Abstract
Objective
To compare outcomes among neonates delivered after documentation of fetal lung maturity prior to 39 weeks and those delivered at 39 or 40 weeks.
Methods
This was a retrospective cohort study of women with singleton pregnancy delivered at 36 0/7 to 38 6/7 weeks after positive fetal lung maturity testing (based on amniotic fluid lecithin to sphingomeylin ratio) or at 39 0/7 to 40 6/7 weeks (without maturity testing) at our center from 1999-2008. Women with major congenital anomalies, cord prolapse, non-reassuring antepartum testing, placental abruption, or oligohydramnios were excluded. A primary composite neonatal outcome included death, adverse respiratory outcomes, hypoglycemia, treated hyperbilirubinemia, generalized seizures, necrotizing enterocolitis, hypoxic ischemic encephalopathy, periventricular leukomalacia, and suspected or proven sepsis.
Results
There were 459 neonates delivered at 36-38 weeks and 13,339 delivered at 39-40 weeks; mean birth weight was 3107±548 and 3362±439 grams, respectively. The risk of the composite adverse neonatal outcome was 6.1% for the 36-38 week group compared to 2.5% for the 39-40 week group; RR 2.4; 1.7-3.5. After multivariable adjustment, early delivery remained significantly associated with an increased risk of the composite outcome (adjusted OR 1.7; 1.1-2.6) as well as several individual outcomes, including respiratory distress syndrome (7.6; 2.2-26.6), treated hyperbilirubinemia (11.2; 3.6-34) and hypoglycemia (5.8; 2.4-14.3).
Conclusions
Neonates delivered at 36-38 weeks after confirmed fetal lung maturity are at higher risk of adverse outcomes than those delivered at 39-40 weeks.
Background
Delivery prior to 39 weeks of gestation is associated with increased neonatal respiratory morbidity compared to delivery at 39 completed weeks (i.e., 39 0/7 to 39 6/7).1-3 Since fetal lung maturity reduces the risk of respiratory morbidity, confirmation of fetal lung maturity is a recognized exception to longstanding recommendations against elective delivery prior to 39 weeks of gestation.4-7 Among a large cohort of US women who underwent elective cesarean at term (i.e., ≥37 weeks), the risks of respiratory morbidity and other adverse neonatal outcomes (including need for CPR, hypoglycemia, seizures, suspected or proven sepsis, and admission to the NICU) nadired at 39-40 weeks’ gestation.3 Despite existing recommendations, in one large study, over a third of elective cesareans were performed before 39 weeks.3 However, since information on fetal lung maturity testing was lacking in that study, it is unclear whether the increased neonatal morbidity was due to failure to confirm lung maturity or whether delivery prior to 39 weeks even after confirmed pulmonary maturity is associated with worse neonatal outcomes than delivery at 39 weeks. Therefore, we undertook this study to evaluate the hypothesis that adverse neonatal outcomes are increased when delivery is undertaken prior to 39 weeks of gestation even after documented fetal lung maturity when compared to delivery at 39-40 weeks.
Methods
We conducted a retrospective cohort study of women receiving prenatal care and delivering at our center from 01/1999 to 12/2008. The study was approved by the IRB of the University of Alabama at Birmingham. The study period represents the time frame for which results of pulmonary maturity testing were available. Data were derived from our electronic obstetric database, supplemented by direct review of the medical records of women and their newborns as detailed below. Our electronic database has been in operation since 1979 and contains more than 1,000 coded antepartum, intrapartum, and postpartum data fields per patient. Dedicated trained personnel are responsible for entering the antepartum data on an ongoing basis and for conducting audits. Immediately after delivery; physicians or nurse practitioners record the intrapartum and postpartum data on standardized forms.8
Pregnancy outcomes for 2 groups of women with singleton pregnancies were compared: a) the early (lung maturity) group included those who delivered between 36 0/7 and 38 6/7 weeks after positive fetal lung maturity testing (based on either the presence of phosphatidyl glycerol or an L/S ratio ≥ 2.0 if non-diabetic or ≥ 3.5 if diabetic).6 This group was identified by review of laboratory records for lung maturity testing and verified by direct review of maternal charts; b) the comparison group included those who delivered at 39 or 40 completed weeks of gestation (without maturity testing). Gestational age was based on the best obstetric estimate used by providers after considering last menstrual period and ultrasound criteria as recommended by the American College of Obstetricians and Gynecologists.9 Mode of delivery in both groups was based on usual obstetric indications. Intended mode of delivery was defined based on the actual mode of delivery and type of labor: intended cesarean included those who had a cesarean without labor; all others delivered either vaginally or by cesarean following induced, augmented or spontaneous labor were coded as intended vaginal deliveries.
Ascertainment of the characteristics of these women and their pregnancy outcomes was through the obstetric database. Those with either multiple births or factors suggesting fetal compromise, including major congenital anomalies, umbilical cord prolapse, non-reassuring antepartum testing, placental abruption, and oligohydramnios were excluded. All admissions to the neonatal ICU (NICU) were identified for direct chart review by two co-authors (EB and MLM) to ascertain neonatal outcomes. Questions or discrepancies regarding outcome abstraction were resolved in consultation with a third author (ATNT).
Several neonatal outcomes were examined, including neonatal death, respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), bronchopulmonary dysplasia (BPD), persistent pulmonary hypertension (PPHN), need for respiratory support (including ventilator support or any other mode of supplemental oxygenation), use of surfactant, metabolic complications including hypoglycemia, hyperbilirubinemia requiring treatment, generalized seizures, necrotizing enterocolitis (NEC), hypoxic ischemic encephalopathy (HIE), periventricular leukomalacia (PVL), feeding difficulties, and suspected or proven sepsis. The primary outcome was the composite of all of these outcomes. In addition, we examined a second composite outcome that excluded suspected sepsis, as this was the neonatal outcome with the highest incidence. Two other neonatal outcomes, NICU admission and prolonged hospitalization (defined as neonatal hospital stay > 4 days), were also examined but were not included in the primary composite outcome. Neonatal outcomes were defined according to standard ICD-9 diagnostic codes assigned by the neonatology attending upon discharge, death, or transfer of the neonate. Although we focused on adverse neonatal outcomes severe enough to warrant a NICU admission, we recognized that mild forms of morbidities such as hyperbilirubinemia and suspected sepsis would not always lead to a NICU admission. Therefore, we also performed post-hoc direct chart review of a random sample of 200 charts of neonates who were not admitted to the NICU (in a ratio of 1 early delivery: 4 at 39-40 weeks). We examined the primary outcome by group to determine whether results were similar to the main findings.
We estimated that there were approximately 450 women delivered prior to 39 weeks after documented lung maturity during the study period. Comparing them with a minimum of 1800 women delivered at 39 to 40 weeks (i.e., 1: 4 ratio), would provide at least 80% power to detect a 4% absolute difference in the primary outcome from a baseline incidence of 7% among births at 39 to 40 weeks with 2-sided alpha of 0.05. Statistical analyses were accomplished using SAS version 9.1 [Cary, NC: SAS Institute Inc., 2002-2003]. Chi square and Student’s t tests were used as appropriate to compare descriptive characteristics. The Mantel-Haenszel chi-square test for trend was used to assess for linear trends in outcomes by gestational age from 36 to 39/40 weeks. Logistic regression was used to adjust for potentially confounding covariates including maternal age, ethnicity, parity, baby gender, intended mode of delivery and any medical complication (including diabetes mellitus and chronic hypertension) for the comparison between the early group and the 39-40 week group. Birth weight and maternal BMI were adjusted for in additional models.
Results
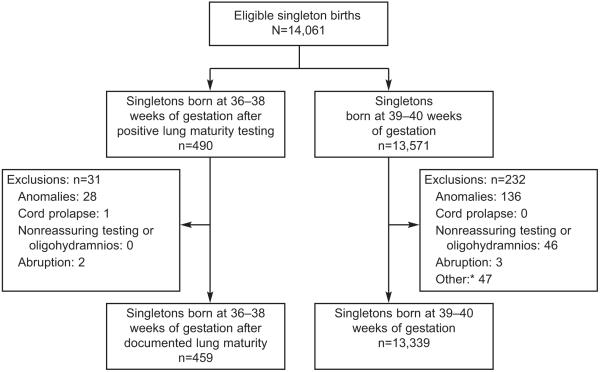
There were a total of 490 singleton deliveries at 36-38 weeks following positive pulmonary maturity testing and 13,571 singleton deliveries at 39 or 40 weeks during the study period. After applying exclusion criteria (Figure 1), a total of 13,798 mother-infant dyads comprised the study population: 459 delivered between 36 and 38 weeks after positive fetal lung maturity testing and 13,339 delivered at 39 to 40 weeks. Reasons for fetal lung maturity testing prior to 39 weeks were diabetes (with or without hypertension) in 37.1%, prior uterine rupture or surgery not restricted to lower segment (22.9%), chronic hypertension (7.2%), preeclampsia (5.2 %), placenta previa (4.1%), elective (4.1%), prior history of fetal death or abruption (2.8%), cholestasis of pregnancy (2.8%) and others (13.8%). The “other” category included a wide range of indications such as suspected growth restriction, polyhydramnios, isoimmunization, and maternal cardiac, pulmonary or other medical conditions.
Figure 1.
Flow chart indicating exclusions. *Unable to confirm or verify gestational age from records.
Among women delivered early after fetal lung maturity, 42.5% were at 36 completed weeks, 40.7% at 37 weeks and only 16.8% at 38 weeks compared to 56.2% at 39 weeks and 43.8% at 40 weeks in the comparison group. Mean gestational age was 37.1±0.7 weeks for the early group compared to 39.8±0.6 weeks. Maternal and infant characteristics for the two study groups are presented in Table 1. Women in the early delivery group were significantly more likely to be older, Caucasian, married and multiparous. Women in the early group initiated prenatal care earlier – mean gestation at first ultrasound was significantly lower and a higher proportion had ultrasounds before 20 completed weeks of gestation. As expected, these women were also more likely to have obstetric or medical complications such as hypertensive disorders or diabetes. Mean birth weight was significantly higher in the 39 to 40 week group, and a higher proportion of infants were small for gestational age.
Table 1. Maternal and neonatal characteristics of the early delivery and the 39–40-week study groups.
| Characteristic* | 36-38 wk (n=459) |
39-40 wk (n=13339) |
P-values |
|---|---|---|---|
|
| |||
| Maternal age (years) | 28 ± 6 | 24 ± 6 | <0.0001 |
| Ethnicity | <0.0001 | ||
| Black | 49.2 (226/459) | 58.5 (7800/13339) | |
| White | 33.8 (155/459) | 23.1 (3077/13339) | |
| Other | 17.0 (78/459) | 18.5 (2462/13339) | |
| Married | 37.1 (158/426) | 25.6 (3269/12775) | <0.0001 |
| Primiparous | 22.9 (105/459) | 41.0 (5467/13336) | <0.0001 |
| BMI | 31 ± 10 | 28 ± 8 | <0.0001 |
| BMI Group | <0.0001 | ||
| <18.5 | 0.5 (1/202) | 4.6 (400/8758) | |
| 18.5 - 24.9 | 29.7 (60/202) | 37.6 (3295/8758) | |
| 25 – 29.9 | 24.8 (50/202) | 25.6 (2239/8758) | |
| ≥30 | 45.1 (91/202) | 32.2 (2824/8758) | |
| GA at initial ultrasound (weeks) | 14 ± 7 | 16 ± 7 | <0.0001 |
| Ultrasound prior to 20 weeks | 72.3% (332/459) | 58.4 (7786/13339) | <0.0001 |
| Hypertensive disease | 20.3 (93/459) | 10.8 (1442/13339) | <0.0001 |
| Diabetes mellitus | 38.2 (175/458) | 5.2 (694/13332) | <0.0001 |
| Current smoker | 26.1 (91/349) | 27.0 (2824/10459) | 0.7013 |
| Alcohol intake | 15.9 (53/334) | 16.0 (1622/10134) | 0.9463 |
| High school education | 58.8 (160/272) | 54.6 (5411/9914) | 0.1654 |
| Intended vaginal delivery | 44.2 (196/443) | 91.7 (12117/13213) | <0.0001 |
| Cesarean delivery | 66.9 (307/459_ | 21.5 (2872/13339)) | <0.0001 |
| Male gender | 49.8 (228/458) | 49.7 (6620/13327) | 0.9637 |
| Birth weight (g) | 3107 ± 548 | 3362 ±439 | <0.0001 |
| Small for gestational age (<10th percentile) | 2.6 (12/459) | 5.4 (716/13339) | 0.0094 |
Values are % (numerator/denominator) except where Mean ± SD
The unadjusted rate of the primary composite adverse neonatal outcome was significantly more common in the early delivery group as was the 2nd composite outcome i.e., excluding suspected sepsis (Figure 2). Both composite adverse outcomes were over 2-fold higher in neonates delivered at < 39 weeks compared to those delivered at 39 or 40 weeks; unadjusted RR 2.4 (1.7-3.5) and 2.8 (1.9-4.2) respectively. The incidence (95% CI) of the primary composite outcome decreased with increasing gestational age (p-value for trend < 0.001): 9.2% (5.9%-14.1%) at 36, 3.2% (1.5-6.8) at 37, 5.2% (2.0-12.6) at 38, and 2.5% (2.2-2.8) at 39-40 weeks.
Figure 2.

Unadjusted incidence and relative risks [RR (95 CI)] for composite adverse outcomes by study groups.
There were no perinatal deaths in the early lung maturity group whereas there were 24 (0.18%) in the 39 to 40 week group of which 22 (91.7%) were stillbirths; this was not significantly different. There was one case of IVH and 7 cases of HIE in the 39 to 40 week group; neither of these outcomes were present in the early group. There was no case of NEC or PVL in either group, and no differences in the incidence of PPHN, generalized seizures, or need for CPR between groups. The unadjusted incidence proportions of other individual adverse outcomes included in the composite, as well as admission to the NICU and prolonged hospitalization were more frequent in the early group compared to the 39-40 week group (Table 2).
Table 2. Unadjusted frequency of Individual adverse neonatal outcomes for the two study groups.
| Neonatal outcome | 36-38 wk (N=459) % (n) |
39-40 wk (N=13339) % (n) |
RR (95% CI) |
|---|---|---|---|
|
| |||
| Suspected or Proven Sepsis * | 5.9 (27) | 2.3 (304) | 2.5 (1.8-3.7) |
| • Proven sepsis | 0 (0) | 0.07 (9) | n/a |
| Need for Respiratory Support * | 3.3 (15) | 1.1 (141) | 3.0 (1.8-4.8) |
| • Ventilator Support | 0.7 (3) | 0.2 (31) | 2.7 (0.9 – 7.9) |
| Hypoglycemia ** | 2.2 (10) | 0.18 (24) | 9.0 (5.3-15.3) |
| Feeding Difficulties (%) ** | 1.5(7) | 0.4 (52) | 3.6 (1.8-7.3) |
| TTN * | 1.5(7) | 0.6 (76) | 2.6 (1.3-5.2) |
| RDS ** | 1.5(7) | 0.04 (6) | 16.4 (9.8-27.4) |
| Treated hyperbilirubinemia ** | 13 (6) | 0.1 (17) | 7.9 (4.0-15.9) |
| Surfactant Use * | 0.4 (2) | 0.05 (7) | 6.7 (2.0-22.8) |
| Perinatal Demise | 0(0) | 0.2 (24) | n/a |
| BPD * | 0.2(1) | 0.0 (0) | n/a |
| Intraventricular Hemorrhage | 0(0) | 0.01 (1) | n/a |
| Hypoxic Ischemic Encephalopathy | 0(0) | 0.05 (7) | n/a |
| Necrotizing Enterocolitis | 0(0) | 0(0) | n/a |
| Periventricular Leukomalacia | 0 (0) | 0 (0) | n/a |
| PPHN | 0.4 (2) | 0.3 (33) | 1.7 (0.4-6.6) |
| Seizures | 0 (0) | 0.07 (10) | n/a |
| Need for CPR | 0 (0) | 0.04 (6) | n/a |
| NICU Admission ** | 6.3 (29) | 2.5 (329) | 2.5 (1.8-3.6) |
| Prolonged Hospitalization ** | 10.5 (39) | 3.3 (366) | 3.2 (2.4-4.4) |
p-value comparing groups < 0.05 or
p <0.01; n/a = not applicable (zero cells)
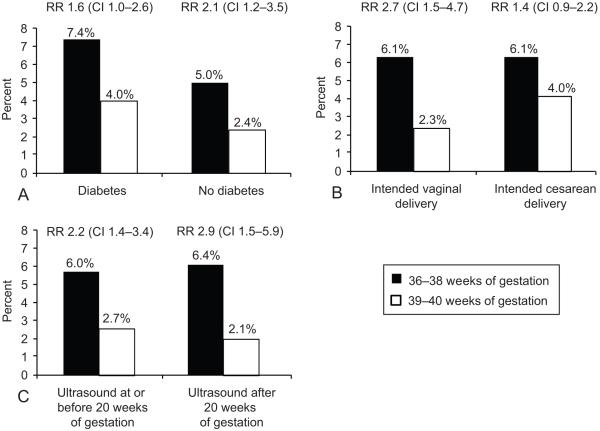
The primary composite adverse outcome was further stratified by each of 3 factors: intended vaginal or cesarean mode of delivery, presence or absence of maternal diabetes and dating ultrasound obtained at or prior to 20 weeks versus after 20 weeks. As shown in Figure 3, the primary outcome was more frequent in the early group for each subgroup of these characteristics. In addition the primary outcome was associated with early delivery regardless of the presence (RR 1.7, 95% CI: 1.1-2.8) or absence (2.4, 1.4-4.2) of any medical complication including diabetes and hypertension.
Figure 3: Association between primary outcome and study groups according to presence or absence of diabetes, intended mode of delivery and dating ultrasound.
Odds ratios for the association between adverse neonatal outcomes (composite or individual) and delivery group (39-40 week group as referent) from logistic regression models adjusting for maternal age, race, parity, medical complications (hypertensive or diabetes), intended mode of delivery and baby gender are presented in Table 3. Earlier delivery after confirmed lung maturity testing was associated with a 1.6-fold increase in the primary outcome and significant increases in other adverse neonatal outcomes by as much as 7.6-fold for RDS and over 11-fold for treated hyperbilirubinemia. Also significantly associated with the primary outcome were maternal age as a continuous variable (OR 1.03; 1.01-1.05), male infant (1.25; 1.01-1.55), nulliparity (2.15; 1.69-2.74), presence of medical complications (1.55; 1.21-2.00) and intended vaginal delivery as compared to intended cesarean delivery (0.49; 0.36-0.68).
Table 3. Adjusted OR (95% CI) for composite adverse outcomes and selected individual outcomes (39-40 week group as referent).
| Outcomes | Adjusted OR (95% CI)* |
|---|---|
|
| |
| Composite adverse outcome | 1.7 (1.1 - 2.6) |
| Composite adverse outcome II | 2.0 (1.2–3.1) |
| RDS | 7.6 (2.2 – 26.6) |
| Respiratory support | 2.0 (1.1 - 3.6) |
| Surfactant use | 6.5 (1.04 - 41) |
| Ventilator support | 2.1 (0.6 – 8.0) |
| Suspected or proven sepsis | 1.7 (1.1 - 2.7) |
| Hypoglycemia | 5.8 (2.4 - 14.3) |
| Treated hyperbilirubinemia | 11.2 (3.6 – 34) |
| NICU admission | 1.7 (1.1 - 2.7) |
| Hospitalization >4 days | 2.6 (1.8 - 3.9) |
Adjusted for maternal age, ethnicity, parity, baby gender, intended mode of delivery and medical complications
When we adjusted for actual (instead of intended) mode of delivery, the results for the outcomes presented in table 3 were materially unchanged. For example, early delivery after lung maturity was associated with a 1.7-fold (95% CI 1.1, 2.7) and 1.9 fold (1.2, 3.1) increase in the primary composite and alternative composite outcomes respectively; the adjusted ORs for cesarean delivery were 3.6 (2.8, 4.6) and 4.1 (3.1, 5.5) respectively. Further adjustment of this model by type of labor (induced, spontaneous, none) gave similar results; compared to no labor, induced (1.43; 0.95-2.15) and spontaneous labor (1.3; 0.89-1.91) were not significantly associated with the primary outcome.
We conducted a series of validity and other analyses. When adjusted further for birth weight, the OR for the association between the primary outcome and early delivery increased slightly to 1.83 (1.16-2.87). In analyses adjusting additionally for BMI in the primary outcome model, BMI was not a significant predictor and the results were limited by the relatively large number of those missing BMI information. Also the primary results were materially unchanged by excluding all infants with low birth weight (<2500 g). An examination of different cut-offs of the L[ckr]S ratio was severely limited by the size of the early group but suggested that a ratio of at least 4 to 5 is required for adverse neonatal outcome to be highly unlikely.
The review of 200 randomly selected charts of neonates who were not admitted to the NICU supported our main results. Neonates in the early group were more likely to experience one or more adverse outcomes compared to the 39-40 week group, RR 1.5, 95% CI 1.0-2.4. The majority of these were mild forms of hyperbilirubinemia or suspected sepsis.
Discussion
A large body of available evidence indicates that delivery prior to 39 weeks of gestation is associated with increased risks of adverse neonatal outcomes compared to delivery at 39 or 40 weeks,1-3,10-11 and documentation of fetal lung maturity before early delivery lowers this risk.4-7 Even so, early delivery even after positive fetal lung maturity was associated with nearly a 2-fold increase in the primary composite adverse neonatal outcome in our large cohort. Furthermore, several individual adverse neonatal outcomes including RDS, treated hyperbilirubinemia, hypoglycemia and suspected or proven sepsis were significantly increased (by up to 7.6-fold for RDS and over 11-fold for treated hyperbilirubinemia) with early delivery. A composite outcome excluding suspected sepsis (the single most frequent adverse neonatal outcome) was also increased, as were admission to the NICU and prolonged hospitalization. The increase in the primary composite outcome persisted regardless of the presence or absence of diabetes, chronic hypertension, or other maternal medical illnesses. As expected the frequency of adverse outcomes was higher among those with diabetes compared to non-diabetics. Adverse outcomes were also increased in the early group regardless of intended vaginal vs. cesarean mode of delivery, and the presence vs. absence of a dating ultrasound prior to 20 weeks. Taken together these findings are consistent with relative immaturity at 36-38 weeks (regardless of lung maturity) compared to 39-40 weeks and lower threshold for admission to the NICU and invasive sepsis work-ups (suspected sepsis).
These findings should be considered in the light of our study’s limitations. First, the retrospective study design limited our ability to identify and correct potential misclassification of neonatal outcomes. However, the extent of such bias would have to differ markedly by study group to negate our findings. Second, direct chart review of adverse neonatal outcomes focused primarily on outcomes associated with a NICU admission. Therefore our results emphasize the more severe spectrum of each adverse outcome. Nonetheless, review of outcomes among the random sample of non-NICU admissions found a consistent increase in adverse neonatal outcomes among those delivered early after positive lung maturity testing. Third, confounding remains a possibility since patients were not randomized into policies of early delivery after fetal lung maturity vs. delaying delivery until 39 weeks. Finally, our study does not fully address the risk of stillbirth associated with either delivery strategy. Although there was no stillbirth in the early delivery group compared to 22 (0.16%) in the 39-40 week group (not statistically significant), this reflects the fact that fetal lung maturity testing is only undertaken when there is a viable fetus. Therefore, the true risk of stillbirth is underestimated in the early group – assuming that 1 stillbirth occurred; the risk would be 0.22%. Multiple comparisons are not a major concern since we specified a primary composite outcome a priori. The results for individual outcomes are internally consistent with our primary finding of increased adverse neonatal outcome with early delivery. We note that severe adverse outcomes such as NEC or IVH that are more likely to be associated with increased risk of death or long-term impairment were not prevalent.
There is a paucity of studies that specifically compare risks of adverse neonatal outcomes between infants delivered early after lung maturity testing to the baseline risks at 39 or 40 weeks. Several reports have examined the risk of adverse outcomes among neonates delivered early with and without maturity testing.12-15 Some reports suggest that, among well-dated pregnancies at or near term (36-38 weeks), the use of fetal lung maturity testing does not reduce the incidence of respiratory distress syndrome,12-13 and is not cost-effective.14 A recent survey of obstetric providers in the US revealed that many do not think amniocentesis for fetal lung maturity prior to near-term (or early term) delivery is beneficial, and this belief has contributed to a decline in amniocentesis for fetal lung maturity.16 However, one study based on the FLM TDX II test showed that delivery gestational age and FLM result were independent determinants of the risk of RDS,15 suggesting that there is no threshold value at which the risks converged. For example, for each reported value of the FLM TDX-II, delivery at 37 weeks was consistently associated with a higher risk of RDS compared to delivery at 39 weeks.15 Our results are consistent with those reported in this study. Quite similar to findings from the recent report from the NICHD MFMU Network3, early delivery was more common among older, white, married women and those who initiated prenatal care early (with earlier dating ultrasound). Ironically, these characteristics may be assumed to place women at lower risk for adverse outcomes.
We believe the differences between our study groups are attributable in large measure to the difference in gestational age rather than to the indications for fetal lung maturity testing. At our institution, antepartum testing is routine for pregnancies with known risk factors for fetal death. Consistent with ACOG recommendations, the fetal lung maturity testing group did not include pregnancies with fetal (or maternal) indications mandating delivery such as non-reassuring antenatal fetal testing.6 We also excluded pregnancies with indications for immediate delivery from the comparison group. There were fewer SGA babies in the early delivery group, likely due to close surveillance and delivery without lung maturity testing. Therefore, we studied pregnancies that were not at immediate fetal risk; fetal lung maturity testing was done in order to prevent future fetal or maternal compromise. Indications for lung maturity testing were present in both study groups; for example among patients with mild preeclampsia diagnosed prior to 37 weeks (when we typically deliver), a proportion accept fetal lung testing and delivery at 36 weeks while others choose to wait until 37 weeks. Overall, our findings suggest that in the absence of ongoing concern about fetal death or maternal well-being if the pregnancy continued, delivery should be delayed until 39 weeks. Although fetal lung maturity testing in our study was based on the L/S ratio, we would expect similar results with the other tests since they have negative predictive values (95% or greater) comparable to the L/S ratio at the cut-offs of 2-3.5 use in this study.6
It is well established that elective delivery prior to 39 weeks is associated with a preventable increase in neonatal morbidity and admissions to the NICU (and increased health care costs).1-3, 10-11,17-18 Moreover, because purely elective deliveries by definition occur in pregnancies at lowest risk of fetal death, the risk of unexplained stillbirth (<0.05 %) is considerably lower than observed in this study.19-20 Therefore, although our study population did not include a sizable proportion of purely elective deliveries (a result of center-specific practices), a natural extension of our findings is that purely elective fetal lung maturity testing and early delivery should be avoided. Replication of these findings in a cohort of women undergoing purely elective delivery is recommended.
Acknowledgments
The authors thank Cherry Neely, UAB Center for Women’s Reproductive Health, who assisted with data acquisition and analysis.
Dr. Tita was a Women’s Reproductive Health Research (WRHR) Advanced Scholar at UAB with Funding from the Eunice Kennedy Shriver NICHD (5 K12 HD01258-09) at the time of the conduct of this study.
Footnotes
Presented in part at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, February 1-6, 2010, Chicago, IL.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective cesarean section. Br J Obstet Gynaecol. 1995;102:101–6. doi: 10.1111/j.1471-0528.1995.tb09060.x. [DOI] [PubMed] [Google Scholar]
- 2.Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective cesarean delivery. Acta Paediatr. 2004;93:643–7. doi: 10.1111/j.1651-2227.2004.tb02990.x. [DOI] [PubMed] [Google Scholar]
- 3.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, Moawad AH, Caritis SN, Meis PJ, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai BM, Langer O, Thorp JM, Ramin SM, Mercer BM. Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists . Fetal maturity assessment prior to elective repeat cesarean delivery. ACOG Committee Opinion Number 98. ACOG; Washington, DC: 1991. [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Cesarean delivery on maternal request. ACOG Committee Opinion Number 394. Obstet Gynecol. 2007;110:1501–4. doi: 10.1097/01.AOG.0000291577.01569.4c. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists Fetal lung maturity. ACOG Practice Bulletin Number 97. Obstet Gynecol. 2008;112:717–26. doi: 10.1097/AOG.0b013e318188d1c2. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists . Induction of labor. ACOG Practice Bulletin 107. ACOG; 2009. [Google Scholar]
- 8.Tita AT, Biggio JR, Chapman V, Neely C, Rouse DJ. Perinatal and maternal outcomes in women with sickle or hemoglobin C trait. Obstet Gynecol. 2007;110:1113–9. doi: 10.1097/01.AOG.0000285995.41769.83. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics and the American College of Obstetricians and Gynecologists . Guidelines for perinatal care. 6th edition ACOG; Washington, DC: 2007. [Google Scholar]
- 10.Bailit JL, Gregory KD, Reddy UM, et al. Maternal and neonatal outcomes by labor onset type and gestational age. Am J Obstet Gynecol. 2010;202:245.e1–12. doi: 10.1016/j.ajog.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilmink FA, Hukkelhoven CWPM, Lunshof S, et al. Neonatal outcome following elective cesarean section beyond 37 weeks of gestation: a 7-year retrospective analysis of a national registry. Am J Obstet Gynecol. 2010;202:250.e1–8. doi: 10.1016/j.ajog.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Kjos SL, Berkowitz KM, Kung B. Prospective delivery of reliably dated term infants of diabetic mothers without determination of fetal lung maturity: comparison to historical control. J Matern Fetal Neonatal Med. 2002;12:433–7. doi: 10.1080/jmf.12.6.433.437. [DOI] [PubMed] [Google Scholar]
- 13.Luo G, Norwitz ER. Revisiting amniocentesis for fetal lung maturity after 36 weeks’ gestation. Rev Obstet Gynecol. 2008;1:61–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Myers ER, Alvarez JG, Richardson DK, Ludmir J. Cost-effectiveness of fetal lung maturity testing in preterm labor. Obstet Gynecol. 1997;90:824–829. doi: 10.1016/S0029-7844(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 15.Parvin CA, Kaplan LA, Chapman JF, McManamon TG, Gronowski AM. Predicting respiratory distress syndrome using gestational age and fetal lung maturity by fluorescent polarization. Am J Obstet Gynecol. 2005;192:199–207. doi: 10.1016/j.ajog.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 16.McGinnis KT, Brown JA, Morrison JC. Changing patterns of fetal lung maturity testing. J Perinatol. 2008;28:20–3. doi: 10.1038/sj.jp.7211880. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg A, van Elburg RM, van Geijn HP, Fetter WP. Neonatal respiratory morbidity following elective caesarean section in term infants. A 5-year retrospective study and a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2001;98:9–13. doi: 10.1016/s0301-2115(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 18.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336:85–7. doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GC, Pell JP, Dobbie R. Caesarean section and risk of unexplained stillbirth in subsequent pregnancy. Lancet. 2003;362:1779–84. doi: 10.1016/s0140-6736(03)14896-9. [DOI] [PubMed] [Google Scholar]
- 20.Wood SL, Chen S, Ross S, Sauve R. The risk of unexplained antepartum stillbirth in second pregnancies following caesarean section in the first pregnancy. BJOG. 2008;115(6):726–31. doi: 10.1111/j.1471-0528.2008.01705.x. [DOI] [PubMed] [Google Scholar]