Abstract
Objective
Necrotizing enterocolitis (NEC) is characterized by macrophage infiltration into affected tissues. Because intestinal macrophages are derived from recruitment and in situ differentiation of blood monocytes in the gut mucosa, we hypothesized that increased recruitment of monocytes to the intestine during NEC reduces the blood monocyte concentration, and that this fall in blood monocytes can be a useful biomarker for NEC.
Patients and methods
We reviewed medical records of very low birth weight (VLBW) infants treated for NEC, and compared them with a matched control group comprised of infants with feeding intolerance but no signs of NEC. Clinical characteristics and absolute monocyte counts (AMC) were recorded. Diagnostic accuracy of AMC values was tested using receiver-operator characteristics (ROC).
Results
We compared 69 cases and 257 controls (median 27 weeks, range 26–29 in both groups). In stage II NEC, AMC decreased from median 1.7 × 109/L (interquartile range (IQR) 0.98–2.4) to 0.8 (IQR 0.62–2.1); p <0.05. In stage III NEC, monocyte counts decreased from median 2.1 × 109/L (IQR 0.1.5–3.2) to 0.8 (IQR 0.6–1.9); p <0.05. There was no change in AMC in control infants. ROC of AMC values showed a diagnostic accuracy (area under the curve) of 0.76. In a given infant with feeding intolerance, a drop in AMC of >20% indicated NEC with sensitivity of 0.70 (95% CI 0.57–0.81) and specificity of 0.71 (95% CI 0.64–0.77).
Conclusions
We have identified a fall in blood monocyte concentration as a novel biomarker for NEC in VLBW infants.
Keywords: Absolute monocyte counts, feeding intolerance, diagnostic test, monocytopenia, diagnosis, neonate
INTRODUCTION
Necrotizing enterocolitis (NEC), an inflammatory bowel necrosis of premature infants, remains a major cause of mortality in infants born before 32 weeks of gestation or with a birth weight <1500 grams.1, 2 The diagnosis of NEC is currently based on radiological findings such as pneumatosis intestinalis, fixed bowel loop(s), or portal venous air,3 characteristic abdominal signs and clinical progression,4 and/or histopathological findings such as coagulative necrosis, pneumatosis, and inflammation.5 Unfortunately, these signs of NEC have low sensitivity and are often recognized late in the course of NEC3, 6, 7 when many infants already have advanced disease requiring surgical resection of affected bowel.8 Cognizant of these difficulties in diagnosis and in the absence of a reliable biomarker of early NEC, clinicians frequently provide presumptive treatment to all at-risk infants with abdominal signs, an approach that is costly and undesirable.9 The lack of a reliable biomarker is also a major limitation in clinical trials; new treatment(s) may not be effective late in the clinical course of NEC when the affected tissue has already lost viability, whereas enrollment of patients before the onset of pathognomonic signs may result in erroneous inclusion of patients who never had NEC in the first place, thereby diluting the effect of the treatment.
Histopathologically, NEC is characterized by the presence of macrophage-rich leukocyte infiltrates,10 which contrasts with other causes of bowel dysfunction in neonates such as dysmotility, sepsis-related ileus, and ischemia-reperfusion.11, 12, 13 We have previously shown that gut macrophage populations are normally maintained through continuous recruitment and in situ differentiation of circulating monocytes in the lamina propria.14, 15 Because preterm infants have a limited circulating monocyte pool16 and lack significant reservoirs of mature monocytes in the bone marrow or elsewhere,17 we hypothesized that a massive influx of circulating monocytes into intestinal tissue during NEC in a preterm infant will result in an acute drop in peripheral blood monocyte counts and may help differentiate early NEC from other causes of feeding intolerance. To investigate this hypothesis, we compared absolute monocyte counts (AMC) in peripheral blood obtained at the time of onset of feeding intolerance in all patients treated for confirmed NEC at our center during the last 10 years and compared these with counts from matched controls with feeding intolerance due to causes other than NEC. We then validated our findings in a small cohort of infants with NEC from another center.
PATIENTS AND METHODS
A retrospective chart review was performed on very-low-birth-weight (VLBW) infants with a diagnosis of NEC at the University of Illinois Hospital during Jan 2001–Jun 2011, after approval by the Institutional Review Board. We used a nested case-control format, where infants with a diagnosis of NEC (Bell stages II or III)4 and for each case, 3–4 controls were identified based on the date of admission (±3 months), gestational age (±1 week), birth weight (±200 g), and the presence of feeding intolerance but lack of suggestive clinical features (tenderness, abdominal wall erythema, or abdominal mass) and radiological signs (pneumatosis, fixed bowel loop, bowel wall thickening, and/or portal venous air) or histopathological evidence (coagulative necrosis, pneumatosis, bacterial overgrowth, and inflammation) of NEC. Feeding intolerance was defined as the presence of ≥2 of the following criteria: abdominal distension, pre-feeding residuals ≥30% of the feeding volume, emesis, diarrhea, or bloody stools, resulting in radiological evaluation and temporary cessation of feedings. Infants with major congenital anomalies and spontaneous bowel perforations in the 1st postnatal week were excluded.
Demographic characteristics including birth weight, gestational age, gender, ethnicity (African-American, Caucasian, Latino, or other), and mode of delivery were noted. We also recorded clinical information including Apgar scores, blood culture-proven sepsis prior to onset of feeding intolerance, central line, patent ductus arteriosus (PDA), indomethacin therapy, intraventricular hemorrhage (IVH), and age of onset of NEC or feeding intolerance. Data retrieved from complete blood counts (CBC) included the date of the test, white cell counts (WCC), absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), and the AMC. These data were obtained from the day of onset of feeding intolerance, from the last available CBC drawn prior to the onset of feeding intolerance, and from 3 follow-up CBCs. All CBCs were performed at the clinical laboratory of the UI hospital using Siemens-Bayer Advia 2120 automated hematology counters (Siemens Medical Solutions, Hoffman Estates, IL).
Statistical Analysis
Statistical analysis was performed using the Sigma Stat 3.1.1 software (Systat, Point Richmond, CA). Data were classified as parametric if 4 conditions were met: (1) continuous scale; (2) equal difference between consecutive data points; (3) normality, evaluated by Shapiro-Wilk test; and (4) equality of variance, evaluated by Levene’s test.18 Clinical characteristics were compared by the Mann-Whitney U test,19 whereas the frequency of risk factors in various groups was compared by the Fisher’s exact test.20 We normalized the WCC, ANC, ALC, and AMC values recorded at onset of feeding intolerance against the last available value prior to the onset of feeding intolerance. Serial blood counts were compared using the Wilcoxon’s signed rank test21 or the Friedman’s repeated measures analysis of variance on ranks.22, 23 AMC data were depicted using Tukey-Koopman box-whisker plots.24 All statistical tests were 2-sided and considered significant at p <0.05. A compound-symmetry form was assumed for repeated measurements.25 Model-based results were accepted as unbiased if missing data were randomly distributed.
We next computed receiver-operating characteristics (ROC) of AMC values by plotting sensitivity vs. 1 – specificity.26 To identify a ‘cut-off’ value for normalized AMC with the best diagnostic effectiveness, we picked the normalized AMC point with the highest sum of sensitivity and specificity (Youden’s J statistic).27 The ability of this cut-off value to discriminate between infants with NEC vs. those with feeding intolerance from other causes was determined by computing sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios from a 2 × 2 confusion matrix.28 Finally, to determine whether the diagnostic accuracy of normalized AMC could be improved by including other clinical characteristics in the ROC model, we performed logistic regression and compared full (with all variables) vs. reduced models to determine whether these predictors were associated with NEC.
Validation of findings in an independent cohort of patients
To test the diagnostic accuracy of AMC as a test for NEC, we reviewed the medical records of infants treated for confirmed NEC at Loyola University Medical Center, Maywood, IL during the period Jan 2007 – Jan 2011 and retrieved data on clinical characteristics and blood counts. We then tested our ROC model in this cohort to determine the percentage of patients who were correctly identified as having NEC.
RESULTS
We reviewed the medical records from all 1654 VLBW infants admitted to the level III NICU at the University of Illinois hospital between Jan 2001- Jun 2011 and identified 72 patients with a diagnosis of NEC. Three infants who developed bowel perforation within the 1st postnatal week were excluded after chart review because a diagnosis of spontaneous intestinal perforation was considered more likely than NEC in these infants. Thus, in our final analysis, we included 69 infants with confirmed NEC. These were compared with 257 control infants, who had a total of 261 episodes of feeding intolerance that were not due to NEC.
Demographic characteristics
The demographic characteristics of infants in the NEC and control groups are summarized in Table 1. Infants in the NEC group were more likely to have been delivered vaginally (59.1 vs. 41% in NEC and control groups, respectively; p = 0.006), transferred from an outside hospital (30.4 vs. 17.5% in NEC and control groups, respectively; p = 0.027), but were less likely to have had a central line (59.4 vs. 73.9% in NEC and control groups, respectively; p = 0.025) or a history of a positive blood culture during hospital stay (17.4 vs. 33.5% in NEC and control groups, respectively; p = 0.012). Infants in the control group developed feeding intolerance at an earlier postnatal age than those with NEC (median 12, range 7–23 days in control group vs. a median of 20, range 12–31 days in infants with NEC; p <0.001).
Table 1.
Demographic characteristics
| Characteristic | Feeding Intolerance (n=257*) | Necrotizing Enterocolitis (n=69) | p-value |
|---|---|---|---|
| Birth weight (g); median (interquartile range) | 968 (771–1186) | 945 (718–1200) | |
| Gestational age (weeks); median (interquartile range) | 27 (26–29) | 27 (26–29) | |
| Male sex – n (%) | 133 (51.8) | 38 (55) | |
| Ethnicity – n (%) | |||
| African-American | 191 (74.3) | 49 (71) | |
| Caucasian | 17 (6.6) | 7 (10.1) | |
| Latino | 38 (14.8) | 13 (18.8) | |
| Mode of delivery | |||
| Cesarean section | 152 (59.1) | 28 (41) | 0.006 |
| Vaginal | 105 (41) | 41 (59.1) | 0.006 |
| Outborn (%) | 45 (17.5) | 21 (30.4) | 0.027 |
| 5-min Apgar <6 – n (%) | 36 (14) | 9 (13.1) | |
| PDA – n (%) | 125 (48.6) | 33 (48) | |
| Indomethacin – n (%) | 79 (30.7) | 19 (27.5) | |
| IVH ≥Grade 2 – n (%) | 24 (9.3) | 11 (16) | |
| Central line† – n (%) | 190 (73.9) | 41 (59.4) | 0.025 |
| Positive blood culture‡ – n (%) | 86 (33.5) | 12 (17.4) | 0.012 |
| Onset of feeding intolerance (postnatal age in days); median (interquartile range) | 12 (7–23) | 20 (12–31) | <0.001 |
PDA, Patent ductus arteriosus; IVH, intraventricular hemorrhage.
Placed before diagnosis of NEC.
Developed before diagnosis of NEC.
261 cases of feeding intolerance in 257 patients
Clinical characteristics
In the NEC group, 25 (36.2%) and 44 (63.8%) infants were classified as Bell stage II and III, respectively. In the NEC group, survivors had a longer length of hospital stay than controls (Table 2). As anticipated, there were more deaths in the NEC group (p <0.001). Pre-feed residuals were recorded more often in the feeding intolerance group (76.6 vs. 60.9% in feeding intolerance and NEC groups, respectively; p =0.004). The NEC group had a higher frequency of respiratory distress, apnea, and acidosis. Frank bleeding per rectum was recorded in 34.8% NEC patients but not in controls (p <0.0001).
Table 2.
Clinical characteristics
| Characteristic | Feeding Intolerance (n=257) | Necrotizing Enterocolitis (n=69) | p-value |
|---|---|---|---|
| Length of Stay (days); median (interquartile range) | 69 (47–93) | 79.5 (34.7–124.7) | |
| Length of stay in survivors (days) | 69 (47–93) | 105 (34.7–142) | <0.01 |
| Died - n (%) | 17 (6.6) | 23 (33.3) | <0.0001 |
| Presentation | |||
| Pre-feed Residuals - n (%) | 197 (76.6) | 42 (60.9) | <0.01 |
| Abdominal Distention - n (%) | 221 (86) | 61 (88.5) | |
| Frank bleeding per Rectum - n (%) | 0 | 19 (27.5) | <0.0001 |
| Other (apnea, respiratory distress, acidosis) - n (%) | 0 | 43 (62.3) | <0.0001 |
| Radiological Signs | |||
| Pneumatosis - n (%) | 0 | 60 (87) | |
| Fixed Bowel Loop - n (%) | 0 | 10 (14.5) | |
| Free Intraperitoneal Air - n (%) | 0 | 11 (16) | |
| Portal Venous Gas - n (%) | 0 | 11 (16) | |
| Surgery | |||
| Exploratory laparotomy - n (%) | Not applicable | 36 (52.1) | |
| Peritoneal drain - n (%) | Not applicable | 16 (23.2) |
Blood counts
In our NEC group, 59/69 (85.5%) patients had a CBC in the chart that was performed median 3.5 days [inter-quartile range (IQR) 1–6 days] prior to the onset of symptoms. Sixty of the 69 (86.9%) cases had a CBC drawn on the day of onset of symptoms. Patients with a missing prior CBC had been transferred from another hospital following onset of NEC. Sixty-seven (97.1%) had a follow-up CBC drawn after median 1 day (IQR 1–1.75 days). A 2nd follow-up CBC was available in 61 (88.4%) patients drawn at median 2 days (IQR 2–3 days) after onset of NEC, whereas 53 (76.8%) had a 3rd follow-up CBC drawn at median 3 days (IQR 3–4 days). In the control group, 258/261 (98.8%) patients had a CBC from median 2 days (IQR 1–4 days) prior to the onset of symptoms. One hundred ninety-five (74.7%) had a CBC drawn on the day of onset of symptoms, whereas 253 (96.9%) had another CBC drawn after median 1.5 days (IQR 1–3 days). A 2nd follow-up CBC was available in 224 (85.8%) infants drawn at median 3.7 days (IQR 2.2–6.5 days) after onset of symptoms, whereas 53 (76.8%) had a 3rd CBC drawn at median 5.7 days (IQR 3.7–9 days).
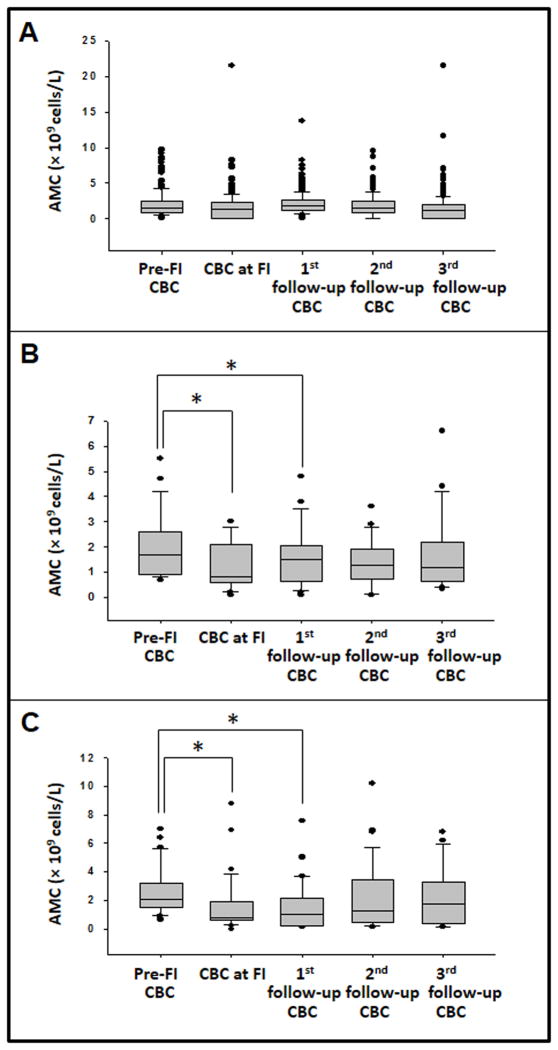
Compared to the pre-symptomatic AMC, monocyte counts were significantly lower in patients with both Bell stage II and III disease on the day of onset of NEC and in the 1st follow-up CBC (Fig. 1). In patients with stage II disease, AMC decreased from median 1.7 × 109 cells/L (IQR 0.98 to 2.4) to median 0.8 (IQR 0.62 to 2.1); p <0.05, whereas in those with stage III NEC, the AMC decreased from median 2.1 × 109 cells/L (IQR 0.1.5 to 3.2) to median 0.8 (IQR 0.6 to 1.9); p <0.05. The WCC, ANC, and ALC did not change significantly (not depicted). In the control group, there were no significant changes in the AMC or the WCC, ANC, and ALC. Within the control group, there was no difference in these counts between infants with positive blood cultures and presumed ileus vs. others with ‘idiopathic’ feeding intolerance.
Figure 1. Longitudinal change in peripheral blood AMC in control and NEC groups.
Box-whisker plots show AMC in (A) controls, (B) infants with NEC stage II, and (C) those with NEC stage III. Data were compared by repeated measures ANOVA on ranks with Dunnett’s test using AMC prior to feeding intolerance as the comparison group.
Receiver-operator characteristics
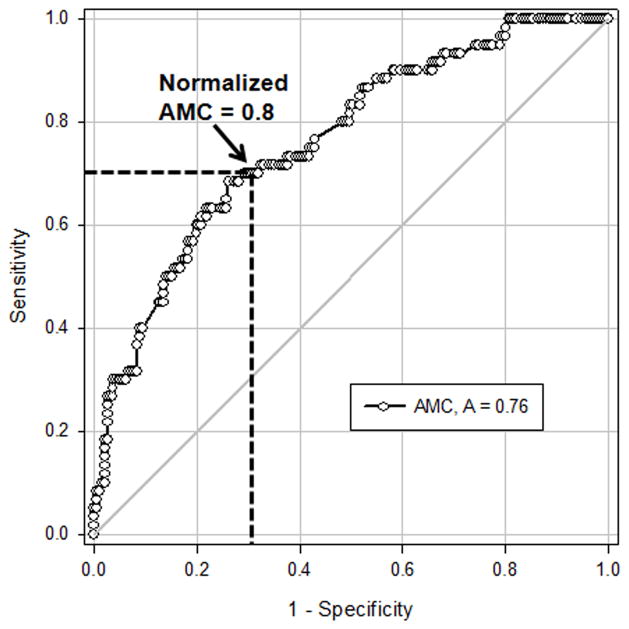
We next investigated whether AMC values obtained at onset of feeding intolerance could discriminate between NEC vs. feeding intolerance from other causes. To account for the variability in blood monocyte counts in neonates,16 we normalized AMC at onset of symptoms against the last available pre-symptomatic AMC for each infant and then computed ROCs curves using normalized AMC (Fig. 2). The area under the curve was 0.76 [95% confidence interval (CI) 0.69 to 0.83; p <0.0001], indicating “fair-to-good” diagnostic accuracy. To determine whether the inclusion of clinical characteristics could improve the diagnostic accuracy of our model, we performed logistic regression to identify covariates associated with NEC. We identified birth weight, ethnicity, and sepsis as significant but the inclusion of these parameters in the ROC model did not improve its diagnostic accuracy.
Figure 2. Diagnostic accuracy of decreased peripheral blood AMC as a test for NEC.
Receiver-operator characteristics of the ratio of AMC in infants at the time of feeding intolerance vs. AMC in the most recent CBC drawn prior to the onset of feeding intolerance show that a >20% drop in AMC correctly identified NEC in 76% cases (depicted by the area under the curve). A cut-off value of 0.8 (marked by broken lines in the figure) provided 70% sensitivity and 70.6% specificity.
To identify a ‘cut-off’ value with maximum diagnostic effectiveness, we computed (sensitivity + specificity) for each normalized AMC and picked normalized AMC = 0.8, which provided the highest summated value (Youden’s J statistic) of 1.4 (Fig. 2). At this cut-off value, the sensitivity was 0.70 (95% CI 0.57–0.81), the specificity was 0.71 (95% CI 0.64 to 0.77), the positive predictive value was 0.43 (95% CI 0.33 to 0.54), and the negative predictive value was 0.88 (95% CI 0.81 to 0.92). Table 3 summarizes the clinical effectiveness of this test.
Table 3.
Diagnostic value of decreased peripheral blood AMC as a test for NEC
| No NEC | NEC | Total | |
|---|---|---|---|
| >20% drop in AMC | 54 | 41 | 95 |
| Increased or ≤20% drop in AMC | 137 | 19 | 156 |
| Total | 191 | 60 | 251 |
| Value (95% CI) | |
|---|---|
| Prevalence | 0.24 (0.19–0.30) |
| Sensitivity | 0.70 (0.57–0.81) |
| Specificity | 0.71 (0.64–0.77) |
| Positive predictive value | 0.43 (0.33–0.54) |
| Negative predictive value | 0.88 (0.81–0.92) |
| Positive likelihood ratio | 2.42 (1.82–3.21) |
| Negative likelihood ratio | 0.44 (0.30–0.64) |
Validation of findings in an independent cohort of patients
We tested our diagnostic cut-off in an independent cohort of VLBW infants treated for confirmed NEC at Loyola University Medical Center. Twenty-three patients were identified, including 17 (74%) and 6 (26%) with Bell stages II and III, respectively. These patients had a median birth weight of 750 g (range 630–1319), gestation 26.5 weeks (range 24.5–30), and except for a higher number of Latino infants (10/23, 43.5%), had clinical characteristics similar to the primary cohort. A CBC was available from prior to onset of NEC in 21 (91%) and in all patients on the day of onset and at median 5 days (IQR 2.5 to 7 days). AMC decreased from median 1.5 × 109 cells/L (IQR 1.1 to 2.8) in the pre-symptomatic CBC to median 0.9 × 109 cells/L (IQR 0.5 to 1.9) at the time of NEC (p = 0.03). A diagnostic cut-off of normalized AMC <0.8 correctly identified 14/23 (66.7%) patients as having NEC, which was consistent with our ROC model.
DISCUSSION
We show that a fall in peripheral blood AMC can be a useful diagnostic marker of NEC in VLBW infants. At the time of onset of feeding intolerance, an acute drop in AMC (from the last available test) correctly discriminated between NEC vs. other causes of feeding intolerance with 76% accuracy. To our knowledge, this is the first study to show that blood monocyte counts can serve as an adjunctive diagnostic test for NEC. Although this information is already available in CBC reports generated from hematology analyzers, most neonatologists currently do not evaluate monocyte counts routinely in their practice. Previous studies have identified several candidate biomarkers of NEC such as the inter-alpha inhibitor protein, intestinal fatty acid-binding protein, hexosaminidase, proapolipoprotein CII, and serum amyloid A.29, 30, 31, 32, 33, 34 However, despite its modest diagnostic accuracy, the AMC is an attractive marker of NEC because (1) the information is already available to the clinician at no extra cost; and (2) its high negative predictive value (88%) can help exclude the diagnosis of NEC in infants with feeding intolerance due to other causes. Because most clinical laboratories now use automated hematology counters, there are additional advantages of rapid turnaround times, a high degree of consistency, and the ease of extrapolation of findings to other centers.
In the present study, our hypothesis that NEC is associated with decreased blood monocyte concentrations emerged from our preclinical observations of macrophage-rich infiltrates in NEC.10 Because macrophages in inflammatory gastrointestinal lesions are derived from blood monocytes,15 we reasoned that the rapid efflux of monocytes to NEC lesions is likely to deplete the limited circulating pool of monocytes in premature neonates.16 Interestingly, decreased blood monocyte counts are likely to be a unique feature of NEC. Growth-restricted preterm infants may have low monocyte counts but most of these infants show a suppression of all leukocyte lineages and not isolated monocytopenia.35 In the NICU, monocytosis may be seen more frequently than monocytopenia and may occur in association with extreme prematurity, repeated RBC transfusions, albumin infusions, and theophylline therapy.36 Monocytosis can also be seen in congenital candidiasis and syphilis,37, 38 and in immune-mediated neutropenia.39, 40
We normalized monocyte counts against the last available pre-symptomatic AMC to account for normal variability in blood monocyte counts in premature neonates. We recently developed reference ranges of AMC in neonates using data retrieved from over 62,000 CBCs,16 where we showed that blood monocyte concentrations increase almost linearly between 22–42 weeks gestation.16 Monocyte concentrations also increased during the first 2 postnatal weeks.16 These data are consistent with kinetic studies in human fetuses, which show a similar maturational increase in monocyte precursors.41, 42
In our study, infants in the NEC group had a later onset of feeding intolerance compared to the control group. Control infants had a higher frequency of blood culture-positive sepsis and of having had a central line, suggesting that the abdominal signs in some of these infants may be explained by sepsis-related ileus, which may peak at an earlier postnatal age than NEC.43, 44 Although monocytosis has been noted in neonatal infections, we did not detect a significant difference in monocyte counts in infants in the control group with feeding intolerance due to sepsis vs. other controls with feeding intolerance of unknown origin. Overall, the NEC group had a higher incidence of systemic signs, gastrointestinal bleeding, and mortality, indicating a higher acuity of illness than controls.
A major limitation of our study is its retrospective design, which increases the risk of bias.45 Considering the limited sample size, our findings need further validation in larger/multi-centric cohorts and in larger infants with NEC. Further study is also needed to evaluate maternal/neonatal covariates known to be associated with NEC such as abnormal fetal umbilical Doppler signatures and chorioamnionitis, feeding practices, anemia, transfusions, and infections.46, 47, 48 The relevance of AMC in spontaneous bowel perforations also remains uncertain. Although most neonatologists view spontaneous perforations and NEC as distinct entities, the two conditions may comprise a clinical continuum and may be difficult to distinguish from each other on the basis of clinical signs, histopathological findings, and even inflammatory markers.49
CONCLUSIONS
We have identified a fall in AMC as a novel biomarker for NEC in VLBW infants. When compared to the last available AMC from prior to onset of feeding intolerance, an acute drop in blood monocyte concentration can identify NEC with 76% accuracy. In a given infant with feeding intolerance, a fall in AMC by >20% indicated NEC with sensitivity of 0.70 (95% CI 0.57–0.81) and specificity of 0.71 (95% CI 0.64 to 0.77). This test offers a high negative predictive value (88%), which can help exclude the diagnosis of NEC in infants with feeding intolerance due to other causes.
Acknowledgments
Funding source: Supported in part by the NIH award R01HD059142 (to A. M.)
Abbreviations
- NICU
neonatal intensive care unit
- AMC
absolute monocyte counts
- NEC
necrotizing enterocolitis
- PDA
patent ductus arteriosus
- IVH
intraventricular hemorrhage
- VLBW
very low birth weight
- FI
feeding intolerance
- IQR
inter-quartile range
- CI
confidence interval
Footnotes
A.M. designed the study and wrote the manuscript, J.I.R., J.K.M., and R.D.C. contributed to study design, J.I.R., R.K., and R.K. collected data. All the authors approved the final manuscript
Financial disclosure statement: The authors have no financial disclosures
Clinical trials registry name/registration: Not applicable
Conflict of interest statement: Dr. Maheshwari’s work has been funded by the NIH. Drs. Remon, Kampanatkosol, Kaul, Muraskas and Christensen declare no conflict of interest.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. 1999;37 (6):1187–1198. vii. doi: 10.1016/s0033-8389(05)70256-6. [DOI] [PubMed] [Google Scholar]
- 4.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6(1):6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coursey CA, Hollingsworth CL, Gaca AM, Maxfield C, Delong D, Bisset G., 3rd Radiologists’ agreement when using a 10-point scale to report abdominal radiographic findings of necrotizing enterocolitis in neonates and infants. AJR Am J Roentgenol. 2008;191(1):190–197. doi: 10.2214/ajr.07.3558. [DOI] [PubMed] [Google Scholar]
- 7.Coursey CA, Hollingsworth CL, Wriston C, Beam C, Rice H, Bisset G., 3rd Radiographic predictors of disease severity in neonates and infants with necrotizing enterocolitis. AJR Am J Roentgenol. 2009;193(5):1408–1413. doi: 10.2214/AJR.08.2306. [DOI] [PubMed] [Google Scholar]
- 8.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 9.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohankumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, et al. Gut Mucosal Injury in Neonates is marked by Macrophage Infiltration in Contrast to Pleomorphic Infiltrates in Adult: Evidence from an Animal Model. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G93–G102. doi: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pender SL, Braegger C, Gunther U, Monteleone G, Meuli M, Schuppan G. Matrix metalloproteinases in necrotising enterocolitis. Pediatr Res. 2003;54(2):160–164. doi: 10.1203/01.PDR.0000072326.23442.C3. [DOI] [PubMed] [Google Scholar]
- 12.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, et al. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51(6):733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G20–26. doi: 10.1152/ajpgi.00168.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, et al. Epithelial Cells in Fetal Intestine Produce Chemerin to Recruit Macrophages. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G1–G10. doi: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, Vu HL, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80(3):492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 16.Christensen RD, Jensen J, Maheshwari A, Henry E. Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63 000 records in a multihospital health-care system. J Perinatol. 2010;30(8):540–545. doi: 10.1038/jp.2009.196. [DOI] [PubMed] [Google Scholar]
- 17.van Furth R, Sluiter W. Distribution of blood monocytes between a marginating and a circulating pool. J Exp Med. 1986;163(2):474–479. doi: 10.1084/jem.163.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MF, Brozovic B, Murphy W, Ouwehand W, Waters AH. Guidelines for platelet transfusions. British Committee for Standards in Haematology, Working Party of the Blood Transfusion Task Force. Transfusion medicine. 1992;2(4):311–318. doi: 10.1111/j.1365-3148.1992.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 19.Giles LG. Evidence-based clinical guidelines submitted to the Australian National Health and Medical Research Council for the management of acute low back pain: a critical review. Journal of manipulative and physiological therapeutics. 2001;24(2):131–139. doi: 10.1067/mmt.2001.112559. [DOI] [PubMed] [Google Scholar]
- 20.Spinella PC, Dressler A, Tucci M, Carroll CL, Rosen RS, Hume H, et al. Survey of transfusion policies at US and Canadian children’s hospitals in 2008 and 2009. Transfusion. 2010;50(11):2328–2335. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 21.Contreras M, Ala FA, Greaves M, Jones J, Levin M, Machin SJ, et al. Guidelines for the use of fresh frozen plasma. British Committee for Standards in Haematology, Working Party of the Blood Transfusion Task Force. Transfusion medicine. 1992;2(1):57–63. doi: 10.1111/j.1365-3148.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 22.Wright SE, McKay R, Taylor KI, Keeffe JE, McCarty CA. Changes in attitudes and practices of optometrists in their management of diabetic retinopathy after the release of NHMRC guidelines. National Health and Medical Research Council. Clinical & experimental ophthalmology. 2001;29(3):121–124. doi: 10.1046/j.1442-9071.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- 23.Shih W. Problems in dealing with missing data and informative censoring in clinical trials. Curr Control Trials Cardiovasc Med. 2002;3(1):4. doi: 10.1186/1468-6708-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84(3):732–747. [PubMed] [Google Scholar]
- 25.Diggle PJ, Liyang KY, Zeger SL. Analysis of longitudinal data. Clarendon Press; Oxford: 1994. [Google Scholar]
- 26.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 27.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab animal. 2011;40(5):155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newcombe RG. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157(5):757–761. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH, Rozycki HJ. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. 1999;34(10):1453–1457. doi: 10.1016/s0022-3468(99)90102-1. [DOI] [PubMed] [Google Scholar]
- 31.Lobe TE, Richardson CJ, Rassin DK, Mills R, Schwartz M. Hexosaminidase: a biochemical marker for necrotizing enterocolitis in the preterm infant. American journal of surgery. 1984;147(1):49–52. doi: 10.1016/0002-9610(84)90033-3. [DOI] [PubMed] [Google Scholar]
- 32.Lobe TE, Schwartz MZ, Richardson CJ, Rassin DK, Gourley WK, Srivastava SK, et al. Hexosaminidase: a marker for intestinal gangrene in necrotizing enterocolitis. J Pediatr Surg. 1983;18(4):449–452. doi: 10.1016/s0022-3468(83)80198-5. [DOI] [PubMed] [Google Scholar]
- 33.Shattuck KE, Richardson CJ, Rassin DK, Lobe TE. Evaluation of hexosaminidase activity as a potential biochemical marker in serum for necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1987;6(2):234–237. doi: 10.1097/00005176-198703000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Ng PC, Ang IL, Chiu RW, Li K, Lam HS, Wong RP, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. The Journal of clinical investigation. 2010;120(8):2989–3000. doi: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirbelauer J, Thomas W, Rieger L, Speer CP. Intrauterine growth retardation in preterm infants </=32 weeks of gestation is associated with low white blood cell counts. Am J Perinatol. 2010;27(10):819–824. doi: 10.1055/s-0030-1254547. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Sperry JL, Ochoa JB, Barclay D, Namas R, Vodovotz Y, et al. Persistence of elevated plasma CXCL8 concentrations following red blood cell transfusion in a trauma cohort. Shock. 2012;37(4):373–377. doi: 10.1097/SHK.0b013e31824bcb72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raabe BM, Artwohl JE, Purcell JE, Lovaglio J, Fortman JD. Effects of weekly blood collection in C57BL/6 mice. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50(5):680–685. [PMC free article] [PubMed] [Google Scholar]
- 38.Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, et al. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. The Journal of biological chemistry. 2011;286(14):12123–12132. doi: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillen U, Cummings JJ, Bell EF, Hosono S, Frantz AR, Maier RF, et al. International survey of transfusion practices for extremely premature infants. Semin Perinatol. 2012;36 (4):244–247. doi: 10.1053/j.semperi.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosebraugh MR, Widness JA, Nalbant D, Veng-Pedersen P. A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion. 2013;53(6):1353–1360. doi: 10.1111/j.1537-2995.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke C, Baghdadi H, Howie AF, Mason JI, Walker SW, Beckett GJ. Selenium supplementation attenuates procollagen-1 and interleukin-8 production in fat-loaded human C3A hepatoblastoma cells treated with TGFbeta1. Biochimica et biophysica acta. 2010;1800(6):611–618. doi: 10.1016/j.bbagen.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Savidge TC, Newman PG, Pan WH, Weng MQ, Shi HN, McCormick BA, et al. Lipopolysaccharide-induced human enterocyte tolerance to cytokine-mediated interleukin-8 production may occur independently of TLR-4/MD-2 signaling. Pediatr Res. 2006;59(1):89–95. doi: 10.1203/01.pdr.0000195101.74184.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Namachivayam K, Blanco CL, MohanKumar K, Jagadeeswaran R, Vasquez M, McGill-Vargas L, et al. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G167–180. doi: 10.1152/ajpgi.00141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maheshwari G, Wiley HS, Lauffenburger DA. Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. The Journal of cell biology. 2001;155(7):1123–1128. doi: 10.1083/jcb.200109060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab animal. 2008;37(1):26–32. doi: 10.1038/laban0108-26. [DOI] [PubMed] [Google Scholar]
- 46.Maheshwari A, Corbin LL, Schelonka RL. Neonatal Necrotizing Enterocolitis. Res Rep Neonatol. 2011;1:39–53. [Google Scholar]
- 47.Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F359–363. doi: 10.1136/adc.2004.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 49.Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS One. 2012;7(5):e36977. doi: 10.1371/journal.pone.0036977. [DOI] [PMC free article] [PubMed] [Google Scholar]