Abstract
Objective
To assess differences in morphological and glycolytic characteristics of primary tumors and locoregional nodal disease between HPV-positive and HPV-negative oropharygeal head and neck squamous cell carcinoma (HNSCC).
Methods
A retrospective analysis of 123 baseline FDG PET/CT scans from patients (age: 57.0 ± 10.6 yrs), newly diagnosed with oropharyngeal SCC between January 2003 and June 2012. There were 98 HPV positive and 25 HPV negative patients. SUVmax, SUVpeak, and SUVmean based on lean body mass, as well as RECIST dimensions, metabolic tumor volume (MTV) (gradient and threshold segmentation methods) and total lesion glycolysis (TLG) were determined for primary and locoregional nodal disease.
Results
HPV negative primary tumors were significantly larger in size as measured by RECIST longest diameter (p=0.002), slightly more heterogenous as meassured by the heterogenity index (HI) (p=0.07), higher SUVmax (p<0.01), SUVpeak (p=0.01), SUVmean (p=0.01), MTV (p=0.002), and TLG (p=0.001), for both segmentation methods. Index parameters of HPV positive nodal disease tends to be larger, but some with no statistical significance (p>0.05). There was no significant difference in the metabolic parameters of primary tumor or nodal metastases for HPV positive patients with and without smoking history.
Conclusion
Index morphologic and glycolytic parameters as measured in FDG PET/CT are significantly larger in HPV negative as compared to HPV positive primary oropharyngeal carcinoma. In contrast, the same parameters trended to be larger in HPV positive regional nodal disease.
Keywords: HPV, FDG PET/CT, metabolic tumor volume, total lesion glycolysis
INTRODUCTION
The head and neck cancers are predominantly of squamous cell carcinoma histology. Tobacco and alcohol are known to be major risk factors for all head and neck of squamous cell cancer (HNSCC) subsites. However, over the past several years HPV infection has been increasingly recognized as a major etiologic factor for a subset of HNSCCs arising from the oropharynx[1]. Greater than 90% of these HPV- HNSCC are associated with a single HPV type, HPV16. HPV positive oropharyngeal sqamous cell cancers (OPSCCs) are epidemiologically distinct from HPV negative ones. HPV-related OPSCCs are characterized by younger age at onset, predominance in males and whites and a strong association with sexual behavior [2, 3].
PET/CT has been increasingly integrated into diagnostic staging and radiation planning for HNSCC, and has been demonstrated to be an accurate and sensitive imaging modality for the post-treatment evaluation of patients with HNSCC compared to clinical exam and CT alone[4-8]. Identification of novel pretreatment imaging biomarkers that potentially predict long-term outcome is of great interest. PET/CT standardized uptake value (SUV) measurements are reproducible imaging biomarkers that have diagnostic and prognostic value in HNSCC in general and in oral and oropharyngeal SCC in particular[7].
Recently, FDG metabolic tumor volume (MTV) and total lesion glyclysis (TLG) have been reported as additional diagnostic and prognostic imaging biomarkers in various human solid tumors. Volumetric indices have been proposed to risk-stratify patients. Studies have reported that the primary tumor metabolic volume correlates with outcomes and survival in patients with HNSCC undergoing curative surgery, radiation, or combined chemoradiation treatments in various head and neck cancer sites[9, 10]. Given the interest in PET-based imaging, the MTV has been recently explored as a combined volumetric and metabolic imaging biomarker[11-13].
The objective of this study is to characterize the FDG PET imaging markers, such as SUVmax, SUVpeak, MTV, and TLG, and heterogenity of the primary tumor and regional nodal metstases in HPV-positive and HPV-negative OPSCC patients.
MATERIALS AND METHODS
Patient selection
The study was conducted as a retrospective review approved by the institutional review board (IRB). Informed consent was waived by the IRB. Patients diagnosed with OPSCC who presented between January 2003 and June 2012 had baseline PET/CT imaging obtained prior to start of any form of therapy were considered. Only patients with HPV status assessed by in situ hybridization were included. All patients were staged according to the AJCC classification (6th edition).
PET/CT protocol
All PET/CT studies were performed using two PET/CT systems: either a Discovery LS (2D), or a Discovery VCT (3D) (General Electric, Milwaukee). All patients were scanned using a dedicated head and neck protocol. Patients were scanned skull vertex to mid-thighs in two separate acquisititons starting from mid-thigh to chin, and then from carina to skull vertex. Head and neck images were acquired with the arms down and body images were obtained with arms up. After at least a 4-hr fast and serum glucose measurement, patients were administered 8.1 MBq (2.2 mCi) of 18 F-FDG per kilogram and incubated for a period of 60 min. Injection-to –scan time for head and neck acquisition was 81.5 ± 19.4 min. Plasma glucose was 102.3 ± 17.7 mg/dL.
The ordered-subsets expectation maximization algorithm was used to reconstruct all PET images. The fully 3-dimensional implementation on the Discovery VCT (RX) used 2 iterations, 21 subsets, a 3.0-mm ostreconstruction gaussian filter, and 4.7-mm pixels and 3.75min for each bed position. All PET data were reconstructed with and without CT-based attenuation correction. Helical CT images for attenuation correction and anatomical correlation (CTAC) were also obtained in two acquisitions: head-and-neck and whole body covering the same regions as PET. Both CTAC acquisitions were obtained with a matrix of 512 x 512. X-ray source voltage was fixed at 120 kVp. Current intensity was modulated via Smart mA on the GE scanner with a minimum of 20 mA and a maximum of 200 mA. Beam collimation was 10mm with a pitch of 0.984, with a rotation speed of 0.5 sec/rev. Reconstruction slice thickness was 3.75 mm. Noise index was fixed at 20 for both head and neck and whole-body acquisitions.
PET/CT image analysis
All PET/CT studies were electronically retrieved and reviewed on a MimVista workstation (version 5.2, MimVista software Inc, Cleveland, OH) by a board-certified nuclear medicine fellow. PET, CT, and fused PET/CT images were displayed in axial, coronal, and sagittal planes. For this study, the relevant imaging parameter measurements included the primary tumor longest diameter, and SUVmax, SUVpeak, SUVmean, MTV and TLG segmented from the PET images. MTV was defined as the tumor volume with FDG uptake segmented with both gradient-based and threshold methods. MIMvista software analysis suite (MIM Software Inc., Cleveland, OH) includes a contouring PET/CT suite. Once the primary tumor (target) was segmented, SUVmax and MTV were automatically calculated by the MIMvista software. The gradient and threshold segmentation methods of volume measurement available in MIMvista software rely on an operator-defined starting point near the center of the lesion. As the operator drags the cursor out from the center of the lesion, six axes extend out, providing visual feedback for the starting point of gradient segmentation. Spatial gradients are calculated along each axis interactively, and the length of an axis is restricted when a large gradient is detected along that axis. The six axes define an ellipsoid that is then used as an initial bounding region for gradient detection. The MTV, TLG, SUVmax, and SUVpeak within the bounding region are automatically calculated. For the threshold method, a 50% of max threshold was used throughout. For nodal metastases, we considered lymph nodes larger than 1 cm in long axis with a minimal SUVmax cut point of 1.5. A quantitative measure of heterogeneity, heterogeneity index (HI)[14] was obtained by dividing SUVmax by SUVmean for primary lesion and nodal disease. To minimize the impact of tumor size on MTV between the HPV positive and HPV negative groups, we further performed analyses with MTV of the primary tumor or the largest nodal metastases divided by the longest tumor diameter (metabolic tumor volume index).
Statistical analysis
We present our summary statistics as the median and range as most parameters do not have a normal distribution. We used the Pearson correlation coefficient to establish the relationship between different segmentation methods. In between group analyses were performed using the Mann Whitney test. We investigated whether there is significant difference between HPV positive and negative groups for longest RECIST diameter, SUVmax, SUVpeak, SUVmean, MTV and TLG, and heterogeneity for primary tumor and nodal metastases. A subgroup analysis was also performed among HPV positive OPSCC patients who were smokers and those who never smoked, as these groups have different outcome with the survival advantage is reduced for those who are HPV positive but also smoked. We used MedCalc (version 12.3,MedCalc Software, Mariakerke, Belgium) and SPSS (version 20; SPSS Inc, Chicago, IL.) statistical packages for all analyses. All hypothesis tests were 2-sided, with a significance level of 0.05.
RESULTS
Patients’ characteristics
A total of 123 patients (age: 57.0 ±10.6 yrs) met the eligibility criteria. These were subdivided into two groups according to HPV status. The HPV positive group had 98 patients (82 male, 16 female); age 57.4 ± 9.7 yrs. The HPV negative group had 25 patients (14 male, 11 female); age 54.1± 13.2 yrs. No statistically significant difference in age between the two groups was found. The HPV positive group had a significantly higher proportion of males (83.7% vs 56%, p = 0.007) and significantly disproportionate number of whites than HPV negative group (88.8% vs 40%, p < 0.0001). The characteristics of each patient group are summarized in table 1.
Table 1.
Study patient characteristics
| HPV+ | HPV− | ||
|---|---|---|---|
| N | 98 | 25 | |
| sex | |||
| Male | 82 | 14 | p = 0.007 |
| Female | 16 | 11 | |
| Age (yrs) | |||
| Range | 29 - 78 | 30 - 76 | |
| Mean ± SD | 57.4 ± 9.7 | 54.1 ± 13.2 | p = 0.16 |
| Ethnicity | 10W | ||
| White | 87 | 10 | p < 0.0001 |
| Black | 5 | 12 | |
| Other | 6 | 3 | |
| Smoking | |||
| Yes | 48 | 17 | p = 0.0001 |
| No | 50 | 8 | |
| Pack-years | |||
| Range | 1 - 88 | 5 - 80 | |
| Mean ± SD | 28.8 ± 19.4 | 35.4 ± 22.5 | p = 0.26 |
Primary tumor
The median SUVmax measurements of the primary tumor site in the oropharynx for the HPV positive and HPV negative groups were 10.4 (range:2.9 – 21.6) and 12.4 (2.7 – 21.5), respectively (p = 0.007). The primary tumor SUVmean measurements for the HPV positive and HPV negative groups were 5.1 (1.9 – 10.5) and 6.4 (1.9 – 13.0), respectively (p = 0.01). The primary tumor SUVpeak measurements for the HPV positive and HPV negative groups were 9.2 (3.9 – 18.1) and 11.9 (5.8 – 19.5), respectively (p = 0.01).
Using segmentation based on the gradient method, the median primary tumor MTV measurements for the HPV positive and HPV negative groups were 8.5 (0.5 – 164.5) and 21.9 (1.9 – 193.1), respectively (p = 0.002). The primary tumor TLG measurements for the HPV positive and HPV negative groups were 41.5 (1.3 – 635.9) and 165.9 (3.6 – 1414.9), respectively (p = 0.001) (figure 1). Similar significant differences were observed for segmentation based on threshold method at 50% of SUVmax.. HPV negative primary lesions are significantly larger in size as measured by the RECIST long axis dimension than their HPV positive counterparts, 3.7 (1.5 – 8.8) vs 2.7 (1.0 – 7.4) respectively, (p=0.02) (Table 2).
Figure 1.
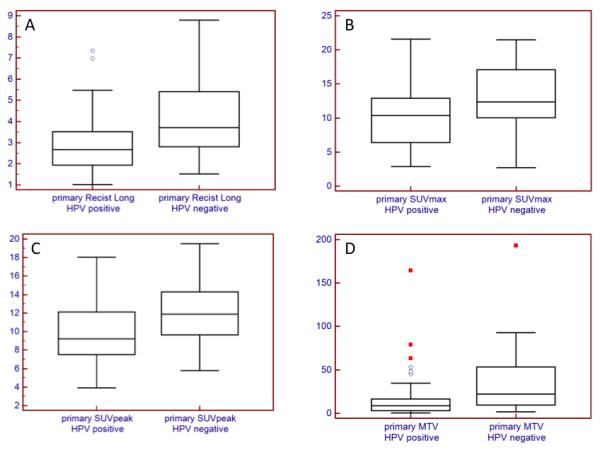
Primary tumor: RECIST long axis length (A), SUVmax (B), SUVpeak (C), and MTV (D). HPV negative primary tumors were significantly larger in size as measured by RECIST longest diameter (p = 0.002), and had higher SUVmax (p = 0.007), SUVpeak (p = 0.01), SUVmean (p = 0.01), MTV (p = 0.002), and TLG (p = 0.001).
Table 2.
Comparison of morphological and metabolic indices on FDG PET between HPV+ and HPV− OPCCs (median and range).
| HPV+ | HPV− | ||
|---|---|---|---|
| Primary | |||
| SUVmax | 10.4 (2.9 – 21.6) | 12.4 (2.7 – 21.5) | p=0.007 |
| SUVmean | 5.1 (1.9 – 10.5) | 6.4 (1.9 – 13.0) | p = 0.01 |
| SUVpeak | 9.2 (3.9 – 18.1) | 11.9 (5.8 – 19.5) | p = 0.01 |
| MTVedge (cm3) | 8.5 (0.5 – 164.5) | 21.9 (1.9 – 193.1) | p = 0.002 |
| TLGedge (cm3) | 41.5 (1.3 – 635.9) |
165. 9 (3.6 – 1414.9) | p = 0.001 |
| MTVthreshold (cm3) | 3.9 (0.4 – 34.7) | 9.4 (0.9 – 68.4) | p = 0.002 |
| TLGthreshold (cm3) | 26.6 (1.1 – 452.0) |
96.4 (1.8 – 757.1) | p = 0.0009 |
| RECISTlong (cm) | 2.7 (1.0 – 7.4) | 3.7 (1.5 – 8.8) | p=0.002 |
| HI | 1.8 (1.3 – 3.2) | 1.9 (1.5 – 2.8) | p = 0.07 |
| MI | 3.1 (0.5 – 22.3) | 6.0 (1.2 – 21.9) | P = 0.002 |
| Nodal | |||
| Highest SUVmax | 8.0 (3.2 – 22.9) | 8.5 (3.0 – 16.6) | p = 0.9 |
| Highest SUVmean | 4.0 (1.4 – 10.7) | 4.2 (1.8 – 8.7) | p =1.0 |
| Highest SUVpeak | 6.8 (2.8 – 30.4) | 6.4 (4.4 – 13.2) | p =0.2 |
| Highest MTVedge (cm3) | 10.0 (0.6 – 148.1) | 4.5 (0.9 – 115.7) | p =0.05 |
| Sum MTVedge (cm3) | 13.5 (0.6 – 188.5) | 5.9 (0.9 – 170.8) | p =0.09 |
| Highest RECISTlong (cm) | 2.8 (1.4 – 7.8) | 2.0 (1.4 – 7.4) | p =0.04 |
| Highest TLGedge | 39.4 (1.4 – 807.1) |
20.9 (1.7 – 565.0) | p =0.1 |
| Sum TLGedge | 43.4 (1.4 – 807.1) | 27.3 (1.7 – 836.3) | p =0.2 |
| Sum MTVthreshold | 5.1 (0.7 – 68.2) | 2.9 (0.5 – 80.3) | p =0.1 |
| Highest MTVthreshold | 3.8 (0.7 – 48.4) | 2.2 (0.5 – 55. 9) | p = 0.04 |
| Highest TLGthreshold | 21.6 (1.7 – 412.3) |
13.6 (1.1 – 360.6) | p = 0.09 |
| Sum TLGthreshold | 26.5 (1.7 – 412.3) |
18.3 (1.1 – 524.0) | p =0.2 |
| Heterogeneity Index | 2.0 (1.4 – 3.0) | 1.9 (1.5 – 3.4) | p =0.4 |
| Metabolic Index | 3.6 (0.4 – 18.9) | 2.2 (0.6 – 15.6) | p = 0.08 |
MTV: metabolic tumor volume, TLG: total lesion glycolysis, HI: heterogeneity index, MI: metabolic index
Comparing heterogeneity indexes as defined in the methods section, primary tumors of HPV negative patients are slightly more heterogeneous than HPV positive lesions, 1.9 (1.5 – 2.8) vs 1.8 (1.3 – 3.2), (p=0.07), with tendency toward statistical significance. The metabolic tumor volume index, defined in the methods section, is significantly higher in HPV negative primary lesions, 6.0 (1.2 – 21.9) vs 3.1 (0.5 – 22.3), (p=0.002). (Table 2, figure 2).
Figure 2.
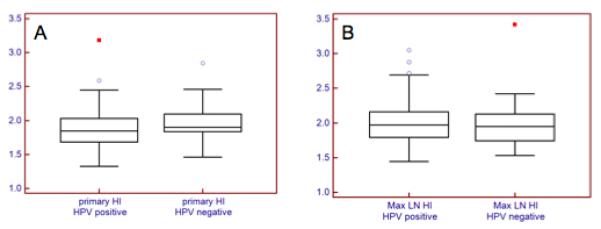
Heterogeneity index (HI) of primary tumor (A), and locoregional lymphadenopthy (B). HPV negative primary tumors tended to be more heterogeneous (p = 0.07), but HPV positive lymph nodes tended to be more heterogeneous (p =0.4)
Regional nodal disease
Comparing the lymph nodes with highest SUVmax for each patient, the highest SUVmax measurements of the nodal disease for the HPV positive and HPV negative groups were 8.0 (3.2 – 22.9) and 8.5 (3.0 – 16.6), respectively (p = 0.9). The nodal SUVmean measurements for the HPV positive and HPV negative groups were 4.0 (1.4 – 10.7) and 4.2 (1.8 – 8.7), respectively (p = 1.0). The nodal SUVpeak measurements for the HPV positive and HPV negative groups were 6.8 (2.8 – 30.4) and 6.4 (4.4 – 13.2), respectively (p = 0.2). Morphological and glycolytic indexes of nodal metastases were generally larger in HPV positive than in HPV negative OPSCC, some without statistical significance.
Using segmentation based on the gradient method, the mean MTV measurements for the HPV positive and HPV negative groups were 10.0 (0.6 – 148.1) and 4.5 (0.9 – 115.7), respectively (p = 0.05). The highest TLG measurements for the HPV positive and HPV negative groups were 39.4 (1.4 – 807.1) and 20.9 (1.7 – 565.0), respectively (p=0.1). The sum MTV measurements for the HPV positive and HPV negative groups was 13.5 (0.6 – 188.5) and 5.9 (0.9 – 170.8), respectively (p = 0.09). The sum TLG for the HPV positive and HPV negative groups was 43.4 (1.4 – 807.1) and 27.3 (1.7 – 836.3), respectively (p = 0.2). HPV positive lymph nodes were larger in size as measured by the RECIST long axis dimension than their HPV negative counterparts, 2.8 (1.4 – 7.8) vs 2.0 (1.4 – 7.4) respectively, p = 0.04 (Table 2).
Comparing the heterogeneity index (HI) of the larger nodes in the two groups of patients, HPV positive nodes tend to be more heterogeneous with no statistical significance, 2.0 (1.4 – 3.0) vs 1.9 (1.5 – 3.4), (p = 0.4) (figure 4). The metabolic tumor volume index tended to also be higher in the HPV positive group, when comparison is made between the largest nodes, 3.6 (0.4 – 18.9) vs 2.2 (0.6 – 15.6), p = 0.08) (Table 2 and Figure 2). An HPV negative and a positive case are illustrated in figures 3 and 4.
Figure 4.
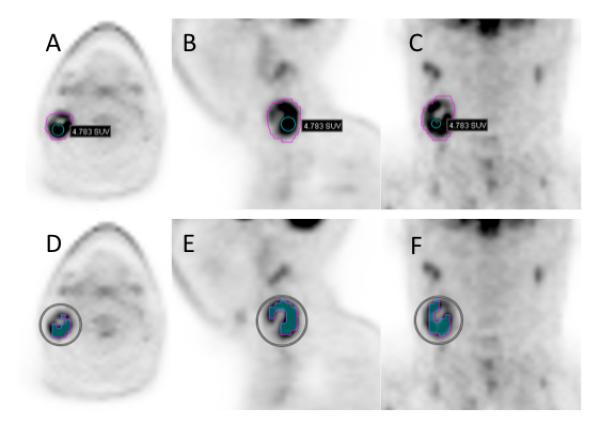
29-year-old man diagnosed with squamous cell carcinoma of the right tonsil that was HPV positive, metastatic to level II right cervical lymph node with central necrosis. Axial (A), sagital (B), and coronal (C) PET slices show edge-segmentation contouring. SUVmax 6.3, SUVmean 2.6, SUVpeak 4.8, MTVedge 19.0, TLGedge 40.5. Axial (D), sagital (E), and coronal (F) PET slices show threshold-segmentation contouring at 50%of % of SUVmax. SUVmean 4.2, MTVthreshold 6.1, TLGthreshold 25.8.
Figure 3.
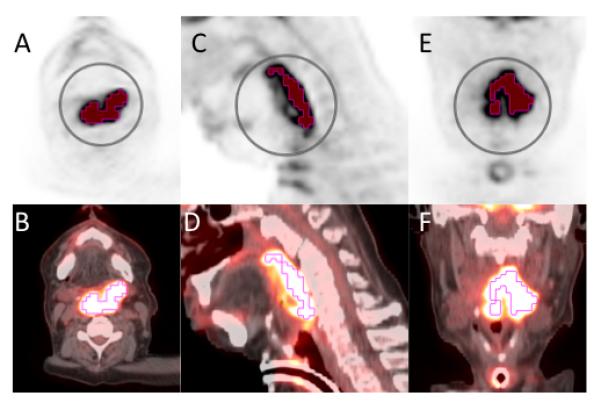
58-year-old African American man with a greater than 60 pack year history of smoking, presented with progressive dysphagia and odynophagia. He was found to have a large left oropharyngeal mass. Biopsy was taken showing invasive moderately differentiated keratinizing squamous cell carcinoma that was negative for P16 and HPV. Staging PET scan with axial PET (A), axial fused (B), sagittal PET (C), sagittal fused (D), coronal PET (E), and coronal fused (E). The primary lesion based is shown with contouring based on threshold method at 50% of SUVmax. SUVmax 16.7, SUVmean 10.9, SUVpeak 13.7, MTVthreshold 35.0, TLGthreshold 382.7.
HPV positive tumors and smoking: Primary tumor and nodal parameters
Taking into consideration the patient group with HPV positive disease, there was no statistically significant difference between morphologic and glycolytic indices whether in the primary tumor or the regional lymph node metastasis between smokers or non-smokers (Table 3).
Table 3.
Comparison of morphological and metabolic indices on FDG PET between HPV positive OPCC patients based on smoking history (median and range)
| smokers | Non-smokers | ||
|---|---|---|---|
| Primary | |||
| SUVmax | 9.7 (2.9 – 19.9) | 10.7 (3.6 – 21.6) | p=0.6 |
| SUVmean | 4.9 (1.9 – 10.0) | 5.5 (2.0 – 10.5) | p = 0.6 |
| SUVpeak | 9.1 (4.2 – 16.9) | 9.2 (3.9 – 18.1) | p = 0.9 |
| MTVedge (cm3) | 7.5 (1.0 – 63.5) | 9.7 (0.5 – 164.5) | p = 0.3 |
| TLGedge (cm3) | 34.5 (2.7 – 635.9) |
55.3 (1.3 – 413.5) | p = 0.4 |
| MTVthreshold (cm3) | 3.3 (0.4 – 34.7) | 4.9 (0.4 – 33.0) | p = 0.6 |
| TLGthreshold (cm3) | 24.1 (1.4 – 452.0) |
34.6 (1.1 – 215.2) | p = 0.5 |
| PERCIST long (cm) | 2.5 (1.3 – 5.5) | 2.8 (1.0 – 7.4) | P = 0.5 |
| HI | 1.8 (1.4 – 2.4) | 1.9 (1.3 – 3.2) | p = 0.4 |
| MI | 2.9 (0.7 – 11.9) | 3.2 (0.5 – 22.3) | p = 0.3 |
| Nodal | |||
| Highest SUVmax | 8.2 (3.5 – 22.9) | 7.4 (3.2 – 16.9) | p = 0.4 |
| Highest SUVpeak | 7.0 (3.3 – 17.5) | 6.2 (2.8 – 30.4) | p = 0.9 |
| highest MTVedge (cm3) | 13.6 (0.6 – 118.2) |
7.2 (1.5 – 148.1) | p = 0.1 |
| Highest RECISTlong (cm) | 3.1 (1.4 – 7.8) | 2.6 (1.4 – 7.8) | p =0.07 |
| Highest TLGedge | 46.9 (1.4 – 339.0) |
28.3 (3.5 – 807.1) | p = 0.06 |
| Sum TLGedge | 50.2 (1.4 – 437.1) |
43.2 (3.5 – 807.1) | p = 0.3 |
| Sum MTVthreshold | 5.0 (0.9 – 68.2) | 5.2 (0.7 – 48.4) | p = 0.7 |
| Sum TLGthreshold | 26.5 (3.5 – 223.2) |
24.6 (1.7 – 412.3) | p = 0.3 |
| HI | 2.0 (1.4 – 2.9) | 1.9 (1.6 – 3.0) | p = 0.3 |
| MI | 3.9 (0.4 – 15.2) | 2.9 (1.0 – 18.9) | p = 0.1 |
MTV: metabolic tumor volume, TLG: total lesion glycolysis, HI: heterogeneity index, MI: metabolic index
DISCUSSION
In this study, we have evaluated a number of morphologic and glycolytic indices based on FDG PET/CT of primary and nodal metastatic disease in OPSCC. We have compared these parameters for two groups of patients based on HPV status assessed by in situ hybridization. We have found that all FDG PET index parameters of the primary lesions are significantly larger in HPV negative compared to patients with HPV positive disease. The primary lesions in the HPV negative patients are significantly larger in size as measured by RECIST longer axis dimension and by MTV, defined both by the edge and the threshold segmentation methods. This is in accord with observations made by previous authors that HPV positive tumors typically present with an earlier T stage at presentation[15-17].
We also found that HPV negative primaries have significantly higher metabolic rates as compared with their HPV positive counterparts. This is indicated by statistically significantly larger SUVmax, SUVpeak, and SUVmean values. Morphological and glycolytic indices of nodal metastases are overall larger in HPV positive than in HPV negative OPSCC. This confirms some earlier observations by different authors [15, 16, 18, 19]. However, statistical significance was not attained for differences in nodal disease PET parameters between HPV negative and HPV positive diseases.
Some authors have suggested that smoking history might have some predictive value for disease outcome[20]. When we sought to find differences in FDG PET indices between patients with smoking history and those who never smoked among the group of patients with HPV disease, we could not find any statistically significant results. However, we found that patients with HPV negative disease had a significant smoking history with significantly higher proportion as compared to HPV positive patients.
We also found that HPV negative primaries are more heterogeneous by comparison of the heterogeneity index defined as the ratio of SUVmax by SUVmean. Using the same measure for nodal disease, we found that HPV positive lymph nodes tended to be more heterogeneous, This is consistent with qualitative observations by previous studies that HPV positive nodes are more heterogeneous as they tend to be more necrotic or cystic [16, 18].
The importance of these morphologic and glytolytic indices stems from their usefulness as prognostic metrics. According to Romesser et al.[21], TLG and MTV demonstrated superior prognostic utility as compared to SUVmax in a study of 41 HNSCC patients, with larger tumor volumes correlating with inferior local control and overall survival in HNSCC patients treated with definitive intensity-modulated radiotherapy. These authors found that SUVmax was not prognostic. However, Schwartz et al.[22] evaluated 54 patients with HNSCC undergoing definitive radiation therapy, and reported that a SUV of greater than 9, the median, significantly correlated with inferior local control and disease-free survival. The same conclusion was reached by other authors [23, 24] using different SUVmax cutoffs. More recently, Lim et al.[25] investigated the prognostic value of staging FDG PET/CT for predicting distant metastases and overall survival in 176 patients after definitive chemoradiotherapy. Primary tumor MTV and TLG were both predictive of distant metastases and overall survival. The primary tumor SUVmax was associated with death but had no relationship with distant metastases.
One of the main limitations of our study was the relatively lower number of subjects with HPV negative disease. We had 25 out of a total of 123 patients included in the study (20.3 %), concordant with observed incidences[26]. We did not investigate the CT volume of the primary tumor in our study because the performance of CT segmentation algorithms may suffer in soft-tissue tumors in which the background soft-tissue radiodensity is similar to tumors, especially when intravenous contrast is not used in all patients, as we only performed intravenous contrast neck CT with FDG PET/CT, when clinicians requested. We used only one, commercially available, software and one reader to segment the volumetric parameters of primary tumors and nodal metastases.
In conclusion, the index morphologic and glycolytic parameters as measured in FDG PET/CT are significantly larger in HPV negative as compared to HPV positive primary OPSCC. The same parameters tend to be larger in HPV positive nodal metastases, without statistical significance. HPV positive primary tumors and HPV negative loco-regional disease tend to be more heterogeneous in FDG uptake suggesting more degree of necrosis or cystic components. No statistically significant differences between morphologic and glycolytic parameters between smokers and non-smoking HPV patients were observed.
ACKNOWLEDGMENT
AKT was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number T32EB006351
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EM, Ritchie JM, Summersgill KF, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766–772. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxyglucose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2010;31:598–604. doi: 10.3174/ajnr.A1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33:210–222. doi: 10.1111/j.1749-4486.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 6.Davison JM, Ozonoff A, Imsande HM, Grillone GA, Subramaniam RM. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. Radiology. 2010;255:578–585. doi: 10.1148/radiol.10091479. [DOI] [PubMed] [Google Scholar]
- 7.Imsande HM, Davison JM, Truong MT, et al. Use of 18F-FDG PET/CT as a predictive biomarker of outcome in patients with head-and-neck non-squamous cell carcinoma. AJR Am J Roentgenol. 2011;197:976–980. doi: 10.2214/AJR.10.4884. [DOI] [PubMed] [Google Scholar]
- 8.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok BH, Setton J, Caria N, et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851–1857. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung MK, Jeong HS, Son YI, et al. Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann Surg Oncol. 2009;16:3111–3117. doi: 10.1245/s10434-009-0621-3. [DOI] [PubMed] [Google Scholar]
- 11.Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 12.Chung MK, Jeong HS, Park SG, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–5868. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 13.La TH, Filion EJ, Turnbull BB, et al. Metabolic tumor volume predicts for recurrence and death in head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–41. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salamon J, Derlin T, Bannas P, et al. Evaluation of intratumoural heterogeneity on (18)FFDG PET/CT for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur J Nucl Med Mol Imaging. 2013;40:685–92. doi: 10.1007/s00259-012-2314-6. [DOI] [PubMed] [Google Scholar]
- 15.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 16.Koch WM. Clinical Features of HPV-Related Head and Neck Squamous Cell Carcinoma: Presentation and Work-Up. Otolaryngol Clin North Am. 2012;45:779–793. doi: 10.1016/j.otc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 19.Lassen P, Eriksen JG, Krogdahl A, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol. 2011;100:49–55. doi: 10.1016/j.radonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza G, Zhang HH, D’Souza WD, Meyer RR, Gillison ML. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010;46:100–104. doi: 10.1016/j.oraloncology.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romesser PB, Qureshi MM, Shah BA, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–34. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz DL, Rajendran J, Yueh B, et al. FDG-PET Prediction of Head and Neck Squamous Cell Cancer Outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–1367. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 23.Torizuka T, Tanizaki Y, Kanno T, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol. 2009;192:W156–160. doi: 10.2214/AJR.08.1429. [DOI] [PubMed] [Google Scholar]
- 24.Machtay M, Natwa M, Andrel J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31:195–201. doi: 10.1002/hed.20942. [DOI] [PubMed] [Google Scholar]
- 25.Lim R, Eaton A, Lee NY, et al. 18F-FDG PET/CT Metabolic Tumor Volume and Total Lesion Glycolysis Predict Outcome in Oropharyngeal Squamous Cell Carcinoma. J Nucl Med. 2012;53:1506–13. doi: 10.2967/jnumed.111.101402. [DOI] [PubMed] [Google Scholar]
- 26.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


