Abstract
Integrins, a family of cell adhesion molecules composed of α and β heterodimeric subunits, are involved in a wide range of cell-extracellular matrix and cell-cell interactions. The study of integrin family members as targets for molecular imaging and therapy has been generally limited with the exception of integrin αvβ3. vβ6, a member of the integrin family, is expressed at low or undetectable levels in normal tissues, but is widely upregulated during many pathological and physiological processes, especially cancer and fibrosis, making it a promising target for molecular imaging. Noninvasive and quantitative imaging of integrin vβ6 expression would be very useful for disease diagnosis, treatment monitoring, and prognosis assessment. Although various molecular probes have been developed for positron emission tomography and single-photon emission computed tomography imaging of integrin vβ6 expression in preclinical animal models, further research efforts are required to optimize integrin vβ6-targeting probes for future potential clinical applications in the fields of oncology and beyond.
Keywords: Molecular probe, noninvasive imaging, positron emission tomography (PET), single-photon emission computed tomography (SPECT), cancer, fibrosis, wound healing
Introduction
Molecular imaging attempts to visualize, characterize, and measure biological processes at the molecular and cellular levels in living subjects [1]. There are a variety of modalities that can be used for molecular imaging, such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), molecular magnetic resonance imaging (mMRI), magnetic resonance spectroscopy, optical bioluminescence, optical fluorescence, targeted contrast-enhanced ultrasound, and photoacoustic tomography [2,3]. Many multimodality systems (e.g., PET/CT and PET/MRI), which combine two or more imaging modalities, are also commercially available or under extensive development. Molecular imaging of key biomarkers in disease progression using specific and sensitive imaging agents (probes) provides unique opportunities not only for the noninvasive determination of biological processes in vivo, but also for early detection of lesions, optimization of therapy, and prediction and assessment of treatment response.
Integrins are a family of cell adhesion receptors that directly bind to the extracellular matrix (ECM) and provide the traction necessary for cell motility and invasion [4]. Integrins are heterodimeric transmembrane receptors consisting of an α subunit and a β subunit. There are 18 α and 8 β subunits in humans that are known to comprise at least 24 different subtypes of integrins [5]. Eight out of the 24 integrins recognize native ligands such as fibronectin, vitronectin, and collagen through the Arg-Gly-Asp (RGD) triple peptide motif, whereas other integrins serve as collagen, laminin, and leukocyte-specific receptors (Figure 1). Several integrin subtypes are highly over-expressed on many types of cancer cells [6-10]. Integrin members such as integrin αvβ3, αvβ5, and α5β1 are also crucial mediators of angiogenesis in solid tumor. Integrins expression is important for tumor progression and metastasis by promoting tumor cell migration, invasion, proliferation, and survival. In addition to cancer, integrins are also highly expressed during many normal and abnormal processes, such as fibrosis, wound healing, and inflammation [5].
Figure 1.
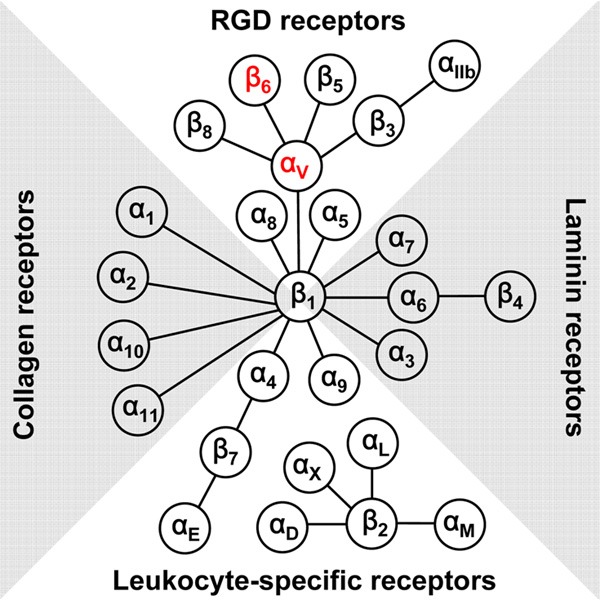
The integrin family in humans: 18 α and 8 β subunits assemble into 24 different functional heterodimers.
In the last decade, noninvasive imaging of integrin αvβ3 using RGD peptides has been extensively investigated for diagnosing cancer and other disorders and monitoring treatment response. Many excellent reviews have been published on this topic [11-15]. In contrast to integrin αvβ3, there are relatively few reports on the molecular imaging of other integrin subtypes. In recent years, emerging evidence indicates that αvβ6, another member of the integrin family, plays pivotal roles in the development of cancer, fibrosis, and other diseases [16,17]. Noninvasive molecular imaging of integrin αvβ6 expression is expected to lead to better disease management. To date, several molecular probes targeting integrin αvβ6 have been developed for PET and SPECT imaging of integrin αvβ6 expression. In this review, we summarize the currently integrin αvβ6-targeting probes and discuss the progress to date in the molecular imaging of αvβ6 expression in living subjects to diagnose and monitor cancer and other diseases.
Integrin αvβ6 expression and signaling
Integrin αvβ6 is a subtype of the integrin family that is expressed exclusively on epithelial cells. The β6 subunit only combines with αv to generate a single heterodimer [16]. Integrin αvβ6 is usually expressed at low or undetectable levels in normal adult tissues but can be highly upregulated during pathological and physiological processes such as wound healing, fibrosis, inflammation, and cancer [4]. Thus, integrin αvβ6 has become a promising target for disease diagnosis and therapy. Integrin αvβ6 has been found to be highly upregulated in various kinds of cancers (Table 1) and is usually a predictor of poor patient outcome [18]. Integrin αvβ6 can also increase cancer metastasis by promoting cancer cell invasion and migration [19-21].
Table 1.
Integrin αvβ6 expression in various cancers
| Cancer Type | Population Size | Integrin αvβ6 expression (%) | Ref. |
|---|---|---|---|
| Endometrial | 126 | 42 | [81] |
| Basal cell | 15 nodular | 7 | [82] |
| 13 morphoeic | 77 | ||
| Oral squamous cell | 30 | 90 | [31] |
| 17 | 100 | [83] | |
| 5 | 80 | [84] | |
| 40 | 100 | [85] | |
| 11 | 100 | [86] | |
| Head and neck squamous cell | 38 | 94.7 | [87] |
| Esophagus | 56 | 68 | [47] |
| Liver | 63 | 71.4 | [88] |
| Colon | 358 | 34 | [88] |
| 488 | 37 | [19] | |
| Gastric | 38 | 71 | [89] |
| 300 | 36.7 | [90] | |
| Pancreas | 34 | 100 | [91] |
| Breast | 90 | 18 | [92] |
| Skin | 49 | 84 | [47] |
| Lung | 51 | 50 | [93] |
| 311 | 54 | [18] | |
| Ovary | 45 | 100 | [94] |
| Cervical squamous | 85 | 59 | [95] |
| 46 | 92 | [47] | |
| Thyroid | 62 papillary | 79.03 | [96] |
| 28 follicular | 78.57 |
Integrin αvβ6 can bind extracellularly to the ECM and intracellularly to the cytoskeleton. Integrins transduce signals from the outside into the cell to modulate invasion, inhibit apoptosis, regulate matrix metalloproteinase (MMP) expression, and activate transforming growth factor-beta (TGF-β) [22-24]. Increasing evidence indicates that there is extensive crosstalk between integrins and TGF-β signaling in many physiological and pathological phenomena. The activation of TGF-β by integrin αvβ6 has been reported in several studies [25-27]. TGF-β is usually secreted as a large latent complex (LLC). LLCs are formed by disulfide covalent binding of latent TGF-β binding proteins to the small latent complexes, which consist of TGF-β noncovalently linked to the latency associated protein (LAP). Integrin αvβ6 binds to the LAP and then to the actin cytoskeleton. This induces a conformational change in the LLC, leading to TGF-β release to its receptors and subsequent pathway activation [28]. TGF-β activation can also upregulate MMPs through the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway [29,30].
In addition to cancer, de novo or increased expression of integrin αvβ6 also occurs during wound healing. During the re-epithelialization of wounds, the expression of integrin αvβ6 is highly upregulated at the wound edge as a consequence of an αvβ5 to αvβ6 switch [31-33]. The absence of integrin αvβ6 has been shown to delay epidermal and corneal wound healing [34,35]. During wound healing, elevated αvβ6 expression is usually accompanied by a concomitant increase in TGF-β, which is important in wound healing for the regulation of re-epithelialization, suppression of inflammation, deposition of ECM, and formation of scar tissue [36]. Several studies have indicated that the role of integrin αvβ6 in wound healing is associated with TGF-β pathway activation [37-39].
The expression of integrin αvβ6 is also dramatically increased during fibrogenesis. Integrin αvβ6-mediated TGF-β activation has been demonstrated to play an important role in animal models of fibrotic kidney, liver, and lung disease [27]. Various groups have reported that β6 knockout mice were partly or completely protected from fibrosis in different organs, and that fibrosis can be inhibited by treatment with TGF-β antagonists and αvβ6 antibodies [37,40-45].
Integrin αvβ6-targeting ligands
Because the expression pattern of integrin αvβ6 is restricted to tumors and other pathological tissues, it has become a promising diagnostic and therapeutic target. Several integrin αvβ6-specific antibodies have significantly blocked αvβ6 binding to the LAP and subsequent TGF-β activation [46]. Studies have also suggested that antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in in vivo animal models [47,48].
Several αvβ6-targeting peptides have also been developed, such as A20FMDV2 (NAVPNLRGDLQVLAQKVART). The sequence of A20FMDV2, a 20-mer peptide, was derived from the G-H loop of an envelope protein of the foot-and-mouth disease virus [49]. Although A20FMDV2 contains the RGD sequence, it has demonstrated a high specificity for αvβ6 but not for other RGD-recognizing integrins [50]. Furthermore, the RGDLXXL (X indicates a nonspecific amino acid) sequence was found to be a key motif for αvβ6 binding, whereas other integrins such as αvβ3 and αvβ5 only minimally interacted with A20FMDV2 [51]. Using a phage display approach, Oyama et al. isolated a 20-mer peptide with the sequence RGDLATLRQLAQEDGVVGVR, named H2009.1, from a panning peptide library of the lung adenocarcinoma cell line H2009 [52]. H2009.1 was shown to deliver chemotherapeutic agents specifically to tumor cells in vitro by targeting integrin αvβ6 [18,53,54] Further studies demonstrated that the tetrameric version of this peptide has a higher affinity for its cellular targets than the corresponding monomers [55]. Furthermore H2009.1 tetrameric peptide-functionalized liposomes [56] and multifunctional micelles encapsulated with superparamagnetic iron-oxide nanoparticles and doxorubicin [57] demonstrated significantly increased tumor cell targeting in MRI and drug-delivery applications. Similar to H2009.1, another αvβ6-targeting peptide, namely HBP-1 (H2N-SPRGDLAVLGHKYCONH2), was also identified via phage display screening against a cell line. HBP-1 showed preferential binding of αvβ6 over αvβ3. 125I/131I-labeled HBP-1 showed relatively stable and high affinity for αvβ6 [58]. As linear peptides may suffer from in vivo instability and rapid in vivo clearance, several engineered cysteine knots were recently designed and developed that exhibit high affinity for αvβ6 but not for the related subtypes αvβ3, αvβ5, and α5β1 [59].
Because of their specific integrin αvβ6-targeting properties, several above mentioned ligands have been labeled with PET or SPECT radionuclides for in vivo imaging in animal models of cancer and other diseases. Representative probes are listed in Table 2.
Table 2.
Selected list of the molecular imaging probes targeted to integrin αvβ6
| Imaging modality | Probe | Targeting sequence | Disease model | Ref. |
|---|---|---|---|---|
| PET | [18F]FBA-A20FMDV2 | NAVPNLRGDLQVLAQKVART | Melanoma | [50] |
| [18F]FBA-PEG28-A20FMDV2 | Pancreatic cancer | [62] | ||
| [18F]FBA-(PEG28)2-A20FMDV2 | ||||
| 64Cu-PEG28-A20FMDV2 (using different chelators) | Melanoma | [67,68] | ||
| [18F]FBA-C6-ADIBON3-PEG7-A20FMDV2 | Melanoma | [64] | ||
| 64Cu-DOTA-R01 | GCILNMRTDLGTLLFRCRRDSDCPGACICRGNGYCG | Pancreatic and epidermoid cancer | [59] | |
| 64Cu-DOTA-S02 | GCRSLARTDLDHLRGRCSSDSDCLAECICLENGFCG | |||
| 18F-fluorobenzoate-R01 | GCILNMRTDLGTLLFRCRRDSDCPGACICRGNGYCG | Pancreatic cancer | [70] | |
| 18F-fluorobenzoate-S02 | GCRSLARTDLDHLRGRCSSDSDCLAECICLENGFCG | |||
| 64Cu-AcD10 | RGDLATLRQL | Non-small cell lung cancer | [69] | |
| SPECT | 125I/131I-HBP-1 | SPRGDLAVLGHKY | Head and neck squamous cell carcinoma | [58] |
| 111In-DTPA-A20FMDV2 | NAVPNLRGDLQV LAQKVART | Breast cancer and melanoma | [71] | |
| Pulmonary fibrosis | [78] | |||
| 123I-IFMDV2 | Pancreatic cancer | [72] | ||
| 111In-DTPA-Streptavidin-biotinylated-D25p | EPRGDLRTLAAREKENFNETLARL QEKGI | Melanoma | [73] | |
| 99mTc-SAAC-S02 | GCRSLARTDLDHLRGRCSSDSDCLAECICLENGFCG | Lung carcinoma | [74] | |
| 99mTc-HYNIC-HK | RGDLATLRQLAQEDGVVGVRK | Pancreatic cancer | [75] |
In vivo imaging of integrin αvβ6 expression in cancer
Integrin αvβ6 is upregulated in numerous cancer types, such as colon, lung, cervical, ovarian, and pancreatic cancers [16], but is expressed at low or undetectable levels in healthy organs. Moreover, high expression of integrin αvβ6 in carcinomas is a prognostic factor for poor patient survival [19]. Thus, molecular imaging agents that target integrin αvβ6 would be a highly useful noninvasive method for cancer detection and monitoring cancer progression. PET and SPECT are the most commonly used modalities for the molecular imaging of integrin αvβ6.
PET
PET imaging offers high sensitivity (10-11~10-12 M [60]) and has been extensively used for the noninvasive clinical management of cancer over the last decade. Traditional PET radionuclides include 18F (t1/2: 109.7 min), 11C (t1/2: 20.3 min), 13N (t1/2: 9.97 min), and 15O (t1/2: 2 min). In recent years, several PET radionuclides such as 68Ga (t1/2: 68 min), 64Cu (t1/2: 12.7 h), 86Y (t1/2: 14.7), and 89Zr (t1/2: 78.41 h) have been under extensive investigation in both preclinical and clinical studies.
Several integrin αvβ6-targeting ligands have been radiolabeled with PET radionuclides for in vivo imaging. The Sutcliffe group has radiolabeled A20FMDV2 with 18F [50,61-66] and 64Cu [67,68] for in vivo cancer imaging. By radiolabeling the N-terminus of A20FMDV2 with 4-[18F]fluorobenzoic acid ([18F]FBA), they demonstrated that A20FMDV2 binding is highly specific for integrin αvβ6 in in vitro cell binding assays [50]. Furthermore, [18F]FBA-A20FMDV2 can be used to selectively image αvβ6-positive tumors in vivo in mice bearing both DX3puro and DX3puroβ6 human melanoma xenografts (Figure 2A) [50]. A20FMDV2 was also labeled with 3 different prosthetic groups, [18F]FBA, [18F]FPA and [18F]FC5. Small-animal PET imaging and biodistribution studies revealed that radiolabeling of the prosthetic groups had a noticeable effect on A20FMDV2 pharmacokinetics, especially tumor uptake and metabolic fate [61]. Although 18F-labeled A20FMDV2 exhibited specific integrin αvβ6 targeting in vivo, low tumor uptake and retention and metabolic instability of the probe limit its general application. To increase tumor uptake and improve in vivo pharmacokinetics of 18F-A20FMDV2, two new radiotracers with polyethylene glycol (PEG) spacers were synthesized. The two modified radiotracers exhibited significantly improved tumor retention without affecting the high specificity for integrin αvβ6 [62]. Recently, a new radiolabeling method, copper-free strain-promoted click chemistry, was used to generate the tracer [18F]FBA-C6-ADIBON3-PEG7-A20FMDV2. Unfortunately, the new tracer altered A20FMDV2 pharmacokinetics, resulting in its unexpected uptake in the gall bladder and gastrointestinal tract [64].
Figure 2.
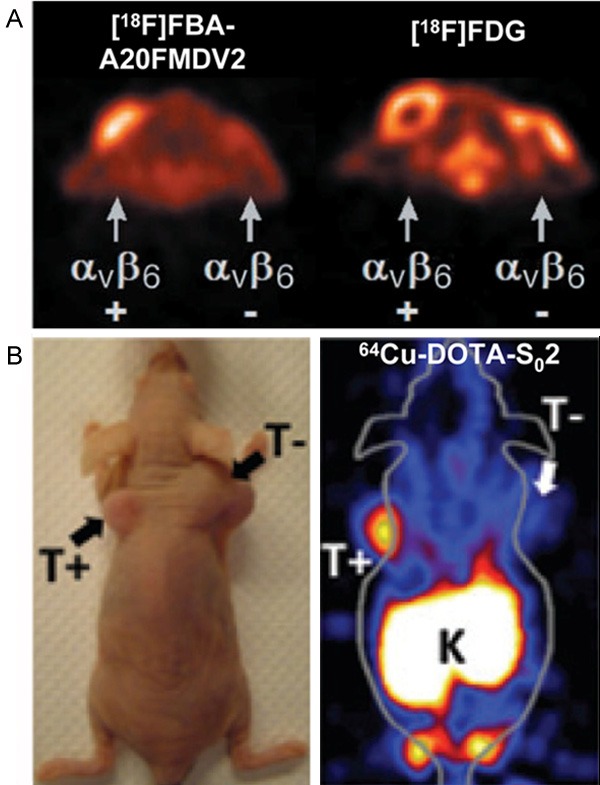
Small-animal PET imaging of integrin αvβ6 expression in cancer. A: Representative transaxial PET images of [18F]FBA-A20FMDV2 and [18F]FDG in the same mouse with the positive (αvβ6-expressing DX3puroβ6) tumors located near the left shoulder and the negative (control DX3puro) tumors near the right shoulder. Adapted with permission from the American Association for Cancer Research: ref. [50]. B: The photograph and PET image of a mouse bearing both BxPC-3 αvβ6-positive (T+) and αvβ6-negative 293 (T-) xenografts injected with 64Cu-DOTA-S02. Adapted with permission from the American Association for Cancer Research: ref. [59].
Because of the relatively rapid radioactive decay of 18F, A20FMDV2 was also labeled with 64Cu, which has a nearly seven times longer half-life compared with 18F. Unfortunately, the 64Cu-labeled tracers showed unexpectedly high and persistent levels of radioactivity in the kidneys and liver, making them less suitable than the 18F-labeled tracers [67]. Four different chelators were further investigated for 64Cu radiolabeling of A20FMDV2; however, the data suggested no clear “best” chelator and no obvious effects of the individual chelators [68].
In addition to A20FMDV2, a 10-mer peptide sequence RGDLATLRQL selected from the αvβ6-specific H2009.1 peptide was also labeled with 64Cu and investigated for its utility in in vivo PET imaging [69]. Two approaches were used to achieve peptide dimerization: dimerization of the peptide followed by conjugation to the chelator and direct presentation of two copies of the peptide on the chelator scaffold. The divalent probes 64Cu-D10 and 64Cu-(M10)2 showed approximately three-fold higher tumor uptake compared with the monovalent probe, indicating the role of multivalency in signal amplification. To abrogate the nonspecific uptake of the divalent probes in the kidneys, the N-terminus of the peptide was acetylated, and the resulting bivalent probe 64Cu-AcD10 exhibited significantly decreased kidney accumulation while maintaining tumor uptake [69].
As liner peptides generally have poor in vivo stability, Kimura et al. [59] engineered several highly stable cysteine knot peptides with high-affinity integrin αvβ6 binding, and labeled them with either 64Cu [59] or 18F [70]. Among the 64Cu-labeled knot peptides, 64Cu-NOTA-S02 showed the best in vivo imaging properties including excellent tumor/background ratio, rapid renal clearance, and high serum stability. Small-animal PET imaging demonstrated that 64Cu-NOTA-S02 targeting of integrin αvβ6 can specifically detect pancreatic tumor xenografts (Figure 2B) [59]. In another study by the same group [70], two cystine knot peptides were radiolabeled with 18F, which was considered to have better matched kinetics with the rapid clearance of the peptides. The results of their study demonstrated the translational promise of the radiotracer 18F-fluorobenzoate-R01 for molecular imaging of integrin αvβ6 overexpression in pancreatic and other cancers.
SPECT
SPECT is the universally available nuclear medicine imaging technique because of the wider availability of gamma cameras and SPECT scanners. Common radionuclides used for SPECT imaging are 99mTc (t1/2: 6.02 h), 111In (t1/2: 2.83 d), 123I (t1/2: 13.2 h), and 131I (t1/2: 8.04 d) [2]. The radionuclides used for SPECT usually have a longer half-life than those used for PET. More than 70% of the radiotracers used in clinics are 99mTc compounds mainly because of their optimal nuclear properties, availability and low cost.
Compared with PET-based radiotracers, SPECT radiotracers for integrin αvβ6-targeting are relatively rare. A20FMDV2 was radiolabeled with 111In-diethylenetriamine pentaacetic acid (DTPA), and the resulting probe 111In-DTPA-A20FMDV2 was tested in SPECT imaging studies. 111In-DTPA-A20FMDV2 was found to efficiently image αvβ6-positive cancers with high resolution (Figure 3A) [71]. However, as 111In-DTPA-A20FMDV2 is degraded in serum, this probe may need to be modified to increase its stability. In a recent study, Man et al. [72] identified a single chain fragment variable, named D25p, using a 3-dimensional geometry-based library designed from ligand-receptor binding. 111In-labeled D25p exhibited similar targeting capabilities to that of A20FMDV2 [73].
Figure 3.
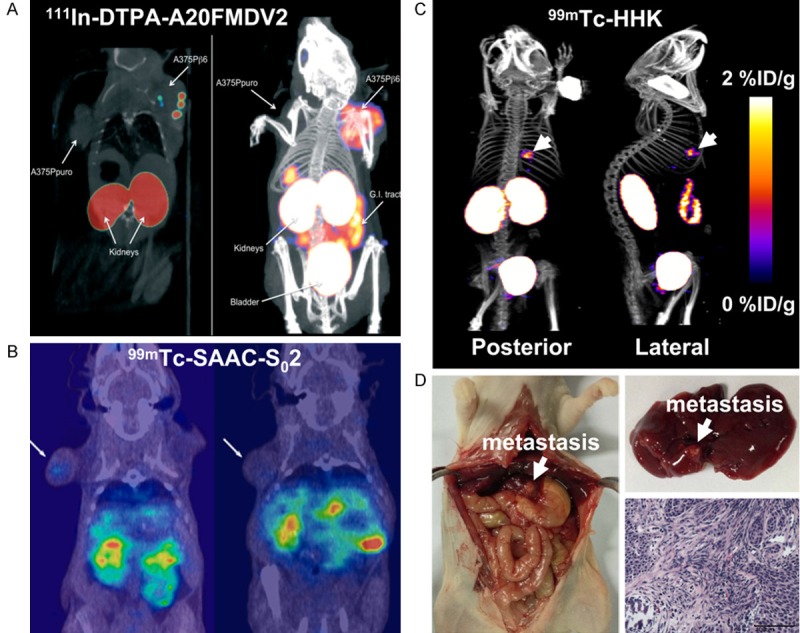
Small-animal SPECT imaging of integrin αvβ6 expression in cancer. (A) Small-animal SPECT (left) and SPECT/CT (right) images of mice bearing αvβ6-negative A375Ppuro (left shoulder) and αvβ6-positive A375Pβ6 (right shoulder) tumors with 111In-DTPA-A20FMDV2. Adapted with permission from ref. [71]. Copyright (2010) Pathological Society of Great Britain and Ireland. (B) Coronal SPECT/CT images of a mouse bearing αvβ6-positive HCC4006 xenograft (left) and a mouse bearing αvβ6-negative H838 xenograft (right) after injection of 99mTc-SAAC-S02. Adapted with permission from ref. [74]. Copyright (2014) American Chemical Society. (C) Whole-body posterior and right lateral SPECT/CT images of nude mice with liver metastasis of αvβ6-positive BxPC-3 tumors after 99mTc-HHK injection. (D) Mouse from (C) was sacrificed, and the liver metastasis was verified. This research was originally published in ref. [75]. Copyright (2014) the Society of Nuclear Medicine and Molecular Imaging, Inc.
Zhu et al. conjugated the cysteine knot peptide S02 with a single amino acid chelate and labeled it with 99mTc [74]. The resulting probe was evaluated in two different tumor models and demonstrated several positive features such as high stability, quick tumor targeting, and rapid renal clearance (Figure 3B). However, because of the high lipophilicity of the 99mTc-(CO)3 core, the probe showed high blood and liver uptake and retention [74]. Recently, we modified the H2009.1 peptide by adding a lysine residue to the C-terminus [75]. The new peptide was conjugated with 6-hydrazinonicotinyl and then labeled with 99mTc to generate the integrin αvβ6-targeting probe 99mTc-HHK. Compared with 18F-FDG, 99mTc-HHK can sensitively distinguish αvβ6-expressing tumors from αvβ6-negative tumors. In addition, SPECT imaging with 99mTc-HHK was found to be an effective approach for the noninvasive detection of liver metastatic lesions of integrin αvβ6-positive tumors (Figure 3C, 3D) [75]. However, a major drawback of 99mTc-HHK is its relatively low tumor uptake, which may be partly due to its in vivo metabolic instability [75].
In vivo imaging of integrin αvβ6 expression in other disorders
Most integrin αvβ6 probes were designed for imaging αvβ6 expression in cancer. Besides cancer imaging, integrin αvβ6 probes can also be used to detect and monitor other pathological processes. Because αvβ6 is also upregulated in fibrogenesis, it has become a promising imaging target for lung and liver fibrosis.
The integrin family member αvβ3 has previously been used for the imaging of liver fibrosis. Li et al. [76] demonstrated that the expression of integrin αvβ3 was markedly upregulated on activated hepatic stellate cells in fibrotic livers and correlated with the stage of fibrosis. They successfully used a 99mTc-labeled RGD peptide, 99mTc-cRGD, to image αvβ3 expression in fibrotic livers. The probe showed potential to noninvasively distinguish different stages of liver fibrosis (Figure 4A) [76]. Because integrin αvβ6-mediated TGF-β activation is essential to the pathogenesis of idiopathic pulmonary fibrosis (IPF), integrin αvβ6 represents a promising therapeutic target for the treatment of this disease [77]. Recently, it has been reported that integrin αvβ6 may be a useful target for imaging IPF. The ability to use SPECT imaging of 111In-labeled A20FMDV2 to monitor and predict the development of lung fibrosis was investigated in a bleomycin model of IPF (Figure 4B). This probe showed the potential to be used to detect the expression of αvβ6, which may be a promising biomarker to facilitate the stratification of IPF treatment [78].
Figure 4.
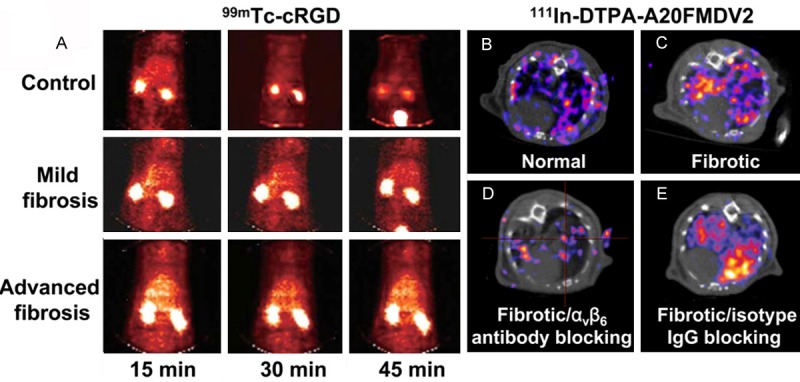
SPECT imaging of fibrosis. (A) SPECT images of rat with or without liver fibrosis after injection of an integrin αvβ3-targeting probe 99mTc-cRGD. Adapted with permission from ref. [76]. Copyright (2011) American Association for the Study of Liver Diseases. (B, C) Transaxial small-animal SPECT/CT images of 111In-DTPA-A20FMDV2 in normal (B) and fibrotic (C) lungs of the mice. (D, E) Transaxial small-animal SPECT/CT images of 111In-DTPA-A20FMDV2 in fibrotic lungs of the mice receiving integrin αvβ6-blocking antibody (D) or IgG isotype control (E). This research was originally published in ref. [78]. Copyright (2013) the Society of Nuclear Medicine and Molecular Imaging, Inc.
Conclusion and future perspectives
In consideration of the important role integrin αvβ6 plays in many pathological and physiological processes, especially in cancer and fibrosis, noninvasive imaging of integrin αvβ6 expression would provide a great deal of information to benefit disease detection, new drug development and validation, and patients management (e.g., treatment monitoring, dose optimization, and patient stratification). Quantitative correlation of probe uptake with αvβ6 expression level and disease progression would be an attractive noninvasive method to monitor clinical response to treatment. Noninvasive quantitative imaging of αvβ6 expression may also play a role in personalized medicine by allowing treatment efficacy to be optimized for each individual patient.
Currently, most αvβ6-targeted probes are used for radionuclide-based imaging modalities PET and SPECT, which have excellent tissue penetration depth and imaging sensitivity. Most developed probes have been peptide based because of the advantages of peptides as targeting agents, such as small molecular weight, minimal immunogenicity, rapid clearance from normal tissues, and easy synthesis, modification, and storage. However, most reported peptidebased integrin αvβ6-targeting probes exhibit limited receptor binding affinity, poor tumor uptake and retention, and metabolic instability. Although some success has been achieved in improving tumor uptake and stability in vivo, unresolved problems still remain, such as the unexpectedly high uptake of modified probes in some normal organs. Optimization strategies [13,15], such as multimerization to increase receptor binding affinity, peptide cyclization to improve stability, and amino acid modifications to improve degradation resistance, may be needed in future studies. After peptide optimization, the next step is to further develop clinically translatable αvβ6-targeted probes.
All the currently investigated integrin αvβ6-targeting probes contain the RGDXXL or DLXXL targeting peptide sequence (Table 2). Previous studies have reported that the interaction of the A20FMDV2 peptide (NAVPNLRGDLQVLAQKVART) with integrin αvβ6 requires the RGD motif and the adjacent DLXXL motif, which forms the C-terminal helix, whereas the residues N-terminal to the RGD motif are not required for binding [49]. Further studies of the structural basis of natural ligand (e.g., fibronectin and TGF-β-LAP) recognition by integrin αvβ6 are needed to elucidate the essential elements required for high specificity and affinity peptide-αvβ6 interactions, thereby allowing for the rational design of targeting peptides for in vivo molecular imaging applications. In addition to peptides, antibody and engineered small molecule proteins could also be developed as they have high binding affinity and long tumor retention. Future research efforts are expected to be directed at the development of such molecular imaging probes for integrin αvβ6 targeting.
Recent advances in nanotechnology have provided numerous strategies for designing nanoplatform-based imaging and theranostic agents that efficiently deliver imaging signals or drugs to tumor sites by overcoming many biological barriers [79]. Nanoparticles have large surface areas to which a variety of targeting ligands and imaging moieties could be incorporated for multivalent and signal amplified molecular imaging. Nanoparticle-based targeting probes can also significantly increase tumor uptake and retention through passive targeting based on the enhanced permeability and retention effect. Nanoparticle-based theranostic agents designed to target integrin αvβ6 may broaden the applications of integrin αvβ6-targeted molecular imaging. However, caution should be taken for in vivo applications of nanoparticle-based therapeutic agents when functionalized by ligands with relatively low affinity such as peptides. For example, in a recent study, Gray et al. [80] studied a liposomal drug platform functionalized with tetramatic H2009.1 peptide for integrin αvβ6-targeted drug delivery for cancer therapy. However, the in vivo targeting of H2009.1 tetrameric peptide liposomal doxorubicin was similar to that of the control peptide and liposomes alone. Therefore, ligand targeting does not guarantee the enhanced efficacy of a liposomal drug, highlighting the complexity of in vivo targeting [80].
Current applications of integrin αvβ6-targeting probes are limited to PET and SPECT. The use of integrin αvβ6-targeting probes for multimodality imaging should also be explored. The combined advantages of different imaging modalities would provide more detailed and accurate information of pathological and physiological processes. This information is essential to better understand the mechanisms of integrin αvβ6 in biological processes. Advances in biology will help to further identify the key role of integrin αvβ6 in normal as well as abnormal biological processes, which may further broaden the application of molecular αvβ6 imaging in living subjects beyond the field of oncology.
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (81222019 and 81125011), the Ministry of Science and Technology of China (2011YQ030114, 2012ZX09102301, and 2012BAK25B03), the Ministry of Education of China (31300 and BMU20110263), the Beijing Natural Science Foundation (7132131 and 7132123), and the Beijing Nova Program (Z121107002512010).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18N, 21N. [PubMed] [Google Scholar]
- 2.Zhang Y, Yang Y, Hong H, Cai W. Multimodality molecular imaging of CD105 (Endoglin) expression. Int J Clin Exp Med. 2011;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson KE, Wang TY, Willmann JK. Acoustic and photoacoustic molecular imaging of cancer. J Nucl Med. 2013;54:1851–1854. doi: 10.2967/jnumed.112.115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor Targeting via Integrin Ligands. Front Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 9.Weis SM, Cheresh DA. AlphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 12.Beer AJ, Schwaiger M. Imaging of integrin alphavbeta3 expression. Cancer Metastasis Rev. 2008;27:631–644. doi: 10.1007/s10555-008-9158-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 14.Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res. 2009;42:969–980. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Wang F. Development of RGD-based radiotracers for tumor imaging and therapy: translating from bench to bedside. Curr Mol Med. 2013;13:1487–1505. doi: 10.2174/1566524013666131111115347. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets. 2009;10:645–652. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, Wistuba II, Roth JA, McGuire MJ, Brown KC. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–5895. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 19.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates RC. Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT) Cell Cycle. 2005;4:1350–1352. doi: 10.4161/cc.4.10.2053. [DOI] [PubMed] [Google Scholar]
- 21.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 22.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 24.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 25.Thomas GJ, Hart IR, Speight PM, Marshall JF. Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells. Br J Cancer. 2002;87:859–867. doi: 10.1038/sj.bjc.6600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 28.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Feldner JC, Brandt BH. Cancer cell motility--on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 32.Clark RA, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MW. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol. 1996;135:46–51. [PubMed] [Google Scholar]
- 33.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark RA, Uitto VJ, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 34.AlDahlawi S, Eslami A, Hakkinen L, Larjava HS. The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 2006;14:289–297. doi: 10.1111/j.1743-6109.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 35.Blanco-Mezquita JT, Hutcheon AE, Stepp MA, Zieske JD. AlphaVbeta6 integrin promotes corneal wound healing. Invest Ophthalmol Vis Sci. 2011;52:8505–8513. doi: 10.1167/iovs.11-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 37.Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 39.Saito T, Izumi K, Shiomi A, Uenoyama A, Ohnuki H, Kato H, Terada M, Nozawa-Inoue K, Kawano Y, Takagi R, Maeda T. Zoledronic acid impairs re-epithelialization through down-regulation of integrin alphavbeta6 and transforming growth factor beta signalling in a three-dimensional in vitro wound healing model. Int J Oral Maxillofac Surg. 2014;43:373–380. doi: 10.1016/j.ijom.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF 3rd, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Zhang L, Chen L, Wang H, Zhang Y, Bie P. Role of integrin alphavbeta6 in the pathogenesis of ischemia-related biliary fibrosis after liver transplantation. Transplantation. 2013;95:1092–1099. doi: 10.1097/TP.0b013e3182884866. [DOI] [PubMed] [Google Scholar]
- 46.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- 47.Van Aarsen LA, Leone DR, Ho S, Dolinski BM, McCoon PE, LePage DJ, Kelly R, Heaney G, Rayhorn P, Reid C, Simon KJ, Horan GS, Tao N, Gardner HA, Skelly MM, Gown AM, Thomas GJ, Weinreb PH, Fawell SE, Violette SM. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism. Cancer Res. 2008;68:561–570. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 48.Eberlein C, Kendrew J, McDaid K, Alfred A, Kang JS, Jacobs VN, Ross SJ, Rooney C, Smith NR, Rinkenberger J, Cao A, Churchman A, Marshall JF, Weir HM, Bedian V, Blakey DC, Foltz IN, Barry ST. A human monoclonal antibody 264RAD targeting alphavbeta6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32:4406–4416. doi: 10.1038/onc.2012.460. [DOI] [PubMed] [Google Scholar]
- 49.DiCara D, Rapisarda C, Sutcliffe JL, Violette SM, Weinreb PH, Hart IR, Howard MJ, Marshall JF. Structure-function analysis of Arg-Gly-Asp helix motifs in alpha v beta 6 integrin ligands. J Biol Chem. 2007;282:9657–9665. doi: 10.1074/jbc.M610461200. [DOI] [PubMed] [Google Scholar]
- 50.Hausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F] fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer Res. 2007;67:7833–7840. doi: 10.1158/0008-5472.CAN-07-1026. [DOI] [PubMed] [Google Scholar]
- 51.Kraft S, Diefenbach B, Mehta R, Jonczyk A, Luckenbach GA, Goodman SL. Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J Biol Chem. 1999;274:1979–1985. doi: 10.1074/jbc.274.4.1979. [DOI] [PubMed] [Google Scholar]
- 52.Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett. 2003;202:219–230. doi: 10.1016/j.canlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhou X, Chang YC, Oyama T, McGuire MJ, Brown KC. Cell-specific delivery of a chemotherapeutic to lung cancer cells. J Am Chem Soc. 2004;126:15656–15657. doi: 10.1021/ja0446496. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Gray BP, McGuire MJ, Brown KC. Synthesis and biological evaluation of a peptide-paclitaxel conjugate which targets the integrin alphavbeta6. Bioorg Med Chem. 2011;19:5480–5489. doi: 10.1016/j.bmc.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, McGuire MJ, Lin M, Liu YH, Oyama T, Sun X, Brown KC. Synthesis and characterization of a high-affinity alphavbeta6-specific ligand for in vitro and in vivo applications. Mol Cancer Ther. 2009;8:1239–1249. doi: 10.1158/1535-7163.MCT-08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray BP, Li S, Brown KC. From phage display to nanoparticle delivery: functionalizing liposomes with multivalent peptides improves targeting to a cancer biomarker. Bioconjug Chem. 2013;24:85–96. doi: 10.1021/bc300498d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guthi JS, Yang SG, Huang G, Li S, Khemtong C, Kessinger CW, Peyton M, Minna JD, Brown KC, Gao J. MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm. 2010;7:32–40. doi: 10.1021/mp9001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nothelfer EM, Zitzmann-Kolbe S, Garcia-Boy R, Kramer S, Herold-Mende C, Altmann A, Eisenhut M, Mier W, Haberkorn U. Identification and characterization of a peptide with affinity to head and neck cancer. J Nucl Med. 2009;50:426–434. doi: 10.2967/jnumed.108.058123. [DOI] [PubMed] [Google Scholar]
- 59.Kimura RH, Teed R, Hackel BJ, Pysz MA, Chuang CZ, Sathirachinda A, Willmann JK, Gambhir SS. Pharmacokinetically stabilized cystine knot peptides that bind alpha-v-beta-6 integrin with single-digit nanomolar affinities for detection of pancreatic cancer. Clin Cancer Res. 2012;18:839–849. doi: 10.1158/1078-0432.CCR-11-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 61.Hausner SH, Marik J, Gagnon MK, Sutcliffe JL. In vivo positron emission tomography (PET) imaging with an alphavbeta6 specific peptide radiolabeled using 18F-“click” chemistry: evaluation and comparison with the corresponding 4-[18F] fluorobenzoyl- and 2-[18F] fluoropropionyl-peptides. J Med Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hausner SH, Abbey CK, Bold RJ, Gagnon MK, Marik J, Marshall JF, Stanecki CE, Sutcliffe JL. Targeted in vivo imaging of integrin alphavbeta6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer Res. 2009;69:5843–5850. doi: 10.1158/0008-5472.CAN-08-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gagnon MK, Hausner SH, Marik J, Abbey CK, Marshall JF, Sutcliffe JL. High-throughput in vivo screening of targeted molecular imaging agents. Proc Natl Acad Sci U S A. 2009;106:17904–17909. doi: 10.1073/pnas.0906925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin alphavbeta6-specific peptide labeled with [18F] fluorine by copperfree, strain-promoted click chemistry. Nucl Med Biol. 2013;40:233–239. doi: 10.1016/j.nucmedbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Hausner SH, Bauer N, Sutcliffe JL. In vitro and in vivo evaluation of the effects of aluminum [18F] fluoride radiolabeling on an integrin alphavbeta6-specific peptide. Nucl Med Biol. 2014;41:43–50. doi: 10.1016/j.nucmedbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 66.White JB, Hausner SH, Carpenter RD, Sutcliffe JL. Optimization of the solid-phase synthesis of [18F] radiolabeled peptides for positron emission tomography. Appl Radiat Isot. 2012;70:2720–2729. doi: 10.1016/j.apradiso.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Hausner SH, Kukis DL, Gagnon MK, Stanecki CE, Ferdani R, Marshall JF, Anderson CJ, Sutcliffe JL. Evaluation of [64Cu] Cu-DOTA and [64Cu] Cu-CB-TE2A chelates for targeted positron emission tomography with an alphavbeta6-specific peptide. Mol Imaging. 2009;8:111–121. [PMC free article] [PubMed] [Google Scholar]
- 68.Hu LY, Bauer N, Knight LM, Li Z, Liu S, Anderson CJ, Conti PS, Sutcliffe JL. Characterization and evaluation of 64Cu-labeled A20FMDV2 conjugates for imaging the integrin alphavbeta6. Mol Imaging Biol. 2014 doi: 10.1007/s11307-013-0717-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh AN, McGuire MJ, Li S, Hao G, Kumar A, Sun X, Brown KC. Dimerization of a phage-dsplay selected peptide for imaging of αvβ6-integrin: two approaches to the multivalent effect. Theranostics. 2014;4:745–760. doi: 10.7150/thno.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hackel BJ, Kathlynn C. Kimura RH, Miao Z, Liu H, Sathirachinda A, Cheng Z, Chin FT, Gambhir SS. 18F-fluorobenzoate-labeled cystine knot peptides for PET imaging of integrin alphavbeta6. J Nucl Med. 2013;54:1101–1105. doi: 10.2967/jnumed.112.110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saha A, Ellison D, Thomas GJ, Vallath S, Mather SJ, Hart IR, Marshall JF. High-resolution in vivo imaging of breast cancer by targeting the pro-invasive integrin alphavbeta6. J Pathol. 2010;222:52–63. doi: 10.1002/path.2745. [DOI] [PubMed] [Google Scholar]
- 72.Ueda M, Fukushima T, Ogawa K, Kimura H, Ono M, Yamaguchi T, Ikehara Y, Saji H. Synthesis and evaluation of a radioiodinated peptide probe targeting alphavbeta6 integrin for the detection of pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun. 2014;445:661–666. doi: 10.1016/j.bbrc.2014.02.086. [DOI] [PubMed] [Google Scholar]
- 73.Man YK, DiCara D, Chan N, Vessillier S, Mather SJ, Rowe ML, Howard MJ, Marshall JF, Nissim A. Structural guided scaffold phage display libraries as a source of bio-therapeutics. PLoS One. 2013;8:e70452. doi: 10.1371/journal.pone.0070452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu X, Li J, Hong Y, Kimura RH, Ma X, Liu H, Qin C, Hu X, Hayes TR, Benny P, Gambhir SS, Cheng Z. 99mTc-labeled cystine knot peptide targeting integrin alphavbeta6 for tumor SPECT imaging. Mol Pharm. 2014;11:1208–1217. doi: 10.1021/mp400683q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, Liu H, Ma T, Sun X, Shi J, Jia B, Sun Y, Zhan J, Zhang H, Zhu Z, Wang F. Integrin alphavbeta6-targeted SPECT imaging for pancreatic cancer detection. J Nucl Med. 2014 doi: 10.2967/jnumed.113.132969. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Li F, Song Z, Li Q, Wu J, Wang J, Xie C, Tu C, Wang J, Huang X, Lu W. Molecular imaging of hepatic stellate cell activity by visualization of hepatic integrin alphavbeta3 expression with SPECT in rat. Hepatology. 2011;54:1020–1030. doi: 10.1002/hep.24467. [DOI] [PubMed] [Google Scholar]
- 77.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alphavbeta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 78.John AE, Luckett JC, Tatler AL, Awais RO, Desai A, Habgood A, Ludbrook S, Blanchard AD, Perkins AC, Jenkins RG, Marshall JF. Preclinical SPECT/CT imaging of alphavbeta6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J Nucl Med. 2013;54:2146–2152. doi: 10.2967/jnumed.113.120592. [DOI] [PubMed] [Google Scholar]
- 79.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 80.Gray BP, McGuire MJ, Brown KC. A liposomal drug platform overrides peptide ligand targeting to a cancer biomarker, irrespective of ligand affinity or density. PLoS One. 2013;8:e72938. doi: 10.1371/journal.pone.0072938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hecht JL, Dolinski BM, Gardner HA, Violette SM, Weinreb PH. Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16:543–547. doi: 10.1097/PAI.0b013e31816bc5ee. [DOI] [PubMed] [Google Scholar]
- 82.Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ. alpha vbeta 6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res. 2008;68:3295–3303. doi: 10.1158/0008-5472.CAN-08-0174. [DOI] [PubMed] [Google Scholar]
- 83.Jones J, Watt FM, Speight PM. Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med. 1997;26:63–68. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 84.Hamidi S, Salo T, Kainulainen T, Epstein J, Lerner K, Larjava H. Expression of alpha(v)beta6 integrin in oral leukoplakia. Br J Cancer. 2000;82:1433–1440. doi: 10.1054/bjoc.1999.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regezi JA, Ramos DM, Pytela R, Dekker NP, Jordan RC. Tenascin and beta 6 integrin are overexpressed in floor of mouth in situ carcinomas and invasive squamous cell carcinomas. Oral Oncol. 2002;38:332–336. doi: 10.1016/s1368-8375(01)00062-8. [DOI] [PubMed] [Google Scholar]
- 86.mpola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, Isaka K, Saarialho-Kere U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol. 2004;202:14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- 87.Hsiao JR, Chang Y, Chen YL, Hsieh SH, Hsu KF, Wang CF, Tsai ST, Jin YT. Cyclic alphavbeta6-targeting peptide selected from biopanning with clinical potential for head and neck squamous cell carcinoma. Head Neck. 2010;32:160–172. doi: 10.1002/hed.21166. [DOI] [PubMed] [Google Scholar]
- 88.Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, Chen R, Niu J. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879–887. doi: 10.1111/j.1349-7006.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawashima A, Tsugawa S, Boku A, Kobayashi M, Minamoto T, Nakanishi I, Nakanishi I, Oda Y. Expression of alphav integrin family in gastric carcinomas: increased alphavbeta6 is associated with lymph node metastasis. Pathol Res Pract. 2003;199:57–64. doi: 10.1078/0344-0338-00355. [DOI] [PubMed] [Google Scholar]
- 90.Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W, Wang JY, Niu WB, Liu EY, Mi YT, Niu J. Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol (R Coll Radiol) 2008;20:61–66. doi: 10.1016/j.clon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, Kalthoff H, Goodman SL, Kosmahl M, Klöppel G. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 92.Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer. 2000;7:19–26. doi: 10.1007/BF02967183. [DOI] [PubMed] [Google Scholar]
- 93.Smythe WR, LeBel E, Bavaria JE, Kaiser LR, Albelda SM. Integrin expression in non-small cell carcinoma of the lung. Cancer Metastasis Rev. 1995;14:229–239. doi: 10.1007/BF00690294. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS. Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–244. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- 95.Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, Weinreb PH, Fleuren GJ. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212:316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 96.Liu S, Liang B, Gao H, Zhang F, Wang B, Dong X, Niu J. Integrin alphavbeta6 as a novel marker for diagnosis and metastatic potential of thyroid carcinoma. Head Neck Oncol. 2013;5:7. [Google Scholar]


