Abstract
Purpose
To determine the safety of intravitreal zoledronic acid (ZA) in the rat eye.
Methods
Twenty eyes of 20 pigmented rats were randomized into five groups to receive an intravitreal injection of 8, 4, 2 and 1 micrograms (mcg) of ZA, or balanced salt solution (BSS). One week and one month after the injections, all eyes were evaluated for intraocular inflammation. Electroretinography (ERG) was performed before, and one week and one month after the injections. All eyes were enucleated one month after the injection for histologic examination.
Results
No significant inflammatory response was observed in any eye. No significant decrease in ERG amplitude (a & b waves) was observed one week and one month after intravitreal ZA injection, as compared to baseline, BSS-treated eyes or non-injected fellow eyes. Histologic examination of the retinal pigment epithelium and neurosensory retina were unremarkable in all groups. Additionally, no significant increase in immune reactivity for glial fibrillary acidic protein was noted in any eye.
Conclusion
Based on clinical, histopathologic and ERG findings in this experimental study, up to 8 mcg of intravitreal zoledronic acid seems to be safe in the rat eye.
Keywords: Zoledronic Acid (ZA), Choroidal Neovascularization (CNV), Age-Related Macular Degeneration (AMD)
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of legal blindness in patients aged 65 and over,1 and is the most common overall cause of blindness in the Western world.2 Choroidal neovascularization (CNV) is the major cause of irreversible blindness in patients with AMD.3 Various factors are suspected to be involved in the development of CNV, such as the vascular endothelial growth factor (VEGF)4 and inflammation.5 Studies have revealed an increased number of lymphocytes, fibroblasts and macrophages within Bruch’s membrane in patients with AMD.5,6 Macrophages play a key role in regulating angiogenesis in the retina and choroid.7 One study showed that macrophage depletion reduced CNV size, cellularity and vascularity.8
Zoledronic acid (ZA) is the most potent form of nitrogen-containing bisphosphonates (BPs).9 Bisphosphonates were developed as powerful inhibitors of osteoclasts and are commonly used to treat and prevent osteoporosis. Recent reports have suggested multiple pharmacologic effects for BPs, such as anti-angiogenic effects via the inhibition of macrophages together with inhibition of production of the pro-angiogenic matrix metalloproteinase (MMP)-9 and VEGF.10-12 Honda et al3 have shown that oral BPs in CNV associated with AMD and pathological myopia significantly decrease CNV size and improve visual acuity. Therefore, ZA may be an effective treatment modality for ocular neovascularization in the future. To achieve a high concentration of ZA in the eye and to reduce its systemic side effects, intravitreal injection of ZA may be superior to systemic administration for treatment of CNV. However, the safety of intraocular injections of ZA has not yet been evaluated; we therefore performed this study to determine the safe dose for intravitreal injection of ZA in a rat model.
METHODS
Intravitreal Injections
Twenty pigmented rats (Razi rats) weighing 250 to 300 grams were divided into five groups (A, B, C, D and E) to receive intravitreal injections of 8, 4, 2 and 1 micrograms (mcg) ZA (Zometa, Novartis, Pharmaceuticals Ltd., UK), or balanced salt solution (BSS) in the left eyes, respectively. The animals were anesthetized using an intraperitoneal injection of ketamine hydrochloride (80 mg/kg) and xylazine hydrochloride (5 mg/kg). Topical anesthesia was achieved with tetracaine. The pupils were dilated with topical tropicamide (0.5%). Intravitreal injections were performed under aseptic conditions using a Hamilton syringe. The animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) guidelines.
Slit lamp and indirect fundus examinations were performed on all animal eyes at baseline, and one week and one month after intravitreal injections. Animals with corneal or lens opacities prior to the study were excluded.
Electroretinogram
All animals were evaluated by electroretinography (ERG) before, and one week and one month after the intravitreal injections. To perform the ERG, the pupils were dilated with 1 drop of tropicamide 0.5%. Standard ERG was recorded in both eyes using corneal electrodes. The reference and ground electrodes were inserted on the frontal area and the ears. The ERG signals were recorded using Retiport/scan 21 electrophysiological diagnostic systems (Roland Consult, Brandenburg an der Havel, Germany). The photopic response, scotopic flash response (maximal combined) and scotopic response after 20 minutes of dark adaptation were recorded simultaneously in both eyes. Amplitudes of "a" and "b" waves were calculated for each recording before, and one week and one month after the injections. ERG changes were considered significant if b wave amplitudes were reduced by at least 30% from baseline.13
Histologic Examination
One month after intravitreal injections, the rats were euthanized with an overdose of phenobarbital and both eyes were enucleated and fixed in 10% formalin. After bisecting the eyes axially into two calottes and embedding them in paraffin blocks, thin tissue sections were prepared at three different tissue levels (100 microns apart). The sections were stained with hematoxylin & eosin (H&E) and examined under light microscopy (BX41, Olympus, Japan) for presence or absence of retinal hemorrhages, inflammation, necrosis and atrophy, and integrity of the retinal pigment epithelium (RPE) and neurosensory retina.
The retinas of all groups were also tested for glial fibrillary acidic protein (GFAP) immune reactivity (polyclonal rabbit anti-glial fibrillary acidic protein, DAKO, Denmark). GFAP immune reactivity in Muller cells in the injected groups was graded as remarkable and unremarkable, and compared to the controls (BSS group).
RESULTS
Among 20 rats, one rat from group B and 2 rats from group D expired before completion of the study and 17 rats were finally evaluated.
Clinical Observations
One week after intravitreal injections, cataracts developed in one eye in group A and 2 eyes in group E, however slit lamp and fundus examination revealed no inflammatory response in any of the eyes.
Electroretinogram
Based on the results of photopic, scotopic and flicker responses, no significant change was observed in ERG amplitudes or latency of a and b waves in eyes receiving intravitreal ZA as compared to baseline values, as well as non-injected eyes and BSS injected eyes. These findings indicate no gross retinal functional damage with up to 8 mcg of intravitreal ZA in rat eyes. ERG scotopic b wave amplitudes at baseline, and 1 and 4 weeks after injection are detailed in Table 1.
Table 1.
Mean scotopic ERG-b wave amplitudes and histologic findings in ZA-injected and control eyes
| Groups | Rats | Baseline ERG | 1 Week ERG | 1 Month ERG | Histologic Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preserved retinal integrity | RH and/or RI | RN and/or RA | VI | VH | Unremarkable GFAP | |||||
| Control (E) | E1 | 356 | 352 | 26 | + | - | - | - | + | + |
| E2 | 398 | 363 | 78 | + | - | - | + | + | + | |
| E3 | 142 | 331 | 37 | + | - | - | + | + | + | |
| E4 | 312 | 113 | 36 | + | - | - | - | - | + | |
| 1 mcg ZA (D) | D1 | 112 | 452 | 4 | + | - | - | + | - | + |
| D2 | 180 | 146 | 69 | + | - | - | + | - | + | |
| 2 mcg ZA (C) | C1 | 145 | 382 | 120 | + | - | - | + | + | + |
| C2 | 118 | 448 | 884 | + | - | - | - | - | + | |
| C3 | 748 | 277 | 293 | + | - | - | + | - | + | |
| C4 | 325 | 238 | 51 | + | - | - | - | + | + | |
| 4 mcg ZA (B) | B1 | 177 | 317 | 75 | + | - | - | + | - | + |
| B2 | 220 | 127 | 38 | + | - | - | + | + | + | |
| B3 | 402 | 531 | 28 | + | - | - | - | + | + | |
| 8µg ZA (A) | A1 | 384 | 135 | 121 | + | - | - | - | - | + |
| A2 | 330 | 224 | 215 | + | - | - | + | - | + | |
| A3 | 104 | 536 | 113 | + | - | - | + | + | + | |
| A4 | 284 | 331 | 125 | + | - | - | + | + | + | |
ZA, zoledronic acid; RH, retinal hemorrhage; RI, retinal inflammation; RN, retinal necrosis; RA, retinal atrophy; VI, vitreous inflammation; VH, vitreous hemorrhage; GFAP, glial fibrillary acidic protein
Histologic Findings
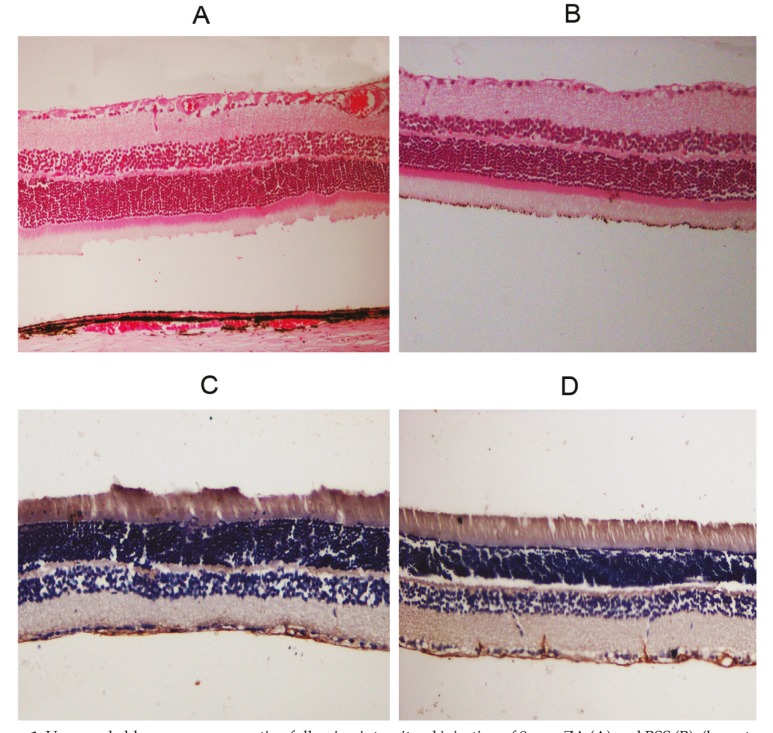
A summary of histopathologic findings in ZA-injected and control eyes is provided in Table 1. None of the eyes in the study groups disclosed intraretinal hemorrhage, inflammation, necrosis or atrophy; furthermore, integrity of retinal layers was well preserved in all cases (Fig. 1). Mild degrees of intravitreal hemorrhage and inflammation were seen in most ZA- as well as BSS-injected eyes (Table-1). GFAP immune reactivity of retinal Muller cells was unremarkable in all ZA-injected eyes as compared to controls (Figure 1).
Figure 1.
Unremarkable neurosensory retina following intravitreal injection of 8 mcg ZA (A) and BSS (B), (hematoxylin & eosin stain, magnification ×200). Immunohistochemical staining for glial fibrillary acidic protein (GFAP) was not remarkable with intravitreal injection of either 8 mcg ZA (C) or BSS (D), (magnification ×200). ZA, zoledronic acid; BSS, basal salt solution
DISCUSSION
Considering ERG findings and microstructural features observed in the current study, no toxic effect was observed following intravitreal injection of ZA up to 8 mcg in rat eyes. Furthermore, the expression of GFAP was not significantly increased in Muller cells of ZA-injected eyes. This observation, in association with unremarkable histologic features, indicates lack of toxicity of intravitraeal ZA to the rat retina. Mild vitreous hemorrhage and inflammation which was observed in all study groups was related to the intravitreal injection procedure.
CNV is a highly complex, dynamic process and so far little is known about its molecular mechanisms. Multiple factors have been related to CNV formation including inflammatory cells such as lymphocytes and macrophages,14 in addition to VEGF and MMP.4,11,12 Current therapy for CNV associated with AMD consists of repeated intravitreal injection of anti-VEGF antibodies.15 Anti-VEGF therapy requires monthly injections of the agents for a long time to maintain vision, which poses a cumulative risk for ocular and systemic complications. Therefore, new treatment modalities and new intravitreal drugs with more prolonged efficacy seem to be necessary.
Zoledronic acid (ZA) or zoledronate (marketed by Novartis) is a bisphosphonate compound used to prevent skeletal fractures in patients with cancers such as multiple myeloma and prostate cancer , as well as to treat osteoporosis .16 Santini et al17 reported that after infusion of ZA, median serum VEGF levels were significantly decreased at days 2, 7 and 21. Serum platelet derived growth factor (PDGF) levels were also significantly decreased by 25% one day after ZA infusion. Recent reports have demonstrated that nitrogen-containing bisphosphonates such as ZA inhibit angiogenesis both in vitro and in vivo.10 Bisphosphonate may suppress CNV via direct inhibition of vascular endothelial cell proliferation, inhibition of VEGF expression, MMP and the integrin families.10,15 In addition, bisphosphonates are known to control the inflammation induced by macrophages and mononuclear cells which are one of the mechanisms involved in CNV formation.8 Inhibition of macrophages was also shown to prevent intraocular tumor growth in a murine model, probably through inhibition of intra-tumoral macrophage-induced angiogenesis.
Two studies revealed that intra-peritoneal bisphosphonate injection decreased the size of experimental CNV lesions.10,18 One human study showed that oral bisphosphonate maintained visual acuity for at least 6 months and significantly reduced the size of the lesion in patients with CNV due to AMD and pathologic myopia.3
As mentioned above, systemic use of ZA has anti-angiogenic effects, however information on ocular penetration of systemically administered ZA and intraocular concentration of ZA after oral intake is limited. Intravitreal injection of ZA presumably achieves higher intraocular concentrations leading to greater drug efficacy. In the field of ophthalmology, bisphosphonates have been regarded as drugs that may cause ocular inflammation.19,20 The current series is the first study reporting the safe dose of intravitreal injection of ZA.
In conclusion, our study showed that intravitreal injection of ZA up to a maximum dose of 8 mcg has no toxic retinal effect in rat eyes. The next step would be evaluation of the efficacy of intravitreal ZA on CNV.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Ferris FL. Senile macular degeneration: review of epidemiologic features. Am J Epidemiol. 1983;118:132–151. doi: 10.1093/oxfordjournals.aje.a113624. [DOI] [PubMed] [Google Scholar]
- 2.Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 3.Honda S, Nagai T, Kondo N, Fukuda M, Kusuhara S, Tsukahara Y, et al. Therapeutic effect of oral bisphosphonates on choroidal neovascularization in the human eye. J Ophthalmol. 2010;doi:10. doi: 10.1155/2010/206837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 5.Loffler KU, Lee WR. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224:493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- 6.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 7.Apte RS. Regulation of angiogenesis by macrophages. Adv Exp Med Biol. 2010;664:15–19. doi: 10.1007/978-1-4419-1399-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 9.Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM, et al. Novel antiangiogenic effects of bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T, Imai H, Honda S, Negi A. Antiangiogenic effects of bisphosphonates on laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2007;48:5716–5721. doi: 10.1167/iovs.07-1023. [DOI] [PubMed] [Google Scholar]
- 11.Rogers TL, Holen I. Tumor macrophages as potential target of bisphosphonates. J Transl Med . 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonates targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond) 1990;4:613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 15.Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16.National Prescribing Service. "Zoledronic Acid for osteoporosis" Medcines Update (2009). [January 27, 2011]. http://www.nps.org.au/consumers/publications/medicine_update/issues/Zoledronic_acid.
- 17.Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti D, et al. Repeated intermittent low dose therapy with zoledronic acid induces an early, sustained and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13:4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 19.Patel DV, Horne A, House M, Reid IR, McGhee CN. The incidence of acute anterior uveitis after intravenous zoledronate. Ophthalmology. 2013;120:773–776. doi: 10.1016/j.ophtha.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Colucci A, Modorati G, Miserocchi E, Di Matteo F, Rama P. Anterior uveitis complicating zoledronic acid infusion. Ocul Immunol Inflamm. 2009;17:267–268. doi: 10.1080/09273940902916111. [DOI] [PubMed] [Google Scholar]