Abstract
Recent success in restoring visual function through photoreceptor replacement in mouse models of photoreceptor degeneration intensifies the need to generate or regenerate photoreceptor cells for the ultimate goal of using cell replacement therapy for blindness caused by photoreceptor degeneration. Current research on deriving new photoreceptors for replacement, as regenerative medicine in general, focuses on the use of embryonic stem cells and induced pluripotent stem (iPS) cells to generate transplantable cells. Nonetheless, naturally occurring regeneration, such as wound healing, involves awakening cells at or near a wound site to produce new cells needed to heal the wound. Here we discuss the possibility of tweaking an ocular tissue, the retinal pigment epithelium (RPE), to produce photoreceptor cells in situ in the eye. Unlike the neural retina, the RPE in adult mammals maintains cell proliferation capability. Furthermore, progeny cells from RPE proliferation may differentiate into cells other than RPE. The combination of proliferation and plasticity opens a question of whether they could be channeled by a regulatory gene with pro-photoreceptor activity towards photoreceptor production. Studies using embryonic chick and transgenic mouse showed that indeed photoreceptor-like cells were produced in culture and in vivo in the eye using gene-directed reprogramming of RPE cells, supporting the feasibility of using the RPE as a convenient source of new photoreceptor cells for in situ retinal repair without involving cell transplantation.
Keywords: Photoreceptor, Regeneration, Replacement, Retina, Retinal Pigment Epithelium
INTRODUCTION
Photoreceptors are the primary neurons in the vertebrate retina and their degeneration underlies many forms of visual impairment. Studies using mouse models of photoreceptor degeneration have demonstrated successful rescue or restoration of visual function through transplantation of photoreceptor cells harvested from young retina or generated in vitro from mouse stem cells.1-3 This has generated great excitement in the vision community and in the general public. At the same time, it has heightened the scientific and societal importance of defining a reliable source of new photoreceptor cells.
Current research on deriving new photoreceptors for replacement, as regenerative medicine in general, centers on using embryonic stem cells and induced pluripotent stem (iPS) cells to generate transplantable cells.4-10 Great strikes have been made towards the ultimate goal of cell replacement using photoreceptor cells derived from the patient’s own somatic cells through iPS cell technology. Nonetheless, nature uses a different approach to repair an injury. It begins with awakening adult cells at the site of a wound to proliferate, followed by activating/reactivating cell differentiation programs to produce the desired cells. If an in vivo regeneration mechanism could be employed to produce new photoreceptor cells, then photoreceptor replacement could be attainable without cell transplantation and associated risks and complications.
CHALLENGES FACING PHOTORECEPTOR REGENERATION IN THE MATURE MAMMALIAN RETINA
Injuries induce photoreceptor regeneration from retinal stem cells residing at the ciliary margin in teleost fish eyes.11 However, this regeneration mechanism seems lacking in mammals.12,13 The previously reported presence of retinal stem cells in adult ciliary epithelium of the mammalian eye14,15 has been contested.16,17 A recent study reported the presence of multipotent stem cells in the retina of 4-8 weeks old mice that could be expanded in vitro for over 35 passages and produced different types of cells including functional photoreceptor cells.18 It would be interesting and important to demonstrate the presence of multipotent retinal stem cells in well matured and aged mice. Muller glia in various species, including mammals, possess certain properties of progenitor cells, but their ability to efficiently give rise to photoreceptors needs to be demonstrated.19-28
ALTERNATIVE OCULAR TISSUES AS A SOURCE OF NEW PHOTORECEPTORS
The rather disappointing outcomes from exploring the neural retina for photoreceptor regeneration, along with the discoveries of multipotent stem cells from various tissues in adult mammals, have spurred interests in non-neural tissues of the eye for photoreceptor genesis. Alternative sources being examined include the iris pigment epithelium,29-34 the ciliary body,14,15,32,35 the limbal epithelium,36 and the retinal pigment epithelium (RPE). Excluding the RPE, none of the alternatives give rise to a significant number of, if any, photoreceptor-like cells. This may reflect the biological nature of these tissues; it may also stem from the used approaches, as few of the studies used a regulatory gene with pro-photoreceptor activity to steer uncommitted cells toward the photoreceptor path or to initiate photoreceptor differentiation program in the otherwise non-neural cells.
ATTRACTION OF THE RPE
The anatomical location places the RPE at an ideal position for providing new photoreceptors to repopulate a damaged retina. The key question upfront is whether or not the RPE is biologically amenable to a reprogramming scheme to give rise to photoreceptor cells. Developmentally, the RPE and the neural retina originate from the same structure, i.e. the optic vesicle. This common origin may facilitate fate changes. Indeed, classic experiments have revealed an intriguing phenomenon, RPE becoming a neural retina, referred to as RPE transdifferentiation. In very young chick embryos, physically separating the RPE from the neural retina,37 or surgically removing most of the neural retina,38 results in the RPE developing into a neural retina. Subsequent investigation led to the discovery of bFGF as a stimulus for this phenomenal transdifferentiation.39 RPE-to-neural retina transdifferentiation also occurs in amphibians.40-42 In culture, rodent RPE from young embryos has been shown to undergo RPE-to-retina transdifferentiation.43,44 Mutations in regulatory factors involved in regulating RPE versus retinal fate or disruptions of bone morphogenetic protein (BMP) and Wnt (Int/wingless) signaling pathways can result in ventral RPE transdifferentiation into neural retina.45-52 Notably, this RPE-to-retina transdifferentiation results in the RPE to be no longer present.
The RPE plays important roles in the well-being and function of photoreceptor cells and the retina as a whole. Dysfunctional RPE is believed to be an underlying pathological condition in age-related macular degeneration, a leading cause of blindness in the elderly in developed countries. Adult mammalian RPE is well-known for two biological properties: proliferation and plasticity. In the mature eye, most cells in the RPE remain quiescent, except a small population in the periphery where cell proliferation has been observed.53 However, RPE cells can re-enter the cell cycle to proliferate upon retinal detachment,54-56 when stimulated physically,57 or under disease conditions.60-62 This proliferative response may result in RPE regeneration/wound healing.61-65 It may also lead to proliferative retinopathy when progeny cells from RPE proliferation differentiate into cells with tractional force causing retinal detachment.66 On the other hand, these very traits of proliferation and plasticity raise an intriguing possibility of exploring the RPE as a source of new photoreceptor cells.
With mounting knowledge on the regulatory guidance of photoreceptor production during retinal development, an alternative approach emerged to produce new photoreceptor cells, i.e. reprogramming the RPE by genes with pro-photoreceptor activities, to channel RPE proliferation and plasticity towards photoreceptor production.
RPE-TO-PHOTORECEPTOR REPROGRAMMING IN EMBRYONIC CHICK CELL CULTURE
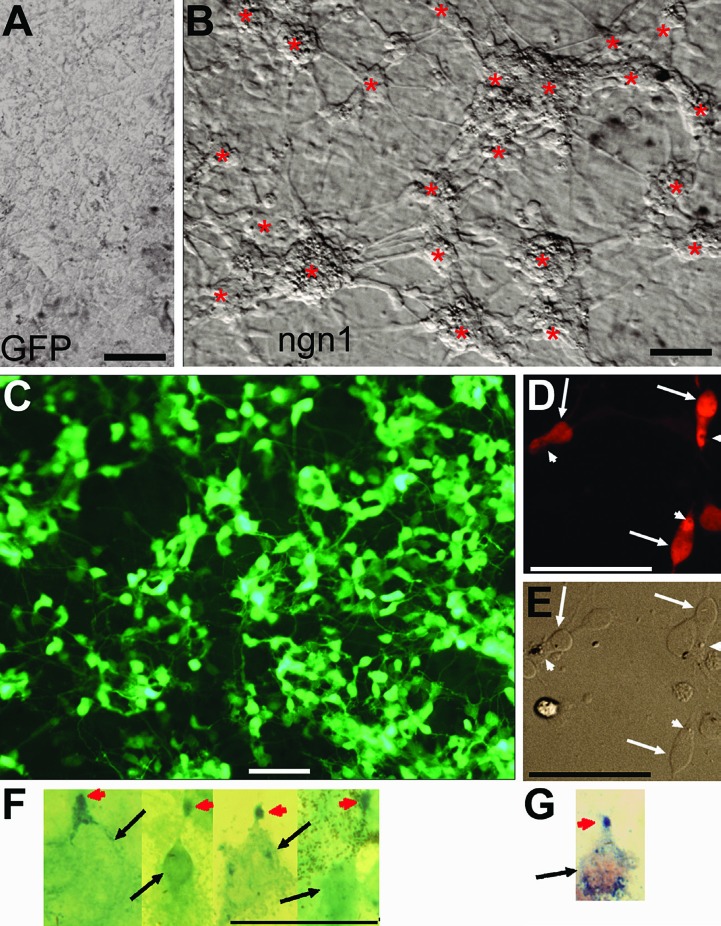
The feasibility of channelling RPE proliferation and plasticity to photoreceptor production using genes that steer unspecified cells toward the path of differentiating into photoreceptors was first tested with the chick system, taking advantage of abundant RPE tissue from chick embryos and readily achievable wide-spread gene transduction from replication-competent retrovirus RCAS. Dissociated RPE cell culture was established from chick embryos at day 6 (E6) and thereafter, stages at which the RPE has already lost its competence to undergo the classic RPE-to-retina transdifferentiation. Cells in the primary RPE cell culture were then infected with RCAS expressing a gene with pro-photoreceptor activities. Using this system, over 20 regulatory genes shown or implicated to be important for the development of the eye, the retina, and/or photoreceptor cells have been screened for activities to guide cultured RPE cells to the path of differentiating towards photoreceptors.67 The screening identified neurogenin1 (ngn1) and ngn3 as the two top-performers for eliciting RPE-to-photoreceptor reprogramming.67 Both genes induced the production of neural clusters from otherwise monolayer RPE cell cultures (Figures 1A and B)67 and de novo generation of large numbers (as high as 80% of the cells present in the culture) of cells (referred to as reprogrammed cells) positive for photoreceptor protein visinin (Figure 1C).67
Figure 1.
Photoreceptor-like cells in reprogrammed RPE cell culture derived from day 6 chick embryos. A: Bright field view of a control culture infected with RCAS-GFP. B: Bright field view of ngn1 reprogrammed culture (infected with RCAS-ngn1) displaying neuron-like clusters (*), which were absent in the control (A). C: Epi-fluorescence view of reprogrammed culture immunostained for photoreceptor protein visinin. D, E: Morphologies of visinin+ cells viewed with bright field (E) and epi-fluorescence (D). Arrows point to the cell body and arrowheads point to a structural feature reminiscent of the lipid-droplet typically present in chick photoreceptors. F: Morphologies of red opsin+ cells in ngn1 reprogrammed culture. Arrows point to cell bodies. Arrowheads point to cells’ apices decorated by anti-red opsin immunostaining. G: A cell double-labeled for visinin (in red) and red opsin (in blue) in ngn1 reprogrammed culture. Scale bars: 50 μm. [Data are from the authors’ laboratories]. [Modified from Yan et al., 2010. Originally published in Journal of Comparative Neurology; DOI 10.1002/cne.22236].67
Molecularly, the reprogrammed cells expressed transcription factors crx, nr2e3, raxL, RXRγ, and neuroD, which participate in initiating the photoreceptor differentiation program. Reprogrammed cells also expressed components of phototransduction, including red opsin, the α-subunit of CNG channels, and cone α-transducin. Red opsin+ cells displayed dot-like immunostaining at the apices of the cells (Figures 1F and G),67 reminiscent of that in the retina, indicating proper localization of red opsin in the reprogrammed cells.
Morphologically, in contrast to the hexagonal RPE cells, visinin+ cells resembled young photoreceptor cells, with an elongated cell body, an axon-like process, an inner segment-like compartment, and a lipid droplet-like structural feature (Figure 1D and E).67 Electron microscopy showed that reprogrammed cells developed a cellular compartment rich in mitochondria, resembling the inner segment of photoreceptor cells.67 On the apex of the inner segment-like structure, reprogrammed cells displayed ciliary expansions, reminiscent of the developing outer segments of retinal photoreceptors in E17 eye or in culture.
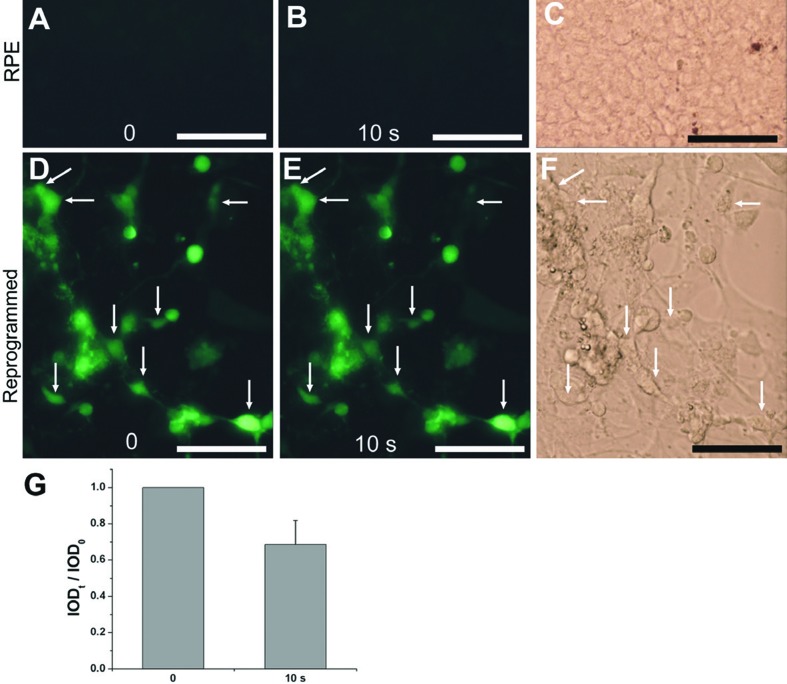
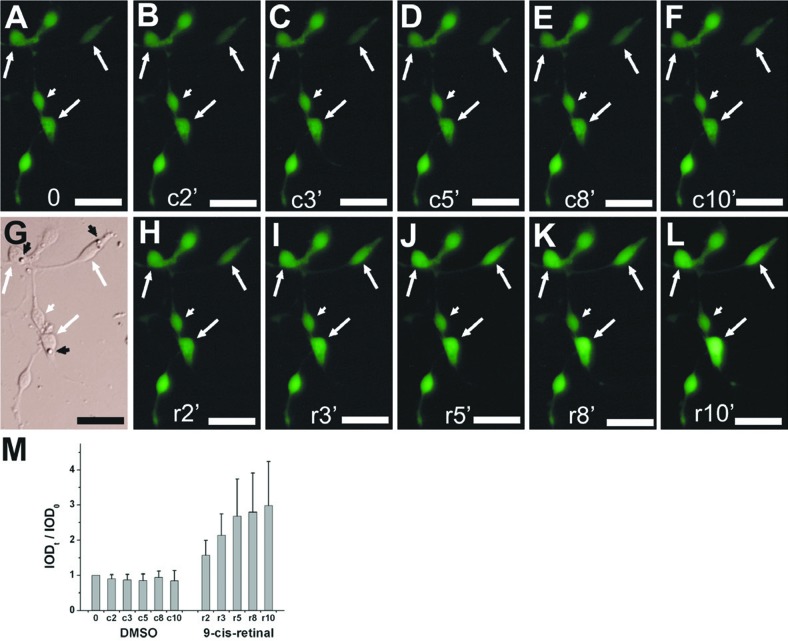
Physiologically, light response and visual recovery are two hallmarks of photoreceptors. Fluorescent calcium imaging showed that reprogrammed cells exhibited both hallmarks: they responded to light by decreasing their Ca2+ levels (Figure 2) and responded to 9-cis-retinal by increasing their Ca2+ levels (Figure 3).67 These results indicate that reprogrammed cells were able to develop advanced photoreceptor traits at molecular, structural, and physiological levels.
Figure 2.
Light responses of reprogrammed cells examined with Ca2+ imaging. A, B: Images before (A) and after 10 seconds (10 s, B) of light exposure of a control RPE cell culture. C: A bright field view of the control culture. D, E: Images of a reprogrammed culture before (D) and after 10 seconds (E) of light exposure. F: A bright field view of the reprogrammed culture. Arrows: cells with noticeable reductions in fluorescence intensities. G: Means and SDs of calculated integrated optical density (IOD) ratios (IODt/ IOD0) of cell’s fluorescent intensity. Scale bars: 50 μm [Data from the authors’ laboratories]. [Modified from Yan et al., 2010. Originally published in Journal of Comparative Neurology; DOI 10.1002/cne.22236].67
Figure 3.
Response to 9-cis-retinal by reprogrammed cells examined with Ca2+ imaging. A-L: Images of reprogrammed cells after light bleaching (A) and at the indicated number of minutes (2’-10’) after sequential administration of vehicle control (c, B-F), replacement of medium (G), and then 9-cis-retinal (r, H-L). Arrows: cells showing increases in fluorescence intensity. Arrowheads: cells lacking such an increase. M: Means and SDs of calculated integrated optical density (IOD) ratios (IODt/IOD0) of cell’s fluorescent intensity. Scale bars: 20 μm. [Data are from the authors’ laboratories]. [Modified from Yan et al., 2010. Originally published in Journal of Comparative Neurology; DOI 10.1002/cne.22236].67
IN OVO RPE-TO-PHOTORECEPTOR REPROGRAMMING IN EMBRYONIC CHICK EYE
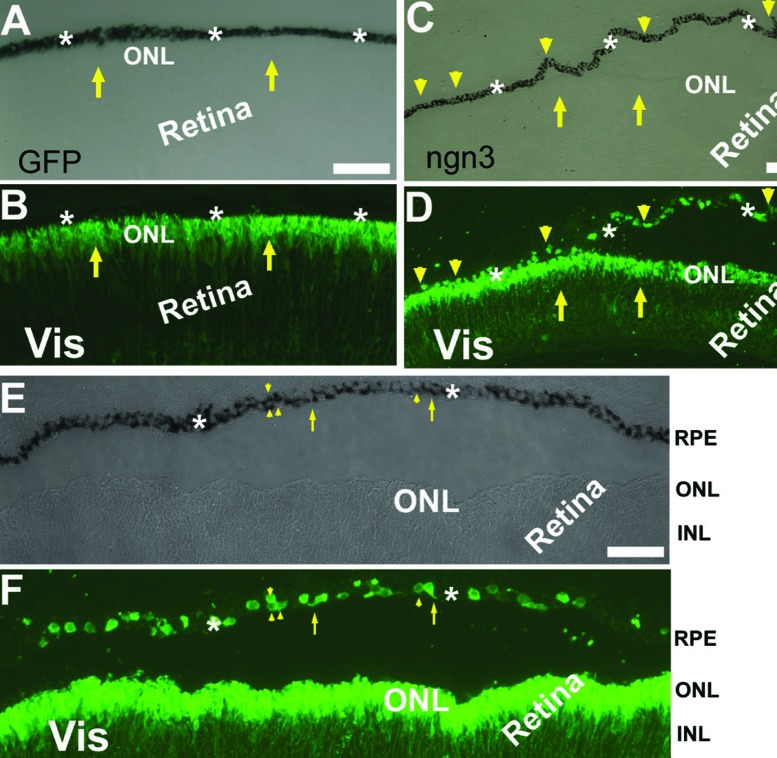
To be a convenient source of new photoreceptor cells, RPE in the eye needs to be responsive to the reprogram scheme. In E7.5 chick eyes, either the wild type or those infected with control retrovirus RCAS expressing GFP (RCAS-GFP), developing photoreceptor cells positive for visinin were confined within the neural retina at the prospective location of the outer nuclear layer (ONL), and the RPE layer lacked visinin+ cells (Figures 4A and B).68 In embryonic chick eyes infected with retrovirus RCAS-ngn3, the RPE layer contained visinin+ cells. This was unequivocally observed in regions where the RPE and the retina were separated (Figures 4C and D).68 The visinin+ cells accounted for ~37.2±4.3% of cells in the RPE layer. A thin process could be seen on some of the visinin+ cells (arrows in Figures 4E and F), indicating they were morphologically more similar to neurons than to RPE cells, while other visinin+ cells were more RPE-like. Some of the visinin+ cells in the RPE layer retained dark pigmentation typical of RPE cells (arrowheads in Figures 4E and F). In addition, visinin+ cells in the RPE predated their presence in the retina.68 Thus visinin+ cells in the PRE layer likely originated from the RPE and possibly were in transitional stages in RPE-to-photoreceptor switching process.
Figure 4.
Visinin+ cells in the RPE layer in chick embryos infected with RCAS expressing ngn3 (RCAS-ngn3). A, B: Bright-field (A) and epi-fluorescence of immunostaining for visinin (Vis, B) of an E7.5 control eye infected with RCAS-GFP. C, D: Bright-field (C) and epi-fluorescence of visinin immunostaining (D) of an E7.5 eye infected with RCAS-ngn3. E, F: Bright-field (E) and epi-fluorescence of visinin immunostaining (F) of a detached region in E7.5 eye infected with RCAS-ngn3. Arrows point to visinin+ cells of the retina, except in E,F, where arrows point to visinin+ cells with a neural-like process in the RPE layer. Arrowheads point to visinin+ cells in the RPE layers. ONL, outer nuclear layer; INL, inner nuclear layer [Data are from the authors’ laboratories]. [Modified from Li et al., 2010. Originally published in Investigative Ophthalmology & Visual Science; DOI:10.1167/iovs.09-3822].68
RPE-TO-PHOTORECEPTOR REPROGRAMMING IN TRANSGENIC MOUSE
The above results provide compelling evidence for the feasibility of reprogramming embryonic chick RPE to produce photoreceptors. Nonetheless, the study used the chick, a non-mammalian vertebrate that is evolutionally more ancient and may manifest phenotypical changes that are lacking in mammals after comparable experimental manipulations. To move forward towards the ultimate therapeutic goal of inducing in situ photoreceptor generation, transgenic mice were created with a DNA construct that would express ngn1 under the control of RPE bestrophin1 (VMD2) promoter69 or ngn3 under the control of RPE65 promoter.70 The logic was to use ngn1/ngn3 to induce in mouse RPE cells the expression of genes that initiate photoreceptor differentiation and suppress the expression of RPE genes, including the transgene itself from the RPE promoter (Figure 5). This would emulate transient ngn1/ngn3 expression in the developing neural retina.71,72 Continuation of the process would lead to the production of mature photoreceptor cells.
Figure 5.
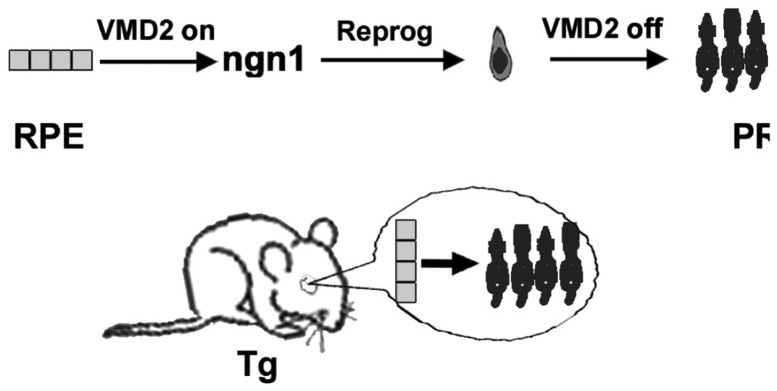
Diagram of the experimental scheme to induce RPE-to-photoreceptor reprogramming by ngn1 in the mouse eye. Reprog, reprogramming; PR, photoreceptor; Tg, transgenic mouse. [The diagram originated from the authors’ laboratories]. [Modified from Yan et al., 2013. Originally published in Investigative Ophthalmology & Visual Science; DOI:10.1167/iovs.13-11936].73
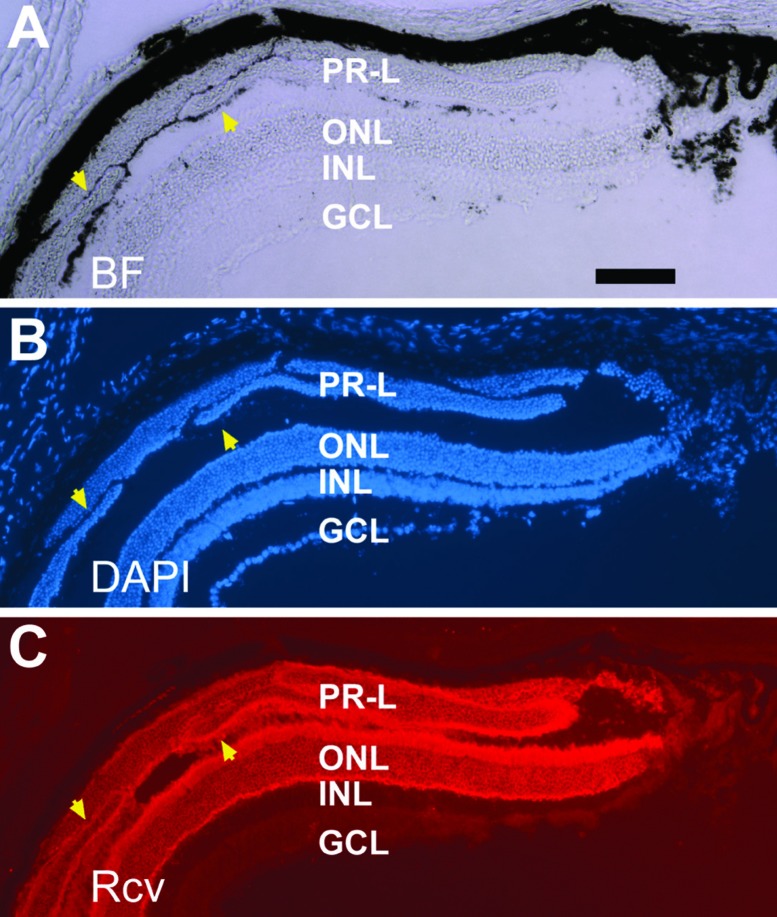
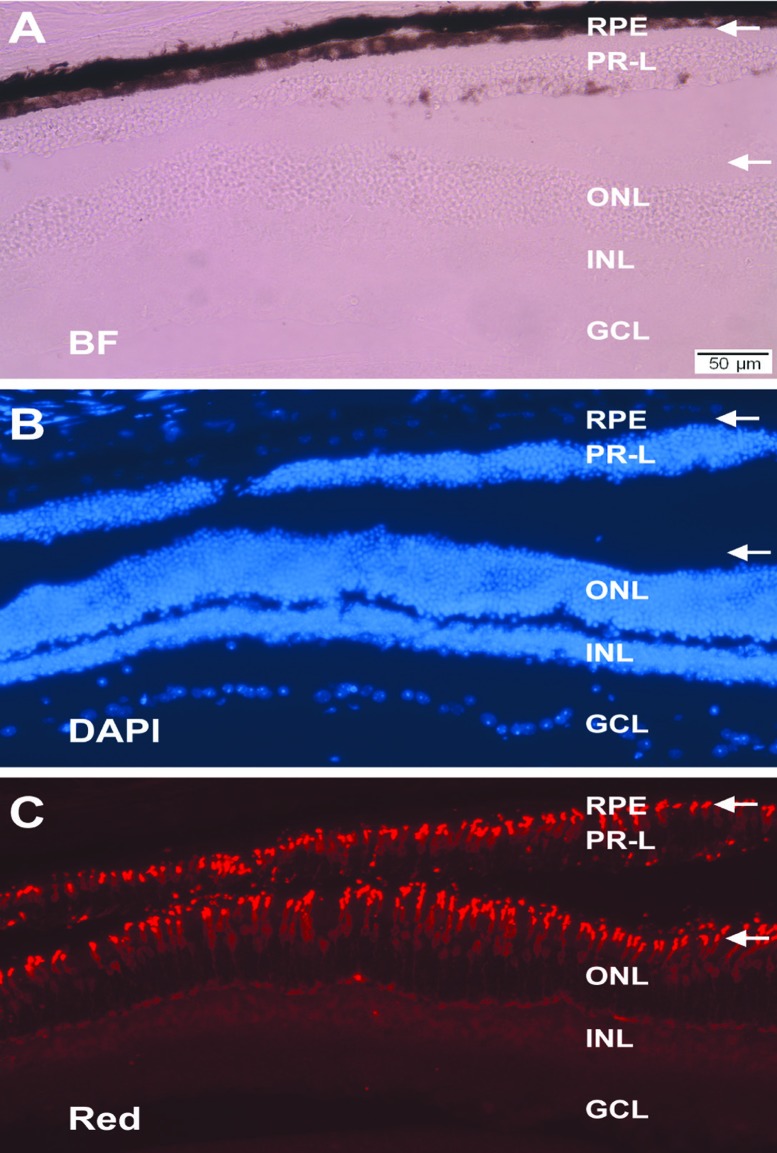
Transgenic animals of PVMD2-ngn1 or PRPE65-ngn3 showed varied degrees of phenotype manifestation. A pronounced phenotypical change was the presence of photoreceptor-like (PR-L) cells in the subretinal space (Figure 6).73 Immunohistologically and morphologically these cells appeared similar to those in the ONL. They displayed outer segments discernible with bright-field microscopy, decorated by anti-red opsin and anti-rhodopsin immunostaining (Figure 7), and containing stacks of electron-dense disc membrane that constitutes the outer segments of photoreceptors.73
Figure 6.
Recoverin+ cells in the subretinal space in a 2.5-month-old PRPE65-ngn3 transgenic mouse. Shown are views of the same sample under bright field (BF, A), counterstaining with nuclear dye (DAPI, B), and immunostaining for photoreceptor protein recoverin (Rcv, C). Arrowheads point to darkly pigmented tissue associated with, as well as demarcating different domains of, recoverin+ (PR-L) cells. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer Scale bar, 100 μm, applies to all panels. [Data are from the authors’ laboratories]. [Modified from Yan et al., 2013. Originally published in Investigative Ophthalmology & Visual Science; DOI:10.1167/iovs.13-11936].73
Figure 7.
Structural resemblance of PR-L cells to photoreceptors and the preservation of the RPE. Shown are histology and immunohistology of the retina from a 1-year-old PRPE65-ngn3 transgenic mouse under bright field view (A), DAPI counterstaining (B), or immunostaining for red opsin (C). In the aging animal, the single-layered RPE and a layer of PR-L cells were apparent. White arrows point to the immunostained outer segments of red cones in the ONL and the apices of similarly oriented PR-L cells. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 50 μm, applies to all panels. [Data are from the authors’ laboratories]. [Modified from Yan et al., 2013. Originally published in Investigative Ophthalmology & Visual Science; DOI:10.1167/iovs.13-11936].73
The very approach of reprogramming the RPE to give rise to photoreceptor cells inherently raises a concern of whether new photoreceptor cells would be produced at the expense of the PRE, an undesirable outcome as the RPE plays essential roles in maintaining the health of the retina, particularly of photoreceptors. This concern was eased by the presence of the monolayer RPE in eyes with PR-L cells (Figure 7)73 and at the place with cells seemingly en route RPE-to-photoreceptor transition.73 It appears that the RPE might have regenerated itself, after some of its cells had taken on the route to becoming PR-L cells. Self-regeneration or wound healing of the mammalian RPE is well documented. After experimental RPE debridement in the pig eye, the RPE heals.62 RPE wound healing has also been reported in aged-related macular degeneration patients after debridement of defective RPE monolayer.65 RPE repair/wound healing initially involves cell sliding migration and subsequently cell proliferation61 and may require the presence of neural retina for the new RPE to structurally and functionally mature.63
A question highly relevant to the potential application of the reprogramming scheme to photoreceptor generation is whether the RPE in an aging mouse eye would be responsive to the gene-directed reprogramming. Cells seemingly en route RPE-to-photoreceptor transition were present in the subretinal space in a 9-month-old PVMD2-ngn1 animal.73 Dark pigment granules were present in these cells, as in cells of the adjacent, monolayer RPE. Yet, these transitional cells were positive for recoverin, a photoreceptor protein involved in phototransduction, and displayed an elongated cell body typical of young photoreceptor cells. Distal to the domain of the transitional cells was a well-defined layer of PR-L cells, which no longer displayed conspicuous RPE marks.73 These observations suggest that the aging RPE in the 9-month-old animal was responsive to the reprograming scheme. Additionally, there were recoverin+/BrdU+ cells in eyecup explants derived from a 6-month-old transgenic mouse, indicating that the eyecup from the animal that was well into adulthood was able to give birth to PR-L cells in vitro.73
In conclusion, results from experiments using chick and mouse systems provided support to the biological feasibility of using gene-directed reprogramming of the RPE to produce new photoreceptor cells in situ in the eye. If applicable to the human eye, gene-directed reprogramming may offer a tantalizing prospect of using the RPE as a convenient source of new photoreceptor cells for retinal repair in situ, without involving cell transplantation and its associated risks and complications. However, enticing as it may seem, it is imperative to carry out rigorous studies to address many issues important to both basic science and potential clinical applications.
Acknowledgments
Work from our laboratories was supported by NIH/NEI grant EY011640, EyeSight Foundation of Alabama Research Grant FY2011-12-276, Research to Prevent Blindness, and NIH/NEI core grant P30 EY003039.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 2.Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 5.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 7.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673–686. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitchcock PF, Raymond PA. Retinal regeneration. Trends Neurosci. 1992;15:103–108. doi: 10.1016/0166-2236(92)90020-9. [DOI] [PubMed] [Google Scholar]
- 12.Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, Ito A, Tanaka H. Neuronal stem/progenitor cells in the vertebrate eye. Dev Growth Differ. 2008;50:253–259. doi: 10.1111/j.1440-169X.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 14.Tropepe V, Coles BL, Chaisson BJ, Horsford DJ, Elia AJ, McInnes RR, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 16.Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009;106:6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gualdoni S, Baron M, Lakowski J, Decembrini S, Smith AJ, Pearson RA, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010;28:1048–1059. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Lewallen M, Chen S, Yu W, Zhang N, Xie T. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013;23:788–802. doi: 10.1038/cr.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 20.Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer AJ, Wang SZ, Reh TA. NeuroD induces the expression of visinin and calretinin by proliferating cells derived from toxin-damaged chicken retina. Dev Dyn. 2004;229:555–563. doi: 10.1002/dvdy.10438. [DOI] [PubMed] [Google Scholar]
- 22.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, et al. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 27.Takeda M, Takamiya A, Jiao JW, Cho KS, Trevino SG, Matsuda T, et al. α-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer AJ, Bongini R. Turning Müller glia into neural progenitors in the retina. Mol Neurobiol. 2010;42:199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- 29.Haruta M, Kosaka M, Kanegae Y, Saito I, Inoue T, Kageyama R, et al. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4:1163–1164. doi: 10.1038/nn762. [DOI] [PubMed] [Google Scholar]
- 30.Akagi T, Akita J, Haruta M, Suzuki T, Honda Y, Inoue T, et al. Iris-derived cells from adult rodents and primates adopt photoreceptor-specific phenotypes. Invest Ophthalmol Vis Sci. 2005;46:3411–3419. doi: 10.1167/iovs.04-1112. [DOI] [PubMed] [Google Scholar]
- 31.Sun G, Asami M, Ohta H, Kosaka J, Kosaka M. Retinal stem/progenitor properties of iris pigment epithelial cells. Dev Biol. 2006;289:243–252. doi: 10.1016/j.ydbio.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 32.MacNeil A, Pearson RA, MacLaren RE, Smith AJ, Sowden JC, Ali RR. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007;25:2430–2438. doi: 10.1634/stemcells.2007-0035. [DOI] [PubMed] [Google Scholar]
- 33.Asami M, Sun G, Yamaguchi M, Kosaka M. Multipotent cells from mammalian iris pigment epithelium. Dev Biol. 2007;304:433–446. doi: 10.1016/j.ydbio.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Seko Y, Azuma N, Kaneda M, Nakatani K, Miyagawa Y, Noshiro Y, et al. Derivation of human differential photoreceptor-like cells from the iris by defined combinations of CRX, RX and NEUROD. PLoS One. 2012;7:e35611. doi: 10.1371/journal.pone.0035611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu P, Harwood LJ, Zhang X, Wylie M, Curry WJ, Cogliati T. Isolation of retinal progenitor and stem cells from the porcine eye. Mol Vis. 2007;13:1045–1057. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Das AV, Bhattacharya S, Thoreson WB, Sierra JR, Mallya KB, et al. Derivation of neurons with functional properties from adult limbal epithelium: implications in autologous cell therapy for photoreceptor degeneration. Stem Cells. 2008;26:939–949. doi: 10.1634/stemcells.2007-0727. [DOI] [PubMed] [Google Scholar]
- 37.Orts-Llorca F, Genis-Galvez JM. Experimental production of retinal septa in the chick embryo. Differentiation of pigment epithelium into neural retina. Acta Anat (Basel) 1960;42:31–70. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- 38.Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 39.Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 40.Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- 41.Vergara MN, Del Rio-Tsonis K. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol Vis. 2009;15:1000–1013. [PMC free article] [PubMed] [Google Scholar]
- 42.Nabeshima A, Nishibayashi C, Ueda Y, Ogino H, Araki M. Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 activation. Genesis. 2013;51:410–419. doi: 10.1002/dvg.22378. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat pigment epithelium to neural retina. Brain Res. 1995;677:300–310. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- 44.Sakami S, Etter P, Reh TA. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech Dev. 2008;125:106–116. doi: 10.1016/j.mod.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bumsted KM, Barnstable CJ. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci. 2000;41:903–908. [PubMed] [Google Scholar]
- 46.Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- 47.Martínez-Morales JR, Dolez V, Rodrigo I, Zaccarini R, Leconte L, Bovolenta P, et al. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J Biol Chem. 2003;278:21721–21731. doi: 10.1074/jbc.M301708200. [DOI] [PubMed] [Google Scholar]
- 48.Bäumer N, Marquardt T, Stoykova A, Spieler D, Treichel D, Ashery-Padan R, et al. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development. 2003;130:2903–2915. doi: 10.1242/dev.00450. [DOI] [PubMed] [Google Scholar]
- 49.Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2alpha knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet. 2010;19:1791–1804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- 51.Fujimura N, Taketo MM, Mori M, Korinek V, Kozmik Z. Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol. 2009;334:31–45. doi: 10.1016/j.ydbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Bharti K, Gasper M, Ou J, Brucato M, Clore-Gronenborn K, Pickel J, et al. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet. 2012;8:e1002757. doi: 10.1371/journal.pgen.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis. 2008;14:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 54.Machemer R, Lauqa H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol. 1975;80:1–23. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- 55.Lauqa H, Machemer R. Clinical-pathological correlation in massive periretinal proliferation. Am J Ophthalmol. 1975;80:913–929. doi: 10.1016/0002-9394(75)90289-5. [DOI] [PubMed] [Google Scholar]
- 56.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–16. [PubMed] [Google Scholar]
- 57.Zhang NL, Samadani EE, Frank RN. Mitogenesis and retinal pigment epithelial cell antigen expression in the rat after krypton laser photocoagulation. Invest Ophthalmol Vis Sci. 1993;34:2412–2424. [PubMed] [Google Scholar]
- 58.Rakoczy PE, Zhang D, Robertson T, Barnett NL, Papadimitriou J, Constable IJ, et al. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am J Pathol. 2002;161:1515–1524. doi: 10.1016/S0002-9440(10)64427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiilgaard JF, Prause JU, Prause M, Scherfig E, Nissen MH, la Cour M. Subretinal posterior pole injury induces selective proliferation of RPE cells in the periphery in in vivo studies in pigs. Invest Ophthalmol Vis Sci. 2007;48:355–360. doi: 10.1167/iovs.05-1565. [DOI] [PubMed] [Google Scholar]
- 60.la Cour M. ACTA-EVER lecture 2007. The retinal pigment epithelium: friend or foe? Acta Ophthalmol. 2008;86:593–597. doi: 10.1111/j.1755-3768.2008.01373.x. [DOI] [PubMed] [Google Scholar]
- 61.Lopez PF, Yan Q, Kohen L, Rao NA, Spee C, Black J, et al. Retinal pigment epithelial wound healing in vivo. Arch Ophthalmol. 1995;113:1437–1446. doi: 10.1001/archopht.1995.01100110097032. [DOI] [PubMed] [Google Scholar]
- 62.Del Priore LV, Hornbeck R, Kaplan HJ, Jones Z, Valentino TL, Mosinger-Ogilvie J, et al. Débridement of the pig retinal pigment epithelium in vivo. Arch Ophthalmol. 1995;113:939–944. doi: 10.1001/archopht.1995.01100070113034. [DOI] [PubMed] [Google Scholar]
- 63.Ozaki S, Kita M, Yamana T, Negi A, Honda Y. Influence of the sensory retina on healing of the rabbit retinal pigment epithelium. Curr Eye Res. 1997;16:349–358. doi: 10.1076/ceyr.16.4.349.10696. [DOI] [PubMed] [Google Scholar]
- 64.Sugino IK, Wang H, Zarbin MA. Age-related macular degeneration and retinal pigment epithelium wound healing. Mol Neurobiol. 2003;28:177–194. doi: 10.1385/MN:28:2:177. [DOI] [PubMed] [Google Scholar]
- 65.Rabenlehner D, Stanzel BV, Krebs I, Binder S, Goll A. Reduction of iatrogenic RPE lesions in AMD patients: evidence for wound healing? Graefes Arch Clin Exp Ophthalmol. 2008;246:345–352. doi: 10.1007/s00417-007-0658-6. [DOI] [PubMed] [Google Scholar]
- 66.Crisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405. [PubMed] [Google Scholar]
- 67.Yan RT, Liang L, Ma W, Li X, Xie W, Wang SZ. Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors. J Comp Neurol. 2010;518:526–546. doi: 10.1002/cne.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Ma W, Zhuo Y, Yan RT, Wang SZ. Using neurogenin to reprogram chick RPE to produce photoreceptor-like neurons. Invest Ophthalmol Vis Sci. 2010;51:516–525. doi: 10.1167/iovs.09-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esumi N, Oshima Y, Li Y, Campochiaro PA, Zack DJ. Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation. J Biol Chem. 2004;279:19064–19073. doi: 10.1074/jbc.M309881200. [DOI] [PubMed] [Google Scholar]
- 70.Boulanger A, Liu S, Henningsgaard AA, Yu S, Redmond TM. The upstream region of the Rpe65 gene confers retinal pigment epithelium-specific expression in vivo and in vitro and contains critical octamer and E-box binding sites. J Biol Chem. 2000;275:31274–31282. doi: 10.1074/jbc.M003441200. [DOI] [PubMed] [Google Scholar]
- 71.Yan RT, He L, Wang SZ. Pro-photoreceptor activity of chick neurogenin1. Invest Ophthalmol Vis Sci. 2009;50:5567–5576. doi: 10.1167/iovs.09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma W, Yan RT, Mao W, Wang SZ. Neurogenin3 promotes early retinal neurogenesis. Mol Cell Neurosci. 2009;40:187–198. doi: 10.1016/j.mcn.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan RT, Li X, Wang SZ. Photoreceptor-like cells in transgenic mouse eye. Invest Ophthalmol Vis Sci. 2013;54:4766–4775. doi: 10.1167/iovs.13-11936. [DOI] [PMC free article] [PubMed] [Google Scholar]