Abstract
The objective of the present work is to extend the applicability of the solution target approach to the production of 68Ga using a low energy cyclotron. Since the developed method does not require solid target infrastructure, it offers a convenient alternative to 68Ge/68Ga generators for the routine production of 68Ga. A new solution target with enhanced heat exchange capacity was designed and utilized with dual foils of Al (0.20 mm) and Havar (0.038 mm) separated by helium cooling to degrade the proton energy to ~14 MeV. The water-cooled solution target insert was made of Ta and its solution holding capacity (1.6 mL) was reduced to enhance heat transfer. An isotopically enriched (99.23%) 1.7 M solution of 68Zn nitrate in 0.2 N nitric acid was utilized in a closed target system. After a 30 min irradiation at 20 μA, the target solution was unloaded to a receiving vessel and the target was rinsed with 1.6 mL water, which was combined with the target solution. An automated module was used to pass the solution through a cation-exchange column (AG-50W-X8, 200-400 mesh, hydrogen form) which efficiently trapped zinc and gallium isotopes. 68Zn was subsequently eluted with 30 mL of 0.5 N HBr formulated in 80% acetone without any measurable loss of 68Ga. 68Ga was eluted with 7 mL of 3 N HCl solution with 92-96% elution efficiency. The radionuclidic purity was determined using an HPGe detector. Additionally, ICP-MS was employed to analyze for non-radioactive metal contaminants. The product yield was 192.5 ± 11.0 MBq/μ·h decay-corrected to EOB with a total processing time of 60-80 min. The radionuclidic purity of 68Ga was found to be >99.9%, with the predominant contaminant being 67Ga. The ICP-MS analysis showed small quantities of Ga, Fe, Cu, Ni and Zn in the final product, with 68Ga specific activity of 5.20-6.27 GBq/μg. Depending upon the user requirements, 68Ga production yield can be further enhanced by increasing the 68Zn concentration in the target solution and extending the irradiation time. In summary, a simple and efficient method of 68Ga production was developed using low energy cyclotron and a solution target. The developed methodology offers a cost-effective alternative to the 68Ge/68Ga generators for the production of 68Ga.
Keywords: 68Ga, cyclotron targetry, solution target
Introduction
In the recent past, a rapid increase has been noted in both clinical and preclinical studies involving 68Ga-labeled radiopharmaceuticals [1-5]. This increase can be attributed to the favorable physical characteristics of 68Ga (Eβmax 1.8 MeV, β+ 89%, T½ = 67.7 min) for imaging various rapidly changing processes (proliferation, apoptosis, angiogenesis) and targets (growth hormones, myocardial and pulmonary perfusion, inflammation and infection), and to some extent, to newer, more reliable production and labeling methods [1-5]. Gallium-68 labeled somatostatin analogs have already shown their superiority over the existing agent 111In-DTPA-octreotide through enhanced sensitivity, specificity, accuracy and cost effectiveness for the diagnosis of patients with neuroendocrine tumors [1,6-9].
The clinical promise of 68Ga-labeled radiopharmaceuticals clearly warrants growth of the supply of 68Ga to meet the increasing demand in various nuclear medicine facilities. Presently, 68Ga can be produced by two different approaches, 1) solid targetry [10,11] and 2) the 68Ge/68Ga generator [12]. The former requires high capital cost and expertise and specialized cyclotron facilities that accommodate solid targets, whereas, the latter is more broadly accessible in nuclear medicine facilities not equipped with an on-site cyclotron. The simplicity and lower capital cost of the 68Ge/68Ga generator have made it more popular among the nuclear medicine facilities with relatively lower number of requirements for 68Ga labeled doses [1,12]. However, the breakthrough of trace quantities of the long-lived 68Ge parent isotope (t½ = 271 d) into the eluted 68Ga remains a concern [13]. Furthermore, with increasing applicability of 68Ga-labeled radiopharmaceuticals, one can foresee a need for alternative production methods to meet the increasing demand especially for the relatively busy nuclear medicine centers having an on-site cyclotron. There have been previous attempts to produce 68Ga using a cyclotron, initially employing a solid target method using 68Zn electrodeposition on a copper substrate [10,14] and more recently using a solution target containing an enriched 68ZnCl2 solution [15]. The solid target methods require a lengthy separation step, which is not optimal for short-lived isotopes like 68Ga, as well as expensive solid target infrastructure.
Our initial attempts to reproduce the solution target method described by Jensen et al. [15] showed that irradiation of aqueous 68ZnCl2 solutions resulted in rapid pressure increased due to radiolysis-mediated release of hydrogen and oxygen. Additionally, we noticed a pinhole in the Havar target window foil, which may be related to a reaction of the Havar foil and with the ZnCl2 solution. We have recently performed an extensive study on the mechanistic aspects of water radiolysis during production of 89Zr in a solution target [16,17]. This study showed that the use of nitrate salts in dilute nitric acid solutions dramatically decreased rates of water radiolysis during radiometal production [17]. Eliminating the use of ZnCl2 could also prolong the life of the Havar foil. In the present work, we extended our solution target approach to the production of 68Ga using a 1.7 M solution of zinc nitrate (Isotopically enriched) in 0.2 N nitric acid for the production of 68Ga in a closed solution target system using 20 μA beam current over 30 min proton irradiation. We also report, an automated separation of 68Ga from 68Zn using cationexchange resin.
Materials and methods
Targetry details
In this study we designed and developed a new solution target having reduced solution capacity (1.6 mL) with a tantalum target body insert having dual foils of Al (0.20 mm) and Havar (0.038 mm) separated by helium cooling to degrade the proton energy to ~14 MeV. The new conical shape target showed enhanced heat exchange capacity, resulting in reduced gas formation during irradiation that enabled it to be run as a closed system, pressurized at ~40 psi with oxygen. The design of the target is depicted in Figure 1. A semi-automated target loading and unloading system was utilized as described in our previous study [17]. In this study, 30-min irradiations were performed with 1.7 M solutions of 68Zn nitrate (99.23% isotopic enrichment) in 0.2 N nitric acid. The proton beam current was 20 μA.
Figure 1.
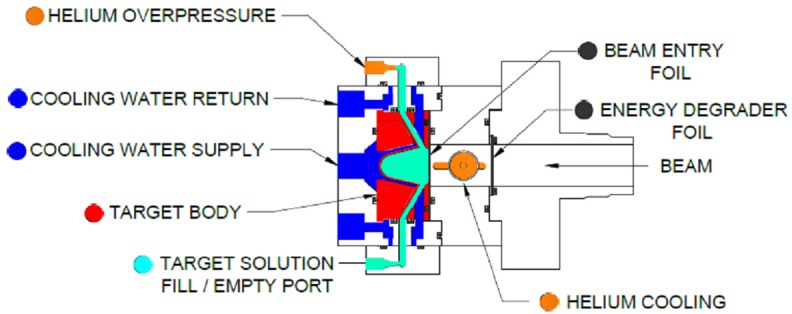
Design of solution target used for 68Ga production.
Chemicals
Zn-68 (99.23%) enriched metal was purchased from Cambridge Isotopes Laboratory (Tewksbury, MA). Hydrochloric acid (34-37% as HCl) and nitric acid (67-70% as HNO3) both trace metal basis were purchased from Fisher Scientific (Suwanee, GA). Hydrobromic acid (48% as HBr) and acetone were purchased from Sigma-Aldrich (St. Louis, MO). AG-50W-X8 and Chelex-100 (50-100 mesh sodium form) resins were purchased from Bio-Rad (Hercules, CA).
Instrumentation
High-purity germanium gamma spectrometer (Canberra, Meriden, CT) counters running Genie 2000 software was utilized to measure the radionuclide purity. The activity readings were measured using a CRC dose calibrator (#416 setting, CRC-55tPET, Capintec, Ramsey, NJ). A Dionex cation analysis HPLC system equipped with an IonPac CS5A analytical column (4 x 250 mm, Dionex) and in-line radioactivity detector (Carroll and Ramsey Associates, Berkeley CA) was employed to analyze for non-radioactive metal contaminants. The flow rate of mobile phase (Dionex MetPac Eluent) was 1.2 mL/min. For anion analysis, a Dionex ICS-2100 Ion Chromatography System by Thermo Scientific was used, employing an IonPac AS19 analytical column with ion suppression, and mobile phase of 70 mM KOH. A Perkin Elmer ELAN DRC II ICP mass spectrometer by Perkin Elmer was also employed to measure trace metal contaminants.
Method for 68Ga separation, determination of specific activity and recovery of 68Zn(NO3)2
Separation of 68Ga from 68Zn(NO3)2
An in-house built automated system was developed for the separation of 68Ga radioisotope from the 68Zn(NO3)2 target solution as outlined in Figure 2. After irradiation, the target dump (1.6 mL) was received in a collection vial along with a 1.6 mL water rinse of the target. The rinse and dump solutions were mixed and passed through a cation-exchange column (AG-50W-X8, 1.3 g, 200-400 mesh, hydrogen form), which was preconditioned by washing with 15 mL of water followed by air. Both 68Ga and 68Zn were effectively trapped on the cation exchange column. After trapping, the column was washed with 5.0 mL of water (chelexed water to avoid any metal contamination) to remove short-lived 11C and 13N isotopes. A cation-exchange guard column (AG-50W-X8, 5.0 g, 200-400 mesh, hydrogen form) was placed before the waste bottle to trap any potential breakthrough of 68Zn during purification. The cation-exchange column was placed inside the dose calibrator to monitor the total radioactivity trapped on the column.
Figure 2.
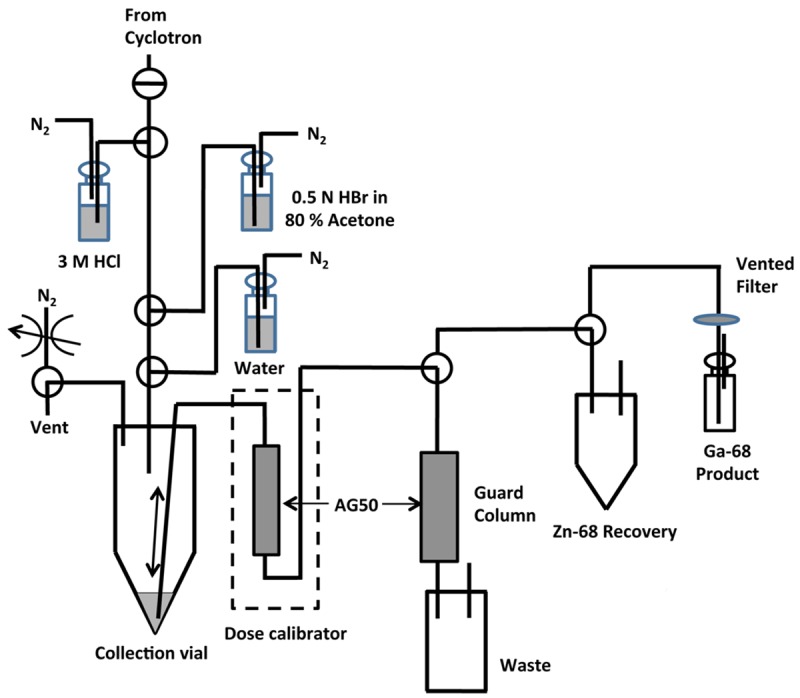
Schematic diagram of 68Ga isolation using a semi-automated system.
Zinc-68 was eluted from the column using 30 mL of 0.5 N HBr in 80% acetone solution and collected in a separate recovery vial followed by a 3 mL water rinse to remove any remaining HBr-acetone. Finally, 68Ga was eluted with 3 N HCl (7 mL) to a product vial.
Recovery of 68Zn(NO3)2 after irradiation was achieved by evaporating the 0.5 N HBr in 80% acetone solution to dryness using a rotary evaporator followed by re-dissolving the residue in concentrated nitric acid (3-5 mL) and evaporating to dryness again on the rotary evaporator. This process was repeated a total of three times to ensure the complete conversion of 68Zn to 68Zn(NO3)2. After conversion, the identity of anionic species was confirmed as nitrate using an HPLC anion chromatography method (Dionex ICS-2100 Ion Chromatography System). The HPLC method did not show the presence of any other anion. The obtained salt was still a viscous material, which on freeze-drying obtained as a solid.
Specific activity and trace metal analysis
Specific activity (GBq/μg) of 68Ga was measured by estimating the total Ga metal present in the in the final product after purification. The other metal contaminants including Zn, Fe, Cu, Ni, and Ga were also analyzed using an ICP-mass spectrometer.
Measurement of radionuclide purity
For the measurement of radionuclidic purity we used a gamma ray spectrometer (Canberra, Meriden, CT, DSA1000) equipped with a high-purity germanium (HPGe) detector.
Results and discussion
Initial experiments, separation and automation
Based on the preliminary study by Jensen et al [15], our first irradiations used 1.7 M 68ZnCl2 in the solution target system. At a beam current of 20 μA, the target pressure rose rapidly to >150 psi within 5 min, and the run had to be aborted. This result was not surprising, as we had observed similar results in our previous studies with yttrium chloride [16]. Based on our previous results, we switched to 68Zn(NO3)2 in 0.2 N nitric acid solution, pressurized with oxygen at ~40 psi. Furthermore, due to the enhanced cooling of the new target design, the target was operated as a closed system. Under these conditions, the target was irradiated for 30 min at 20 μA. The in-target pressure was maintained below 100 psi over the run.
To accomplish the separation of 68Ga from 68Zn, we modified the cation-exchange method developed by Strelow [18] in the early 1980s for the separation of non-radioactive Ga salts from zinc and other metal salts. A column of 1.3 g of AG-50W-X8 resin was used. 68Ga was trapped at more than 99% efficiency. We did not observe loss of 68Ga during the elution of 68Zn with 0.5 N HBr in 80% acetone or during subsequent rinsing of the column with water. Finally, 68Ga was eluted using 3 N HCl solution (7 mL) with 92-96% elution efficiency. The elution efficiency can be increased by increasing the amount of 3 N HCl solution, but at the cost of increasing the volume of acid in the subsequent processing steps. The isotope separation process was automated using an in-house radiochemistry module with actuated valves.
Production conditions of the solution target were 1.7 M 68Zn(NO3)2 in 0.2 N HNO3 solution at 20 μA beam current over a 30 min irradiation. The production yield of 68Ga was found to be 192.5 ± 11.0 MBq/μA·h after isotope separation having specific activity in the range of 5.20-6.27 GBq/μg (Table 1). These values are decay-corrected to end of bombardment (EOB).
Table 1.
Optimized yields of 68Ga in solution target (n = 3)
| Uncorrected Yield (GBq) | Corrected Yield (GBq) | Yield (MBq/μA·h) | Specific activity (GBq/μg) | Specific activity (mCi/μg) | ICP-MS determined quantities of Ga and other trace metals in product* (μg, n = 2) |
|---|---|---|---|---|---|
| 0.96 ± 0.10 | 1.93 ± 0.11 | 192.5 ± 11.0 | 5.20-6.27 | 140-169 | Ga (0.32, 0.39), Fe (17.0, 95.1), Cu (51.4, 12.6), Ni (3.5, 33.7), Zn (1.9, 1.9) |
These amounts were estimated for the total 7 mL of the product solution.
At 2 h after EOB, a purified sample of 68Ga was subjected to HPGe spectrometry and only two peaks were evident, at 511 keV and 1077 keV both corresponding to 68Ga. Therefore, the radionuclidic purity of 68Ga was >99.9% (Figure 3). However, when same sample was reanalyzed 36 h after EOB additional peaks were observed corresponding 67Ga, which on decay correction to EOB, was estimated to be <0.07% of the total 68Ga (Figure 4). The Ga-67 emissions were predominated by the Ga-68 emissions at 2 h after EOB. The presence of 67Ga impurity can be attributed to two possible nuclear reactions: 68Zn(p,2n)67Ga and 67Zn(p,n)67Ga [19,20]. We would anticipate ~0.1% 67Ga impurity in the 68Ga product for a 60 min irradiation. Radiochemical purity, as measured by cation-exchange HPLC, was >99.9% (Figure 5).
Figure 3.
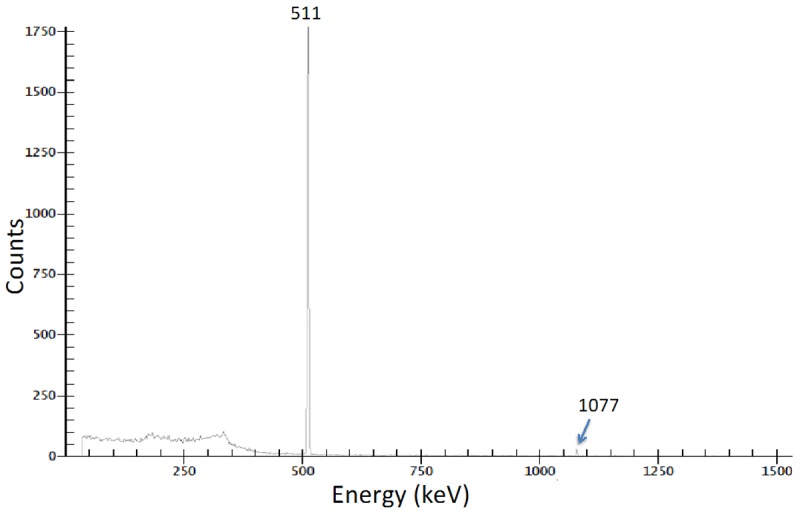
Characterization of 68Ga by high-purity germanium (HPGe) radiation detection after production.
Figure 4.
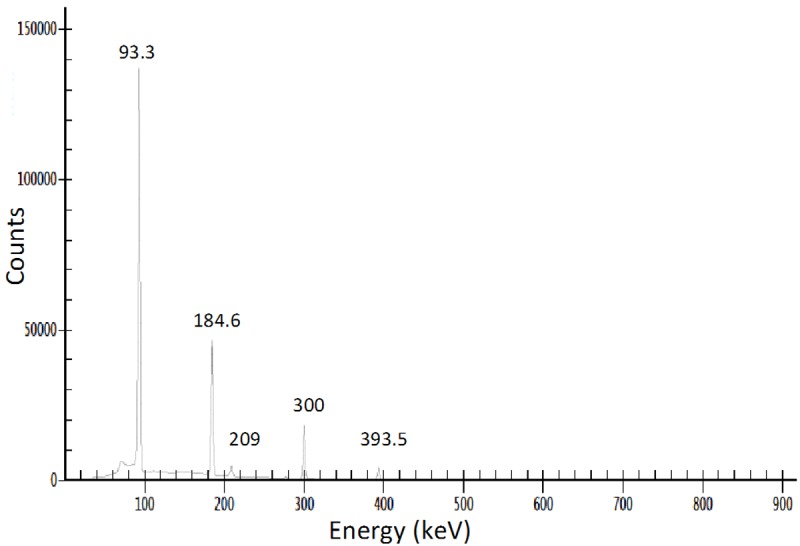
Trace quantity of 67Ga (0.07%) produced during 68Ga production and was characterized by high-purity germanium (HPGe) spectroscopy 36 h after EOB.
Figure 5.
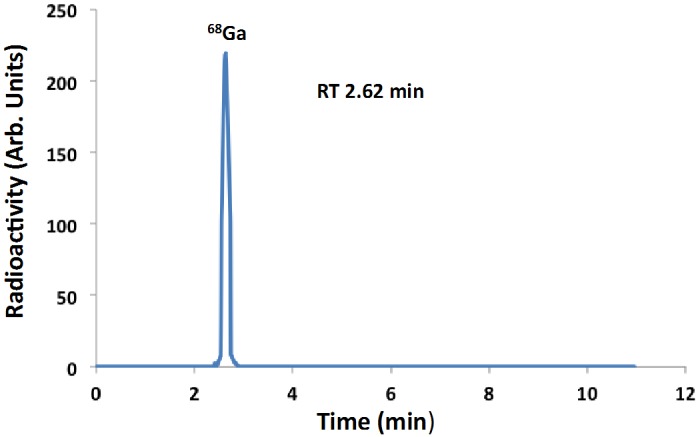
HPLC chromatogram of the purified 68Ga (Retention Time 2.62 min).
The saturation yields for 68Ga before and after the separation of isotopes were found to be 0.43 ± 0.01 GBq/μA and 0.36 ± 0.02 GBq/μA, respectively (Table 2). We attempted to make a comparison with the saturation yield using the solid target method as reported by Sadeghi et al [11]. These authors reported production yield as 5.032 GBq/μA·h, presumably without taking the isotope separation into consideration. The calculated saturation yield from their data is 35.3 GBq/μA. Thus, our yields were approximately 83 times less than the solid target yields. However, Sadeghi et al [11] did not specify the time of isotope separation so we cannot compare isolated product yields. We would anticipate that the comparison of isolated yields would be somewhat more favorable for the solution target method because it does not require a target dissolution step.
Table 2.
Saturation yields of 68Ga*
| Before separation (mCi/μA) | Before separation (GBq/μA) | After separation (mCi/μA) | After separation (GBq/μA) |
|---|---|---|---|
| 11.5 ± 2.8 | 0.43 ± 0.01 | 9.8 ± 0.57 | 0.36-0.02 |
n = 3 and all values decay corrected to EOB.
Considering that the primary end-use of the 68Ga would be in for labeling of molecular targeted peptides, we also analyzed for the presence of trace metal contamination in the final product using ICP-MS. Although very low quantities of Ga were found (0.32-0.39 μg), relatively higher amounts of Fe, Cu and Ni were observed (Table 1). If necessary, these contaminants can be further reduced before labeling using SnCl2/TiCl3 and Amberchrom CG-161m resin as described by Van der Meulen et al [21]. Furthermore, in order to achieve the optimum labeling conditions (pH) for various chelators, the final 68Ga solution (7 mL, 3 N HCl) can be concentrated using an anionexchange column, where [68GaCl4]- will be effectively trapped and can be further eluted with water as previously described [1,22]. The final pH can be readjusted using the buffer required to meet the labeling conditions. We estimate the cost of a single dose (370 MBq) of the 68Ga produced from a cyclotron using the 68Zn(NO3)2 in 0.2 N HNO3 solution target method to be $20-25 assuming 85-90% recovery of 68Zn, which we found in our initial attempts.
The unoptimized separation time was in the range of 60-80 min, but we would anticipate reduction of this time to ~45 min with further optimization. We were able to make ~25 mCi (0.92 GBq) 68Ga at end of separation. By increasing 68Zn nitrate concentration (2X) and beam current (2X), we anticipate that >100 mCi (3.7 GBq) can be practically achieved. This would serve the need for 2-4 patients, depending on labeling yields.
Conclusions
A solution target approach for production and automated separation of 68Ga was successfully developed employing a solution of 68Zn nitrate in 0.2 N nitric acid. The production yield was found to be 192.5 ± 11.0 MBq/μA·h decay-corrected to EOB with a specific activity in the range 5.20-6.27 GBq/μg. Radiochemical and radionuclidic purities were both >99.9%. Increasing the target solution concentration of 68Zn and irradiation time may further increase the production yield. The isotope separation method employed AG 50W-X8 resin eluted with a solution of 0.5 N HBr in 80% acetone to remove the zinc isotopes, followed by elution of 68Ga in 3 N HCl. The new target design with reduced target volume (1.6 mL) and enhanced heat transfer allowed irradiation as a closed system. Gallium-68 can, therefore, be produced on a low energy cyclotron in sufficient quantities to provide a viable alternative to the 68Ge/68Ga generator for those facilities that have an on-site cyclotron.
Acknowledgements
The authors are thankful to Mark Jacobson and Ray Steichen at Mayo Clinic for their technical assistance. This work was financially supported by DOE grant DE SC-0008947.
Disclosure of conflict of interest
None.
References
- 1.Velikyan I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee SR, Pomper MG. Clinical applications of Gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman BE. Current status and future needs for standards of radionuclides used in positron emission tomography. Appl Radiat Isot. 2013;76:31–37. doi: 10.1016/j.apradiso.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Smith DL, Breeman WAP, Sims-Mourtada J. The untapped potential of Gallium 68-PET: The next wave of 68Ga-agents. Appl Radiat Isot. 2013;76:14–23. doi: 10.1016/j.apradiso.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Baum RP, Kulkarni HR. Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy-the Bad Berka experience. Theranostics. 2012;2:437–47. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Öberg K. Gallium-68 somatostatin receptor PET/CT: Is it time to replace 111Indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine. 2012;42:3–4. doi: 10.1007/s12020-012-9681-4. [DOI] [PubMed] [Google Scholar]
- 7.Schreiter NF, Brenner W, Nogami M, Buchert R, Huppertz A, Pape UF, Prasad V, Hamm B, Maurer MH. Cost comparison of 111In-DTPA-octreotide scintigraphy and 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:72–82. doi: 10.1007/s00259-011-1935-5. [DOI] [PubMed] [Google Scholar]
- 8.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–7. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsson H, Larsson P, Jonsson C, Jussing E, Gryback P. Normal uptake of 68Ga-DOTA-TOC by the pancreas uncinate process mimicking malignancy at somatostatin receptor PET. Clin Nucl Med. 2012;37:362–5. doi: 10.1097/RLU.0b013e3182485110. [DOI] [PubMed] [Google Scholar]
- 10.Engle JW, Lopez-Rodriguez V, Gaspar-Carcamo RE, Valdovinos HF, Valle-Gonzalez M, Trejo-Ballado M, Severin GW, Barnhart TE, Nickles RJ. Avila-Rodriguez MA. Very high specific activity 66/68Ga from zinc targets for PET. Appl Radiat Isot. 2012;70:1792–1796. doi: 10.1016/j.apradiso.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadeghi M, Kakavand T, Rajabifar S, Mokhtari L, Nezhad AR. Cyclotron production of 68Ga via proton-induced reaction on 68Zn target. Nukleonika. 2009;54:25–28. [Google Scholar]
- 12.Rosch F. Past, present and future of 68Ge/68Ga generators. Appl Radiat Isot. 2013;76:24–30. doi: 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Belosi F, Cicoria G, Lodi F, Malizia C, Fanti S, Boschi S, Marengo M. Generator breakthrough and radionuclidic purification in automated synthesis of 68Ga-DOTANOC. Curr Radiopharm. 2013;6:72–7. doi: 10.2174/1874471011306020002. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghi M, Kakavand T, Mokhtari L, Gholamzadeh Z. Determination of 68Ga production parameters by different reactions using ALICE and TALYS codes. Pramana J Phys. 2009;72:335–341. [Google Scholar]
- 15.Jensen M, Clark J. Direct production of Ga-68 from proton bombardment of concentrated aqueous solutions of [Zn-68] zinc chloride. Proceedings of 13th International Workshop on Targetry and Target Chemistry. 2011:288–292. [Google Scholar]
- 16.DeGrado TR, Byrne JP, Packard AB, Belanger AP, Rangarajan S, Pandey MK. A solution target approach for cyclotron production of Zr-89: Understanding and coping with in-target electrolysis. J Labelled Compd Radiopharm. 2011;54:S248–S248. [Google Scholar]
- 17.Pandey MK, Engelbrecht HP, Byrne JP, Packard AB, DeGrado TR. Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: Effect of solution composition on in-target chemistry. Nucl Med Biol. 2014;41:309–316. doi: 10.1016/j.nucmedbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Strelow FEW. Quantitative separation of Gallium from Zinc, Copper, Indium, Iron (III) and other elements by cation-exchange chromatography in hydrobromic acid-acetone medium. Talanta. 1980;27:231–236. doi: 10.1016/0039-9140(80)80049-x. [DOI] [PubMed] [Google Scholar]
- 19.Szelecsenyi F, Boothe TE, Takacs S, Tarkanyi F, Tavano E. Evaluated cross section and thick target yield data bases of Zn+p processes for practical applications. Appl Radiat Isot. 1998;49:1005–1032. [Google Scholar]
- 20.Takacs S, Tarkanyi F, Hermanne A. Validation and upgrade of the recommended cross section data of charged particle reactions to produce gamma emitter radioisotopes. Nucl Instrum Methods Phys Res. 2005;240:790–802. [Google Scholar]
- 21.Meulen NPV, Walt TNV. The separation of Fe from Ga to produce ultrapure 67Ga. Z Naturforsch. 2007;62:483–486. [Google Scholar]
- 22.Patrascu I, Niculae D, Lungu V, Ursu I, Iliescu M, Tuta C, Antohe A. The purification and quality control of 68Ga eluates from 68Ge/68Ga generator. Rom Rep Phys. 2011;63:988–996. [Google Scholar]


