Abstract
Purpose
To determine corneal biomechanical changes following major glaucoma procedures.
Methods
In a prospective comparative case series, corneal biomechanical properties were assessed using the Ocular Response Analyzer (ORA, Reichert Inc., Depew, New York, USA) before and 3 months after surgery in 89 eyes of 89 patients undergoing trabeculectomy + mitomycin C (MMC) (23 eyes, group 1), phacotrabeculectomy + MMC (23 eyes; group 2), Ahmed glaucoma valve (AGV) implantation (17 eyes; group 3) or phacoemulsification (PE) alone (26 non-glaucomatous eyes; group 4). Patients with history of contact lens use, previous intraocular surgery, any chronic corneal disease, central corneal thickness ≥580 microns or ≤500 microns, post-operative intraocular pressure (IOP) > 21 mmHg or ≤ 5 mmHg, and any surgical complication were excluded. Main outcome measures included changes in corneal hysteresis (CH) and corneal resistance factor (CRF).
Results
Preoperatively, CH was lower in glaucomatous versus non-glaucomatous eyes (5.4, 5.3, 5.2 and 8.1 mmHg in groups 1, 2, 3 and 4 respectively, p<0.001). Three months after surgery, mean CH increased by 2.16, 2.29 and 2.30 mmHg in groups 1, 2 and 3 respectively (P<0.001) but only by 0.11 mmHg in group 4 (p=0.704). The postoperative increase in CH in glaucomatous eyes was more significant when IOP was reduced by more than 10 mmHg. CRF also showed a significant increase in all study groups postoperatively (P<0.001).
Conclusion
CH and CRF increased significantly 3 months after glaucoma procedures. Alterations in corneal biomechanical properties should be considered when measuring IOP after successful glaucoma surgery.
Keywords: Ocular Response Analyzer, Corneal Biomechanics, Corneal Hysteresis, Glaucoma Surgery
INTRODUCTION
Intraocular pressure (IOP) measurement is influenced by corneal biomechanical properties such as elasticity and viscoelasticity.1 It has also been shown that the rate of glaucoma progression is faster in eyes with lower corneal hysteresis.2 Multiple factors may affect corneal biomechanical properties which consequently influence IOP measurements. It has been shown that corneal incisions for cataract surgery may alter CH,3 however corneal biomechanical changes after glaucoma surgery have not been evaluated.
The Ocular Response Analyzer (ORA, Reichert Inc., Depew, New York, USA) measures corneal biomechanical properties in vivo.4 It also provides corneal compensated IOP (IOPcc) which is less affected by central corneal thickness (CCT), Goldmann correlated IOP (IOPg), and corneal resistance factor (CRF) which is an indicator of overall corneal resistance.5
Corneal hysteresis indicates the viscous dampening property of the cornea and has a normal range of 9.6 to 12.2 mmHg.5 This parameter seems to be independent of IOP in normal eyes but is negatively correlated with IOP in glaucomatous eyes.5,6 It has been shown that CH is significantly below normal in eyes with primary open angle glaucoma (POAG) and normal tension glaucoma,5,7 and partially recovers after IOP reduction in primary angle closure glaucoma.8
It is not clear how corneal biomechanical properties are influenced by intraocular surgery although it is known that these properties have an impact on IOP measurement by certain instruments; for example Goldmann applanation tonometry (GAT) underestimates IOP when CH is low.1,7 Knowledge about these changes can be helpful regarding IOP measurement after intraocular procedures.
The current study was aimed to evaluate changes in corneal biomechanical properties following trabeculectomy, phaco-trabeculectomy (PT), Ahmed glaucoma valve (AGV) implantation and phacoemulsification (PE).
METHODS
In this prospective comparative case series, 89 patients including 63 glaucomatous and 26 non-glaucomatous eyes were recruited from the Glaucoma Clinic at Labbafinejad Medical Center from November 2010 to August 2011. Glaucomatous eyes underwent trabeculectomy + mitomycin C (MMC) (23 eyes, group 1), PT+ MMC (23 eyes, group 2) and AGV implantation (17 eyes, group 3), while non-glaucomatous eyes underwent PE alone (26 eyes, group 4).
The study was approved by the Ethics Committee (equivalent to Institutional Review Board) of the Ophthalmic Research Center. After giving adequate explanation about the study, written informed consent was obtained from all patients prior to enrollment. Only patients with POAG were selected for trabeculectomy and phacoemulsification, in the AGV group all patients had neovascular glaucoma, patients in the phacoemulsification group were non-glaucomatous and took no topical ocular medications. Exclusion criteria were history of contact lens wear, previous intraocular surgery, any corneal disease such as keratoconus, corneal dystrophies or corneal scars, CCT ≥580 or ≤500 microns, post-operative IOP >21 or ≤5 mmHg and occurrence of any surgical complications.
Complete ophthalmological examinations including IOP measurement using a Goldmann applanation tonometer (GAT, Haag-Streit, Konig, Switzerland), CCT measurement using NIDEK UP-1000 ultrasonic pachymeter (Nidek Technologies, Gamagori, Japan), and evaluation of corneal biomechanical properties using the ORA (Reichert Inc., Depew, New York, USA) were performed before the operations. At each visit, a mean of 4 measurements for CH, CRF, IOPg, and IOPcc were recorded. GAT and ORA were repeated 3 months after the operation. The preoperative number of topical medications and duration of MMC application were analyzed to determine any correlation with CH. Primary outcome measures were changes in corneal hysteresis (CH) and corneal resistance factor (CRF) after the procedures.
Surgical Technique
All procedures were performed at the same center using the same technique by one of two glaucoma specialists (MP and SY), or under their direct supervision by a glaucoma fellow.
In group 1, trabeculectomy was performed using a fornix-based conjunctival flap and a half-thickness trapezoid scleral flap 4×3×3 mm in size in the superonasal or superotemporal quadrant. After preparation of the scleral flap, MMC (0.2 mg/ml) was applied by soaked methylcellulose sponge pieces for 1.5 to 2.5 minutes. A clear cornea block 1×1.5 mm in size was fashioned using a Kelly punch; then basal iridectomy was performed with Vannas scissors. The scleral flap was closed with two 10-0 nylon releasable sutures, and the conjunctiva was closed with 10-0 nylon wing sutures.
In group 2, phacotrabeculectomy was performed from one site; trabeculectomy technique was similar to that described above and after preparation of the half-thickness scleral flap in the superotemporal quadrants of right eyes, and superonasal quadrants of left eyes, MMC (0.2 mg/ml) was applied for 2-3 minutes; after thorough irrigation of the area with normal saline, phacoemulsification was performed and a one-piece acrylic foldable intraocular lens was implanted. After removing the viscoelastic material, the corneal block was fashioned followed by iridectomy as described for the trabeculectomy procedure. The scleral flap was closed with two 10-0 nylon releasable sutures, and the conjunctiva was closed with 10-0 nylon wing sutures.
In group 3, conjunctival peritomy was performed 4 mm posterior to the limbus in the superotemporal quadrant and MMC (0.2 mg/ml) was applied using a soaked cotton tipped applicator for 3 minutes. AGV priming was performed by gentle irrigation of the tube with a 27-gauge needle. The plate of the implant was then secured to the sclera 8 to 10 mm posterior to the surgical limbus using two 7-0 silk sutures. The tube was trimmed to an appropriate length with the bevel facing anteriorly and after injection of methyl cellulose into the anterior chamber via a clear cornea stab incision, it was inserted into the anterior chamber through a corneoscleral track created with a 23-gauge needle. The tube was fixed to the episclera with a 10-0 nylon mattress suture. A quadrangular donor scleral patch graft (4×7mm) was fashioned and secured over the exposed part of the tube and fixed with two or more 8-0 vicryl sutures. Then, the conjunctiva was closed with 10-0 nylon running mattress sutures.
In group 4, standard phacoemulsification was performed via a temporal clear cornea approach using a 2.8 mm keratome; a one- piece acrylic foldable intraocular lens implanted and no sutures were applied.
Betamethasone 4 mg and cephazolin 100 mg were injected subconjunctivally at the conclusion of all procedures. The postoperative regimen included topical chloramphenicol 0.5% eye drops 4 times a day for 1 week and topical betamethasone 0.1% eye drops 6 times a day which was usually tapered over 4 to 8 weeks, except in cases with severe inflammation requiring a longer period of treatment.
Statistical analysis was performed using SPSS statistical software (version 17.0.1; SPSS Science Inc., Chicago, IL, USA) by an independent statistician. Student paired t-test and analyses of variance were used. Data was presented in mean and standard deviations and p-values less than 0.05 were considered as statistically significant.
RESULTS
A total of 89 eyes of 89 patients (including 62.2% male subjects) were operated in 4 groups; these included 23 eyes in group 1 (trabeculectomy + MMC), 23 eyes in group 2 (PT + MMC), 17 eyes in group 3 (AGV implantation), and 26 non-glaucomatous eyes in group 4 (PE). Mean patient age was 57.9±21.1 years, which was comparable among the study groups (P= 0.15). The type of glaucoma was POAG in groups 1 and 2, and neovascular in group 3 including 12 cases of proliferative diabetic retinopathy and 5 cases of central retinal vein occlusion.
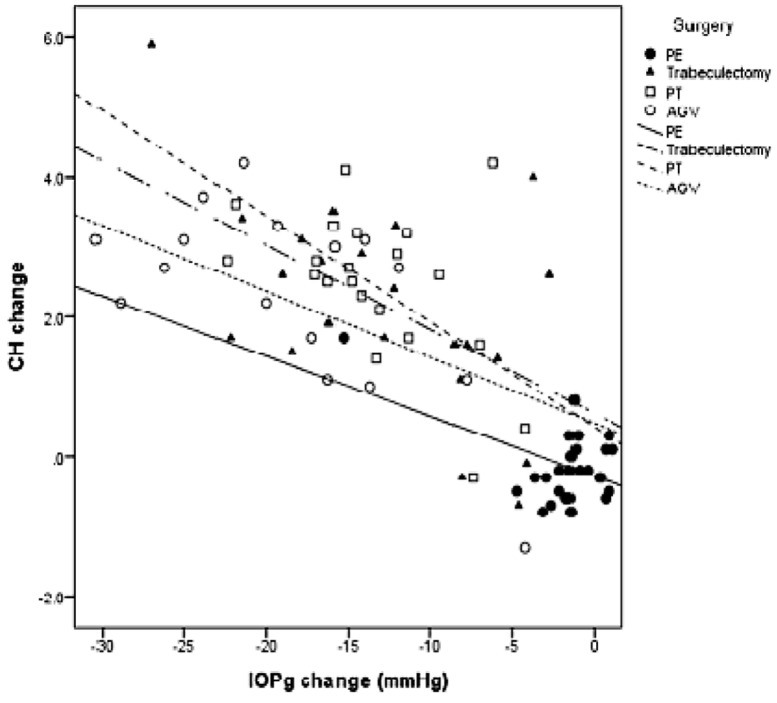
Mean preoperative CH was lower in glaucomatous eyes (5.4±1.8, 5.3±1.7 and 5.2±2.6 mmHg in groups 1, 2 and 3, respectively) as compared to non-glaucomatous eyes (8.1±1.8 mmHg) (p<0.001, Table 1). Postoperatively, CH was increased in glaucomatous eyes (p<0.001), especially when IOP reduction exceeded 10 mmHg (Fig 1) but remained relatively stable in non-glaucomatous eyes (P=0.704; Table 2). Mean preoperative CRF was comparable between glaucomatous and non-glaucomatous eyes (p=0.835; Table 1) and was significantly increased after surgery in all study groups (all P values < 0.001).
Table 1.
Baseline patient characteristics
| Total | Surgery | P-Value | ||||
|---|---|---|---|---|---|---|
| Trabeculectomy | PT | AGV | PE | |||
| Eye | ||||||
| OD | 48 (53.9%) | 12 (52.2%) | 13 (57%) | 10 (58.8%) | 13 (50%) | 0.948* |
| OS | 41 (46.1%) | 11 (47.8%) | 10 (43%) | 7 (41.2%) | 13 (50%) | |
| Sex | ||||||
| Male | 56 (63%) | 15 (65.2%) | 18 (78%) | 13 (76.5%) | 13 (50%) | 0.088* |
| Female | 34 (37%) | 8 (34.8%) | 5 (22%) | 4 (23.5%) | 13 (50%) | |
| Age | 57.9±21.1 | 54.7±24.2 | 60.2±20.2 | 46.9±22.1 | 66.2±16.2 | 0.150 |
| VCDR | 0.74±0.27 | 0.91±0.07 | 0.88±0.1 | 0.92±0.1 | 0.37±0.17 | <0.001‡ |
| Pre-op CRF | 8.4±1.5 | 7.7±1.9 | 8.5±0.8 | 8.7±1.7 | 8.7±1.2 | 0.835‡ |
| Pre-op CH | 6.2±2.3 | 5.4±1.8 | 5.3±1.7 | 5.2±2.6 | 8.1±1.8 | <0.001‡ |
| CCT | 540±23 | 536±23 | 546±26 | 547±27 | 537±19 | 0.655‡ |
Based on Chi-Square test
Based on ANOVA
PT, phaco-trabeculectomy; AGV, Ahmed glaucoma valve implantation; PE, phacoemulsification; OD, right eye; OS, left eye; VCDR, vertical cup to disc ratio; CRF, corneal resistant factor; CH, corneal hysteresis; CCT, central corneal thickness
Table 2.
Corneal hysteresis (CH) and corneal resistance factor (CRF) before and three months after surgery
| Mean ± Standard Deviation | ||||
|---|---|---|---|---|
| Trabeculectomy | PT | AGV | PE | |
| Pre-operative CH (mmHg) | 5.4±1.8 | 5.3±1.7 | 5.2 ±2.6 | 8.1±1.8 |
| Post-operative CH ( mmHg) | 7.5±1.1 | 7.6±0.9 | 7.5±1.7 | 8.1±1.6 |
| P (within group) | <0.001 | <0.001 | <0.001 | 0.704 |
| Pre-operative CRF | 7.7±1.9 | 8.5 ± 0.8 | 8.7±1.7 | 8.7±1.2 |
| Post-operative CRF | 9±1.6 | 8.9±0.9 | 9.3±2 | 9.1±1.3 |
| P (within group) | <0.001 | <0.001 | <0.001 | <0.001 |
PT, Phacotrabeculectomy; AGV, Ahmed glaucoma valve implantation; PE, phacoemulsification; CH, corneal hysteresis; CRF, corneal resistance factor
In glaucomatous eyes, mean preoperative GAT, IOPg, and IOPcc were 25.9±6.6, 24.4±7.6, and 27.4±8.1 mmHg, respectively which reached 11.10±2.3, 11.7±2.7, and 12.7±2.6 mmHg respectively, three months postoperatively. Corresponding values in group 4 were 16.4±2.8, 15.7±3.1, and 16.9±3.9 mmHg preoperatively; and 14.8±2.1, 14±2.4 and 14.9±2.5 mmHg, postoperatively (Table 3).
Table 3.
Comparison of IOPg and IOPcc values before and after operations
| Mean ± SD | ||||
|---|---|---|---|---|
| Trabeculectomy | PT | AGV | PE | |
| Pre-operative IOPg (mmHg) | 22.8±6.4 | 23.3±5.4 | 31.2±7.2 | 15.7±3.1 |
| Post-operative IOPg (mmHg) | 10±2.1 | 10.6±1.8 | 11.9±2.2 | 14±2.4 |
| IOPg change mmHg | 12.9±6.6 | 12.7±5.6 | 19.3±7.9 | 1.8±3.4 |
| P (within group) | <0.001 | <0.001 | <0.001 | 0.012 |
| Pre-operative IOPcc (mmHg) | 24.7±7 | 24.8±6.6 | 32.4±7.1 | 16.9±3.9 |
| Post-operative IOPcc (mmHg) | 11.4±2 | 11.3±1.8 | 12.7±2.2 | 14.9±2.5 |
| IOPcc change (mmHg) | 13.3±7.2 | 13.5±6.8 | 19.6±7.9 | 2±4.3 |
| P (within group) | <0.001 | <0.001 | <0.001 | 0.021 |
PT, Phacotrabeculectomy; AGV, Ahmed glaucoma valve; PE, Phacoemulsification; IOPg, Goldman correlated intraocular pressure; IOPcc, corneal compensated intraocular pressure
There was no significant correlation between the duration of MMC application and CH changes in the three glaucoma subgroups; Spearman correlation coefficients were rs=0.129 (p=0.599), rs=-0.366 (p=0.123) and rs=0.512 (p=0.107) in groups 1, 2, and 3 respectively. The mean number of preoperative glaucoma medications in glaucomatous eyes was 2.78±1, which was significantly correlated with preoperative CH (rs=-0.550, p<0.001). CCT was comparable among the different study subgroups (p=0.655).
DISCUSSION
Corneal biomechanical properties are important in many fields of ophthalmology and their evaluation can provide insight to pathologic changes of the cornea. It has been shown that CH is not correlated with corneal curvature, astigmatism, or axial length.4 However, the influence of intraocular surgery is not very well understood. In this prospective comparative case series we evaluated the influence of certain intraocular procedures on corneal biomechanics in 63 glaucomatous and 26 non-glaucomatous eyes.
We found lower CH values in glaucomatous eyes preoperatively with significant elevation after glaucoma surgery, especially when the procedure was successful and IOP was reduced by more than 10 mmHg. We also observed that CRF was significantly increased after the operation in all groups.
The ORA is an instrument that measures dynamic aspects of corneal remolding by air pulses. A metered air pulse is directed onto the cornea until applanation is achieved; after reaching a peak, air pressure is steadily reduced until it is completely removed.9 The ORA makes two measurements, the first is the force required to applanate the cornea (P1= force-in applanation) and second the force at which the cornea flattens again as the air pulse falls (P2=force-out applanation). P2 occurs at a lower pressure and is related to viscoelastic dampening effect of the cornea. The difference between these two forces (P1- P2) is named CH.10 CH is a direct measure of corneal biomechanical properties and better describes the contribution of corneal resistance to IOP measurements than CCT alone.10 CRF is derived from the formula (P1- kP2) where k is a constant. CH predominantly reflects viscous properties of the cornea while CRF reflects the elastic properties of the cornea. The ORA also provides measurements called cornea compensated intraocular pressure (IOPcc), which is obtained from the difference between the two applanation pressures using the formula P2-kP1. The IOPcc is supposed to represent a measure of IOP that is free of corneal influence. The constant k has a value of 0.43, which was derived from a study on intraocular pressure changes before and after refractive surgery.10
Sun8 and Luce11 reported that glaucomatous eyes with elevated IOP have much lower CH than normal eyes. Our findings are in agreement with their observations. CCT values did not account for differences in CH between glaucomatous and non-glaucomatous eyes. Sustained IOP elevation does not appear to cause changes in CCT; glaucomatous corneal changes may better be reflected by microstructural changes that affect CH rather than macrostructural alterations that can affect CCT.8 In a series of 230 patients, Congdon and associates2 reported that glaucomatous eyes have lower CH; they also found that lower CH is associated with more visual field progression which has also been described in another study by De Moraes.12
There are few publications showing that clear cornea phacoemulsification leads to changes in ocular biomechanical properties. De Freitas et al13 found that CH and CRF both decrease after phacoemulsification. In our study however, CH increased after glaucoma surgery but remained unchanged after PE; CRF was increased after all types of surgeries.
In our series, CH was significantly increased after successful glaucoma surgery especially when IOP reduction exceeded 10 mmHg. Multiple factors may play a role in CH changes in glaucoma patients after IOP reduction. Higher IOP prior to glaucoma surgery might be the first factor; Sun et al8 also detected partial recovery and an increase in CH after successful IOP control, which is in agreement with our observations. However in another study, Martines14 stated that CH is independent of IOP changes measured by the Goldmann device. There may be other factors in glaucoma that alter corneal biomechanics. Even though it has been reported that no correlation exists between CH and keratometric values in non-pathologic corneas;4,15 the surgical incision and changes in corneal curvature especially due to scleral flap dissection into clear cornea may be responsible for CH changes. If IOP remains constant, an incision may weaken ocular tissues leading to a decrement in CH; after successful IOP control, the effect of IOP reduction on CH is probably larger than the impact of the incision and the net result could be an increase in CH.
Treatment with topical glaucoma medications may also induce changes in corneal biomechanics, in addition to the effect on IOP. It has been shown that instillation of eye drops containing preservatives modifies the ocular surface. Theoretically this modification could influence viscoelastic properties of the cornea.16,17 Topical application of prostaglandin F2a analogs over the cornea reduces CCT and might have an effect on corneal biomechanics.18 We looked at this issue and found a negative correlation between the numbers of consumed medications and preoperative CH. Discontinuation of anti-glaucoma drops after the operation may be another reason for increased CH. We also considered MMC as a possible source of influence but found no correlation between duration of MMC application and CH in any of the study subgroups (P>0.11).
The current study is, to our knowledge, the first to demonstrate an increase in CH after successful glaucoma procedures. One limitation of our study is the sample size in each subgroup which may not have been large enough to reflect the real magnitude of CH changes following intraocular surgery.
In summary, our study revealed that CH is significantly lower in glaucomatous eyes as compared to non-glaucomatous counterparts, and that CH and CRF increase after successful glaucoma procedures. Awareness of corneal biomechanical changes is important and should be kept in mind especially when one measures IOP with regular tonometers following glaucoma procedures. Further studies are warranted to account for these observations.
Figure 1.
Scatter plot demonstrating the correlation between changes in corneal hysteresis (CH) against postoperative intraocular pressure (IOP) reduction. CH change was negatively correlated with IOP reduction in all groups. PE, phacoemulsification; PT, phacotrabeculectomy; AGV, Ahmed glaucoma valve implantation
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Wang J, Cayer MM, Descovich D, Kamdeu-Fansi A, Harasymowycz PJ, Li G, et al. Assessment of factors affecting the difference in intraocular pressure measurement between dynamic contour tonometry and Goldmann applanation tonometry. J Glaucoma . 2011;20:482–487. doi: 10.1097/IJG.0b013e3181efbe8f. [DOI] [PubMed] [Google Scholar]
- 2.Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141:868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Hager A, Loge K, Füllhas MO, Schroeder B, Grossherr M, Wiegand W. Changes in corneal hysteresis after clear corneal cataract surgery. Am J Ophthalmol. 2007;144:341–346. doi: 10.1016/j.ajo.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Shah S, Laiquzzaman M, Cunliffe I, Mantry S. The use of the Reichert ocular response analyzer to establish the relationship between ocular hysteresis, corneal resistance factor and central corneal thickness in normal eyes. Cont Lens Anterior Eye. 2006;29:257–262. doi: 10.1016/j.clae.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. Ocular response analyzer in subjects with and without glaucoma. Optom Vis Sci. 2008;85:463–470. doi: 10.1097/OPX.0b013e3181784673. [DOI] [PubMed] [Google Scholar]
- 7.Broman AT, Congdon NG, Bandeen-Roche K, Quigley HA. Influence of corneal structure, corneal responsiveness, and other ocular parameters on tonometric measurement of intraocular pressure. J Glaucoma. 2007;16:581–588. doi: 10.1097/IJG.0b013e3180640f40. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Shen M, Wang J, Fang A, Xu A, Fang H. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am J Ophthalmol. 2009;147:1061–1066. doi: 10.1016/j.ajo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF. Corneal thickness and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2006;47:5337–5347. doi: 10.1167/iovs.06-0557. [DOI] [PubMed] [Google Scholar]
- 11.Luce D, Taylor D. Reichert ocular response analyzer measures corneal biomechanical properties and IOP: provides new indicators for corneal specialties and glaucoma management. Ocular Response Analyzer white paper. [March, 2006]. http://www.ocularresponseanalyzer.com/downloads.html.
- 12.De Moraes CE, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21:209–213. doi: 10.1097/IJG.0b013e3182071b92. [DOI] [PubMed] [Google Scholar]
- 13.de Freitas Valbon B, Ventura MP, da Silva RS, Canedo AL, Velarde GC, Ambrósio R. Central corneal thickness and biomechanical changes after clear corneal phacoemulsification. J Refract Surg. 2012;28:215–219. doi: 10.3928/1081597X-20111103-02. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, Mendez-Hernandez C, Garcia-Sanchez J. Ocular Response Analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Invest Ophthalmol Vis Sci. 2006;47:4410–4414. doi: 10.1167/iovs.06-0158. [DOI] [PubMed] [Google Scholar]
- 15.Montard R, Kopito R, Touzeau O, Allouch C, Letaief I, Borderie V, et al. Ocular Response Analyzer: feasibility study and correlation with normal eyes. J Fr Ophthalmol. 2007;30:978–984. doi: 10.1016/s0181-5512(07)79273-2. [DOI] [PubMed] [Google Scholar]
- 16.Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48:4123–4128. doi: 10.1167/iovs.07-0266. [DOI] [PubMed] [Google Scholar]
- 17.Baudouin C, Liang H, Hamard P, Riancho L, Creuzot-Garcher C, Warnet JM, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-Helper 1 and T-Helper 2 pathways. Ophthalmology. 2008;115:109–115. doi: 10.1016/j.ophtha.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Viestenz A, Martus P, Schlötzer-Schrehardt U, Langenbucher A, Mardin CY. Impact of prostaglandin-F (2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness- a cross-sectional study on 403 eyes. Klin Monbl Augenheilkd. 2004;221:753–756. doi: 10.1055/s-2004-81361. [DOI] [PubMed] [Google Scholar]