Abstract
Atlantic killifish (Fundulus heteroclitus) inhabiting the Atlantic Wood Superfund site on the Elizabeth River (Portsmouth, VA, USA) are exposed to a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from former creosote operations, but are resistant to the acute toxicity and cardiac teratogenesis caused by PAHs. The resistance is associated with a dramatic recalcitrance to induction of cytochrome P450 (CYP1) metabolism enzymes following exposure to aryl hydrocarbon receptor (AHR) agonists, along with an elevated antioxidant response and increased expression of several other xenobiotic metabolism and excretion enzymes. However, the heritability of the resistance in the absence of chemical stressors has been inconsistently demonstrated. Understanding the heritability of this resistance will help clarify the nature of population-level responses to chronic exposure to PAH mixtures and aid in identifying the important mechanistic components of resistance to aryl hydrocarbons. We compared the response of Atlantic Wood F1 and F2 embryos to benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), 3,3’,4,4’,5-pentachlorobiphenyl (PCB-126), and a mixture of BkF and fluoranthene (Fl) to that of F1 embryos of reference site killifish. Resistance to cardiac teratogenesis and induction of CYP mRNA expression and CYP activity was determined. We found that both Atlantic Wood F1 and F2 embryos were highly resistance to cardiac teratogenesis. However, the resistance by Atlantic Wood F2 embryos to induction of CYP mRNA expression and enzyme activity was intermediate between that of Atlantic Wood F1 embryos and reference embryos. These results suggest that resistance to cardiac teratogenesis in Atlantic Wood fish is conferred by multiple factors, not all of which appear to be fully genetically heritable.
Keywords: Fundulus heteroclitus, aryl hydrocarbon pathway, polycyclic aromatic hydrocarbons, pollution adaptation, cardiac teratogenesis
1. Introduction
As with any stressor, chronic pollutant stress has the potential to drive an adaptive response in exposed populations. Multiple populations of fish living in polluted estuaries in eastern North America are resistant to the toxic effects of the contaminants they face, and several have likely developed heritable adaptations (reviewed by Wirgin & Waldman 2004). In particular, several populations of fish are resistant to the toxic effects of various aryl hydrocarbons, including polychlorinated biphenyls (PCBs), dioxins, and polycyclic aromatic hydrocarbons (PAHs) (Bello et al. 2001, Meyer & Di Giulio 2002, Nacci et al. 1999, Ownby et al. 2002, Prince & Cooper 1995). One adapted population of Fundulus heteroclitus (the Atlantic killifish or mummichog, hereafter referred to as killifish) is found in an inlet adjacent to the Atlantic Wood Industries Superfund site (Elizabeth River, Virginia, USA) in the southern portion of the Chesapeake Bay watershed. Former wood-treatment operations contaminated the site and sediments with creosote, a complex mixture consisting primarily of unsubstituted polycyclic aromatic hydrocarbons (PAHs), as well as heterocyclic and phenolic PAHs (Mulvey et al. 2002, Walker et al. 2004).
Classically, PAHs are known as carcinogenic, immunosuppressive, and as non-specific narcotic toxicants (Samanta et al. 2002). In addition to these well-established toxicities, recent work has shown that some PAHs cause early life stage toxicity and teratogenesis in fish. Many PAHs are aryl hydrocarbon receptor (AHR) agonists, but others are antagonistic or do not have great affinity for the receptor (Billiard et al. 2004, Billiard et al. 2002, Denison & Nagy 2003). Various PAHs cause developmental toxicity in both an AHR-independent (Incardona et al. 2006, Incardona et al. 2005, Incardona et al. 2004) and AHR-dependent manner (Billiard et al. 2006, Clark et al. 2010, Incardona et al. 2006). In many cases, PAH teratogenesis manifests as cranio-facial and cardiac malformations, reminiscent of the “blue-sac syndrome” observed with the related and highly studied planar halogenated aromatic hydrocarbons (pHAHs; e.g. 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD)) (Hahn 2002, Prasch et al. 2003a, Toomey et al. 2001). While initially it was noted that killifish inhabiting the Atlantic Wood Superfund site had high rates of liver lesions (Vogelbein et al. 1990), it became apparent that the population had developed remarkable resistance to the acute effects and teratogenesis caused by aryl hydrocarbons and Elizabeth River sediments (Meyer & Di Giulio 2002, Meyer et al. 2002, Ownby et al. 2002, Van Veld & Westbrook 1995).
Perhaps the most dramatic biochemical or molecular difference between Atlantic Wood killifish and naïve fish is recalcitrance to induction of cytochrome P450 (CYP) metabolic enzymes by AHR agonists (Meyer & Di Giulio 2002, Meyer et al. 2002, Van Veld & Westbrook 1995). Lack of CYP induction by AHR agonists is generally considered to be a marker of down-regulation of the AHR pathway. In Atlantic Wood killifish and other fish populations exposed over multiple generations to aryl hydrocarbon pollution, recalcitrance to CYP induction is correlated with marked resistance to the toxic effects of the contaminants (Bello et al. 2001, Meyer et al. 2002, Nacci et al. 2002, Powell et al. 2000, Prince & Cooper 1995, Roy et al. 2002).
However, previous investigations into the heritability of the various aspects of the resistance have yielded conflicting results. Ownby et al. (2002) showed that both F1 embryos and F2 embryos from laboratory-reared F1 adults were resistant to teratogenesis due to Elizabeth River sediments. Likewise, Nacci et al. (2010) found heritable resistance to induction of CYP activity and early life stage toxicity caused by PCB-126 (3,3’,4,4’,5-pentachlorobiphenyl) in F1 and F2 Atlantic Woodkillifish embryos. In contrast, investigation by Meyer and co-workers found more complicated patterns of heritability. They found that toxicity resistance was less marked in the F2 generation, although still evident (Meyer & Di Giulio 2003). In addition, they found that the recalcitrance to CYP induction faded somewhat in later generations (Meyer & Di Giulio 2002, Meyer & Di Giulio 2003, Meyer et al. 2002). It is notable that some data obtained by Meyer and colleagues support a conclusion of genetic heritability, but other data do not. In general, the strongest evidence for full genetic heritability was obtained for resistance to teratogenesis in embryos, while studies of heritable resistance in larvae and adults yielded mixed results. Refractory CYP response and resistance to toxicity tended to fade with age, perhaps indicating that components of the adaptation are developmental stage specific.
The patterns of heritability of various adaptive traits in Atlantic Wood killifish and their lab-reared offspring are summarized in Table 1. To date, it is unclear in some cases what the role of each alteration is in tolerance to contamination, which are the result of acclimation, and which are genetically heritable adaptations. Understanding the heritability of the resistance and its underlying components can help us better understand the nature of population-level responses to chronic contaminant exposure. Furthermore, elucidating if the adaptation is genetically heritable will aid in identifying the important mechanistic components of resistance to aryl hydrocarbons in Atlantic Wood killifish.
Table 1.
Pattern of heritability of various adaptive parameters in Atlantic Wood killifish compared to reference fish
| Stage | Generation | Response of Atlantic Wood killifish | "Strength" of heritability† |
Reference |
|---|---|---|---|---|
| Embryo | F1, F2 | Resistant to toxicity and EROD induction due to PCB-126 | + + + | Nacci et al. 2010 |
| F1 | Recalcitrant to CYP mRNA induction by BaP, BkF, & PCB-126 | + | Wills et al. 2010 | |
| F1 | Resistant to cardiac teratogenesis & EROD induction from BaP/Fl mixture | + | Wills et al. 2009 | |
| F1, F2 | Resistant to cardiac abnormalities from ER sediments | + + + | Ownby et al. 2002 | |
| F1, F2 | Fewer developmental abnormalities with exposure to ERSE | + + + | Meyer & Di Giulio 2003 | |
| F1, F2 | Lack of EROD induction by 3-MC in F1, but not F2 | + / − | Meyer et al. 2002 | |
| F1, F3 | Lack of EROD induction by BNF in F1, but not F3; hybrids (regardless of sex of ER parent) had low, but intermediate EROD | + + | Meyer et al. 2002 | |
| F1, F3 | Resistant to CYP1A activity & protein induction by PCB-126 in F1, not F3 | + / − | Meyer & Di Giulio 2002 | |
| F1, F3 | Resistant to teratogenesis from PCB-126; at highest dose F1 resistant, F3 intermediate | + + | Meyer & Di Giulio 2002 | |
| F1 | Recalcitrant to induction of mitochondrial CYP by BaP, BkF | + | Jung et al. 2010 | |
| Larvae | F1 | Higher basal mtDNA & nDNA damage, resistant to induction of DNA damage by BaP | + | Jung et al. 2009 |
| F1, F2 | Reduced induction of EROD activity by BNF in F1; F2 intermediate | + + | Meyer et al. 2002 | |
| F1, F2 | Increased survival in t-BOOH exposure (F1 and F2 same) | + + | Meyer et al. 2003b | |
| F1, F2 | Elevated TOSC (F1 and F2 same) | + + | Meyer et al. 2003b | |
| F2 | Slightly elevated total GSH | + | Meyer et al. 2003b | |
| F1 | Elevated MnSOD protein, lack of induction by ERSE | + | Meyer et al. 2003b | |
| F1, F2 | Increased survival in ERSE (F2 intermediate to ref and F1) | + + | Meyer & Di Giulio 2003 | |
| F1 | Hybrids (regardless of sex of ER parent) show intermediate tolerance to ERSE | + + | Meyer & Di Giulio 2003 | |
| Juvenile | F1 | Elevated total GSH in liver, no difference in gill | + | Meyer et al. 2003b |
| Adult | F1, F2 | Reduced EROD activity in F1 (3-year old) and F2 (2-year old) exposed to ERSE (F1 not different on day 2, but different on day 5) | + | Meyer et al. 2002 |
| F1, F2 | Total GSH (basal and induction by ERSE) roughly same as reference | − − | Meyer et al. 2003b | |
| F1, F2 | No difference from reference in glutamate cysteine ligase activity | − − | Meyer et al. 2003b | |
| F1, F2 | Basal level and induction of GPx activity roughly same as reference | − − | Meyer et al. 2003b | |
| F1, F2 | Reduced induction of glutathione reductase activity | − | Meyer et al. 2003b |
Plus signs indicate evidence that the response is heritable, minus signs indicate evidence the response was not heritable; the number of signs indicates the relative strength of the evidence for heritability.
Abbreviations: PCB-126 – 3,3',4,4',5 pentachlorobiphenyl, BaP – benzo[a]pyrene, BkFbenzo[k]fluoranthene, Fl – fluoranthene, ERSE – Elizabeth River sediment extract, EROD – ethoxyresorufin-o-deethylase assay, 3-MC – 3-methylcholanthrene, BNF – β-napthoflavone, ER – Elizabeth River, CYP – cytochrome P450, mtDNA – mitochondrial DNA, nDNA – nuclear DNA, t-BOOH – t-butyl hydroperoxide, TOSC – total oxyradical scavenging capability, GSH – glutathione, MnSOD – manganese superoxide dismutase, GST – glutathione S-transferase, GPx – glutathione peroxidase, P-gp – p-glycoprotein, AHR – aryl hydrocarbon receptor, ARNT – aryl hydrocarbon receptor nuclear translocator, AHRR – aryl hydrocarbon receptor repressor
In the current study we compared the response to various aryl hydrocarbons of Atlantic Wood F1 embryos (offspring of wild-caught parents), Atlantic Wood F2 embryos (offspring of lab-reared F1 adults), and embryos of fish from a reference population. Embryos were exposed to benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), PCB-126, and a mixture of BkF and fluoranthene (Fl). Resistance to aryl hydrocarbon-mediated induction of CYP activity, expression of CYP1A, CYP1B1, and CYP1C1 mRNA, and cardiac teratogenesis was determined. This study focused on these endpoints for several reasons. Due to the probability of close contact with contaminated sediments and the potential for increased sensitivity in early life stages, effects on embryos are of particular importance. Furthermore, it is likely that heritable PAH adaptation is driven by acute toxicity and early life stage effects that prevent survival to reproduction. This investigation focused on CYP induction because of the aforementioned importance of CYP and the AHR pathway in the toxicity of and resistance to many aryl hydrocarbons.
2. Methods
2.1. Fish
Adult killifish from the PAH-adapted Atlantic Wood population were collected with wire mesh minnow traps at the Atlantic Wood Industries Superfund Site (36°48’27.2” N, 76°17’38.1” W). Adult killifish from a reference population were collected from King’s Creek, a relatively uncontaminated tributary of the Severn River in Virginia (37°18’16.2”N, 76° 24’58.9”W). In the laboratory, adults were maintained in flow-through systems consisting of a series of 30-L or 40-L tanks containing 20‰ artificial sea water (ASW; Instant Ocean, Foster & Smith, Rhinelander, WI, USA). The system was maintained at 23–25 °C on a 14:10 light:dark cycle. Adults were fed pelleted feed ad libidum (Aquamax ® Fingerling Starter 300, PMI Nutritional International, LLC, Brentwood, MO, USA). Eggs were obtained by manual spawning of females and fertilized in vitro by expressing sperm from males into a beaker containing eggs in ASW. Following spawning, embryos were set aside for a minimum of one hour to allow fertilization, then washed briefly with 0.3% hydrogen peroxide solution.
To obtain F1 Atlantic Wood adults, mixed breedings of >100 females and >20 males were conducted. This resulted in several thousand eggs and approximately 1000 were maintained in petri dishes (VWR International, West Chester, PA, USA) lined with absorbent filter paper (No. 3MM chromatography paper, Whatman International Ltd., Maidstone, England). Enough ASW was added to the dishes to keep the eggs moist but not completely submerged. They were maintained in an incubator for 12–14 days at 27 °C. For hatching, more ASW was added to the petri dishes, the absorbent paper was removed, and the dishes were gently rocked in a shaker. After hatching, larvae were maintained in 2-L beakers of ASW in an incubator at 27 °C and fed Artemia nauplii. Larvae were maintained in beakers for several weeks, then moved to a 19-liter aquarium in the same room as the adult colonies (23–25 °C on a 14:10 light:dark cycle) for several months. Finally, juveniles were moved into dedicated tanks in the flow-through system described previously. The Atlantic Wood F1 generation fish began reproducing approximately 6 months after hatching. The experiments described in the current paper were conducted with F2 embryos obtained while the Atlantic Wood F1 fish were 1–2 years old.
All care, reproductive, and rearing techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee (A234-07-08).
2.2. Chemicals
Ethoxyresorufin, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), and fluoranthene (Fl) were purchased from Absolute Standards, Inc. (Hamden, CT, USA) and 3,3’,4,4’,5-pentachlorobiphenyl (PCB-126) was purchased from AccuStandard (New Haven, CT, USA). Chemical stocks were prepared by dissolving the appropriate mass of BkF, BaP, Fl, or PCB-126 in DMSO.
2.3 Dosing
Adult King’s Creek (reference), Atlantic Wood F1s, and Atlantic Wood F2s were spawned as described previously. Embryos were dosed individually in 20-mL glass scintillation vials (VWR, West Chester, PA) beginning at 24 hours post fertilization (hpf). Embryos were exposed in 10 mL of dosing solution (20‰ ASW) containing the following: 100 µg/L BaP, 300 µg/L BkF, or 1 µg/L PCB-126. Embryos were also exposed to 20 µg/L BkF plus 100 µg/L Fl, which is representative of field conditions where killifish are exposed to a mixture of PAHs consisting of AHR agonists (i.e., BkF) and CYP inhibitors (i.e., Fl); the combination of AHR agonists and CYP inhibitors causes a synergistic increase in cardiac toxicity (Wassenberg & Di Giulio 2004). The doses were chosen based on previous work (Clark et al. 2013, Clark et al. 2010) because they were known to both cause deformities and induce mRNA expression and EROD activity in the reference embryos. Control embryos were exposed to DMSO at a concentration (v/v) equal to that in the dosed group; DMSO concentrations were held at <0.03% across all treatments. All exposure groups also received 21 µg/L ethoxyresorufin. Embryos in the dosing solution were maintained in at 27 °C from 24 hpf. CYP1 activity was measured using the in ovo EROD (ethoxyresorufin-o-deethlyase) assay at 96 hpf (section 2.4), and cardiac deformities were assessed at 144 hpf (section 2.5). EROD was not assessed in the BkF plus Fl treatment because Fl inhibits EROD activity at the doses used and cardiac deformities were not assessed in the BaP exposure because BaP does not induce cardiac deformities without co-exposure to a CYP inhibitor. After deformity screening, embryos exposed to DMSO, BkF, or PCB-126 were flash frozen in liquid nitrogen and stored at −80 °C for later mRNA analysis. This timepoint was chosen for mRNA analysis to allow comparison to previous work with Atlantic Wood F1 embryos (Wills et al. 2010). All exposures consisted of three experimental replicates with n = 10 embryos per treatment group.
2.4 EROD assay
CYP1 activity was measured via the in ovo EROD assay modified from Nacci et al. (1998), in which embryos are co-exposed to ethoxyresorufin in the dosing solutions. Resorufin, the fluorescent product of CYP1 activity on ethoxyresorufin, collects in the urinary bladder of the embryo. At 96 hpf, this fluorescence was visualized using fluorescent microscopy (50× magnification, rhodamine red filter set; Axioskop, Zeiss, Thornwood, NY, USA). EROD activity was measured as the intensity of fluorescence within the bladder normalized to the intensity within a region outside the bladder and quantified using IPLab software (BD Biosciences, Rockville, MD, USA). All EROD values are expressed as percent of the King’s Creek (reference) population DMSO-dosed control group response.
2.5 Deformity assessment
Embryos were scored for cardiac deformities at 144 hpf. Deformity assessment was performed blind using a scale shown in detail previously (Clark et al. 2010, Matson et al. 2008). The scale consists of three scores categorized as normal (0), mild deformities (1), and severe (2) deformities. Embryos receiving a score of 0 had hearts with a normal appearance including properly aligned and sized chambers, no visible pericardial edema, and unrestricted blood flow. Embryos receiving a score of 1 had slightly elongated hearts, with generally distinct but misaligned chambers, and visible pericardial edema. Embryos with a score of 2 had greatly elongated hearts often with no identifiable chambers and an extremely reduced or complete absence of blood flow.
2.6 Quantitative real-time PCR
Paired embryos were thawed on ice, homogenized with RNA-Bee for 30 seconds, and mRNA was extracted by modified phenol-chloroform extraction according to the RNA-Bee protocol (Tel-Test, Inc., Friendswood, Texas, USA). RNA quantity and quality was analyzed using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). Using the Omniscript cDNA synthesis kit for Reverse Transcription (Qiagen, Valencia, CA, USA), cDNA was prepared according to manufacturer’s instructions using 500 ng of RNA, random hexamers, and RNAse inhibitor, and carried out for 1 h at 37 °C in a Biometra T1 thermocycler (Göttingen, Germany).
QPCR was performed in a 25 µL reaction containing 200 nM of each primer, 12.5 µL of 2X SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA), 9.5 µL dH20, and 4 ng cDNA template. The β-actin (F – 5’-ACCACACATTTCTCATACACTCGGG-3’, R – 5’-CGCCTCCCTTCATCGTTCCAGTTT-3’), CYP1A (F – 5’-AAGAATGGAGGACACTGGATGACC-3’, R – 5’-AGATTACAGGACAACACGACAGCG-3’), and CYP1B1 (F – 5’-CCAAAGAATACACAGAGGCAACGG-3’, R – 5’-ATGAAGGCATCCAGGTAAGGCAT-3’) primers used were previously reported by Wills et al. (2010) and the CYP1C1 (F – 5’-TCTGGACGCCTTCATCTACGA-3’, R – 5’-GTGACGTCCGATGTGGTTGA-3’) primers reported by Wang et al. (2006). The reactions were carried out on an Applied Biosystems 7300 Real-Time PCR system with the following profile: 10 min at 95 °C and 40 cycles of 15 s at °C followed by 1 min at 60 °C. Dissociation curves were calculated for each sample at the end of the run to confirm formation of a single product. Each sample was run in duplicate wells, a minimum of six biological replicates (consisting of paired individuals) was analyzed per experiment, and embryos from duplicate experiments were analyzed. Data analyses were performed using ABI PRISM 7300 Sequence Detection System Software (Applied Biosystems). Expression was calculated using relative quantification by the 2−ΔΔCT. Target gene expression was normalized to β-actin and then compared to the population control treatment to determine average fold induction.
2.7 Statistical analyses
All analyses were performed using JMP 8.0 (SAS Institute Inc, Cary, NC, USA). For all EROD and deformity analyses, the individual was the unit of replication. To measure mRNA, two individuals were pooled. Therefore, a pair of embryos was the unit of replication. EROD data were rank-transformed and analyzed by non-parametric analysis of variance (ANOVA), followed by least square means (LSMeans) procedures. As stated previously, experiments were replicated three times; no differences between experimental replicates were observed for any test. For post hoc comparisons Tukey-adjusted pairwise comparisons were conducted to determine differences between all groups. Statistical significance was accepted at p≤0.05 for all tests.
3. Results
3.1 Heritability of resistance to cardiac teratogenesis due to aryl hydrocarbons
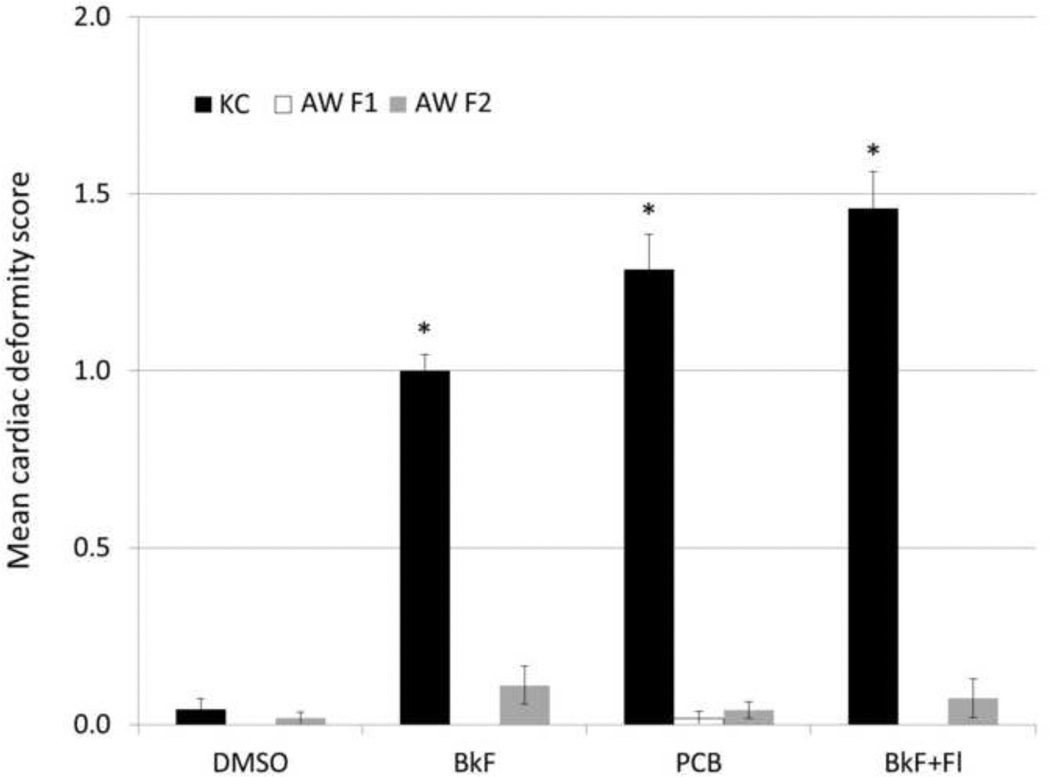
Compared to King’s Creek (reference) embryos, both Atlantic Wood F1 and F2 embryos were highly resistant to the teratogenic effects of each of the aryl hydrocarbon exposures (Figure 1). The mean deformity score of King’s Creek embryos was significantly elevated above that of controls (0.04±0.03) for exposure to BkF (1.0±0.05; p<0.0001), PCB-126 (1.3±0.10; p<0.0001), and the mixture of BkF and Fl (1.5±0.10; p<0.0001). In contrast, Atlantic Wood F1 embryos exhibited no differences from controls (0.0±0.0) in mean deformity score for exposure to BkF (0.0±0.0, p=1.000), PCB-126 (0.02±0.02, p=1.000), and the mixture of BkF and Fl (0.0±0.0, p=1.000). Likewise, Atlantic Wood F2 embryos exhibited no statistical differences from controls (0.02±0.02) in mean deformity score for exposure to BkF (0.11±0.05, p=0.9733), PCB-126 (0.04±0.02, p=1.000), and the mixture of BkF and Fl (0.08±0.06, p=0.9995). The deformity scores of Atlantic Wood F1 and F2 embryos were not statistically different for any exposure tested (all p>0.9).
Figure 1. Mean deformity score of F1 and F2 Atlantic Wood and King’s Creek killifish embryos exposed to aryl hydrocarbons.
Mean deformity score (±SEM) of King’s Creek (black bars), Atlantic Wood F1 (white bars), and Atlantic Wood F2 (grey bars) embryos exposed to 300 µg/L benzo[k]fluoranthene (BkF), 1 µg/L 3,3’4,4’,5-pentachlorobiphenyl (PCB-126), or a mixture of 20 µg/L BkF and 100 µg/L fluoranthene (Fl). Values marked by * are significantly different from the DMSO-dosed population-matched control group at p<0.05 (ANOVA, Tukey-adjusted LSMeans). n = 30 individuals per treatment group.
3.2 Heritability of resistance to induction of CYP1 activity by aryl hydrocarbons
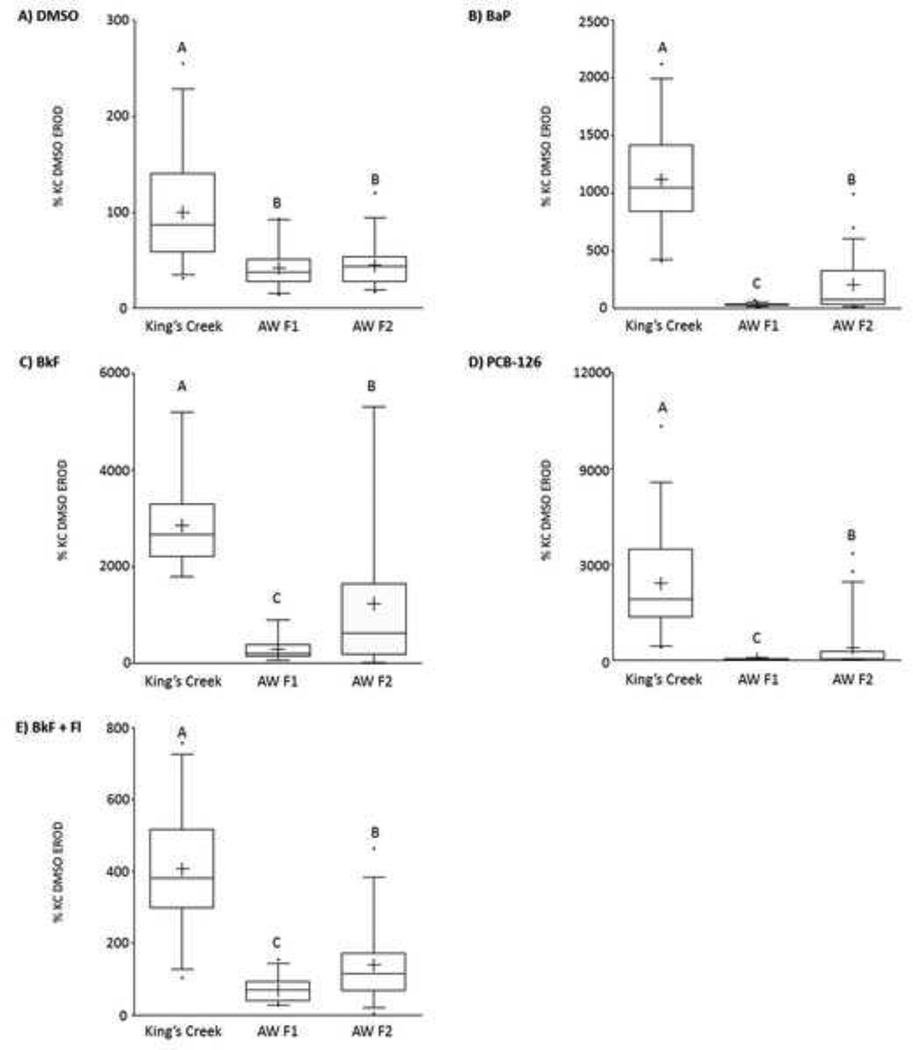
Aryl hydrocarbon exposure consistently induced CYP1 activity (as measured by EROD response) in the King’s Creek (reference) embryos (Figure 2). The mean EROD response for King’s Creek embryos was 1118±85% for BaP, 2847±281%, 3215±350% for PCB-126, and 407±33% for the mixture of BkF and Fl. For all exposures of King’s Creek embryos, EROD activity was statistically different from DMSO-dosed controls (p<0.0001). In contrast, the F1 Atlantic Wood embryos exhibited very little increase in EROD activity with any exposure. The EROD response of Atlantic Wood F1 embryos was not significantly different from that of DMSO-dosed F1s (34±2.7%) for exposure to BaP (32±2.9%; p=1.000), BkF (220±43%; p=1.000), PCB-126 (51±4.6%; p=1.000), or the mixture of BkF and Fl (70±6.7%; p=1.000). For Atlantic Wood F2 embryos, EROD activity was significantly different from DMSO-dosed F2s (35±2.6%) for exposure to BaP (204±53%; p=0.0302), BkF (1462±366%; p=0.001), PCB-126 (532±150%; p=0.0294) and BkF and Fl (139±18%; p<0.0001). Furthermore, the EROD response of Atlantic Wood F2s was significantly different from that of Atlantic Wood F1s for BaP (p=0.0302), BkF (p=0.0323), PCB-126 (p=0.0253), and BkF and Fl (p=0.0233), but not for DMSO alone (p=1.000). However, the response was also much lower than that of the King’s Creek embryos. Both Atlantic Wood F1 and F2 embryos exhibited statistically lower EROD activity than King’s Creek embryos for all treatments (p<0.0001).
Figure 2. Mean ethoxyresorufin-o-deethylase (EROD) activity of F1 and F2 Atlantic Wood and King’s Creek killifish embryos exposed to aryl hydrocarbons.
Percent of KC DMSO EROD activity for King’s Creek, Atlantic Wood F1, and Atlantic Wood F2 embryos exposed to A) DMSO, B) 100 µg/L benzo[a]pyrene (BaP), C) 300 µg/L benzo[k]fluoranthene (BkF), D) 1 µg/L 3,3’4,4’,5-pentachlorobiphenyl (PCB-126), or E) a mixture of 20 µg/L BkF and 100 µg/L fluoranthene (Fl). Box plots represent median with upper and lower quartiles. Whiskers show the 5th and 95th percentiles. Mean is represented by + and outliers are represented by dots. Within a given panel, groups not marked by the same letter are statistically different at p < 0.05 (ANOVA, Tukey-adjusted LSMeans). n = 30 individuals per treatment group.
3.3 Heritability of resistance to induction of CYP1 mRNA expression by aryl hydrocarbons
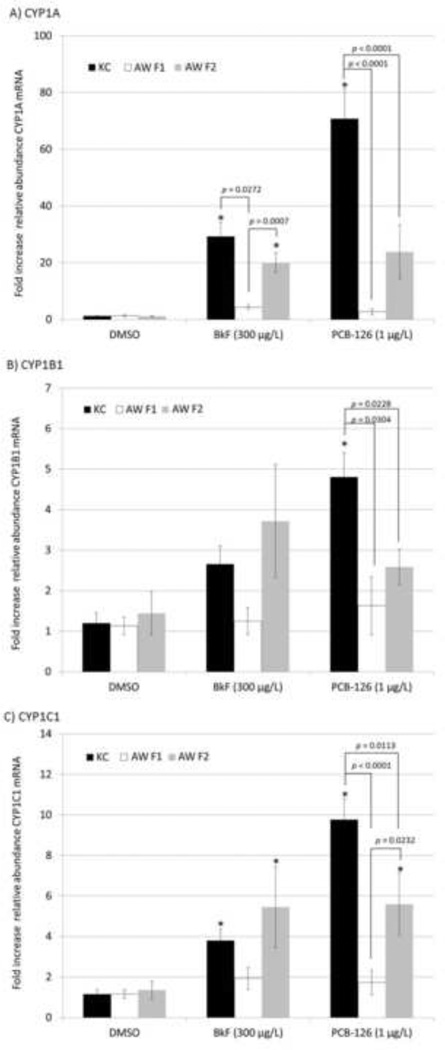
Expression of three CYP1 mRNAs (Figure 3) exhibited a similar pattern to that observed for EROD activity. The fold change in expression of CYP1A, CYP1B1, and CYP1C1 in BkF- and PCB-126-dosed King’s Creek embryos was significantly elevated above controls (p<0.05), except for CYP1B1 expression in the BkF treatment (p=0.5363). In contrast, expression of CYP1A, CYP1B1 and CYP1C1 was not elevated above controls in Atlantic Wood F1 embryos for any treatment group (p<0.05). Furthermore, Atlantic Wood F1 embryos exhibited significantly lower BkF-induced expression of CYP1A (p=0.0272) and PCB-126-induced expression of CYP1A (p<0.0001), CYP1B1 (p=0.0304) and CYP1C1 (p<0.0001) than that observed for reference embryos.
Figure 3. Mean CYP mRNA expression of F1 and F2 Atlantic Wood and King’s Creek killifish embryos exposed to benzo[k]fluoranthene (BkF) or 3,3’4,4’,5-pentachlorobiphenyl (PCB-126).
mRNA expression of A) CYP1A, B) CYP1B1, C) CYP1C1 of King’s Creek (black bars), Atlantic Wood F1 (white bars), and Atlantic Wood F2 (grey bars) embryos exposed to 300 µg/L benzo[k]fluoranthene (BkF) and 1 µg/L 3,3’4,4’,5-pentachlorobiphenyl (PCB-126). Values marked by * are significantly different from the DMSO-dosed population-matched control group at p<0.05 (ANOVA, Tukey-adjusted LSMeans). All other statistically different post hoc pairwise comparisons are labeled on the figure (ANOVA, Tukey-adjusted LSMeans). n = 8 pairs of individuals per treatment group.
The expression pattern in Atlantic Wood F2 embryos appeared to be intermediate between that of the F1s and the King’s Creek embryos. Unlike in F1 embryos, CYP1A expression was elevated with BkF treatment (p<0.0001) and CYP1C1 expression was elevated with both BkF (p=0.0292) and PCB-126 (p=0.0098). Furthermore, F2 embryos exhibited significantly greater induction than F1s of CYP1A with BkF treatment (p=0.0007) and CYP1C1 with PCB-126 treatment (p=0.0232). However, PCB-126 treated F2 embryos still exhibited significantly lower expression of CYP1A (p<0.0001), CYP1B1 (p=0.0228) and CYP1C1 (p=0.0113) than that of reference embryos.
4. Discussion
Resistance of Atlantic Wood killifish embryos to cardiac teratogenesis and AHR pathway activation by aryl hydrocarbons was found to be heritable for two laboratory-raised generations, suggesting a genetically inherited adaptive response. However, Atlantic Wood F2s demonstrated less resistance to induction of CYP mRNA and EROD activity, perhaps indicating that some part of the resistance is rapidly lost and not inherited genetically.
4.1 Resistance to cardiac teratogenesis
In the current study, Atlantic Wood F1 and F2 embryos were highly resistant to cardiac teratogenesis caused by aryl hydrocarbon exposure. This agrees with much of the previous work investigating resistance of Atlantic Wood embryos to aryl hydrocarbons. Ownby et al. (2002) showed that F2 offspring of laboratory-reared F1 Atlantic Wood killifish were highly resistant to Elizabeth River sediments. Previous work in our laboratory also showed that Atlantic Wood F1and F2 embryos were also resistant to developmental abnormalities and mortality caused by Elizabeth River pore water (Meyer & Di Giulio 2003, Meyer et al. 2002). In addition, a recent study showed that both F1 and F2 Atlantic Wood killifish were even more resistant to PCB-126 toxicity than adapted killifish from sites in Newark Bay, NJ and New Bedford Harbor, MA, where contamination is dominated by PCBs and dioxins (Nacci et al. 2010). In contrast, Meyer and Di Giulio (2002) found that F1 Atlantic Wood embryos were highly resistant to cardiac teratogenesis generated by PCB-126 exposure, but at the highest doses resistance by F3 embryos (F2 embryos were not studied) was intermediate between that of F1 embryos and susceptible reference embryos (Meyer & Di Giulio 2002). It is not clear why this study showed a loss of resistance in F3 embryos whereas all other studies, including the current study, found resistance to toxicity to be inherited for at least two laboratory-reared generations. However, the heritability of resistance to embryonic toxicity in Atlantic Wood killifish has been fairly consistently observed across studies. This suggests that resistance to embryonic toxicity is a major driving force in adaptation to contaminated sediments in the Elizabeth River habitat. Because of close contact with contaminated sediments and increased sensitivity in early life stages, it is likely that heritable PAH adaptation is driven by acute toxicity and early life stage effects that prevent survival to reproduction, rather than chronic effects associated with PAH exposures, such as carcinogenesis.
4.2 Resistance to induction of CYP1 mRNA expression and EROD activity
For all exposures in the current study, EROD activity was suppressed in both Atlantic Wood F1 and F2 embryos compared to that exhibited by reference embryos. However, the response of Atlantic Wood F2 embryos was consistently higher than that of Atlantic Wood F1s. This consistent pattern suggests that although the resistance to induction of CYP activity may be genetically heritable, it is not brought about solely by genetically inherited mechanisms.
In previous studies in our laboratory, F1 Atlantic Wood embryos were highly resistant to induction of CYP1 activity generated by PCB-126 exposure (Meyer & Di Giulio 2002). In addition, Atlantic Wood F1 embryos were recalcitrant to induction of CYP1A by the AHR agonist-type PAHs 3-MC and β-naphthoflavone (BNF). However in these studies, the resistance was not consistently heritable to subsequent generations. PCB-126-induced EROD activity in F3 embryos (F2 were not tested) returned to levels similar to those of reference embryos, Likewise, recalcitrance to CYP induction was largely lost in F3 embryos and F2 larvae dosed with 3-MC, BNF, or sediment pore water (Meyer & Di Giulio 2003, Meyer et al. 2002). In addition, hepatic EROD activities in adult Atlantic Wood F1s and F2s exposed to Elizabeth River sediments were found to be similar to those of adult reference fish. This contrasts with the degree of resistance to CYP induction by Atlantic Wood F2 embryos observed in the current study. Furthermore, Nacci et al. (2010) found that Atlantic Wood F2 individuals were highly resistant to induction of EROD activity by PCB-126. They did not test Atlantic Wood F1 individuals, so it is unknown if they would have observed a difference between F1 and F2 response similar to that seen in the current study. However, they were unable to calculate an exact EC50 for F2 EROD response because there was no change in response even at 200 µg/L of PCB-126.
Although, EROD activity is frequently used as a measure of CYP1A activity, other CYP1s have been shown to metabolize ethoxyresorufin, although often at a lower rate (Scornaienchi et al. 2010). As would be expected given the observed EROD activity, Atlantic Wood F1s and F2s were recalcitrant to expression of multiple CYP1s. Atlantic Wood F1 embryos were highly resistant to induction of CYP mRNA expression by both BkF and PCB-126. This agrees with previous work demonstrating that Atlantic Wood F1 embryos were resistant to induction of CYP1A, CYP1B1, and CYP1C1 mRNA expression by multiple compounds, including BkF and PCB-126 (Wills et al. 2010). However, similar to the EROD response, the expression pattern in Atlantic Wood F2 embryos appeared to be intermediate between that of the F1s and the King’s Creek embryos. Overall, these data and work by Wills et al. (2010) show that mRNA expression of multiple CYPs is highly suppressed in Atlantic Wood F1 embryos and to a lesser degree in F2 embryos, suggesting at least partially heritable suppression via a shared upstream regulator such as the AHR. Interestingly, the suppression of CYP expression in Atlantic Wood F2 embryos appears to be stronger for exposure to PCB-126 than for BkF, which concurs with the strong suppression of CYP activity in PCB-exposed Atlantic Wood F2s observed by Nacci et al. (2010). It is possible that the greater level of expression induced by PCB-126 made it easier to resolve differences between the F1 and F2 embryos, but these data do not demonstrate any resistance to BkF-induced CYP mRNA induction in Atlantic Wood F2s.
The current work and studies by Meyer and colleagues provide evidence that resistance to induction of CYP mRNA expression and enzyme activity is heritable but may not be fully genetic. Meyer and coworkers proposed that this might be achieved through epigenetic regulation of CYP1A. However, Timme-Laragy et al. (2005) found no difference between the methylation status of CpG sites in the CYP1A promoter of Atlantic Wood and reference fish. As discussed previously, suppression of mRNA expression of multiple CYPs indicates suppression via a shared regulator. This suggests that it would be more useful to look at epigenetic regulation of a factor upstream of CYP, such as the AHR.
It is difficult to reconcile the results of Meyer and coworkers with those of the current study and Nacci et al. (2010), although it is notable that some data obtained by Meyer and coworkers did support a conclusion of genetic heritability. As stated previously, the strongest evidence for full genetic heritability was obtained for resistance to teratogenesis in embryos, while studies of heritable resistance in larvae and adults yielded mixed results. Refractory CYP response and resistance to toxicity tended to fade with age, perhaps indicating that components of the adaptation were developmental stage specific. Interestingly, hybrid embryos generated by crossing Atlantic Wood fish of either sex with reference fish demonstrated a BNF-induced EROD response intermediate between those of reference and Atlantic Wood embryos (Meyer et al. 2002). Furthermore, the response of the two hybrid lines was nearly indistinguishable, regardless of the sex of the parent from the Atlantic Wood population. These results seem to be more consistent with a hypothesis of genetically heritable resistance, transmitted by both male and female Atlantic Wood fish.
It is noteworthy that some of the elevated EROD response of Atlantic Wood F2 embryos observed could be attributed to a subset of highly responsive individuals, as shown in Figure 2. Little evidence of such a group was observed for Atlantic Wood F1 embryos. Meyer and Di Giulio (2003) discussed the possibility that because resistant fish were less fit under clean conditions, the laboratory population could have undergone “reverse” selection, yielding less resistant offspring in later generations. However, it is hard to imagine that the adaptive response could be selected against in only one generation, especially when the observed response of Atlantic Wood F1 embryos does not appear to yield much variation on which to select.
It is also possible that the contaminant resistance is conveyed by multiple adaptive changes, and some components of the resistance are fully heritable, while others are not. Atlantic Wood F1 embryos might have all components of the resistance, both genetically heritable and non-genetically heritable, so their response is very low. In contrast, some Atlantic Wood F2 individuals may have lost non-genetically heritable components of resistance, yielding a subset of higher-responding individuals. If this is the case, it is interesting that loss of some of these components did not affect the overall resistance to toxicity of Atlantic Wood F2 embryos.
4.4. Role of AHR pathway in resistance of Atlantic Wood killifish to aryl hydrocarbons
As described previously, the AHR pathway plays a pivotal role in the toxicity of many aryl hydrocarbons, including some PAHs, and is a likely target for resistance in aryl hydrocarbon-adapted fish populations (reviewed in Wirgin & Waldman 2004). Furthermore, aryl hydrocarbon-generated cardiac teratogenesis in fish is mediated at least in part through the AHR pathway (Billiard et al. 2006, Clark et al. 2010, Incardona et al. 2006, Prasch et al. 2003b), so it seems likely that down-regulation of the AHR pathway is an important target for adaptation by killifish to the PAH-contaminated Elizabeth River habitat. However, resistance to activation of the AHR pathway (as measured by CYP mRNA expression, protein, and enzyme activity) was not consistently heritable past the F1 generation in many studies by Meyer and coworkers previously discussed (Table 1). Several conclusions could be drawn from these data and the incomplete resistance to CYP induction of Atlantic Wood F2 embryos observed in the current study. One possibility is that suppression of AHR pathway activity is not fully genetically heritable. In addition, induction of CYP family members has been shown to be regulated by the constitutive androstane receptor, the pregnane X receptor, the retinoic acid receptor, and the peroxisome proliferators-activated receptor (Xu et al. 2005, Monostory and Pascussi 2008); therefore, non-genetically heritable alteration of any of these pathways could also play a role in the observed pattern of heritability of CYP suppression. Alternatively, the degree of AHR pathway suppression could be consistent in F1 and F2 embryos, but the up-regulation of other protective factors observed in Atlantic Wood killifish might fade in laboratory-reared generations. Feral Atlantic Wood killifish have elevated levels of glutathione S-transferase (Armknecht et al. 1998), hepatic P-glycoprotein (Cooper et al. 1999), UDP-glucuronosyl transferase (UGT), and sulfotransferase (Gaworecki et al. 2004). Many of these enzymes enhance removal of xenobiotics from the body. In fact, some may have contributed to observed resistance of Atlantic Wood F1 larvae to pesticides that could not easily be attributed to AHR downregulation (Clark et al. 2012). It is possible that some of these changes are non-genetically heritable acclimatory changes and their absence in Atlantic Wood F2 embryos resulted in the apparent increase observed in AHR pathway activation. To our knowledge, heritability of these protective factors has not been reported.
In any case, a complete lack of CYP activity and suppression of the AHR pathway do not appear to be required for strong resistance to aryl hydrocarbons at the doses tested. Similarly, investigation of killifish from throughout the Elizabeth River found that some subpopulations were highly resistant to teratogenesis while still demonstrating significant induction of CYP activity (Clark et al. 2013). These experiments clearly demonstrate that while suppression of the AHR pathway may play a major role in resistance of Elizabeth River killifish to aryl hydrocarbons, it is not the only important factor. This suggests instead that multiple alterations play an important role in PAH-adaptation.
5. Conclusion
This study confirmed that both resistance to cardiac teratogenesis and induction of CYP were heritable for two generations in the absence of PAH stress. However, the suppression of the AHR pathway response was weaker in F2 embryos and therefore did not appear to be fully heritable. There is still much to be determined about the mechanisms by which resistance has occurred, although these results support the conclusion that heritable resistance is conferred by multiple alterations in adapted fish. The existence of genetically heritable resistance to pollutants in this and other fish populations demonstrates the potential influence of anthropogenic contamination at a population and perhaps evolutionary scale.
Acknowledgements
We thank Dr. Cole Matson, Dr. Lauren Wills, Dr. Dawoon Jung, Dr. Lindsey Garner, and Daniel Brown for their assistance with fish collection and exposure. This work was supported by the National Institute of Environmental Health supported Superfund Research Program (P42ES10356) and Duke University Integrated Toxicology Program (T32ES007031).
Literature Cited
- Armknecht SL, Kaattari SL, Van Veld PA. An elevated glutathione S-transferase in creosote-resistant mummichog (Fundulus heteroclitus) Aquat. Toxicol. 1998;41:1–16. [Google Scholar]
- Bacanskas LR, Whitaker J, Di Giulio RT. Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar Environ Res. 2004;58:597–601. doi: 10.1016/j.marenvres.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs) Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2002;133:55–68. doi: 10.1016/s1096-4959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Bols NC, Hodson PV. In vitro and in vivo comparisons of fish-specific CYP1A induction relative potency factors for selected polycyclic aromatic hydrocarbons. Ecotoxicol. Environ. Saf. 2004;59:292–299. doi: 10.1016/j.ecoenv.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Clark BW, Di Giulio RT. Fundulus heteroclitus adapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology. 2012;21(2):465–474. doi: 10.1007/s10646-011-0807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat. Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and Mixture-Specific Differences in Resistance to Polycyclic Aromatic Hydrocarbons and PCB-126 among Fundulus heteroclitus Subpopulations throughout the Elizabeth River Estuary (Virginia, USA) Environ. Sci. Technol. 2013;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PS, Vogelbein WK, Van Veld PA. Altered expression of the xenobiotic transporter P-glycoprotein in liver and liver tumours of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Biomarkers. 1999;4:48–58. doi: 10.1080/135475099230994. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Gaworecki KM, Rice CD, van den Hurk P. Induction of phenol-type sulfotransferase and glucuronosyltransferase in channel catfish and mummichog. Mar Environ Res. 2004;58:525–528. doi: 10.1016/j.marenvres.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem.-Biol. Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Incardona J, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer JS, Zheng J. The sources, transport, and fate of PAHs in the marine environment. In: Douben P, editor. PAHs: An Ecotoxicological Perspective. West Sussex, UK: John Wiley & Sons; 2003. pp. 9–33. [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat. Toxicol. 2008;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Di Giulio R. Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res. 2002;54:621–626. doi: 10.1016/s0141-1136(02)00170-8. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol. Sci. 2002;68:69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in killifish (Fundulus beteroclitus) inhabiting a polluted estuary. Ecol. Appl. 2003;13:490–503. [Google Scholar]
- Meyer JN, Smith JD, Winston GW, Di Giulio RT. Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model prooxidants: short-term and heritable responses. Aquat. Toxicol. 2003;65:377–395. doi: 10.1016/j.aquatox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Newman MC, Vogelbein W, Unger MA. Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat. Toxicol. 2002;61:195–209. doi: 10.1016/s0166-445x(02)00055-3. [DOI] [PubMed] [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, Cooper K. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ. Toxicol. Chem. 1998;17:2481–2486. [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, Munns WR, Specker JL, Cooper KR. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar. Biol. 1999;134:9–17. [Google Scholar]
- Nacci D, Champlin D, Jayaraman S. Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs) Estuar Coasts. 2010;33:853–864. [Google Scholar]
- Nacci DE, Gleason TR, Munns WR. Evolutionary and ecological effects of multi-generational exposures to anthropogenic stressors. Hum. Ecol. Risk Assess. 2002;8:91–97. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ. Toxicol. Chem. 2002;21:1897–1902. [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue-specific expression of AHR1, AHR2, and ARNT2 in dioxin-sensitive and -resistant populations of the marine fish Fundulus heteroclitus. Toxicol. Sci. 2000;57:229–239. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Carney SA, Heideman W, Peterson RE. Morpholino knockdown of AHR2 in the zebrafish embryo protects against TCDD developmental toxicity. Toxicological Sciences. 2003a;72:366–366. [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003b;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimpacted subpopulations of Fundulus heteroclitus .1. Tcdd toxicity. Environ. Toxicol. Chem. 1995;14:579–587. [Google Scholar]
- Roy NK, Courtenay S, Maxwell G, Yuan ZP, Chambers RC, Wirgin I. Cytochrome P4501A1 is induced by PCB 77 and benzo[a] pyrene treatment but not by exposure to the Hudson River environment in Atlantic tomcod (Microgadus tomcod) post-yolk sac larvae. Biomarkers. 2002;7:162–173. doi: 10.1080/13547500110113981. [DOI] [PubMed] [Google Scholar]
- Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 2002;20:243–248. doi: 10.1016/s0167-7799(02)01943-1. [DOI] [PubMed] [Google Scholar]
- Scornaienchi ML, Thornton C, Willett KL, Wilson JY. Functional differences in the cytochrome P450 1 family enzymes from Zebrafish (Danio rerio) using heterologously expressed proteins. Arch Biochem Biophys. 2010;502:17–22. doi: 10.1016/j.abb.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Meyer JN, Waterland RA, Di Giulio RT. Analysis of CpG methylation in the killifish CYP1A promoter. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2005;141:406–411. doi: 10.1016/j.cbpc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Toomey BH, Bello S, Hahn ME, Cantrell S, Wright P, Tillitt DE, Di Giulio RT. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptotic cell death and cytochrome P4501A expression in developing Fundulus heteroclitus embryos. Aquat. Toxicol. 2001;53:127–138. doi: 10.1016/s0166-445x(00)00161-2. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ. Sci. Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Westbrook DJ. Evidence for depression of cytochrome P4501A in a population of chemically resistant mummichog (Fundulus heteroclitus) Environ Sci. 1995;3:221–234. [Google Scholar]
- Vogelbein WK, Fournie JW, Vanveld PA, Huggett RJ. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Walker SE, Dickhut RM, Chisholm-Brause C. Polycyclic aromatic hydrocarbons in a highly industrialized urban estuary: Inventories and trends. Environ. Toxicol. Chem. 2004;23:2655–2664. doi: 10.1897/03-628. [DOI] [PubMed] [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ. Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat. Toxicol. 2010;99:33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR. Resistance to contaminants in North American fish populations. Mutat. Res.-Fundam. Mol. Mech. Mutag. 2004;552:73–100. doi: 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]