Abstract
Although distal forearm fractures (DFFs) are common during childhood and adolescence, it is unclear whether they reflect underlying skeletal deficits or are simply a consequence of the usual physical activities, and associated trauma, during growth. Therefore, we examined whether a recent DFF, resulting from mild or moderate trauma, is related to deficits in bone strength and cortical and trabecular bone macro- and microstructure compared with nonfracture controls. High-resolution peripheral quantitative computed tomography was used to assess micro-finite element-derived bone strength (ie, failure load) and to measure cortical and trabecular bone parameters at the distal radius and tibia in 115 boys and girls with a recent (<1 year) DFF and 108 nonfracture controls aged 8 to 15 years. Trauma levels (mild versus moderate) were assigned based on a validated classification scheme. Compared with sex-matched controls, boys and girls with a mild-trauma DFF (eg, fall from standing height) showed significant deficits at the distal radius in failure load (−13% and −11%, respectively; p<0.05) and had higher (“worse”) fall load-to-strength ratios (both þ10%; p<0.05 for boys and p=0.06 for girls). In addition, boys and girls with a mild-trauma DFF had significant reductions in cortical area (−26% and −23%, respectively; p<0.01) and cortical thickness (−14% and −13%, respectively; p<0.01) compared with controls. The skeletal deficits in the mild-trauma DFF patients were generalized, as similar changes were present at the distal tibia. By contrast, both boys and girls with a moderate-trauma DFF (eg, fall from a bicycle) had virtually identical values for all of the measured bone parameters compared with controls. In conclusion, DFFs during growth have two distinct etiologies: those owing to underlying skeletal deficits leading to fractures with mild trauma versus those owing to more significant trauma in the setting of normal bone strength.
Keywords: BONE STRUCTURE, HRPQCT, FOREARM FRACTURE, TRAUMA LEVELS, CHILDREN
Introduction
Approximately one in three otherwise healthy children will fracture.(1–4) Distal forearm fractures (DFFs) are most common, and their incidence peaks during the early adolescent growth spurt.(1–4) Moreover, the incidence of childhood DFFs has increased markedly over the past 30 years.(5) Thus, there is an urgent need to better characterize the underlying skeletal basis for childhood DFFs and to identify high-risk individuals so that they can be targeted for interventions.
A complex interplay of genetic, hormonal, environmental, and behavioral factors determines skeletal development,(6) and some of these factors have been associated with childhood fractures.(7) These seemingly cluster into precipitating events (eg, obesity, falls) and underlying predisposition (eg, low bone density, impaired bone structure), and the latter are of interest with respect to long-term consequences for bone strength. In particular, although there is evidence that childhood DFFs may be related to skeletal fragility,(8) not all studies found this to be the case,(9) and it is also possible that these fractures are simply a consequence of the usual physical activities, and associated trauma, during growth.(10) Furthermore, the relationship between fracture and skeletal fragility has been suggested to be independent of trauma severity.(11) However, previous studies have been confounded by reliance on self-reported fractures, have included patients with diverse fractures, and most have relied on dual-energy X-ray absorptiometry (DXA), which has inherent limitations in assessing bone density and structure during growth. This is because two-dimensional areal bone mineral density (aBMD) measured by DXA is influenced by bone size, and the rapid changes that occur during growth confound interpretation.(12) In addition, DXA cannot differentiate between trabecular and cortical bone, which limits our understanding of the specific skeletal phenotype associated with childhood fracture. By contrast, high-resolution peripheral quantitative computed tomography (HRpQCT) allows for safe in vivo measurements (“noninvasive bone biopsy”) of bone macro-and microstructure of cortical and trabecular bone compartments, separately, as well as of micro-finite-element-derived biomechanical bone strength at the distal radius and tibia.
Therefore, to better characterize the underlying skeletal phenotype associated with childhood DFF, we used HRpQCT to examine whether a recent DFF is related to deficits in bone strength and structure compared with nonfracture controls. In addition, we rigorously classified the severity of the associated trauma to test whether this clinical variable, which is rarely accounted for in current clinical practice, may serve to identify children or adolescents who may have specific skeletal deficits and therefore warrant closer follow-up.
Materials and Methods
Study subjects
This cross-sectional study was approved by the Mayo Clinic Institutional Review Board. In accordance with Declaration of Helsinki, all guardians and subjects >12 years of age provided written informed consent; informed written assent was obtained from subjects≤12 years of age. Between October 2009 and April 2012, we continuously recruited healthy boys and girls between ages 8 and 15 years (which spans the peak in childhood DFF in the surrounding population5) from Olmsted County, MN, USA. Our cohort included patients who had sustained a recent (<1 year) DFF (63 boys and 52 girls) and subjects with no fracture history and similar age distribution (58 boys and 50 girls). Exclusion criteria included diseases that could affect bone metabolism and treatment with anti-epileptic drugs or history of oral corticosteroid use ≥4 weeks or ≥4 courses of oral or inhaled corticosteroids in a year. None of the girls had a history of oral contraceptive use. In addition, none of the subjects had a chronic illness or dietary restrictions. Clinical details in the medical records were reviewed to determine if potential subjects met study criteria.
Computerized diagnostic and procedure indices as part of the Mayo Clinic electronic medical records linkage system(13) were used to enumerate the local population and generate reports that were reviewed by trained research study personnel and cross-checked for agreement with the original reports to determine whether candidate DFF patients met established study criteria. Additional details regarding the medical records linkage system are provided in the Supplementary Methods. We verified from the original radiology reports that the DFF occurred at the distal metaphysis of the radius. Patients who presented at the Emergency Department or other clinical settings with an injury that resulted in either a fracture of the distal radius or in fractures of both the distal radius and ulna were eligible for the study. Candidates were excluded if the injury only resulted in a fracture of the ulna, and not the distal radius, because isolated ulnar fractures are less common and may result from different mechanisms of injury.(14,15) Fractures of the epiphyseal plate or cortical shaft of the radius were also excluded. In addition, we only recruited patients with a distal radius fracture owing to mild or moderate trauma based on a thorough review of redundant inpatient and outpatient data sources. We did not recruit any candidate patients with a distal radius fracture resulting from severe trauma (eg, falls >3 meters, motor vehicle accidents, etc.) or those with a history of bilateral DFFs. Patients with a DFF resulting from severe trauma were excluded because we hypothesized that these fractures likely occurred as a result of the trauma rather than because of underlying skeletal fragility. After enrollment in the study, mild (boys, n=30; girls, n=27) versus moderate (boys, n=33; girls, n=25) trauma was ascertained, blind to the bone imaging results, using Landin’s modified criteria11 (Table 1) based on specific details regarding the mechanism of injury that were obtained during an interview with the guardian and child. The nonfracture control subjects were simultaneously recruited using flyers as well as local newspaper and website advertisements from an age-stratified (8 to 15 years) random sample of Olmsted County, MN, residents. Clinical details in the medical records were reviewed to determine if potential control subjects met study criteria. This community is highly characteristic of the United States white population but underrepresented with respect to persons of African or Asian ancestry.(13) Reflecting the ethnic composition of the population of Olmsted County, MN, 97% of the sample was white.
Table 1.
Descriptive Categories of Landin's Modified Trauma Levelsa
| Descriptives indicating mild trauma |
| Falling onto the ground from standing height or less (<0.5 m) |
| Falling onto a resilient surface (eg, rubber, grass, or sand) from 0.5 m to 3 m |
| Falling from a bed or couch |
| Playing injuries including playground scuffles |
| Falling while moving at slow speed on a scooter, skateboard, skis, rollerblades, or skates |
| Lower-energy collisions with an object moving at slow speed |
| Lower-energy sport injuries (eg, basketball and soccer) |
| Descriptives indicating moderate trauma |
| Falling onto concrete or other nonresilient surface from 0.5 m to 3 m |
| Falling onto another person resulting in a moderate-energy collision |
| Falling from a bunk bed |
| Falling down stairs |
| Falling from a bicycle or horseback |
| Falling from a swing or slide or similar playground equipment |
| Falling while moving at fast speed on a scooter, skateboard, skis, rollerblades, or skates |
| Moderate-energy collisions with an object moving at fast speed |
| Moderate-energy collisions between two moving objects (eg, football and hockey) |
| Descriptives indicating severe trauma |
| Falling from a height exceeding 3 m (~10 feet) |
| Traffic accidents |
| Being hit by a moving heavy object |
Adapted from Clark and colleagues.(11)
Study protocol
Skeletal maturity (bone age) was assessed from plain X-rays of the left hand and wrist (ie, radius-ulna-small bones) using the RUS-TW3 method delineated by Tanner and Whitehouse.(16) This technique is considered the “gold standard” for assessing skeletal maturation in children and adolescents, and is possible even with a fracture and cast. Anthropometric data were collected on all subjects wearing light clothing and no shoes; weight was obtained using an electronic scale (Model 5002, Tronic, Inc., White Plains, NY, USA) and height was measured at full inhalation using a customized stadiometer (Mayo Section of Engineering). Diet(17) and physical activity(18) were assessed using validated questionnaires specific for children and adolescents; details are provided in the Supplementary Methods. Bone density and macro- and microstructure of the distal radius and tibia were assessed by HRpQCT as described previously,(19) but data from 13 scans (10 radius; 3 tibia) were excluded because of motion artifact. The nondominant distal tibia of all subjects was scanned, whereas in fracture patients, the nonfractured distal radius (and in control subjects, a randomly assigned radius) was scanned. Bone biomechanical strength was assessed from the HRpQCT images using micro-finite element (µFE) analysis.(20) Regional bone mass and body composition were assessed by DXA. Morning fasting blood samples were obtained for batch analyses and stored at −80°C. Sufficient serum was available on 119 boys and 98 girls, and biochemical assays were performed on these 217 subjects. Assay methods for the various biochemical parameters are detailed in the Supplementary Methods.
HRpQCT imaging
The HRpQCT device and in vivo image processing and analysis protocols used in our laboratory have been described previously.(19) The Xtreme-CT (Scanco Medical AG, Brüttisellen, Switzerland) was used to assess cortical and trabecular bone macro- and microstructure at the distal radius and tibia. From a digital image (scout view) of the distal forearm/ankle, a reference line was set at the proximal limit of the epiphyseal growth plate. For subjects whose epiphyseal plates were fused, the still visible remnant of the plate was used to set the reference line. An automated program was subsequently used to acquire high-resolution scans of the distal radius and tibia. Each 9.02-mmscan consisted of a fixed three-dimensional stack of 110 CT slices starting at 2mm proximal to the reference line, thereby minimizing concerns regarding radiation exposure to the growth plate. Total scan time was 2.8 minutes for each site, with an isotropic voxel size and slice thickness of 82µm, a field of view of 125.9 mm, and an image matrix of 1536 × 1536 pixels. Radiation exposure to subjects from HRpQCT is minimal, with a local absorbed dose of 0.065 Gy and total radiation exposure of <0.01 mSv. A single operator performed all HRpQCT scans. Short-term precision (CVs) of the HRpQCT device in our laboratory has been reported previously.(21) The validity of this approach has been rigorously tested, and excellent correlations (r≥0.96) have been shown between this technique and the “gold standard” ex vivo µCT technique.(22,23)
Trabecular bone volume fraction (bone volume/tissue volume [BV/TV]), trabecular area (Tb.A, mm2), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm) and trabecular separation (Tb.Sp, mm) were derived as previously described.(24,25) For the cortical parameters, the cortex was segmented from the grayscale image with a Gaussian filter and threshold.(25) Recognizing that the default cortical bone analysis performs poorly for subjects with thin cortices,(26,27) we used the extended cortical analysis available from the manufacturer to obtain cortical volumetric BMD (Ct.vBMD, mg/cm3), cortical area (Ct.A, mm2), cortical thickness (Ct.Th, mm), endocortical circumference (EC, mm), and periosteal circumference (PC, mm). Furthermore, we derived apparent cortical porosity (Ct.Po, %), which is a measure of the volume of the intracortical pore space normalized by the sum of the pore and cortical bone volumes, using a method described in detail by Burghardt and colleagues.(27)
µFE analysis
To evaluate bone strength, linear µFE models20 of the distal radius were created directly from the HRpQCT images using software provided by the manufacturer (µFE analysis solver v.1.15, Scanco Medical AG) as described previously.(28) A Young’s modulus of 10 GPa(29) and a Poisson’s ratio of 0.3(30) was assigned to each element. Bone strength (ie, failure load [N, newtons]) based on biomechanical properties was derived by scaling the resulting load from a test simulating 1% compression, such that 2% of all elements had an effective strain >7000 microstrain.(20) Failure loads calculated from such µFE models have been shown to correlate highly (r=0.87) with compressive loads producing a DFF in cadaveric forearms.(20) The fall load applied to the wrist was estimated from predicted impact forces on the upper extremity during loading conditions for a forward fall on the outstretched hand.(31) We assessed the ratio of fall load to failure load, as determined by µFE analysis, as an estimate of the fall load-to-strength ratio, or factor of risk (Ф).(32)
DXA measurements
Because bone mineral content (BMC) has been proposed by the International Society for Clinical Densitometry (ISCD) as the most appropriate DXA-derived skeletal measure in children and adolescents,(33) we measured BMC of the radius (ultradistal [UD] and total), lumbar spine (L1 to L4), total body less head (TBLH), and nondominant total hip, femoral neck (FN), trochanter, and diaphysis regions using DXA (Lunar Prodigy System; GE Healthcare, Madison, WI, USA). Lean mass, total body fat mass (TBFM), and percent body fat were obtained from DXA whole-body scans. Relative appendicular skeletal muscle mass (ASM, kg/m2) was calculated as the ratio of the lean mass of the arms and legs (kg) to height (m2), as previously described.(34) Similarly, relative TBFM (kg/m2) was calculated as the ratio of the TBFM (kg) to height (m2).
Statistical analyses
Data are expressed as mean±SE unless otherwise specified. All variables were tested for skewness and kurtosis; plots and multivariable regression models were used to check the data for normality, linearity, outliers, and potential influential observations. Because all variables satisfied the requirements for parametric statistics, transformations were not performed. Accordingly, descriptive, anthropometric, body composition, physical activity, and dietary characteristics were compared between the control and DFF groups (mild trauma, moderate trauma, and all patients) using one-way ANOVA. Further comparisons of the bone and biochemical parameters between the control and DFF groups were made using an ANCOVA model adjusted for skeletal maturation (ie, bone age). For all parameters, the Dunnett’s test was used to account for multiple comparisons when comparing the mild- or moderate-trauma DFF groups with the respective sex-matched control group. Separate analyses were performed for boys and girls because of known differences in the timing of growth and maturation between sexes.(16) To explore the role of potential confounders, the ANCOVA analyses were repeated after additional adjustment for the following variables: ethnicity, height, weight, vigorous-intensity physical activity, physical activity load score, calcium intake, and vitamin D intake. To address the primary objective, we assessed bone strength of the distal radius using µFE analysis of HRpQCT images. Secondary outcomes included the cortical and trabecular bone parameters of the distal radius and tibia obtained by HRpQCT and the DXA-derived measurements. All testing was performed at a significance level of p < 0.05 (two-tailed). Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
According to Landin’s modified criteria (Table 1),(11) 57 DFFs were classified as resulting from mild trauma (boys, n = 30; girls, n = 27), whereas the other 58 DFFs were attributed to moderate trauma (boys, n = 33; girls, n = 25). In all female and male fracture patients combined, 54% of DFFs occurred at the left forearm. The DFF was the only fracture suffered by 81% (n = 93) of patients, whereas 15% (n = 17) of DFF patients had one other earlier fracture and 4% (n = 5) of DFF patients had two or more other earlier fractures. Four patients had suffered an earlier DFF on the same side. Other earlier fracture sites included the clavicle (n=2), finger/hand (n=9), humerus (n=5), ulna shaft (n=3), fibula (n=4), and toe/foot (n=2). DFF patients completed the study 3.9 (0.7–11.9) [median (range)] months post DFF; 43% of DFF patients completed the study within 3 months of the DFF event.
Table 2 shows the descriptive characteristics of the study subjects. In this cohort of healthy children and adolescents, consistent with data from our previous analysis of DFF incidence in boys and girls in Rochester, MN,(5) we observed higher DFF rates in children and adolescents with bone ages in the range of 9 to 14 years (Supplemental Fig. S1). As evident in the figure, there were no differences in proportion of nonfracture controls and DFF patients (either combined or stratified by mild and moderate trauma) in each bone age category. Thus, the nonfracture controls and DFF patients were well matched for skeletal maturation (bone age). In addition, the DFF patients and controls were similar in chronological age, height, and weight. However, the male mild-trauma DFF patients had significantly lower relative ASM compared with controls. Furthermore, the female moderate-trauma DFF patients and the combined group of all female DFF patients tended to have higher BMIs and body fat compared with controls. Time spent in vigorous-intensity physical activity and physical activity load score tended to be higher in the DFF patients, but were not significantly different among the groups. Furthermore, calcium and vitamin D intakes did not differ among the groups.
Table 2.
Descriptive, Anthropometric, Body Composition, Physical Activity, and Dietary Characteristics of the Distal Forearm Fracture Patients (Mild Trauma, Moderate Trauma, All) and Nonfracture Controls
| Controls | Mild trauma | Moderate trauma | All patients | pa | pb | pc | |
|---|---|---|---|---|---|---|---|
| No. of subjects | |||||||
| Boys | 58 | 30 | 33 | 63 | |||
| Girls | 50 | 27 | 25 | 52 | |||
| DFF side (%; left) | |||||||
| Boys | NA | 50 | 52 | 51 | |||
| Girls | NA | 56 | 60 | 58 | |||
| Bone age (years) | |||||||
| Boys | 12.3 ±0.3 | 12.4 ±0.4 | 12.1 ±0.4 | 12.3 ±0.3 | 0.939 | 0.959 | 0.981 |
| Girls | 12.0 ± 0.3 | 11.5 ±0.4 | 12.0 ±0.4 | 11.8 ±0.3 | 0.646 | 0.999 | 0.625 |
| Chronological age (years) | |||||||
| Boys | 11.9 ± 0.2 | 12.1 ±0.3 | 11.7 ±0.3 | 11.9 ±0.2 | 0.880 | 0.919 | 0.973 |
| Girls | 11.9 ± 0.3 | 11.6 ± 0.4 | 11.7 ±0.4 | 11.6 ±0.3 | 0.845 | 0.886 | 0.571 |
| Height (cm) | |||||||
| Boys | 153 ± 1.8 | 154 ±2.5 | 153 ±2.4 | 154 ± 1.7 | 0.936 | 0.982 | 0.935 |
| Girls | 152 ± 1.6 | 151 ±2.1 | 151 ±2.2 | 151 ±1.5 | 0.902 | 0.954 | 0.686 |
| Weight (kg) | |||||||
| Boys | 48.5 ±2.1 | 47.3 ±2.9 | 49.3 ±2.8 | 48.3 ± 2.0 | 0.922 | 0.965 | 0.952 |
| Girls | 44.4 ± 1.7 | 44.2 ±2.3 | 48.4 ± 2.4 | 46.2 ±1.7 | 0.997 | 0.315 | 0.450 |
| BMI (kg/m2) | |||||||
| Boys | 20.0 ± 0.6 | 19.5 ±0.8 | 20.7 ±0.7 | 20.1 ±0.5 | 0.812 | 0.722 | 0.911 |
| Girls | 19.0 ± 0.5 | 19.4 ±0.7 | 20.9 ±0.8 | 20.1 ±0.5 | 0.876 | 0.076 | 0.135 |
| Relative ASM (kg/m2) | |||||||
| Boys | 6.4 ±0.14 | 6.1 ±0.20 | 6.5 ±0.19 | 6.3 ±0.14 | 0.283 | 0.948 | 0.515 |
| Girls | 5.7 ±0.10 | 5.7 ±0.14 | 5.9 ±0.15 | 5.8 ±0.10 | 0.993 | 0.351 | 0.487 |
| Relative TBFM (kg/m2) | |||||||
| Boys | 4.6 ± 0.43 | 4.8 ± 0.60 | 5.0 ±0.57 | 4.9 ±0.41 | 0.965 | 0.858 | 0.663 |
| Girls | 5.0 ±0.40 | 5.7 ±0.54 | 6.6 ± 0.56 | 6.2 ±0.39 | 0.492 | 0.043 | 0.045 |
| Percent body fat | |||||||
| Boys | 21.9 ±1.4 | 23.0 ±1.9 | 22.5 ±1.8 | 22.7 ±1.3 | 0.855 | 0.958 | 0.659 |
| Girls | 25.9 ±1.2 | 29.2 ±1.6 | 30.1 ±1.6 | 29.7 ±1.1 | 0.166 | 0.069 | 0.021 |
| Vigorous PA (hours/week) | |||||||
| Boys | 6.8 ± 0.6 | 7.7 ± 0.8 | 7.8 ± 0.8 | 7.8 ± 0.6 | 0.634 | 0.516 | 0.263 |
| Girls | 3.8 ±0.5 | 4.9 ± 0.6 | 4.2 ± 0.6 | 4.5 ± 0.4 | 0.284 | 0.855 | 0.243 |
| PA load score | |||||||
| Boys | 13.0 ± 0.6 | 12.4 ±0.8 | 12.8 ±0.8 | 12.6 ±0.6 | 0.791 | 0.985 | 0.659 |
| Girls | 11.1 ±0.7 | 12.3 ±1.0 | 11.6 ± 1.012.0 | 12.0 ±0.7 | 0.559 | 0.899 | 0.408 |
| Calcium intake (mg/day) | |||||||
| Boys | 1276 ±62 | 1260 ± 86 | 1477 ±82 | 1373 ± 60 | 0.984 | 0.098 | 0.262 |
| Girls | 1276 ±60 | 1286 ±81 | 1376 ± 85 | 1329 ±59 | 0.993 | 0.545 | 0.524 |
| Vitamin D intake (IU/day) | |||||||
| Boys | 433 ± 36 | 427 ±50 | 513 ±48 | 472 ±35 | 0.994 | 0.315 | 0.431 |
| Girls | 432 ±31 | 393 ±42 | 449 ± 43 | 420 ±30 | 0.687 | 0.931 | 0.782 |
DFF = distal forearm fracture; NA = not applicable; BMI = body mass index; ASM = appendicular skeletal muscle mass; TBFM = total body fat mass; PA = physical activity.
Values are presented as mean ±SE unless otherwise specified. Significant p values are in bold.
p = controls versus mild-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus moderate-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus all patients.
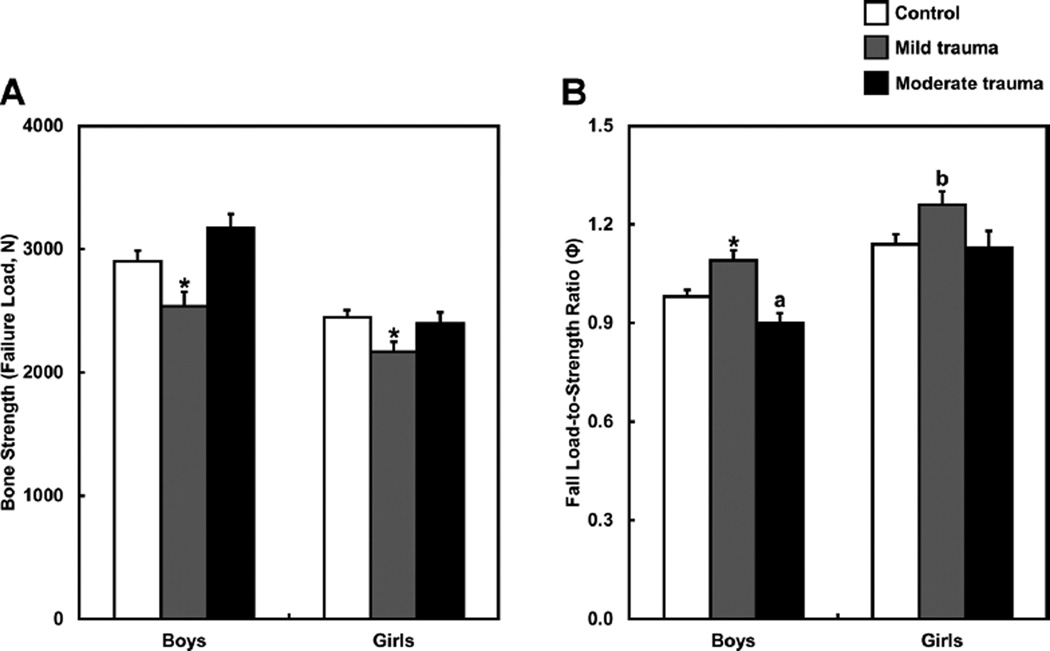
Fig. 1 shows the µFE-derived failure load and fall load-to-strength ratios of the distal radius for the controls and fracture patients within each sex, stratified by mild- or moderate-trauma DFFs. Compared with controls, male and female mild-trauma DFF patients showed reductions in failure load and increases in the fall load-to-strength ratios of the distal radius. By contrast, relative to controls, female moderate-trauma DFF patients had similar values for these parameters, whereas male moderatetrauma DFF patients actually had higher failure loads and lower fall load-to-strength ratios of the distal radius. In fact, as shown in Fig. 1B, male moderate-trauma DFF patients tended to have a reduced (“better”) load-to-strength ratio, the so-called factor of risk (Ф), relative to controls.
Fig. 1.
(A) Bone strength (failure load [N, newtons]) and (B) fall load-to-strength ratio (factor of risk [Ф]) of the distal radius in controls and the mild- and moderate-trauma distal forearm fracture groups separately by males and females. Values are presented as mean±SE adjusted for bone age. *p < 0.05; ap = 0.075; bp = 0.060 compared with the respective nonfracture control group, using the Dunnett adjustment for multiple comparisons.
Detailed macro- and microstructural analyses of the distal radius (Tables 3 and 4) revealed that compared with controls, male and female mild-trauma DFF patients had significant reductions in cortical bone area and thickness, whereas moderate-trauma DFF patients had similar values for these parameters compared with controls. In addition, male mild-trauma DFF patients had a lower trabecular bone volume fraction, reduced trabecular number, and increased trabecular separation, whereas female mild-trauma DFF patients showed a reduction in trabecular thickness at the distal radius. By contrast, endocortical/periosteal circumferences, cortical vBMD, and cortical porosity at the distal radius did not differ among the groups. The skeletal alterations were not confined to the distal radius, as similar findings were observed at the distal tibia (Tables 3 and 4). Similar to the data in Fig. 1, none of the cortical or trabecular bone parameters at any skeletal site differed between the moderate-trauma DFF patients and controls (Tables 3 and 4).
Table 3.
Cortical and Trabecular Bone Parameters of the Distal Radius and Tibia for the Male Distal Forearm Fracture Patients (Mild Trauma, Moderate Trauma, All) and Nonfracture Controls
| Male distal forearm fracture patients |
|||||||
|---|---|---|---|---|---|---|---|
| Male controls | Mild trauma | Moderate trauma | All patients | ||||
| (n = 58) | (n = 30) | (n = 33) | (n = 63) | pa | pb | pc | |
| Distal radius cortical parameters | |||||||
| Ct.A (mm2) | 30.7 ±1.3 | 22.7 ±1.9 | 32.7 ±1.8 | 27.8 ± 1.4 | 0.001 | 0.601 | 0.142 |
| Ct.Th (mm) | 0.796 ±0.022 | 0.682 ± 0.030 | 0.852 ± 0.030 | 0.768 ± 0.023 | 0.005 | 0.241 | 0.387 |
| EC (mm) | 45.7 ±0.7 | 45.6 ±1.0 | 46.7 ± 0.9 | 46.1 ±0.7 | 0.982 | 0.649 | 0.689 |
| PC (mm) | 58.2 ±0.7 | 57.0 ±1.0 | 59.2 ± 1.0 | 58.1 ±0.7 | 0.566 | 0.658 | 0.943 |
| Ct.vBMD (mg/cm3) | 707 ±5.6 | 697 ±7.8 | 713 ±7.7 | 705 ± 5.5 | 0.519 | 0.734 | 0.854 |
| Ct.Po (%) | 3.08 ±0.19 | 2.76 ±0.26 | 3.43 ±0.25 | 3.10±0.18 | 0.520 | 0.446 | 0.934 |
| Distal radius trabecular parameters | |||||||
| BV/TV | 0.146 ±0.004 | 0.134 ±0.005 | 0.151 ±0.005 | 0.143 ±0.004 | 0.067 | 0.667 | 0.435 |
| Tb.A (mm2) | 172 ±5.3 | 169 ±7.3 | 178 ± 7.2 | 174 ±5.1 | 0.944 | 0.694 | 0.778 |
| Tb.N (1/mm) | 2.02 ±0.03 | 1.89 ± 0.05 | 2.03 ± 0.04 | 1.96 ± 0.03 | 0.038 | 0.981 | 0.195 |
| Tb.Th (mm) | 0.072 ±0.001 | 0.070 ±0.001 | 0.074 ±0.001 | 0.072 ±0.001 | 0.414 | 0.501 | 0.945 |
| Tb.Sp (mm) | 0.429 ±0.010 | 0.478 ±0.015 | 0.425 ±0.014 | 0.451 ±0.010 | 0.015 | 0.963 | 0.149 |
| Distal tibia cortical parameters | |||||||
| Ct.A (mm2) | 59.8 ±2.6 | 40.4 ± 3.6 | 58.9 ± 3.4 | 50.3 ±2.6 | <0.001 | 0.972 | 0.013 |
| Ct.Th (mm) | 0.757 ±0.021 | 0.641 ±0.029 | 0.763 ± 0.028 | 0.706 ±0.021 | 0.003 | 0.981 | 0.089 |
| EC (mm) | 93.0 ±1.2 | 92.5 ±1.7 | 93.9 ± 1.6 | 93.2 ±1.2 | 0.960 | 0.876 | 0.889 |
| PC (mm) | 109.4 ±1.3 | 107.8 ±1.9 | 111.0 ± 1.8 | 109.5 ±1.3 | 0.719 | 0.717 | 0.969 |
| Ct.vBMD (mg/cm3) | 704 ±5.1 | 683 ± 7.2 | 696 ± 6.7 | 690 ± 4.9 | 0.038 | 0.602 | 0.056 |
| Ct.Po (%) | 4.74 ±0.21 | 4.01 ±0.30 | 5.22 ±0.28 | 4.65 ±0.21 | 0.090 | 0.323 | 0.759 |
| Distal tibia trabecular parameters | |||||||
| BV/TV | 0.159 ±0.003 | 0.145 ±0.004 | 0.157 ±0.003 | 0.151 ±0.003 | 0.003 | 0.850 | 0.034 |
| Tb.A (mm2) | 705 ±18.5 | 696 ±26.2 | 715 ±24.5 | 706 ± 17.8 | 0.946 | 0.925 | 0.960 |
| Tb.N (1/mm) | 2.12 ±0.03 | 2.00 ± 0.05 | 2.13 ±0.04 | 2.07 ±0.03 | 0.064 | 0.996 | 0.236 |
| Tb.Th (mm) | 0.075 ±0.001 | 0.073 ± 0.002 | 0.074 ±0.001 | 0.073 ±0.001 | 0.327 | 0.818 | 0.263 |
| Tb.Sp (mm) | 0.404 ± 0.008 | 0.441 ±0.012 | 0.405 ±0.011 | 0.422 ± 0.008 | 0.019 | 0.996 | 0.123 |
Ct.A = cortical area; Ct.Th = cortical thickness; EC = endocortical circumference; PC = periosteal circumference; Ct.vBMD = cortical volumetric bone mineral density; Ct.Po = cortical porosity; BV/TV = bone volume/total volume; Tb.A = trabecular area; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation.
Values are presented as mean ± SE adjusted for bone age. Significant p values are in bold.
p = controls versus mild-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus moderate-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus all patients.
Table 4.
Cortical and Trabecular Bone Parameters of the Distal Radius and Tibia for the Female Distal Forearm Fracture Patients (Mild Trauma, Moderate Trauma, All) and Nonfracture Controls
| Female distal forearm fracture patients |
|||||||
|---|---|---|---|---|---|---|---|
| Female controls | Mild trauma | Moderate trauma | All patients | ||||
| (n = 50) | (n = 27) | (n = 25) | (n = 52) | pa | pb | pc | |
| Distal radius cortical parameters | |||||||
| Ct.A (mm2) | 27.6 ±1.1 | 21.4 ±1.5 | 26.6 ± 1.723.7 | 23.7 ±1.2 | 0.003 | 0.850 | 0.021 |
| Ct.Th (mm) | 0.765 ±0.019 | 0.669 ± 0.026 | 0.760 ± 0.029 | 0.710 ±0.020 | 0.007 | 0.986 | 0.051 |
| EC (mm) | 42.2 ±0.6 | 44.2 ± 0.9 | 42.7 ±0.9 | 43.5 ± 0.6 | 0.122 | 0.871 | 0.144 |
| PC (mm) | 53.7 ±0.6 | 55.3 ±0.9 | 54.2 ±0.9 | 54.8 ± 0.6 | 0.250 | 0.852 | 0.212 |
| Ct.vBMD (mg/cm3) | 727 ±5.9 | 714 ±7.9 | 724 ± 8.8 | 718 ±5.9 | 0.304 | 0.937 | 0.284 |
| Ct.Po (%) | 2.10±0.14 | 2.02 ±0.19 | 2.41 ±0.21 | 2.20 ±0.14 | 0.926 | 0.395 | 0.640 |
| Distal radius trabecular parameters | |||||||
| BV/TV | 0.132 ±0.003 | 0.122 ±0.005 | 0.126 ±0.005 | 0.124 ±0.003 | 0.147 | 0.547 | 0.089 |
| Tb.A (mm2) | 145 ±4.3 | 157 ±5.8 | 148 ±6.4 | 153 ±4.3 | 0.160 | 0.863 | 0.166 |
| Tb.N (1/mm) | 1.87 ± 0.03 | 1.82 ± 0.05 | 1.83 ± 0.05 | 1.83 ± 0.03 | 0.682 | 0.824 | 0.419 |
| Tb.Th (mm) | 0.071 ±0.001 | 0.067 ±0.001 | 0.069 ± 0.002 | 0.068 ±0.001 | 0.050 | 0.534 | 0.044 |
| Tb.Sp (mm) | 0.478 ±0.012 | 0.489 ±0.016 | 0.484 ±0.017 | 0.486 ±0.012 | 0.813 | 0.951 | 0.602 |
| Distal tibia cortical parameters | |||||||
| Ct.A (mm2) | 44.0 ± 1.9 | 36.3 ± 2.6 | 45.8 ±2.7 | 40.9 ± 1.9 | 0.038 | 0.808 | 0.271 |
| Ct.Th (mm) | 0.659 ±0.018 | 0.593 ±0.024 | 0.661 ±0.025 | 0.626 ±0.018 | 0.061 | 0.997 | 0.200 |
| EC (mm) | 90.0 ± 1.0 | 94.1 ±1.4 | 89.0 ± 1.4 | 91.6 ±1.0 | 0.030 | 0.795 | 0.256 |
| PC (mm) | 105.0 ±1.1 | 108.9 ±1.5 | 103.6 ±1.5 | 106.3 ±1.1 | 0.077 | 0.708 | 0.404 |
| Ct.vBMD (mg/cm3) | 707 ±6.1 | 693 ± 8.2 | 707 ± 8.5 | 700 ± 5.9 | 0.340 | 0.999 | 0.422 |
| Ct.Po (%) | 3.46 ±0.19 | 3.27 ±0.26 | 3.59 ±0.26 | 3.42 ±0.18 | 0.780 | 0.903 | 0.884 |
| Distal tibia trabecular parameters | |||||||
| BV/TV | 0.145 ±0.003 | 0.138 ±0.004 | 0.143 ±0.004 | 0.140 ±0.003 | 0.317 | 0.902 | 0.287 |
| Tb.A (mm2) | 651 ±14.5 | 709 ± 19.4 | 637 ±20.1 | 675 ±14.3 | 0.037 | 0.806 | 0.269 |
| Tb.N (1/mm) | 2.06 ± 0.04 | 1.99 ±0.05 | 1.98 ± 0.05 | 1.98 ± 0.04 | 0.453 | 0.344 | 0.146 |
| Tb.Th (mm) | 0.070 ±0.001 | 0.070 ± 0.002 | 0.072 ± 0.002 | 0.071 ±0.001 | 0.900 | 0.497 | 0.712 |
| Tb.Sp (mm) | 0.424 ±0.010 | 0.438 ±0.013 | 0.442 ±0.013 | 0.440 ± 0.009 | 0.607 | 0.482 | 0.241 |
Ct.A = cortical area; Ct.Th = cortical thickness; EC = endocortical circumference; PC = periosteal circumference; Ct.vBMD = cortical volumetric bone mineral density; Ct.Po = cortical porosity; BV/TV = bone volume/total volume; Tb.A = trabecular area; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation.
Values are presented as mean ±SE adjusted for bone age. Significant p values are in bold.
p = controls versus mild-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus moderate-trauma patients, using the Dunnett adjustment for multiple comparisons.
p = controls versus all patients.
The generalized nature of the skeletal deficits in the mild-trauma DFF patients was also evident in the DXA BMC measurements in the boys (Supplemental Table S1), as these patients, but not the moderate-trauma DFF patients, had deficits at all of the DXA sites assessed. A similar pattern was observed for DXA measures in the mild-trauma DFF girls, although these differences did not reach statistical significance in minimally adjusted (bone age) analyses (Supplemental Table S2). However, some deficits in DXA measures in the mild-trauma DFF girls were significant after additional adjustment for confounders (Supplemental Table S6). Furthermore, in the boys and girls, serum biochemical/hormonal parameters did not differ between fracture patients and controls, either combined or separated by mild- and moderate-trauma DFFs (Supplemental Tables S1 and S2).
Collapsing the mild- and moderate-trauma DFF patients into a single group resulted in lesser differences in cortical and trabecular bone parameters and regional BMC measures between the DFF patients and nonfracture controls (Tables 3 and 4). Nonetheless, some between-group differences remained statistically significant. Lastly, similar trends were observed after additional adjustment for potential confounders (Supplemental Tables S3 to S6).
Discussion
Using a validated approach to assess bone strength from in vivo HRpQCT images,(20) the present study provides evidence that children and adolescents with a DFF attributable to mild, but not moderate, trauma have compromised bone strength at the distal radius. This is because of thinner bone cortices and deficits in trabecular bone microstructure. Moreover, boys and girls with a mild-trauma DFF have higher (“worse”) fall load-to-strength ratios (factor of risk [Ф]) at the distal radius, whereas girls with a moderate-trauma DFF have a similar Ф, and boys with a moderate-trauma DFF actually have a lower (“better”) Ф compared with nonfracture controls. The skeletal deficits in the mild-trauma DFF patients are not confined to the distal radius, but rather are generalized, given that similar alterations were present for the HRpQCT parameters at the distal tibia as well as for the DXA measures at peripheral and central skeletal sites.
It is of interest that we previously showed in children and adolescents without prior fracture that there is a transient peak in cortical porosity and a thinning of the cortex at the distal radius that occurs during mid to late puberty in both sexes.(19) These findings are supported by data from a study by Wang and colleagues.(35) As first hypothesized by Parfitt,(10) the transient weakening of the metaphyseal cortex of long bones may develop in response to greater calcium demand and intracortical bone turnover during peak linear growth. This transient cortical weakening is mirrored by the increase in the incidence of DFFs during puberty.(1–4) In the present study, however, we did not observe any differences among the groups in cortical porosity at the distal radius or tibia, suggesting that the transient peak in cortical porosity during puberty is common to all children and that the magnitude of this peak is similar in those with and without DFF. Moreover, although all children experience cortical thinning at the distal metaphysis of long bones during peak linear growth,(19,35) the present study demonstrates that boys and girls who fracture in the setting of mild, but not moderate, trauma have more severe cortical thinning at the distal radius and tibia than those without fracture.
Our findings are consistent with evidence from a systemic review and meta-analysis,(8) suggesting that there is a significant relationship between fractures and skeletal fragility in children and adolescents. However, it should be noted that a study by Beccard and colleagues(9) used standard pQCT in children and adolescents and found no differences between subjects with and without prior fracture. Although contradictory to our findings, differences in bone-imaging technology and study design likely explain why Beccard and colleagues(9) found no differences between the fracture and nonfracture groups. For example, a limitation of standard pQCT is that with a voxel size of approximately 400µm, this technique is less reliable for assessing cortical bone compared with HRpQCT.(36) In addition, this relatively low resolution prevents the ability to assess bone microstructure. Furthermore, Beccard and colleagues(9) did not classify the severity of the associated trauma to test whether this clinical variable serves to identify children or adolescents who have specific skeletal deficits. Although there have been previous HRpQCT studies in children and adolescents, to our knowledge, our study is the first to use this technique in boys and girls to characterize the underlying skeletal phenotype associated with a childhood DFF in the setting of mild versus moderate trauma.
One previous HRpQCT study in boys showed that prior fracture was associated with lower trabecular bone volume fraction and trabecular number and higher trabecular separation at the distal tibia but not at the distal radius.(37) In the present study, we found similar results after combining the male mild- and moderate-trauma DFF groups. However, whereas Chevalley and colleagues(37) did not report results according to fracture trauma severity, we observed much greater deficits in cortical and trabecular bone parameters at both the distal radius and tibia after separating fracture patients according to trauma severity and comparing the mild-trauma DFF patients with sex-matched nonfracture controls.
The relationship between DXA-derived bone measures and fracture risk in children has been previously shown to be independent of the level of trauma preceding the injury, as defined by Landin’s modified criteria.(11) Indeed, perhaps surprisingly, Clark and colleagues(11) reported that humeral estimated vBMD (derived from DXA) was reduced to a similar extent in children with fractures resulting from mild or moderate/ severe trauma compared with nonfracture controls. Contrary to these findings, we observed virtually identical DXA- and HRpQCT-derived bone parameters between moderate-trauma DFF patients and nonfracture controls. The reason(s) for the discrepancies in findings likely stem from differences in study design. For example, Clark and colleagues(11) measured bone parameters using DXA in the right humerus. The choice of this skeletal site may have been a confounder in the findings because the majority (71.4%) of fractures in their cohort occurred in the upper limb. Therefore, assuming a 50/50 split of left versus right upper limb fractures, casting/immobilization likely resulted in a significant reduction of bone mass in the right arm in a subset of patients in the moderate/severe-trauma fracture group. Our study avoided this limitation by specifically measuring bone parameters in the nonfractured forearm and excluding subjects with a history of bilateral DFFs. Additional potential limitations of the study by Clark and colleagues(11) include the self-report of the majority of fractures (radiology reports were only available on 40% of the sample), inclusion of subjects with any miscellaneous fracture, the assignment of trauma levels in only ~50% of children reporting fractures because of the limited number of children returning fracture questionnaires, and the previously noted concerns of relying on DXA for skeletal measurements, especially in children.(12)
One of the findings from our study was that compared with nonfracture controls, the male mild-trauma DFF patients had significantly lower appendicular skeletal muscle mass. Furthermore, the female DFF patients had significantly higher body fat relative to nonfracture controls, which has been previously implicated as a risk factor for fracture during growth.(38–40) Thus, our study provides further support for the potential roles of low muscle mass and obesity in increasing fracture risk in children and adolescents. Future analyses in this cohort should shed light on how body composition influences µFE-derived bone strength at weight-bearing versus nonweight-bearing skeletal sites.
Perhaps surprisingly, our study did not show any differences between the mild-trauma DFF patients and controls in any of the biochemical parameters, physical activity, or in calcium and vitamin D intakes. We acknowledge that our sample size may not have been sufficient to detect statistically significant differences in these parameters. Future studies with greater numbers of subjects will be necessary to further define the key biochemical parameters and lifestyle factors that determine bone strength and fracture risk in children and adolescents who suffer DFFs resulting from mild or moderate trauma.
Our findings raise the clinically important question of whether the skeletal deficits we observed in the children and adolescents with mild-trauma DFFs persist into adult life and predispose them to osteoporotic fractures later in life. Relevant to this question, using data from Olmsted County, MN, we recently found that a DFF in childhood increased the risk for a subsequent osteoporotic fracture in aging men, but not in women, with the sex difference perhaps being attributable to the fact that menopause in women may represent such a significant skeletal insult so as to obscure an association with childhood fractures.(41) Consistent with these data, observational studies have consistently shown that bone mass, size, and shape tend to track throughout life in the percentile of origin during growth(42–44) so that skeletal deficits, such as those found in mild-trauma DFF patients in the present study, could be an important determinant of fracture risk later in life.
We recognize several limitations of our study. First, our findings are cross-sectional; thus, they need to be confirmed prospectively. Second, there are well-known difficulties in obtaining data from questionnaires in children and adolescents, although we attempted to minimize the impact of this limitation by encouraging guardian assistance. A third issue is the generalizability of these data from a relatively small Midwestern community that is predominantly white. Nevertheless, our results can be reasonably extrapolated to a large part of the general pediatric population. A fourth issue is that the majority, but not all, of the DFF patients were seen in the Emergency Department versus other clinical settings; thus, we cannot completely exclude the possibility that this may have introduced selection bias. Lastly, a potential concern with the HRpQCT imaging, particularly in children and adolescents, is the choice of scan site. Unlike earlier pQCT imaging, the Xtreme-CT does not provide a scout film of the entire bone. Thus, to scan at a proportional distance of the radius (or tibia) in each subject requires reliance on external landmarks, which is potentially inaccurate. Moreover, this approach may end up including (and thus exposing to radiation) the growth plate in a subset of subjects. Minimizing concerns regarding radiation exposure to the growth plate, our procedure ensures that, despite differences in arm length, scans are performed as close as possible to the anatomic site where childhood DFFs most commonly occur.(1–4) It should be noted, however, that this approach could result in a scan site that would differ in subjects of differing ages. Nevertheless, this issue would be expected to have little impact on the findings of the present study because the DFF patients and nonfracture controls were well matched for bone age (Supplemental Fig. S1).
Given the mounting evidence suggesting that the skeletal deficits we observed in the patients who suffered a DFF resulting from mild trauma will likely track into adulthood,(42–44) our findings are concerning. The most current guidelines(45) established by the ISCD for fracture prediction and the definition of osteoporosis in children do not consider the level of trauma preceding the injury. However, because association does not prove causality, we suggest caution because only cross-sectional data are currently available. Therefore, well-designed prospective studies that rigorously classify trauma severity are needed to definitively establish whether this clinical variable, which is rarely accounted for in current clinical practice, can lead to improved diagnostic and therapeutic decisions for children and adolescents who present with a fracture resulting from mild trauma.
In conclusion, the present study provides evidence that children and adolescents with a DFF resulting from mild, but not moderate, trauma have suboptimal bone strength compared with nonfracture controls. We infer from these findings that DFFs during growth have two distinct etiologies: those attributable to underlying skeletal deficits leading to fractures with mild trauma versus those attributable to more significant trauma in the setting of normal bone strength. This study highlights the need for clinicians to consider the level of trauma preceding the injury when treating children and adolescents who present with fracture. Our findings further suggest that because individuals who fracture in the setting of mild trauma likely have underlying skeletal deficits, they may benefit from interventions to optimize lifestyle and nutritional factors related to bone health. Moreover, if additional studies validate that the skeletal deficits in children and adolescents with mild-trauma DFFs persist into adult life and predispose them to osteoporotic fractures during aging, then individuals with a history of such fractures may need to be more aggressively evaluated for osteoporosis later in life.
Supplementary Material
Acknowledgments
We thank the parents and the boys and girls for their participation in this study. The authors also thank James Peterson for data management, Margaret Holets for performing the HRpQCT scans, Sara Achenbach for statistical support, Sue Demaray for sample processing, and the Mayo Immunochemical Core Laboratory for performing the biochemical and hormonal assays.
This work was supported by NIH Grants R01 AR027065, P01 AG004875, and UL1 TR000135 (Mayo Center for Translational Science Activities). JNF is supported by T32 DK007352: Diabetes and Metabolism.
Authors’ roles: Study design: SA, LJM, SKi, BJA, RM, and SKh. Study contact: SKh. Study conduct: JNF and LKM. Data collection: JNF and LKM. Data analysis: BJA. Data interpretation: JNF, SA, LJM, BJA, and SKh. Drafting manuscript: JNF and SKh. Revising manuscript content: JNF, SA, LJM, SKi, LKM, BJA, RM, and SKh. Approving final version of manuscript: JNF, SA, LJM, SKi, LKM, BJA, RM, and SKh. SKh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand. 1983;202(Suppl):1–109. [PubMed] [Google Scholar]
- 2.Kramhoft M, Bodtker S. Epidemiology of distal forearm fractures in Danish children. Acta Orthop Scand. 1988;59:557–559. doi: 10.3109/17453678809148784. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg. 1989;71-A(1):1225–1231. [PubMed] [Google Scholar]
- 4.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19(12):1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Melton LJ, III, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290(11):1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 6.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 7.Goulding A. Risk factors for fractures in normally active children and adolescents. In: Daily RM, Petit MA, editors. Optimizing bone mass and strength: the role of physical activity and nutrition during growth. Basel, Switzerland: Karger; 2007. pp. 102–120. [Google Scholar]
- 8.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:291–297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beccard R, Land C, Semler O, et al. Do bone mineral density, bone geometry and the functional muscle-bone unit explain bone fractures in healthy children and adolescents? Horm Res Paediatr. 2010;74(5):312–318. doi: 10.1159/000313380. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4(6):382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 11.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23(2):173–179. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachrach LK. Dual energy X-ray absorptiometry (DEXA) measurements of bone density and body composition: promise and pitfalls. J Pediatr Endocrinol Metab. 2000;13(Suppl 2):983–988. [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Dymond IW. The treatment of isolated fractures of the distal ulna. J Bone Joint Surg Br. 1984;66(3):408–410. doi: 10.1302/0301-620X.66B3.6725352. [DOI] [PubMed] [Google Scholar]
- 15.Szabo RM, Skinner M. Isolated ulnar shaft fractures. Retrospective study of 46 cases. Acta Orthop Scand. 1990;61(4):350–352. doi: 10.3109/17453679008993534. [DOI] [PubMed] [Google Scholar]
- 16.Tanner JM, Healy MJR, Goldstein H, Cameron N. Assessment of skeletal maturity and prediction of adult height (TW3 method) Saunders; Philadelphia: 2001. [Google Scholar]
- 17.Rockett HRH, Breitenbach M, Frazier AL, et al. Validation of a youth/ adolescent food frequency questionnaire. Prev Med. 1997;26(6):808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 18.Rifas-Shiman SL, Gillman MW, Field AE, et al. Comparing physical activity questionnaires for youth: seasonal vs annual format. Am J Prev Med. 2001;20(4):282–285. doi: 10.1016/s0749-3797(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 19.Kirmani S, Christen D, van Lenthe GH, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24(6):1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–848. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 21.Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24(1):35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 24.Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: a new parameter for in vivo bone structure analysis. Bone. 1997;21(6):541–546. doi: 10.1016/s8756-3282(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 25.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6(5–6):329–337. [PubMed] [Google Scholar]
- 26.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–515. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farr JN, Charkoudian N, Barnes JN, et al. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012;97(11):4219–4227. doi: 10.1210/jc.2012-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech. 1995;28(1):69–81. doi: 10.1016/0021-9290(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 30.van Rietbergen B, Odgaard A, Kabel J, Huiskes R. Direct mechanics assessment of elastic symmetries and properties of trabecular bone architecture. J Biomech. 1996;29(12):1653–1657. doi: 10.1016/0021-9290(96)00093-0. [DOI] [PubMed] [Google Scholar]
- 31.Chiu J, Robinovitch SN. Prediction of upper extremity impact forces during falls on the outstretched hand. J Biomech. 1998;31(12):1169–1176. doi: 10.1016/s0021-9290(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 32.Melton LJ, 3rd, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22(9):1442–1448. doi: 10.1359/jbmr.070514. [DOI] [PubMed] [Google Scholar]
- 33.Gordon CM, Bachrach LK, Carpenter TO, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clin Proc. 2000;75:S10–S13. [PubMed] [Google Scholar]
- 35.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25(7):1521–1526. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 36.Augat P, Gordon CL, Lang TF, Iida H, Genant HK. Accuracy of cortical and trabecular bone measurements with peripheral quantitative computed tomography (pQCT) . Phys Med Biol. 1998;43:2873–2883. doi: 10.1088/0031-9155/43/10/015. [DOI] [PubMed] [Google Scholar]
- 37.Chevalley T, Bonjour JP, van Rietbergen B, Ferrari S, Rizzoli R. Fractures during childhood and adolescence in healthy boys: relation with bone mass, microstructure, and strength. J Clin Endocrinol Metab. 2011;96(10):3134–3142. doi: 10.1210/jc.2011-1445. [DOI] [PubMed] [Google Scholar]
- 38.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20(12):2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 39.Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res. 2010;25(3):527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 40.Dimitri P, Bishop N, Walsh JS, Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50(2):457–466. doi: 10.1016/j.bone.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Amin S, Melton LJ, Achenbach SJ, et al. A distal forearm fracture in childhood increases the risk for fracture during adulthood in men, but not in women. J Bone Miner Res. 2013;28(8):1751–1759. doi: 10.1002/jbmr.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrari S, Rizzoli R, Slosman D, Bonjour JP. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83(2):358–361. doi: 10.1210/jcem.83.2.4583. [DOI] [PubMed] [Google Scholar]
- 43.Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85(10):3908–3918. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Cheng S, Alen M, Seeman E. Bone’s structural diversity in adult females is established before puberty. J Clin Endocrinol Metab. 2009;94(5):1555–1561. doi: 10.1210/jc.2008-2339. [DOI] [PubMed] [Google Scholar]
- 45.Rauch F, Plotkin H, DiMeglio L, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2007 Pediatric Official Positions. J Clin Densitom. 2008;11(1):22–28. doi: 10.1016/j.jocd.2007.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.