zero probability values, resulting in a cluster of 698 voxels. Note that unlike our functionally-defined ROIs, which we defined separately in each hemisphere, this VTA ROI consists of a single midline region. Within the anatomically-defined VTA ROI, activity tended to be greater during high-value cues than during low-value cues; this difference approached, but did not reach, significance, t(19) = 1.84, p = .08. Thus, it seems that there was generally more activity in reward-sensitive brain regions during high-value cues, relative to low-value cues.
Word-encoding period
We also examined differences in brain activity as a function of value during the word-encoding period (Figure 3B; Supplemental Table 2). A whole-brain analysis revealed greater BOLD signal during high-value encoding in a large cluster that included almost the entirety of the left inferior frontal gyrus (LIFG), including both the pars triangularis (peak voxel: −44, 32, 6), and the pars opercularis (peak voxel: −42, 8, 18). Whole brain analysis also showed greater activity during high-value encoding in the left superior temporal gyrus, and throughout the posterior portion of the left lateral temporal cortex (peak voxel: −46, −52, −12). Similar patterns of brain activity are apparent in homologous right-hemisphere regions, but these effects were weaker and less extensive than their left-hemisphere counterparts. In addition, during encoding of high-value words, there was less activity in bilateral posterior cingulate cortex and in right angular gyrus, suggesting greater deactivation of the default mode network during encoding of these items, relative to low-value words.
We also observe increased activity in dopaminergic striatal and midbrain regions during the word-encoding period for high-value words. Whole-brain analysis revealed clusters centered in the caudate/putamen bilaterally (L peak voxel: −16, 10, 10; R peak voxel: 22, 6, −8). In addition, we examined how value affected activity in NAcc/ventral striatal and midbrain reward-sensitive regions during word encoding using the same ROIs described above. We find significantly greater activity during high-value encoding in bilateral NAcc/ventral striatum, t(19) = 4.23, p < .001. We also find a significant effect of value in our reward-sensitive midbrain ROI, t(19) = 3.02, p = .007. Finally, we find a significant effect of value in our anatomically-defined VTA ROI, t(19) = 2.26, p = .036. Overall, we can conclude that these reward-sensitive brain regions were generally more active on high-value items, during the word-encoding period as well as during the cue period.
Correlation with Selectivity Index
Our primary question of interest concerns how value contributes to subsequent recall. Because many of the participants remembered few low-value words or forgot few high value words, it was not possible to construct a viable contrast representing the interaction between value and recall. We instead used an individual differences approach to examine the relationship between item value and memory success. Specifically, we correlated each individual’s mean selectivity index with effects of value in the brain (i.e., the difference between activity on high value and low value trials in each voxel). Selectivity index reflects how close participants were to achieving an optimal point score, independent of the actual number of items recalled. We can thus infer that participants who were more selective in the words that they remembered on the recall test were engaging more strongly the processes that yield relatively better memory for high-value items in this task.
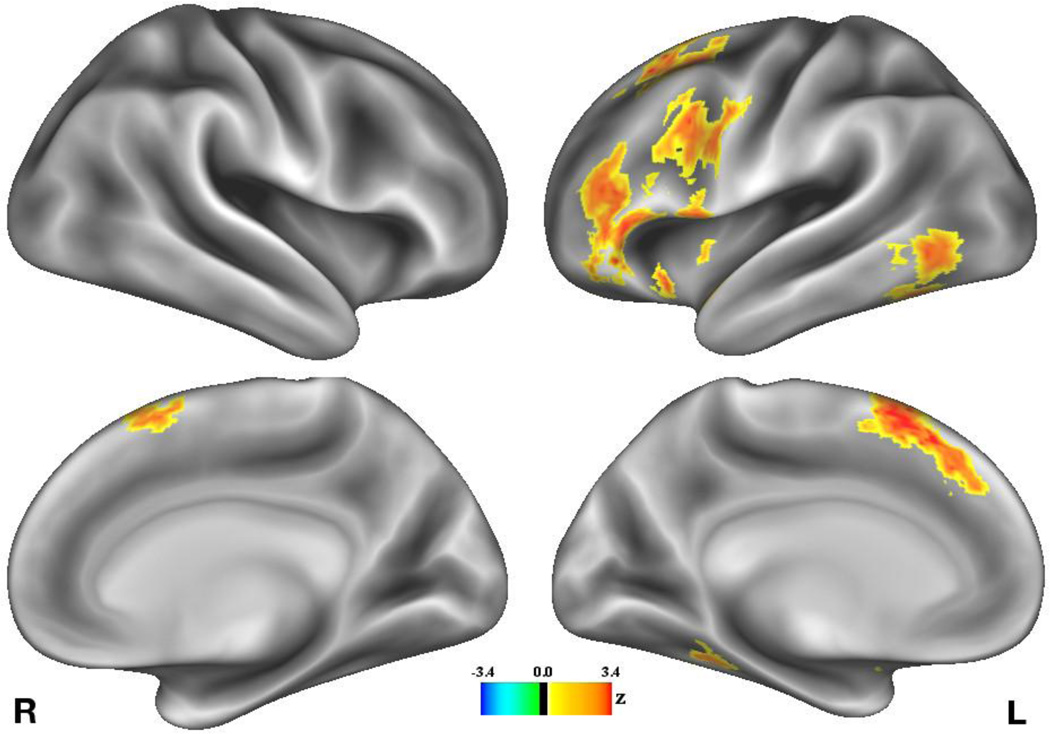
We first looked for regions in which the effect of value on BOLD signal during the cue period correlates with selectivity index. Whole-brain analysis yielded no significant correlations with selectivity index during the cue period. When using a whole-brain analysis to examine brain activity during the word-encoding period, however, a number of significant clusters emerged (Figure 4; Table 2). Most notably, we found a correlation between selectivity index and value-related activity in a cluster that included the anterior portion of the left IFG and ventral portions of the left middle frontal gyrus (peak voxel: −46, 20, −6), and in a second cluster that included the left posterior IFG (peak voxel: −38, 6, 28). Another notable cluster is apparent in the posterior portion of the middle and inferior temporal gyri (peak voxel: −52, −64, −2).
Figure 4.
Map depicting regions demonstrating a significant positive correlation between selectivity index and effects of value on BOLD signal during the word encoding period. No regions demonstrated a significant negative correlation between these variables.
Table 2.
Activation peaks for regions showing significant correlations between selectivity index and value effects (High > Low contrast) during word encoding period.
| Cluster | Peak | BA | z-stat | X | Y | Z | Voxels |
|---|---|---|---|---|---|---|---|
| 1 | L superior frontal gyrus | 8 | 4.13 | −4 | 26 | 52 | 1612 |
| L paracingulate gyrus | 32 | 3.97 | −2 | 34 | 38 | ||
| L supplementary motor area (SMA) |
6 | 3.78 | −4 | 14 | 64 | ||
| L premotor cortex | 6 | 3.60 | −28 | 10 | 58 | ||
| R SMA | 6 | 3.26 | 6 | 12 | 60 | ||
| 2 | L IFG pars orbitalis | 47 | 4.02 | −46 | 20 | −6 | 1397 |
| L middle frontal gyrus | 46 | 3.80 | −50 | 40 | 10 | ||
| L IFG pars triangularis | 45 | 3.76 | −46 | 22 | 16 | ||
| L IFG pars opercularis | 44 | 3.75 | −54 | 12 | −2 | ||
| L frontal pole | 10 | 3.73 | −40 | 42 | −10 | ||
| L precentral gyrus | 4 | 3.24 | −48 | 6 | 10 | ||
| L insula | 13 | 2.92 | −40 | 2 | 0 | ||
| 3 | L putamen | -- | 3.7 | −20 | 4 | −4 | 562 |
| L insula | 13 | 3.54 | −30 | 20 | −6 | ||
| L caudate | -- | 3.23 | −12 | 12 | 8 | ||
| 4 | L inferior occipital/ posterior middle temporal gyrus |
19 | 3.5 | −52 | −64 | −2 | 457 |
| L inferior temporal gyrus |
37 | 3.49 | −48 | −56 | −14 | ||
| L middle temporal gyrus | 21 | 3.36 | −60 | −56 | −4 | ||
| L fusiform gyrus | 37 | 3.17 | −34 | −42 | −14 | ||
| 5 | L precentral gyrus | 4 | 3.86 | −38 | 6 | 28 | 388 |
| L IFG pars opercularis | 44 | 3.77 | −48 | 8 | 28 | ||
| L premotor cortex | 6 | 3.76 | −42 | 8 | 38 | ||
We also examined how selectivity index correlated with value-related changes in activity in the mesolimbic dopamine system. During the cue period, none of the three reward-sensitive ROIs described above showed significant correlations with selectivity index (NAcc: r = −.01, functionally-defined midbrain: r = −.27, anatomical VTA: r = −.11). During the word-encoding period, however, we found a positive correlation between selectivity index and value-related activity in nucleus accumbens/ventral striatum (r = .495, p = .026). After applying a Bonferroni-Holm correction, the corrected p value for this correlation is .052, narrowly missing our cut-off for significance; we nonetheless believe this trend is noteworthy. We did not find a correlation in our functionally-defined midbrain ROI (r = .12) during the word encoding period, but we do find a positive correlation between selectivity index and value-related in the anatomically-defined VTA ROI (r = .534, p = .015), and this correlation does survive a Bonferroni-Holm correction. Thus, while it seems clear that effects of value on activity in dopaminergic reward regions during the cue period do not positively correlate with memory selectivity, activation of dopaminergic reward regions during word encoding may make some contribution to greater selectivity.
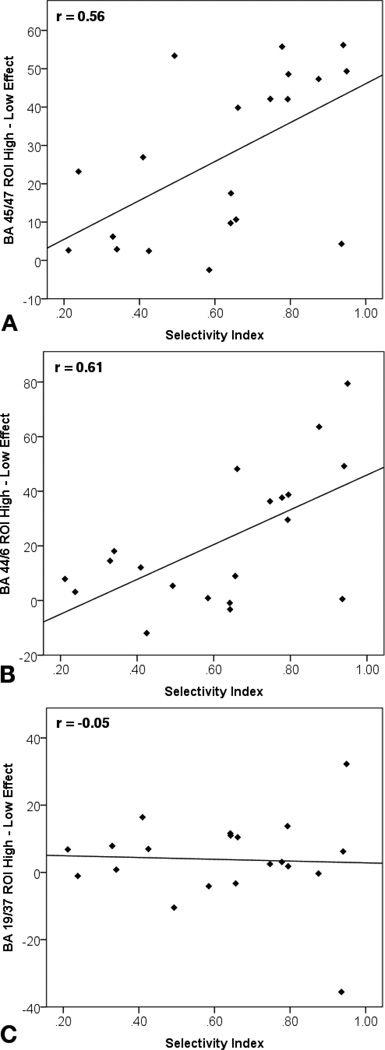
To provide additional evidence for inferences about the use of cognitive strategies at encoding, we also examined value effects in three different a priori regions from a prior fMRI study of strategy use during encoding (Kirchhoff & Buckner, 2006). The three relevant peaks were in left anterior IFG (BA 45/47), left posterior IFG (BA 44/6), and extrastriate cortex (BA 19/37). Kirchhoff and Buckner found that activity in both IFG clusters correlated positively with use of verbal elaboration strategies during encoding. Activity in the extrastriate cortex correlated instead with the use of a visual inspection strategy, which would likely not be useful for our verbal materials. Thus, if participants were using elaborative verbal encoding strategies to selectively remember the high-value words in our study, we would expect to find correlations between selectivity index and effects of value on BOLD signal in the two L IFG ROIs, but not in the extrastriate ROI.
To test this hypothesis, we converted the activation peaks reported by Kirchhoff and Buckner from Talairach to MNI space (Lancaster et al., 2007), and drew a sphere with an 8 mm radius around each of those peaks. During the cue period, there were no significant main effects of value in any of the 3 ROIs, all ts < 1.76, nor was there a correlation with selectivity index in any of the 3 ROIs, all rs < 0.13. During the word-encoding period, there was a main effect of value in both the anterior L IFG ROI, t(19) = 5.65, p < .001, and in the posterior L IFG ROI, t(19) = 3.96, p = .001, but not in the extrastriate ROI, t(19) = 1.33. In addition, during word encoding, selectivity index correlated significantly with value effects in the anterior L IFG ROI, r = 0.56, p = .010, and with value effects in the posterior L IFG ROI, r = 0.61, p = .005, but not with value effects in the extrastriate ROI, r = −0.05 (Figure 5). Thus, our results are consistent with the idea that participants who exhibit more memory selectivity may be preferentially engaging prefrontally-mediated verbal elaboration strategies during encoding of high vs. low value words.
Figure 5.
Correlations between selectivity index and value effects on BOLD signal in 3 ROIs from Kirchhoff and Buckner (2006):
(A) Anterior L IFG (BA 45/47)
(B) Posterior L IFG (BA 44/6)
(C) Extrastriate visual cortex (BA 19/37)
Regions A and B, which have been associated with verbal strategy use, show significant correlations between selectivity index and value effects on BOLD signal. Region C, a visual association area that has been associated with non-verbal encoding strategies (BA 19/37), does not show a significant correlation.
Individual Differences in Self-Reported Strategies, Selectivity, and Working Memory
To further enhance our understanding of how people tend to strengthen encoding of high-value items, we examined responses to the post-study questionnaires. We first examined and categorized self-reported strategy use at encoding. All participants reported using some type of verbal strategy to try to remember the words. Of these, 14 participants described strategies that would seem to rely on the meaning of the words (e.g., generating stories or images that combined multiple words). The remaining 6 participants described strategies that did not rely on meaning (e.g., rote rehearsal or alphabetizing). Selectivity index did not reliably vary between the groups using these two different strategy types, t(18) < 1. Individuals who used meaning-based strategies did recall more high-value words (M = 9.21, SD = 1.57) than those who used other verbal strategies (M = 7.48, SD = 1.95), t(18) = 2.10, p = .050, while not differing on the number of low-value words recalled, t(18) < 1. In addition, individuals who used meaning-based strategies tended to have higher working memory (WM) composite span scores (M = .26, SD = .70) than those who used non-semantic verbal strategies (M = -.61, SD = .85), t(18) = 2.40, p = .027.
Another result that speaks to strategy use is based on whether individuals reported limiting rehearsal exclusively (or nearly so) to high-value items. These reports largely came from people’s descriptions of what they were doing during fixation and vowel-consonant periods. We assume that the distinction between those who exclusively rehearsed high-value words and those who merely preferred rehearsing high-value words during these periods of “down time” reflected similarly divergent strategy use during the word-encoding period. Twelve participants reported largely or entirely ignoring the low-value items, while seven participants reported trying harder on high-value items, but did not appear to ignore low-value items. Finally, one participant reported ignoring value completely. An independent-samples t-test comparing the first two groups (excluding the one person who reported being indifferent to value) showed that selectivity index was significantly higher for individuals who reported that they ignored low-value items (M = .74, SD = .19) than for those who just tried to focus more on high-value items (M = .47, SD = .22), t(17) = 2.80, p = .012. Perhaps unsurprisingly, individuals who reported ignoring low-value items recalled significantly fewer of these items per list (M = 1.87, SD = 1.85) as compared to those who did not report ignoring low-value items (M = 4.34, SD = 2.96), t(17) = 2.25, p = .038. The two groups did not reliably differ on the number of high-value items recalled, however, t(17) = 0.91.
These findings led us to further examine individual differences in high-value vs. low-value recall. We found that selectivity index shows a highly significant negative correlation with low-value recall (r = −.72, p < .001), while the expected positive correlation between selectivity index and high-value recall does not reach significance (r = .26). We compared the absolute values of these r coefficients via a test of dependent correlation coefficients (Stieger, 1980), and found that the correlation between selectivity index and low-value recall is significantly stronger than the correlation with high-value recall, t(18) = 2.40, p = .03. Thus, our selectivity index measure is more strongly driven by the number of low-value items recalled than the number of high-value items recalled.
We also examined more closely the relationship between selectivity and WM span. We find that WM span score does not significantly correlate with selectivity index (r = .25), similar to the null effect shown by Castel et al. (2009). We also see dissociations in how selectivity and WM affect memory as a function of value. We used linear regression analyses to determine the degree to which selectivity and WM jointly predict high-value recall and, separately, low-value recall. We find that WM span is a strong positive predictor for high-value recall (β = .66, p = .002), but selectivity index is not (β = .09, p = .61). In contrast, WM span is a positive predictor of low-value recall (β = .33, p = .048), while selectivity index is a strongly negative predictor (β =−.81, p < .001). Thus, it seems that higher WM span is generally associated with better recall, consistent with prior work (e.g., Rosen & Engle, 1997; Unsworth, Brewer, & Spillers, 2013). At the same time, selectivity seems to be primarily associated with the degree to which people avoid encoding low-value items. These findings suggest that selectivity relies on strategic control processes that are, at least to some extent, separable from working memory.
Discussion
Prior neuroimaging studies have demonstrated the functional contributions of left ventrolateral prefrontal cortex to deep semantic processing and to the use of verbal elaboration strategies during memory encoding. Here, we demonstrate that activity in this region (specifically in left inferior gyrus and ventral portions of the left middle frontal gyrus) is greater during encoding of high-value words. We also demonstrate a correlation between neural effects of value in this region and a behavioral expression of memory selectivity.
An association between effects of value on BOLD signal and memory selectivity is specifically apparent in regions of L IFG for which individual differences in activity have previously been associated with individual differences in the use of verbal encoding strategies (Kirchhoff & Buckner, 2006). Others have additionally shown that L IFG is specifically involved in control processes related to semantic retrieval (e.g., Thompson-Schill et al., 1997; Badre et al., 2005; see Badre & Wagner, 2007 for review). Our findings thus provide suggestive evidence that people who selectively encode the most valuable items tend to do so by being more selective in the degree to which they engage semantic encoding strategies when encoding items deemed to be more valuable, relative to items that are less valuable. Subjects with high selectivity frequently reported that they tried to ignore low value items, and this was reflected in greater differences in brain activity in these left hemisphere regions during encoding of high vs. low-value words.
The effect of value on activity in posterior portions of the middle temporal gyrus (pMTG) also correlated with individual differences in memory selectivity. There is prior evidence relating this region with controlled retrieval of semantic knowledge as well. For instance, Wagner, Paré-Blagoev, Clark, & Poldrack (2001) found that searching for a weak semantic associate led to increased activity in pMTG, as well as increased activity in both anterior and posterior portions of left IFG, compared to searching for a strong semantic associate. Badre et al. (2005) observed a similar effect of semantic relatedness on both MTG and LIFG, but also found evidence suggesting that MTG activity reflects retrieval of semantic knowledge, but that only activity in LIFG mediates semantic control processes per se. More recent work has supported a somewhat different viewpoint that both regions play a necessary role in control processes related to retrieval of semantic knowledge, rather than pMTG activity only reflecting retrieval of semantic knowledge itself. For instance, Whitney, Kirk, O’Sullivan, Lambon Ralph, & Jeffries (2011) found that virtual lesions temporarily induced by transcranial magnetic stimulation (TMS) in either left IFG or left pMTG led to similar impairments to performance when judging weak semantic associates, but did not impair performance in judging strong semantic associates. The fact that the degree of increased activity during high-value encoding in both LIFG and pMTG is associated with memory selectivity in the present task, then, provides additional evidence that successfully enhancing memory for high-value items in our value-directed remembering task depends on strategic engagement of semantic processing.
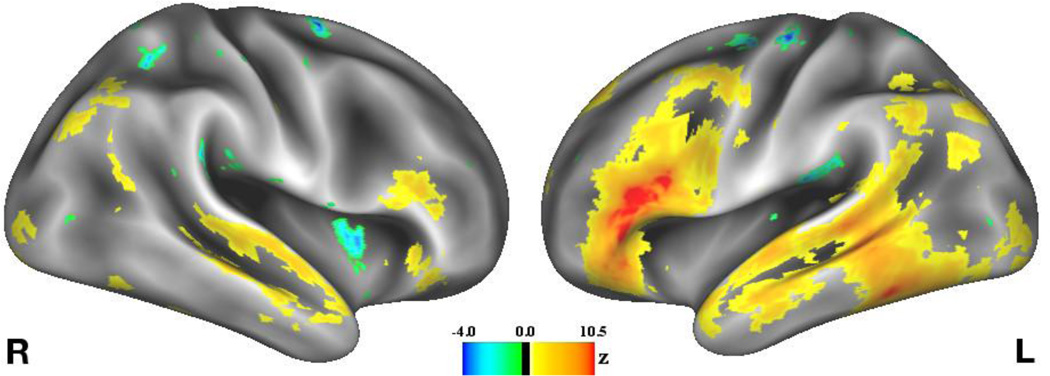
An automated meta-analysis using Neurosynth (http://neurosynth.org; Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) provides further evidence suggesting that the regions in which activity is modulated by value in the present study are typically involved in semantic processing. Specifically, a “reverse inference” statistical map generated from peak coordinates from the 670 neuroimaging studies in the Neurosynth database that most heavily utilized the term “semantic”, and which formally quantifies the probability that the term “semantic” would be associated with activation in these regions (Figure 6), looks strikingly similar to the regions associated with encoding of high-value words in the present study (Figure 3B). The meta-analytic map also reflects many of the same regions in which the degree of increased activity during encoding of high-value words correlates with memory selectivity. Thus, the automated meta-analysis supports the view that memory selectivity arises from differential semantic processing of valuable items.
Figure 6.
Automated Neurosynth meta-analysis of semantic processing. Voxel intensity values reflect the statistical likelihood that any given study reporting an effect in that voxel would be a study that heavily utilized the term “semantic.” Note the correspondence between the left PFC and posterior lateral temporal regions that emerged in this meta-analysis and our effects reported in Figures 3B & 4.
Selective encoding could potentially be mediated via a selective increase in the use of verbal strategies during encoding of high-value words, or via a selective reduction in verbal strategy use during encoding of low-value words. We observe that selectivity is reliably associated with the degree to which people self-report ignoring low-value words, and that selectivity index is more strongly associated with reduced memory for low-value words than with increased memory for high-value words. Thus, it seems likely that, at least in young adults, selectivity is primarily modulated by the degree to which people disengage semantic processing during encoding of low-value items, rather than by how effectively they encode high-value items.
At the same time, we observe that memory for high-value words and self-reported engagement of semantic encoding strategies is reliably associated with WM span, but these measures are not reliably associated with selectivity. One possible reason for the association between high-value recall and WM capacity is that individuals with high WM span may be better able to implement deep encoding of new high-value items while also simultaneously maintaining previous items. High WM span individuals may also be better able to maintain important items in memory while simultaneously performing the vowel-consonant task that occurred between successive word encoding trials. While these WM mechanisms do seem to be related to higher point totals, they do not seem to be a major factor in selective encoding.
It is also worth noting that we did tend to find greater activity in reward-sensitive regions (specifically, functionally-defined NAcc/ventral striatum and midbrain regions, and an anatomically-defined VTA region) on high-value trials than on low-value trials across participants. The VDR paradigm differs from most studies of reward-motivated learning in that we incentivize high-value items with higher point values, rather than using rewards that have external value (e.g., money). The observation that high point values still lead to increased activity in dopaminergic reward regions, similar to that observed with monetary rewards, supports our assumption that points are sufficiently rewarding to motivate changes in behavior. Indeed, memory performance was very sensitive to point value. This finding is similar to what is observed in a number of real-world contexts (e.g., video games, sports), in which people are motivated by the prospect of a high score. Our findings do, however, differ from past work in that the strength of dopamine-driven reward effects during the anticipatory cue period did not correlate significantly with individual differences in memory selectivity, Rather, in our data, this relationship was only apparent during the phase of the task when participants actually encountered the words. Previous work also suggests that the effects of activity in mesolimbic dopamine regions on subsequent memory are most apparent after a delay (e.g., Spaniol et al., in press; Murayama and Kuhbander, 2011), perhaps due to their dependence on off-line consolidation mechanisms. Such findings imply that the role of mesolimbic dopamine regions on value-induced memory enhancement should not be apparent in the immediate free recall measure used in the VDR. We believe that our findings of strategic enhancement of encoding and free recall relate to a second mechanism for value-related memory enhancement. This additional mechanism may be complementary to the dopaminergic enhancement of memory consolidation that has been demonstrated by others (e.g., Adcock et al., 2006; Murty et al., 2012; Wolosin et al., 2012), but the two different mechanisms appear to make varying contributions to memory performance based on the time scale and the type of information to-be-remembered.
Finally, our results suggest important potential implications for research on cognitive aging. Castel et al. (2002, 2007, 2009, 2013) found that healthy older adults generally show an excellent ability to be selective in the VDR task. Indeed, older adults often have equivalent memory to young adults for the most valuable items, despite recalling fewer items overall. This pattern of data often yields a higher selectivity index for older adults than that shown by young adults for tests of short-term memory. Thus, whatever older adults do to selectively encode high-value items in the VDR paradigm, they clearly seem to be relying on processes that are not substantially degraded by healthy aging. It may be that older adults retain the ability to be selective in their engagement of the semantic encoding strategies mediated by left PFC, which would provide important evidence about the type of processing that older adults are typically able to engage successfully. Thus, an important direction for future research is to examine age-related differences and similarities in the neural mechanisms of value-directed remembering.
While dopaminergic reward systems play an important role in memory formation, it is also important to consider how the strategic control of frontally-mediated encoding processes serves to selectively enhance memory for valuable items. Particularly in situations in which the delay between study and recall is relatively short, and when the items that need to be memorized are amenable to selective use of verbal encoding strategies, we might expect differential strategy use to be a more important contributor to memory performance than dopaminergic modulation of hippocampal activity. We anticipate that future work will help to determine the specific situations that preferentially engage these respective mechanisms, and whether they independently or interactively contribute to memory performance.
Supplementary Material
Acknowledgments
Funding was provided by NSF grant BCS-0848246 to B.J.K., and a grant from the Scientific Research Network for Decision Neuroscience and Aging (SRNDNA) (subaward under NIH grant AG039350) to B.J.K., M.S.C., J.R., and A.D.C. We thank Susan Bookheimer, Martin Monti, Gregory Samanez-Larkin, Michael Vendetti, and Aimee Drolet Rossi for helpful suggestions related to the design and analysis of this study. We thank Brian Knutson and Gregory Samanez-Larkin for providing scripts to run the MID task, and Vishnu Murty for providing an anatomical VTA atlas. We also thank Shruti Ullas for assistance with running participants.
Footnotes
Portions of this work were presented at the 20th annual meeting of the Cognitive Neuroscience Society, San Francisco, CA, and at the Mechanisms of Motivation, Cognition, and Aging Interactions (MoMCAI) conference, Washington, DC.
Instead of using a more stringent threshold, we felt that it was preferable to present a more complete picture of activity represented in a given contrast, while also employing dynamic range in the figures to highlight the regions that would emerge with a stronger threshold.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Ariel R, Castel AD. Eyes wide open: Enhanced pupil dilation when selectively studying important information. Experimental Brain Research. 2014;232:337–344. doi: 10.1007/s00221-013-3744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler R, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vegleichende Lokalisationslehre der Grosshirnde. Barth: Leipzig; 1909. [Google Scholar]
- Castel AD. The adaptive and strategic use of memory by older adults: Evaluative processing and value-directed remembering. In: Benjamin AS, Ross BH, editors. The psychology of learning and motivation. Vol. 48. San Diego: Academic Press; 2008. [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: Impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology. 2009;23:297–306. doi: 10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Benjamin AS, Craik FIM, Watkins MJ. The effects of aging on selectivity and control in short-term recall. Memory and Cognition. 2002;30:1078–1085. doi: 10.3758/bf03194325. [DOI] [PubMed] [Google Scholar]
- Castel AD, Farb N, Craik FIM. Memory for general and specific value information in younger and older adults: Measuring the limits of strategic control. Memory & Cognition. 2007;35:689–700. doi: 10.3758/bf03193307. [DOI] [PubMed] [Google Scholar]
- Castel AD, Humphries KL, Lee SS, Galvan A, Balota DA, McCabe DP. The development of memory efficiency and value-directed remembering across the life span: A cross-sectional study of memory and selectivity. Developmental Psychology. 2011a;47:1553–1564. doi: 10.1037/a0025623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Lee SS, Humphreys KL, Moore AN. Memory capacity, selective control, and value-directed remembering in children with and with- out attention-deficit/hyperactivity disorder (ADHD) Neuropsychology. 2011b;25:15–24. doi: 10.1037/a0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, McGillivray S, Friedman MC. Metamemory and memory efficiency in older adults: Learning about the benefits of priority processing and value-directed remembering. In: Naveh-Benjamin M, Ohta N, editors. Memory and aging: Current issues and future directions. New York: Psychology Press; 2012. pp. 245–270. [Google Scholar]
- Castel AD, Murayama K, Friedman MC, McGillivray S, Link I. Selecting valuable information to remember: Age-related differences and similarities in self-regulated learning. Psychology and Aging. 2013;28:232–242. doi: 10.1037/a0030678. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin and Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Research. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann KG, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neuroscience Letters. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Chapman SB, Swank P, Gamino JF, Roberson G, Levin HS. Development of verbal selective learning. Developmental Neuropsychology. 2007;32:585–596. doi: 10.1080/87565640701361112. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences USA. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progress in Neurobiology. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FIM, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proceedings of the National Academy of Sciences USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbrick-Oldfield EH, Brooks JCW, Wise RJS, Padormo F, Hajnal JV, Beckmann CF, Ungless MA. Identification and characterisation of midbrain nuclei using optimised functional magnetic resonance imaging. NeuroImage. 2012;59:1230–1238. doi: 10.1016/j.neuroimage.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Wickens TD. Effect of incentive on storage and retrieval processes. Journal of Experimental Psychology: General. 1970;85:141–147. [Google Scholar]
- McGillivray S, Castel AD. Betting on memory leads to metacognitive improvement in younger and older adults. Psychology and Aging. 2011;26:137–142. doi: 10.1037/a0022681. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MG, Iaki S, Amaro E., Jr Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K, Kuhbandner C. Money enhances memory consolidation—But only for boring material. Cognition. 2011;119:120–124. doi: 10.1016/j.cognition.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. Journal of Neuroscience. 2012;32:8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Adcock RA. Enriched encoding: Reward motivation organizes cortical networks for hippocampal detection of unexpected events. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht063. E-publication prior to print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RGM. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learning and Memory. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Functional contributions of the ventromedial prefrontal cortex in value-based decision making. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. pp. 302–315. [Google Scholar]
- O’Neill ME, Douglas VI. Rehearsal strategies and recall performance in boys with and without Attention Deficit Hyperactivity Disorder. Journal of Pediatric Psychology. 1996;21:73–88. doi: 10.1093/jpepsy/21.1.73. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Shermohammed M, Murty VP, Smith DV, Carter RM, Huettel S, Adcock RA. Resting-state analysis of the ventral tegmental area and substantia nigra reveals differential connectivity with the prefrontal cortex; Poster presented at the 19th annual meeting of the Cognitive Neuroscience Society; Chicago, IL. 2012. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom NC, McCabe DP. The interplay between value and relatedness as bases for metacognitive monitoring and control: Evidence for agenda-based monitoring. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:1236–1242. doi: 10.1037/a0023548. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Schain C, Bowen HJ. Reward-enhanced memory in younger and older adults. The Journals of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbt044. in press. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proceedings of the National Academy of Sciences USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toglia MP, Battig WF Handbook of semantic word norms. Republished online as supplement to: Toglia, M.P. Withstanding the test of time: The 1978 semantic word norms. Behavior Research Methods. 1978;41:531–533. doi: 10.3758/BRM.41.2.531. 2009. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Brewer GA, Spillers GJ. Working memory capacity and retrieval from long-term memory: the role of controlled search. Memory & Cognition. 2013;41:242–254. doi: 10.3758/s13421-012-0261-x. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of American Medical Informatics Association. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cerebral Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;288:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Watkins MJ, Bloom LC. Selectivity in memory: An exploration of willful control over the remembering process. 1999 Unpublished manuscript. [Google Scholar]
- Whitney C, Kirk K, O’Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. Journal of Cognitive Neuroscience. 2012;24:1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.