Abstract
The cognitive effects of stress are profound, yet it is unknown if the consequences of concurrent multiple stresses on learning and memory differ from those of a single stress of equal intensity and duration. We compared the effects on hippocampus-dependent memory of concurrent, hours-long light, loud noise, jostling and restraint (multimodal stress) with those of restraint or of loud noise alone. We then examined if differences in memory impairment following these two stress types might derive from their differential impact on hippocampal synapses, distinguishing dorsal and ventral hippocampus. Mice exposed to hours-long restraint or loud noise were modestly or minimally impaired in novel object recognition, whereas similar-duration multimodal stress provoked severe deficits. Differences in memory were not explained by differences in plasma corticosterone levels or numbers of Fos-labeled neurons in stress-sensitive hypothalamic neurons. However, although synapses in hippocampal CA3 were impacted by both restraint and multimodal stress, multimodal stress alone reduced synapse numbers severely in dorsal CA1, a region crucial for hippocampus-dependent memory. Ventral CA1 synapses were not significantly affected by either stress modality. Probing the basis of the preferential loss of dorsal synapses after multimodal stress, we found differential patterns of neuronal activation by the two stress types. Cross-correlation matrices, reflecting functional connectivity among activated regions, demonstrated that multimodal stress reduced hippocampal correlations with septum and thalamus and increased correlations with amygdala and BST. Thus, despite similar effects on plasma corticosterone and on hypothalamic stress-sensitive cells, multimodal and restraint stress differ in their activation of brain networks and in their impact on hippocampal synapses. Both of these processes might contribute to amplified memory impairments following short, multimodal stress.
Keywords: brain networks, dendritic spines, hippocampus, salience, stress-complexity, synapses
Introduction
Stress is common and unavoidable, and exposure to stress of various forms can significantly alter brain function and promote cognitive and emotional disorders.1, 2, 3 Effects of stress on cognition are complex and depend on factors including the nature, duration and timing of stress, and the specific cognitive task employed.4, 5, 6 The hippocampus contributes greatly to memory,7,8 and it has been increasingly recognized that processing of sensory signals into memories in rodents involves dorsal hippocampus networks.9,10 In contrast, ventral hippocampus is endowed with distinct afferent/efferent connections9,11 including robust pathways to amygdala,12,13 which may enhance the emotional salience of memories.9,14 Stress and stress hormones influence dorsal and ventral hippocampus differentially: chronic stress attenuates long-term potentiation in dorsal, but not ventral CA1, suggesting that discrete mechanisms exist for the effects of stress mediators on dorsoventral components of the hippocampal formation.15
The effects of stress on memory have received intense investigation, showing that the duration of stress, for example, acute vs chronic, crucially influences its effect on memory function.16, 17, 18, 19, 20, 21 However, the potential importance of the complexity of stress has not been clarified. Regarding chronic stress, habituation occurs to a single stress, and sequential different stresses are required to maintain stress responses.22,23 In the context of hours-long stress, it is unclear if the effects of combined or multimodal stress on learning and memory differ from those of a discrete stress, independent of stress severity. This is important, because modern-life stress often involves multiple concurrent psychological, social and physical stresses. Here, we tested the hypothesis that a relatively short (hours) multimodal stress has more profound impact on memory compared with a unimodal stress of equal duration and severity. We further queried if multimodal and unimodal stresses influenced dorsal and ventral hippocampus differentially. Using novel object recognition which involves hippocampal and parahippocampal structures,24 we found that memory problems of mice exposed to multimodal stress were significantly greater than those of mice exposed to restraint alone, although hormonal and hypothalamic stress responses did not differ following each stress. Analyses of hippocampal dendritic spines suggested that the greater memory impairment might reflect greater loss of synapses in dorsal CA1, a hippocampal region crucially important for long-term memory,25 with relative preservation of ventral synapses. Further, brain structures and networks were differentially activated by the qualitatively different forms of stress, with augmented connectivity of ventral-hippocampus-amygdala circuits after multimodal stress. Together, these data suggest that multimodal stress adversely impacts dorsal versus ventral hippocampal circuits, with distinct consequences for memory function.
Materials and Methods
A complete description is found in the Supplementary Information.
Animals
Three-month-old C57BL/6J male mice were used. Animals were group-housed in a quiet, uncrowded facility on a 12 h light/dark cycle, with ad libitum access to lab chow and water. In addition, to examine if postsynaptic density protein 95 (PSD-95) was a reasonable marker for mature synapses, 3-month-old yellow fluorescent protein-expressing mice (n=3, Jackson Labs, Bar Harbor, ME, USA) were used to visualize the localization of PSD-95-ir puncta on dendritic spines of hippocampal pyramidal cells. Experiments were performed in accordance with the NIH guidelines on laboratory animal welfare and approved by the Institutional Animal Care and Use Committee.
Stress paradigms
Mice were assigned to one of several experimental groups: unimodal (restraint only), multimodal or stress-free control. For restraint stress, mice were placed in a restrainer fashioned from a 50 ml plastic tube in an empty, clean cage. For multimodal stress, mice were restrained and placed five per cage (social stress) on a laboratory shaker and jostled in a brightly lit room bathed in loud rap music. Both restraint-only and multimodal stress conditions lasted for 1, 2 or 5 h. For additional behavioral comparisons, another group of mice (n=5) was exposed to the loud music (noise) alone for 5 h. These mice were transported to the lab, but remained in their home cage under dim lights. Following the stress, mice were returned to their home cages (learning and memory experiments), killed by decapitation for plasma corticosterone levels, or anesthetized for perfusion.
Learning/memory testing
Restraint, noise (n=5), multimodal or control mice (n=8 per group) were tested in a novel object recognition task 90 min after the termination of the stress as described previously.26 A 90-minute delay period was chosen because previous studies demonstrated that it sufficed for stressed mice to recover, enabling them to explore the two objects for the same duration as non-stressed cohorts.26 Objects were counterbalanced across groups, and all objects were cleaned with ethanol between trials. Six hours after training, mice were exposed to one of the previously encountered objects and a novel object and allowed to explore for 5 min. Both training and testing phases were video-recorded and scored blindly without the knowledge of stress condition. Recognition memory of the familiar object was assessed using a discrimination index (DI).27
As measures of anxiety following stress, we analyzed exploration times as well as several locomotion parameters in a large open field apparatus (dimensions: 30 × 23 × 21.5 cm). To consider the potential confounding effects of anxiety on learning, the assessment was performed during the training phase of the novel object recognition test. A computerized video tracking system (Noldus Ethovision) was used to calculate the total distance traveled, as well as the time spent in, and frequency of entries into, the inner ‘anxiogenic' regions of the apparatus.
Quantification of hippocampal synapses
A separate cohort of restraint stress, multimodal stress and control mice (n=5–9 mice per group) were used to assess the effects of each stress on the number of hippocampal synapses. The postsynaptic density 95 protein (PSD-95), which is localized primarily to dendritic spine heads, was used as a reliable marker for mature synapses.28, 29, 30 Mice were perfused immediately after the stress, and brains were postfixed and processed for immunohistochemistry as described in previous studies.31 Briefly, brains were sectioned coronally (for dorsal hippocampus) or horizontally into 20 μm thick slices. Immunohistochemistry was performed on free-floating sections and mouse anti-PSD-95 antibody (1:2000; Affinity BioReagents, Golden, CO, USA) was used. Antibody binding was visualized with anti-mouse IgG conjugated to Alexa Fluor 568 (1:200, Molecular Probes, Eugene, OR, USA). The restraint experiments and the multimodal stress experiments were run separately and each included a control group. Sections from each stress group and its control group were run concurrently in the same conditions, and analyzed without knowledge of treatment group (blindly). Deconvolution analysis was performed on wide-field three-dimensional images as described previously.26,31,32
Radioimmunoassay
Plasma corticosterone levels were measured in a subset of mice at one (n=3 per group) or five hours (n=4 per group) from stress onset, compared with stress-free controls (n=9), using a commercial kit.33 Assay sensitivity was 0.16 μg dl−1, and all samples were run in a single assay.
Neuronal activation
A separate cohort of restraint and multimodal stress mice (n=5 per group) were anesthetized after termination of the 5-hour stress (or under stress-free conditions, n=5) and perfused for Fos analyses. To obviate the possibility that the 5-hour time-point was not representative of patterns of Fos expression during earlier time points, additional cohorts of mice were perfused following 2 h of restraint or multimodal stress. Immunohistochemistry was carried out and sections from all experimental groups were run concurrently.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 and GraphPad Prism 5.0 software. For DI values, anxiety measures, corticosterone, PSD-95-ir, and Fos-ir cells, separate one-way analyses of variance (ANOVAs) were performed, followed by Bonferroni post hoc comparisons. To study the linear relationship of Fos-activation among 12 selected brain regions, we calculated pair-wise Pearson correlation coefficients (r) separately for each stress group, correlating numbers of Fos-ir cells within each region to other regions. To identify significant differences in the strengths of these correlations, Fisher r to z transformations were used to directly compare r-coefficients between restraint and multimodal stress conditions. Mean DIs for each group were compared with chance levels using one-sample t-tests.
Results
Multimodal stress affects learning and memory to a greater degree than a unimodal stress
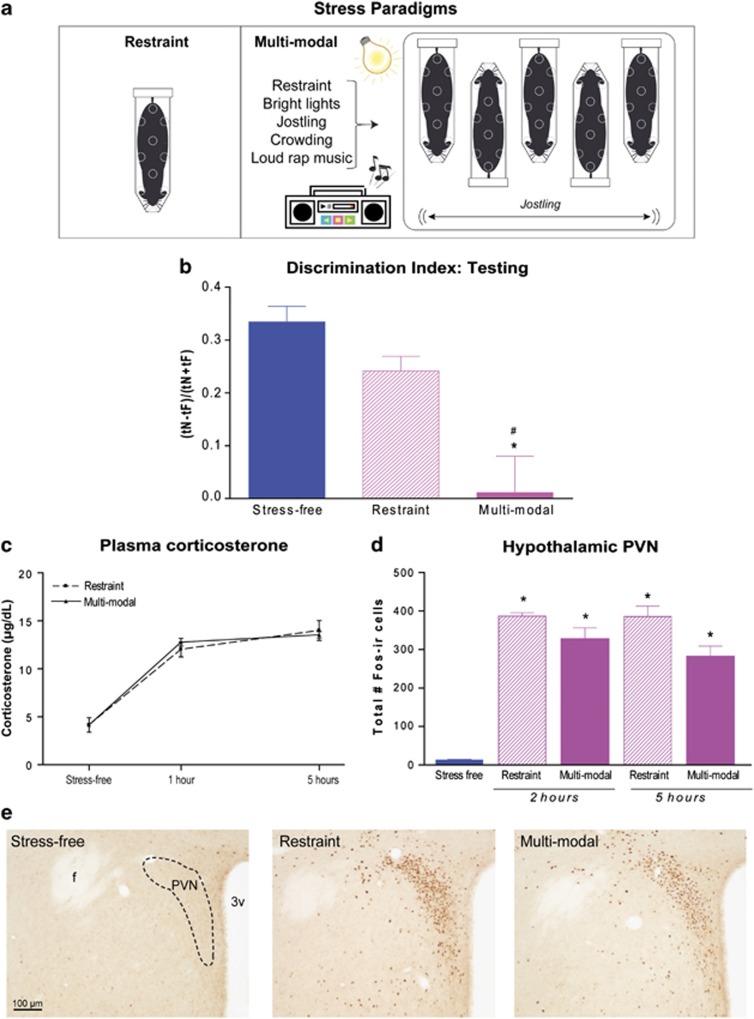
To study if the complexity of stress influences brain function, we developed a model of multimodal stress.26,34 Mice were subjected to concurrent psychological and physical stresses including restraint, jostling, bright light and unpredictable loud noise (rap music) for several hours (Figure 1a).35 The effects of this multimodal stress on memory were compared with those of a typical restraint paradigm or of rap music, of equal duration (five hours). We compared learning and memory of groups of mice subjected to either of these paradigms with memory of unstressed controls using a novel object recognition task.24,26 This task, which involves both hippocampal and cortical circuits,24,36,37 is advantageous because it requires little training, is not stressful in itself, and can be assessed in a relatively short time frame.38 Although restraint stress led to modest impairment in this task (Figure 1b), and the loud noise stress had no appreciable effect (Supplementary Figure 1a), the multimodal stress resulted in significantly worse memory of a familiar object. Specifically, after a recovery period of 90 min, the exploration time of all stress groups was similar to that of unstressed mice (one-way ANOVA, F2,21=0.01, P=0.99; Supplementary Figure 2a) and each group explored two identical objects for equal durations during the training phase (one-sample t-tests, all P>0.05; Supplementary Figure 2b). However, when tested for memory of these objects 6 h later, the discrimination index, reflecting differential object exploration while adjusting for potential differences in total exploration duration, demonstrated overall group differences in object recognition (F2,21=13.04, P<0.001). Post hoc comparisons revealed that multimodal stress reduced DI compared with both control- and restraint-stressed mice (all P<0.01; Figure 1b). Although restraint alone tended to reduce DI, these mice were not significantly worse than controls (P>0.05) and their object recognition index was above chance (t7=8.7, P<0.001; Figure 1b). As mentioned, the group of mice (n=5) exposed to the loud noise (rap music) alone for 5 h performed as well as the non-stressed control group (Supplementary Figure 1). Together, these memory tests in the restraint, loud noise and the multimodal stress groups demonstrate distinct impacts on learning and memory of hours-long exposure to a combined, multimodal stress as compared with more discrete (‘uni-modal') stresses. They suggest that the underlying neural circuits of memory functions may be differentially impacted by a combined vs single stress.
Figure 1.
A multimodal stress impacted memory differently than a more discrete stress. (a) Mice exposed to five hours of restraint stress were compared with those exposed to the same duration of a multimodal stress consisting of restraint, light, loud music and jostling. (b) During the testing phase of the novel object recognition task, the Discrimination index (DI) demonstrated that mice exposed to multimodal stress failed to distinguish the familiar object from a novel one. (c) Hormonal stress responses were comparable following restraint or multimodal stress. The levels and trajectories of plasma corticosterone at several time points after a restraint or a multimodal stress were indistinguishable. This was the case both at 1 h after the onset of stress, and at the 5-h time point (when stresses were terminated). n=3–5 per group per time point. (d) The total numbers of activated (Fos-ir) cells within the hypothalamic paraventricular nucleus were similar following restraint or multimodal stress, either 2 h from the onset of stress or at its termination (n=3–5 per group per time point). (e) Photomicrographs showing typical Fos staining within the PVN are quantified in d. Together, these data indicate that the two stress paradigms stimulate the neuroendocrine stress response system to a similar degree, that is, they do not differ in overall intensity. * signifies compared with stress-free controls, # signifies compared with restraint. 3v, third ventricle; f, fornix; PVN, paraventricular nucleus of the hypothalamus.
Stronger memory effects of multimodal stress are not associated with differential stress or anxiety responses
Differences in memory between the two stress paradigms might result if the multimodal paradigm simply elicited a much stronger physiological or behavioral stress response. To test for a potentially larger hormonal stress response following multimodal stress, plasma levels of the stress hormone corticosterone were measured at several time points following either stress, and the pattern and trajectory of corticosterone levels were compared. Restraint and multimodal stress elicited similar corticosterone levels (Figure 1c), both at one hour after the onset of the stress and at the 5 h time point when stress was terminated (all P>0.05), suggesting that the two stress paradigms stimulated the physiological stress response to a similar degree. Corticosterone release is driven by the activation of corticotropin-releasing hormone-expressing cells within the hypothalamic paraventricular nucleus (PVN) and subsequent release of hypothalamic corticotropin-releasing hormone and pituitary adrenocorticotropic hormone. In line with the comparable plasma corticosterone levels, the numbers of Fos-immunoreactive (ir) cells in PVN, either two hours from the onset of stress39 or at its termination (Figures 1d and e), did not distinguish multimodal from unimodal stress (restraint vs multimodal). These data indicate that the two forms of stress stimulated physiological responses governed by the neuroendocrine hypothalamic–pituitary adrenal stress system to a comparable degree, and suggest that the two stress paradigms did not differ appreciably in their overall intensity or magnitude.
In addition, to examine if the stress paradigms provoked different levels of anxiety during the learning task, we analyzed several independent measures of anxiety. First, exploration durations during the training phase (90 min post-stress termination) did not differ appreciably between the restraint and multimodal stresses (Supplementary Figure 2a). These findings excluded anxiety-induced poor exploration as a source of the memory defects. In addition, we examined locomotion in a large, open-field arena. We assessed distance traveled and the classical anxiety measures of the duration of time spent in the inner region of the apparatus and the frequency of entries into the inner region (Supplementary Figure 3). There were no differences across stress conditions in total distance traveled during the training phase that followed the stresses, as well as the duration of time spent in the inner region of the apparatus or the frequency of entries into the inner region (one-way ANOVAs, main effect of stress: all P>0.05). Although there was a main effect of stress on total exploration time during the testing period (7.5 h after stress termination, F2,21=4.94, P<0.05), there was no difference between the two stress groups (P>05; Supplementary Figure 2c). Thus, hours-long multimodal and restraint stress did not lead to differential levels of anxiety-like behavior when the animals learned the task.
Greater memory impairment is associated with differential loss of hippocampal excitatory synapses following multimodal versus restraint stress
What might be the mechanisms underlying the more severe memory impairment provoked by short multimodal stress? Stress has been shown to influence the number of excitatory synapses on dendritic spines already within hours26,40 in brain regions including the hippocampus.34,41,42 A differential loss of hippocampal synapses would be a plausible mechanism for the effects of multimodal stress on memory.
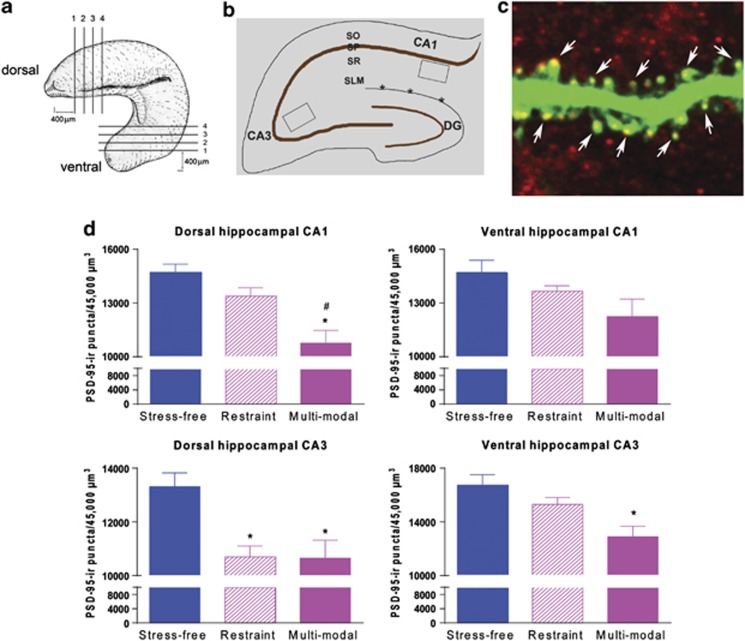
We tested this hypothesis by quantifying PSD-95, a marker for mature synapses, in hippocampal regions harboring the apical dendrites of CA3 and CA1 pyramidal cells.28, 29, 30 We used wide-field imaging followed by 3-dimensional deconvolution tomography, a method enabling analysis of hundreds of thousands of synapses.43,44 We sampled systematically dorsal (septal) and ventral (temporal) hippocampus, and compared the effects of restraint and multimodal stresses (Figures 2a and d). PSD-95 localized to the heads of GFP-expressing dendritic spines of hippocampal pyramidal neurons, (Figure 2c), so that counts of PSD-95 puncta provided a measure of dendritic spines and synapses.31
Figure 2.
A differential loss of synapses in the hippocampus following restraint vs multimodal stress. (a) Diagramatic representation of the analyzed sections: serial sections (20 μm) were collected from the dorsal hippocampus (commencing at 400 μm from the septal pole) and ventral hippocampus at the coronal and horizontal plan, respectively, with intersection intervals of 100 μm. (b) Diagram illustrating the location of regions analyzed for synapse and spine density. Boxed areas in the stratum radiatum of CA1, CA3 were imaged for the deconvolution tomography analysis of PSD-95 (postsynaptic density protein 95)-ir puncta. *, hippocampal fissure. DG, dentate gyrus; SLM, stratum lacunosum-moleculare; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. (c) Illustration of combined methodologies employed to establish that PSD-95 puncta are reasonable markers of dendritic spine heads (arrows). This is shown by confocal microscopy of dendrite from a 3-month-old YFP-expressing mouse, subjected to immunohistochemistry for the synaptic marker. The thickness of the optical section is 0.2 μm. (d) Quantification of the number of PSD-95-ir puncta as measures of excitatory synapses. Restraint stress resulted in a modest reduction of PSD-95 in dorsal CA3, but not in ventral CA3 nor in dorsal or ventral CA1. In contrast, multimodal stress provoked a robust loss of PSD-95-ir puncta in the CA3 area and a selective loss in the dorsal CA1, a hippocampal area implicated as crucial for long-term memory (see McQuown et al.25). Scale bar, C, 1 μm. * signifies compared with stress-free controls, # signifies compared with restraint.
There were significant differences in the effects of restraint and multimodal stresses on overall hippocampal synaptic integrity, which varied along the dorsoventral hippocampal axis. Restraint stress reduced PSD-95-ir puncta only in the dorsal CA3 (P<0.05), sparing the ventral CA3 synapses (P>0.05); restraint stress had no significant effects on either the dorsal or the ventral CA1 synapses (all P>0.05; Figure 2d). In contrast, multimodal stress provoked spine loss throughout CA3 (dorsal: P<0.05; ventral: P<0.01; Figure 2d). Remarkably, within hippocampal area CA1, synapse loss varied dramatically along the dorsoventral axis of the hippocampus; multimodal stress reduced PSD-95 puncta counts drastically, but only in the dorsal CA1 (P<0.001), a region crucial to normal memory function,25 with preserved synapses within the ventral CA1 (P>0.05). Of note, in the dorsal CA1, multimodal stress led to significantly more synapse loss compared with restraint (P<0.05).
Unimodal and multimodal stresses of comparable magnitude lead to distinct neuronal activation patterns
The data above suggest that the perception and processing within the brain of multimodal and restraint stresses of similar magnitudes might differ. Therefore, we employed Fos, a widely used marker, to map neuronal activation throughout the brain after the two types of stress.16,17,19,45 We analyzed Fos expression at the time point when differential learning and memory effects of the two stress paradigms were observed (end of the five-hour stress).
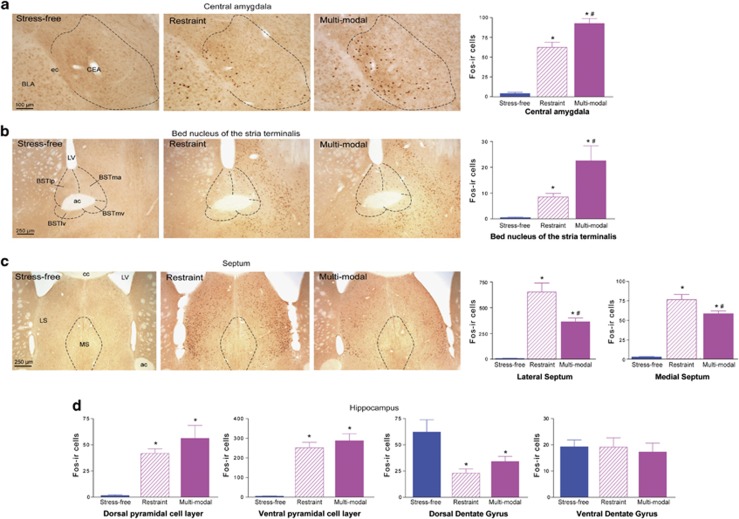
Analysis of Fos expression throughout the brain revealed that most regions were similarly activated by restraint or multimodal stress (Supplementary Table 1), consistent with the comparable overall intensity of the two stresses. However, differential patterns of activation were observed within key brain structures involved in stress processing and memory networks. Specifically, within the central amygdala nucleus (CEA) (F2,12=71.9, P<0.001) and lateral-posterior region of the bed nucleus of the stria terminalis (BST) (F2,12=10.4, P=0.002), there were more Fos-ir cells following multimodal compared to restraint stress (Figures 3a and b; Supplementary Table 1). The CEA is the principal output of the amygdala, a region involved in emotional functions including anxiety and fear,46 and BST is afferent to stress-sensitive hypothalamic neurons.19,47 Interestingly, the opposite pattern was observed in the lateral (F2,12=34.4, P<0.001) and medial (F2,12=74.2, P<0.001) septum, where restraint stress led to higher numbers of Fos-expressing cells than multimodal stress (Figure 3c; Supplementary Table 1). The septum is a primary link from hippocampus to both cortical and subcortical regions48 and may gate sensory input to/from hippocampus, contributing to memory.
Figure 3.
Brain regions preferentially activated by the multimodal vs restraint stress. Within the central amygdala (a) and lateral-posterior region of the bed nucleus of the stria terminalis (b), there were more Fos-ir cells following a 5 h multimodal compared with restraint stress. (c) The lateral and the medial nuclei of the septum contained more Fos-ir cells following the restraint stress (note different scales for the two subdivisions). (d) Hippocampal activation by restraint vs multimodal stress. Within the pyramidal cell layer and dentate gyrus, restraint or multimodal stress led to similar numbers of Fos-ir cells. Of note, the pyramidal cell layers of the ventral hippocampus were activated to a much greater degree than those in the dorsal hippocampus (Restraint: dorsal=41.8±4.3; ventral=251.9±28.1; t4=7.39, P<0.001; multimodal: dorsal=56.2±12.4; ventral=287.4±35.8; t8=6.1, P<0.001). Activation of the dorsal dentate gyrus was suppressed by either stress. * signifies compared with stress-free controls, # signifies compared with restraint. ac, anterior commissure; BLA, basolateral amygdala; BST, bed nucleus of the stria terminalis; BSTlp, lateral-posterior BST; BSTlv, lateral-ventral BST; BSTma, medial-anterior BST; BSTmv, medial-ventral BST; CC, corpus callosum; CEA, central amygdala; ec, external capsule; LS, lateral septum; LV, lateral ventricle; MS, medial septum.
Restraint and multimodal stresses activated similar numbers of cells within the hippocampal pyramidal cell layers (all P>0.05; Figure 3d; Supplementary Table 1), suggesting that the hippocampus itself may not distinguish the complexity of stress. Fos-ir neurons were strikingly more numerous in ventral compared with dorsal hippocampal pyramidal cell layer of stressed mice (Figure 3d; note different y axes). These findings are consistent with the functional distinctions between dorsal and ventral hippocampus,9 and the involvement of the latter in stress-sensitive ‘emotional' networks.49 Interestingly, Fos expression within dentate gyrus, the origin of afferents to the CA3 pyramidal cells appeared to be suppressed by both stresses (F2,12=6.7, P=0.011; Figure 3d).
Measures of functional connectivity of the hippocampus differ after restraint versus multimodal stress
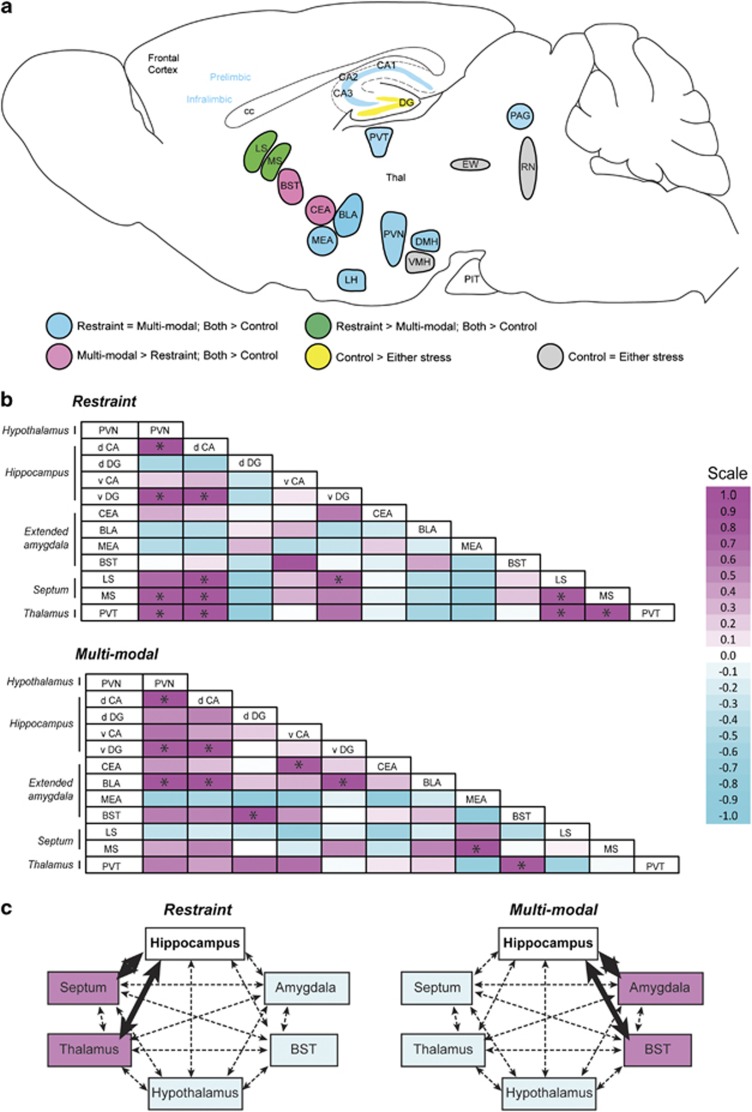
The initial analysis of Fos expression described above established the general patterns of neuronal activation across stress-activated and information-processing circuits (Figure 4a). However, these data did not provide information about interactions among these regions. Indeed, rather than working independently, these structures are organized into functional networks50 that interact and contribute to the perception and processing of sensory input and subsequent emotional, behavioral and neurohormonal responses to stress.51,52 We employed Pearson cross-correlation matrices of regional Fos activation as surrogate parameters for functional connectivity among selected brain regions (Table 1).53 Pair-wise correlation values between two regions (ranging from −1 to 1) provided an index of the strength and nature of interactions among them in response to either multimodal or restraint stresses.
Figure 4.
Patterns of network activation by restraint or multimodal stress. (a) Analysis of Fos expression throughout the brain revealed distinct patterns of neural activation, summarized in the schematic. Although most regions were equally activated by restraint or multimodal stress, differential patterns of activation were observed within central amygdala, BST and septum, suggesting that these regions may discriminate the relative complexity of stress. (b) Measures of functional network connectivity differ following restraint or multimodal stress. Cross-correlation matrices of regional Fos activation levels were generated for each stress condition (Restraint, top; multimodal, bottom). Pearson r-values are color coded to reflect the direction and strength of each correlation, and significant correlations are indicated with an asterisk. These analyses revealed that both stress paradigms resulted in significant pair-wise correlations in activity and that the patterns of these correlations were influenced by stress complexity. (c) Focusing on the hippocampus, restraint stress led to strong correlations with the septum and thalamic paraventricular nucleus (left), whereas the multimodal stress shifted functional connectivity measures of the hippocampus away from the septum/thalamus, and increased correlations between hippocampus and amygdala and BST (right). Bold lines in (b) indicate significant correlations. BLA, basolateral amygdala; BST, bed nucleus of the stria terminalis; CA, pyramidal cell layer of hippocampus; CEA, central amygdala; d CA, dorsal CA; DG, dentate gyrus of hippocampus; d DG, dorsal DG; DMH, dorsomedial hypothalamus; EW, Edinger–Westphal nucleus; LH, lateral hypothalamus; LS, lateral septum; MEA, medial amygdala; MS, medial septum; PAG, periaqueductal gray; PIT, pituitary gland; PVN, paraventricular nucleus of hypothalamus; PVT, paraventricular thalamic nucleus; RN, raphe nucleus; Thal, thalamus; v CA, ventral CA; v DG, ventral DG; VMH, ventromedial hypothalamus.
Table 1. Pearson correlation matrices for activation levels following restraint or multimodal stress.
| Correlations with hypothalamic periventricular nucleus | |||||||||||||
| d CA1 | d CA3 | d DG | v CA1 | v CA3 | v DG | CEA | BLA | MEA | BST | LS | MS | PVT | |
| Restraint | r=0.271 P=0.659 | r=0.960* P=0.010 | r=−0.264 P=0.668 | r=0.600 P=0.284 | r=0.583 P=0.303 | r=0.946* P=0.015 | r=0.674 P=0.212 | r=−0.168 P=0.787 | r=−0.156 P=0.802 | r=0.587 P=0.299 | r=0.848 P=0.070 | r=0.897* P=0.039 | r=0.895* P=0.040 |
| Multimodal | r=0.993* P=0.001 | r=0.937* P=0.019 | r=0.750 P=0.144 | r=0.796 P=0.107 | r=0.923* P=0.025 | r=0.899* P=0.038 | r=0.722 P=0.168 | r=0.960* P=0.010 | r=−0.471 P=0.529 | r=0.856 P=0.064 | r=-0.047 P=0.940 | r=0.689 P=0.198 | r=0.789 P=0.113 |
| Correlations with dorsal CA1 | |||||||||||||
| d CA3 | d DG | v CA1 | v CA3 | v DG | CEA | BLA | MEA | BST | LS | MS | PVT | ||
| Restraint | r=0.133 P=0.832 | r=−0.031 P=0.960 | r=0.015 P=0.981 | r=0.062 P=0.921 | r=0.561 P=0.326 | r=0.541 P=0.347 | r=−0.253 P=0.682 | r=0.345 P=0.569 | r=−0.296 P=0.629 | r=0.490 P=0.402 | r=0.073 P=0.908 | r=0.259 P=0.674 | |
| Multimodal | r=0.952* P=0.013 | r=0.756 P=0.139 | r=0.723 P=0.167 | r=0.878 P=0.050 | r=0.912* P=0.031 | r=0.636 P=0.248 | r=0.970* P=0.006 | r=−0.258 P=0.742 | r=0.872 P=0.054 | r=0.054 P=0.931 | r=0.771 P=0.127 | r=0.768 P=0.129 | |
| Correlations with dorsal CA3 | |||||||||||||
| d DG | v CA1 | v CA3 | v DG | CEA | BLA | MEA | BST | LS | MS | PVT | |||
| Restraint | r=−0.418 P=0.484 | r=0.703 P=0.185 | r=0.644 P=0.241 | r=0.860 P=0.061 | r=0.484 P=0.408 | r=−0.074 P=0.905 | r=−0.392 P=0.514 | r=0.787 P=0.114 | r=0.891* P=0.043 | r=0.982* P=0.003 | r=0.943* P=0.016 | ||
| Multimodal | r=0.767 P=0.130 | r=0.584 P=0.301 | r=0.877 P=0.051 | r=0.935* P=0.020 | r=0.620 P=0.264 | r=0.965* P=0.008 | r=−0.189 P=0.811 | r=0.836 P=0.078 | r=0.261 P=0.672 | r=0.748 P=0.146 | r=0.607 P=0.278 | ||
| Correlations with dorsal dentate gyrus | |||||||||||||
| v CA1 | v CA3 | v DG | CEA | BLA | MEA | BST | LS | MS | PVT | ||||
| Restraint | r=0.034 P=0.957 | r=0.200 P=0.747 | r=−0.170 P=0.784 | r=0.451 P=0.446 | r=0.558 P=0.328 | r=0.892* P=0.042 | r=−0.521 P=0.368 | r=−0.531 P=0.357 | r=−0.567 P=0.319 | r=−0.659 P=0.227 | |||
| Multimodal | r=0.591 P=0.294 | r=0.721 P=0.169 | r=0.503 P=0.388 | r=0.483 P=0.410 | r=0.626 P=0.259 | r=−0.764 P=0.236 | r=0.967* P=0.007 | r=0.010 P=0.987 | r=0.510 P=0.380 | r=0.826 P=0.085 | |||
| Correlations with ventral CA1 | |||||||||||||
| v CA3 | v DG | CEA | BLA | MEA | BST | LS | MS | PVT | |||||
| Restraint | r=0.984* P=0.002 | r=0.556 P=0.331 | r=0.444 P=0.454 | r=0.646 P=0.239 | r=−0.169 P=0.786 | r=0.768 P=0.129 | r=0.637 P=0.248 | r=0.669 P=0.217 | r=0.500 P=0.391 | ||||
| Multimodal | r=0.864 P=0.059 | r=0.540 P=0.348 | r=0.892* P=0.042 | r=0.634 P=0.251 | r=−0.755 P=0.245 | r=0.637 P=0.247 | r=−0.637 P=0.247 | r=−0.631 P=0.254 | r=0.835 P=0.079 | ||||
| Correlations with ventral CA3 | |||||||||||||
| v DG | CEA | BLA | MEA | BST | LS | MS | PVT | ||||||
| Restraint | r=0.566 P=0.320 | r=0.559 P=0.327 | r=0.694 P=0.194 | r=0.010 P=0.988 | r=0.650 P=0.235 | r=0.569 P=0.316 | r=0.569 P=0.316 | r=0.409 P=0.494 | |||||
| Multimodal | r=0.815 P=0.093 | r=0.916* P=0.029 | r=0.965 P=0.058 | r=−0.753 P=0.247 | r=0.750 P=0.144 | r=−0.192 P=0.757 | r=0.392 P=0.514 | r=0.703 P=0.186 | |||||
| Correlations with ventral dentate gyrus | |||||||||||||
| CEA | BLA | MEA | BST | LS | MS | PVT | |||||||
| Restraint | r=0.797 P=0.106 | r=−0.166 P=0.789 | r=0.032 P=0.960 | r=0.397 P=0.508 | r=0.874 P=0.053 | r=0.776 P=0.123 | r=0.824 P=0.086 | ||||||
| Multimodal | r=0.603 P=0.282 | r=0.984* P=0.002 | r=0.295 P=0.705 | r=0.633 P=0.252 | r=0.264 P=0.667 | r=0.750 P=0.145 | r=0.446 P=0.452 | ||||||
| Correlations with central amygdala | |||||||||||||
| BLA | MEA | BST | LS | MS | PVT | ||||||||
| Restraint | r=0.116 P=0.853 | r=0.607 P=0.277 | r=−0.034 P=0.956 | r=0.447 P=0.450 | r=0.315 P=0.606 | r=0.327 P=0.591 | |||||||
| Multimodal | r=0.638 P=0.247 | r=−0.749 P=0.251 | r=0.475 P=0.419 | r=−0.469 P=0.425 | r=0.015 P=0.981 | r=0.558 P=0.329 | |||||||
| Correlations with basolateral amygdala | |||||||||||||
| MEA | BST | LS | MS | PVT | |||||||||
| Restraint | r=0.208 P=0.737 | r=0.246 P=0.690 | r=−0.130 P=0.835 | r=−0.114 P=0.856 | r=−0.334 P=0.583 | ||||||||
| Multimodal | r=0.131 P=0.869 | r=0.750 P=0.144 | r=0.177 P=0.775 | r=0.769 P=0.128 | r=0.594 P=0.291 | ||||||||
| Correlations with medial amygdala | |||||||||||||
| BST | LS | MS | PVT | ||||||||||
| Restraint | r=−0.732 P=0.160 | r=−0.389 P=0.518 | r=−0.559 P=0.328 | r=−0.532 P=0.357 | |||||||||
| Multimodal | r=−0.679 P=0.321 | r=0.739 P=0.261 | r=0.961 P=0.039* | r=−0.655 P=0.345 | |||||||||
| Correlations with bed nucleus of the stria terminalis | |||||||||||||
| LS | MS | PVT | |||||||||||
| Restraint | r=0.661 P=0.224 | r=0.855 P=0.065 | r=0.715 P=0.174 | ||||||||||
| Multimodal | r=0.030 P=0.962 | r=0.675 P=0.211 | r=0.880 P=0.049* | ||||||||||
| Correlations with lateral septum | |||||||||||||
| MS | PVT | ||||||||||||
| Restraint | r=0.895* P=0.040 | r=0.932* P=0.021 | |||||||||||
| Multimodal | r=0.535 P=0.353 | r=−0.420 P=0.482 | |||||||||||
| Correlations with medial septum | |||||||||||||
| PVT | |||||||||||||
| Restraint | r=0.964* P=0.008 | ||||||||||||
| Multimodal | r=0.438 P=0.461 | ||||||||||||
Abbreviations: BLA, basolateral amygdala; BST, bed nucleus of the stria terminalis; CA, pyramidal cell layer of hippocampus; CEA, central amygdala; d CA, dorsal CA; DG, dentate gyrus of hippocampus; d DG, dorsal DG; DMH, dorsomedial hypothalamus; EW, Edinger–Westphal nucleus; LS, lateral septum; MEA, medial amygdala; MS, medial septum; PVT, paraventricular thalamic nucleus; v CA, ventral CA; v DG, ventral DG.
Pearson correlation values (r) and corresponding P-valuesfor each of the correlations assessed. Total numbers of Fos-immunoreactive (Fos-ir) cells within each brain area were correlated with the numbers of Fos-ir cells in each of the other areas of the brain. The resulting correlation values provide a proxy measure of the nature and strength of interactions between brain regions during stress processing (that is, measures of functional connectivity). Significant correlations are indicated with an asterisk, and significant differences in the r-values between stress conditions are bolded (Fisher's r to z transformations).
Both multimodal and restraint stress resulted in several significant pair-wise correlations of activation throughout brain structures as would be expected in a functional network (Figure 4b; Table 1). However, the patterns of these correlations, and in particular the significant correlations of the hippocampus, were influenced by the complexity of the stress. Following restraint stress, hippocampal activation was strongly correlated to activation within septum and the paraventricular region of the thalamus (PVT) (Figure 4b, top; 4c, left). Following multimodal stress of comparable duration and magnitude, the pattern of hippocampal connectivity shifted, reducing correlations with septum and PVT, and increasing correlation among hippocampus, amygdala and BST (Figure 4b, bottom; 4c, right). This shift in measures of functional connectivity was supported by directly comparing Pearson coefficients between the stress conditions (Table 1). These results suggest that hippocampal network-connectivity patterns distinguish between the two forms of stress.
Discussion
The principal findings of this study are (a) hours-long, multimodal stress provokes severe memory problems compared with restraint or to loud, unpredictable noise stress of comparable duration and hypothalamic–pituitary adrenal stress system axis stimulation; (b) the memory defects may be partially explained by the greater loss of synapses in hippocampus, and particularly in dorsal hippocampal CA1, following multimodal stress; (c) Fos mapping demonstrates that the brain distinguishes a stress that combines multiple components from a more discrete restraint stress. Together, these data indicate that the immediate effects of short stress on cognitive function are influenced by the complexity of the stress: combined psychological, social and physical stress lasting merely hours may lead to unexpectedly severe memory defects, with important implications to the function of individuals experiencing emotional trauma together with physical or social duress. The findings are also important to our understanding of stress-related disorders, such as posttraumatic stress disorder, where memory processes are deranged.
More severe memory problems follow hours-long multimodal vs restraint stress
Because of the prevalence of stress, its effects on cognitive function including memory have been extensively studied.54, 55, 56, 57 A major body of work has demonstrated the importance of stress duration. In general, acute stress, lasting seconds to minutes, enhances learning and memory.58, 59, 60, 61, 62, 63, 64, 65 By contrast, chronic stress, lasting weeks, generally impairs these processes.3,5,66, 67, 68, 69, 70, 71 In addition, distinct effects of specific modalities of stress (physical or psychological) have been well studied.19,20,35,72,73
Given that many stressful experiences are not unitary or discrete, this study sought to understand the effects of multiple concurrent stresses, and to determine whether the effects on the brain are distinguishable from those following single stress of similar intensity. We chose a duration of hours, because many stressful episodes may last hours rather than seconds or weeks, and are thus neither acute (minutes) nor chronic (days/weeks). Surprisingly, we found highly divergent effects of the multimodal compared with less complex stresses such as restraint or loud unpredictable noise on memory: these had only modest effects on object memory, whereas an equal duration of the multimodal stress abrogated object recognition.
Differential loss of hippocampal apical dendritic synapses may underlie impairment of memory after multimodal versus unimodal stress
Because general measures of the severity of the two stresses, including a time-course of plasma corticosterone and activation of stress-integrating centers in the PVN,73 did not differ appreciably (Figure 1), we examined alternative sources for the profound differential effects of these stresses on memory. We found major distinctions between multimodal and unimodal stress on excitatory synapses in hippocampal areas CA3 and CA1. Notably, the overall degree of synapse loss was far greater after the multimodal stress. There were also important variations between CA regions, as well as the dorsal-ventral plane of the hippocampus. Indeed, synaptic loss following restraint stress was mild, consistent with modest memory defects observed after this stress (Figure 1) and was confined to dorsal CA3 neurons, which are known to have high sensitivity to stress.2,23,74 In response to the combined stress, synaptic loss spread throughout the CA3 (dorsal and ventral) and into the dorsal CA1, leaving ventral CA1 intact.
These data seem to suggest a progressive loss of synapses from CA3 to CA1, corresponding with increased stress complexity. Interestingly, these data are in line with the greater role of memory functions subserved by dorsal hippocampus, and in particular by CA1 region25,75 and suggest that synapses within these circuits are specifically targeted in mice exposed to multimodal stress. This differential loss of dorsal CA1 synapses may lead to disrupted function of the memory networks associated with dorsal hippocampus.9,15
The current findings are congruent with previous studies showing that stress can influence the number of synapses already within hours40,55 in brain regions including the hippocampus.26,34,42 Neurotransmitters, including norepinephrine,76, 77, 78 serotonin79, 80, 81 and glutamate,82,83 as well as stress-induced release of neuropeptides26,34,84,85 and steroids,86, 87, 88 can influence synapse number and function. Building on the existing body of information, the current findings suggest that rapid, stress-induced changes in the structure and function of synapses may provide a mechanism for fine-tuning of specific neuronal networks in response to ever-changing environmental conditions.2 Indeed, selective loss of synapses in networks involved in memory may serve an adaptive role, attenuating memories of severe, adverse experiences.
The differential impairment of memory after multimodal vs unimodal stress is accompanied by a differential pattern of regional neuronal activation
Clues into the distinct brain responses that may explain the higher impact on memory of multimodal compared with restraint stress emerge from the activation patterns of specific brain regions and the measures of their interconnectivity. Expression of the immediate early-gene c-fos provides a useful tool to examine activation of neurons and identify brain regions recruited by stress.45,89,90 Indeed, various models of stress have been shown to activate neurons throughout the brain, including hypothalamus, amygdala, hippocampus and cortex.72,90, 91, 92, 93, 94, 95 Much of this work has categorized different forms of stress (physical, psychological, social) and has identified distinct and partially overlapping circuits recruited by different stress modalities.16,18, 19, 20,35,96,97
Here, Fos mapping revealed distinctive activation of specific neuronal populations and networks by a multimodal compared with a more discrete stress. Within the amygdala, we found higher levels of Fos expression in the CEA after the multimodal vs restraint stress. The CEA is rich in stress hormones and their receptors,98, 99, 100, 101 and contributes to processing of stress signals.102, 103, 104 Functionally, CEA has been implicated in learning and expression of fear responses,105, 106, 107 as well as the expression of stress-induced anxiety behaviors.108, 109, 110, 111, 112, 113 As the major outflow of the amygdala complex,46 higher activation of CEA might indicate a convergence of the several stressful components inherent in the multimodal stress and a potential for overwhelming of emotional processing/stress-coping pathways.
In the hippocampal formation, a key region involved in memory,7,32,37 we found specific changes of Fos expression in both afferent structures and outflow targets. First, stress of both types reduced activity in the dentate gyrus, considered a gateway to the hippocampus proper,11 suggesting that sensory signals to the hippocampus may be gated or filtered during either stress. In addition, both types of stress activated ventral pyramidal cells to a much larger degree than dorsal hippocampus counterparts. It is interesting to speculate about the function of hippocampal neuron activation by stress: like every experience, stressful experiences are accompanied by learning. It is conceivable that the type of learning evoked by the restraint vs multimodal stress might differ, as a larger number of hippocampal pyramidal cell-layer neurons were activated by the latter.
Here, we employed Fos mapping, a powerful tool for delineating stress-induced neural activation. There are known limitations to this technique.89,90,114 Most notably the fact that not all cell types express the gene product, and thus the absence of Fos does not necessarily mean a cell was not activated or involved in stress processing. Therefore, there has been a need to validate this method, and other immediate early gene products have been used to map stress responses; in many, but not all, of these studies there is considerable overlap in activated areas.90,114 In the context of hours-long stress, we have previously examined pCREB activation115 and found that although the distribution of activated cells was similar to that labeled by Fos, pCREB activation followed a rapid and transient time-course which is not optimal for the hours-long stress models employed here. More recently, the activity-regulated cytoskeletal-associated protein (Arc) has emerged as an alternative tool to examine activity-dependent transcription, with particular relevance for mechanisms of plasticity. However, Arc has a much more restricted pattern of expression than Fos in response to stress: Arc appears to be expressed primarily within telencephalic areas116 and is therefore problematic for mapping global network patterns of activation, including hypothalamic and midbrain structures. Arc might be an interesting future target of studies in the context of hippocampal plasticity following multimodal stress.
Multimodal and unimodal stress influence dorsal and ventral hippocampal connectivity differentially
Distinct functions and connectivity of ventral versus dorsal hippocampus are being increasingly recognized.9 Dorsal hippocampus in the rodent, and primarily CA1, has emerged as the principal location of memory encoding and processing, whereas ventral hippocampus contributes to a network involved in salience and emotionality.9,10,25,117 These ideas are in line with the differing connectivity of hippocampal subregions: dorsal hippocampus primarily projects to cortical structures, whereas outflow from ventral hippocampus targets subcortical regions including amygdala and hypothalamus.9,11,13 In addition, stress and glucocorticoids affect synaptic plasticity differentially along the hippocampal dorsoventral axis, impairing long-term potentiation in dorsal hippocampus, but enhancing this process in ventral hippocampus.15,49,118 The current results further support the distinct roles of dorsal and ventral hippocampus and indicate that multimodal stress may influence them selectively in a manner unique from that of restraint stress.
Specifically, restraint stress enhanced functional connectivity of hippocampus to septum and to the PVT. The dorsal hippocampus-septal network contributes to cognitive functions,119,120 and PVT is considered a ‘stress memory' center.22 Thus, the restraint effects may reflect processes of memory designed to aid in coping with future stress. In contrast, multimodal stress reduced correlation of hippocampus and septum and increased significantly correlations with amygdala and BST. Amygdala-hippocampal networks, mediated primarily via ventral hippocampal pathways, have been strongly implicated in emotional responses to stress, including pathological anxiety and stress-related disorders.104,121 Hence, these changes may reflect reduced ability for objective memory and increased emotional salience of the stress.103 Previous animal studies have shown that stress can alter relative activation patterns within the limbic system,122 as well as modulate the nature of interactions among brain regions.123,124 Similar shifts in functional connectivity have also been observed in humans in response to emotionally salient stimuli.77,125
Overall, these data indicate that increased stress complexity causes a shift in network connectivity from cognitive processing of basic sensory input toward emotional and stress-coping circuits. Functional connectivity results from activity of synapses, and is hence modulated by the number of synapses and the robustness of their function.126,127 The current studies find both an apparent connectivity shift towards ventral hippocampus and a preferential loss of synapses in dorsal-hippocampus pyramidal cells, a fact that will lessen dorsal-hippocampus septum connections while maintaining ventral-hippocampus amygdala connectivity (Supplementary Figure 4). Although the precise causal relationships of these two phenomena are not fully clear at this point, both of these processes might contribute to the augmented memory impairment following short, multimodal stress.
Acknowledgments
Supported by National Institutes of Health Grants NS28912, MH73136 and P50 MH096889 (to TZB), T32-GM08620 (to SGJ, A. Goldin, PI), and a grant from the George E Hewitt Foundation for Medical Research (to PMM). We thank Mrs Barbara Cartwright for editorial assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Liberzon I, Morilak D, Ressler K. Stress modulation of cognitive and affective processes. Stress. 2011;14:503–519. doi: 10.3109/10253890.2011.596864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2012;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures. Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. The Hippocampus Book. Oxford University Press: New York, NY, US; 2007. [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Lucas M, Richter-Levin G. Emotional tagging—a simple hypothesis in a complex reality. Prog Neurobiol. 2011;94:64–76. doi: 10.1016/j.pneurobio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Steroid modulation of hippocampal plasticity: switching between cognitive and emotional memories. Front Cell Neurosci. 2012;6:12. doi: 10.3389/fncel.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–182. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM, et al. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections. Neuroscience. 2012;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, et al. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese RR, Chen LY, Cox CD, Schulz D, Babayan AH, Bunney WE, et al. Synaptic abnormalities in the infralimbic cortex of a model of congenital depression. J Neurosci. 2013;33:13441–13448. doi: 10.1523/JNEUROSCI.2434-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald N. Inhibitory function of the dorsomedial hypothalamic nucleus on the hypothalamic-pituitary-adrenal axis response to an emotional stressor but not immune challenge. J Neuroendocrinol. 2013;25:48–55. doi: 10.1111/j.1365-2826.2012.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Segal M, Richter-Levin G, Maggio N. Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus. 2010;20:1332–1338. doi: 10.1002/hipo.20751. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, et al. Stress Impact on Resting State Brain Networks. PLoS One. 2013;8:e66500. doi: 10.1371/journal.pone.0066500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Linear and non-linear dose-response functions reveal a hormetic relationship between stress and learning. Dose Response. 2009;7:132–148. doi: 10.2203/dose-response.08-015.Zoladz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Lucassen PJ, Karst H, Joels M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:24. doi: 10.3389/fnsyn.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci. 2002;22:6282–6289. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Haettig J, Sun Y, Wood MA, Xu X. Cell-type specific inactivation of hippocampal CA1 disrupts location-dependent object recognition in the mouse. Learn Mem. 2013;20:139–146. doi: 10.1101/lm.027847.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Alves SE, Hoskin E, Lee SJ, Brake WG, Ferguson D, Luine V, et al. Serotonin mediates CA1 spine density but is not crucial for ovarian steroid regulation of synaptic plasticity in the adult rat dorsal hippocampus. Synapse. 2002;45:143–151. doi: 10.1002/syn.10093. [DOI] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Luthi A, Savic N, Stierli B, Streit P, Gahwiler BH, et al. Synaptic modifications at the CA3-CA1 synapse after chronic AMPA receptor blockade in rat hippocampal slices. J Physiol. 2007;581:129–138. doi: 10.1113/jphysiol.2006.120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, De Simoni A, Velzing EH, Bracey E, Joels M, Edwards FA, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki Y, Hatanaka Y, Murakami G, Mukai H, Hojo Y, Saito M, et al. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS One. 2012;7:e34124. doi: 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all 'fos-ed out'. J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Arnold FJ, De Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KA, Ginsberg AB, Maksimova E, Wieseler Frank JL, Johnson JD, Spencer RL, et al. Stress-induced sensitization of the hypothalamic-pituitary adrenal axis is associated with alterations of hypothalamic and pituitary gene expression. Neuroendocrinology. 2004;80:252–263. doi: 10.1159/000082876. [DOI] [PubMed] [Google Scholar]
- Schreiber SS, Tocco G, Shors TJ, Thompson RF. Activation of immediate early genes after acute stress. Neuroreport. 1991;2:17–20. doi: 10.1097/00001756-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci USA. 1989;86:9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol. 2008;583:204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR. Localization of mineralocorticoid receptors at mammalian synapses. PLoS One. 2010;5:e14344. doi: 10.1371/journal.pone.0014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain—and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol. 2011;336:2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- Tsoory MM, Vouimba RM, Akirav I, Kavushansky A, Avital A, Richter-Levin G. Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD. Prog Brain Res. 2008;167:35–51. doi: 10.1016/S0079-6123(07)67003-4. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R, Shallow T. Rodent anxiety and kindling of the central amygdala and nucleus basalis. Physiol Behav. 2000;70:177–187. doi: 10.1016/s0031-9384(00)00250-x. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, et al. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J Neurochem. 2004;89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus. 2007;17:10–25. doi: 10.1002/hipo.20237. [DOI] [PubMed] [Google Scholar]
- Colom LV, Garrido-Sanabria E. Modulation of normal and altered hippocampal excitability states by septal networks. J Neurosci Res. 2007;85:2839–2843. doi: 10.1002/jnr.21276. [DOI] [PubMed] [Google Scholar]
- Niewiadomska G, Baksalerska-Pazera M, Riedel G. The septo-hippocampal system, learning and recovery of function. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:791–805. doi: 10.1016/j.pnpbp.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Kogan I, Richter-Levin G. Activation pattern of the limbic system following spatial learning under stress. Eur J Neurosci. 2008;27:715–722. doi: 10.1111/j.1460-9568.2008.06034.x. [DOI] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.