Abstract
Caspase-8, an executioner enzyme in the death receptor pathway, has previously been shown to initiate apoptosis and suppress necroptosis. Here, we identify a novel, cell death-independent role for caspase-8 in dendritic cells (DCs); namely, DC-specific expression of caspase-8 prevents the onset of systemic autoimmunity. Failure to express caspase-8 has no effect on the life-span of DCs but instead leads to an enhanced intrinsic activation and subsequently more mature and autoreactive lymphocytes. Uncontrolled toll-like receptor activation in a RIPK1-dependent manner is responsible for the enhanced functionality of caspase-8-deficient DCs, as deletion of TLR signaling mediator, MyD88, ameliorates systemic autoimmunity induced by caspase-8 deficiency. Taken together, these data demonstrate that caspase-8 functions in a cell-type-specific manner and acts uniquely in DCs to maintain tolerance.
INTRODUCTION
Activation of cell surface death receptors (DRs) can initiate two essential death pathways responsible for cell turnover, apoptosis or necroptosis, depending on the cytosolic milieu. Aggregation of a DR (Fas, TNFR1) with its ligand facilitates recruitment of Fas-associated death domain protein (FADD). FADD then recruits the cysteine-aspartic acid enzyme pro-caspase-8, which becomes catalytically active by forming a homodimer that initiates the degradative phase of apoptosis through subsequent activation of caspase 3/7 (1). In the absence of FADD or caspase-8, apoptosis is prevented, but under these conditions receptor-interacting serine-threonine kinase (RIPK) 1-RIPK3 signaling proceeds unchecked, leading to necroptosis (2). However, when FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein (cFLIP) is present at sufficient levels, this catalytically inactive homolog of caspase-8 forms a heterodimer with caspase-8 that not only prevents apoptosis but also limits RIPK1-RIPK3 necrosome activity (2).
While caspase-8 is known to function in cell death, conditional deletion studies implicate caspase-8 in a number of cell death-independent activities including cell motility (3), metastasis (4), suppression of inflammation (5, 6) and NFκB activation (7). The current paradigm for these alternate roles for caspase-8 is that they are the consequences of unleashed necroptosis (8, 9). However, a number of recent studies point at the idea that caspase-8 may function in an entirely cell death-independent manner. Toll-like receptor (TLR) engagement can provoke RIPK signaling independent of DR activation, thereby leading to formation of a ripoptosome, a complex containing similar proteins involved in necroptosis including caspase-8, RIPK1, cFLIP and FADD (10). Additionally, ripoptosome and RIPK3 activity have been shown to induce production of pro-inflammatory cytokine IL-1β in a caspase-8-dependent manner (11) independent of cell death. Activation of most TLRs requires the adaptor myeloid differentiation primary response gene 88 (MyD88), which may lead to the phosphorylation and nuclear translocation of transcription factors IFN regulatory factors, causing up-regulation of proinflammatory gene expression (12). Previous studies have shown that caspase-8 cleaves IRF3 targeting it for degradation and dampening TLR-dependent downstream gene induction (13). Taken together, these data suggest that heightened IRF3 transcriptional activity in the absence of caspase-8, which may lead to hyperexpression of deleterious downstream IRF3 specific genes.
The vast majority of studies on the Fas signaling pathway in the immune system and its role in apoptosis and necroptosis have focused on lymphocytes. Loss of Fas in lymphocytes has led to conflicting results (14–16), while deletion of caspase-8 yields lymphopenic mice due to a failure in proliferation and increased necroptosis (17). Although the phenotype of global and T-cell-specific caspase-8 deletion is reversed by RIPK3 deficiency which suggests that necroptosis is the underlying cause (18), a systemic autoimmunity develops that is similar to germline knockout of Fas (2, 17, 19). Since conditional deletion of Fas or caspase-8 in lymphocytes results in opposite phenotypes, and loss of Fas in dendritic cells (DCs) or over-expression of the general caspase inhibitor p35 in DCs induces a systemic autoimmune disease (14, 20) we investigated the role that caspase-8 plays in DC development and in maintaining tolerance.
Specific deletion of caspase-8 in DCs (CreCD11cCasp8fl/fl) is sufficient to induce a systemic autoimmune disease reminiscent of systemic lupus erythematosus (SLE) that is not a consequence of unleashed necroptosis, as this break in tolerance is neither ameliorated nor exacerbated by RIPK3 deletion. CreCD11cCasp8fl/fl DCs do not display a survival advantage indicating that defective DC apoptosis is not the underlying cause of the observed inflammation. However, these DCs possess a heightened costimulatory capacity and an elevated response to TLR signaling that is abrogated by RIPK1 inhibition. Interestingly, IRF3 deletion in CreCD11cCasp8fl/fl mice exacerbates the observed break in tolerance. In contrast, concurrent deletion of caspase-8 and MyD88 in DCs abates both the lymphoproliferative and end-organ disease in MyD88fl/flCreCD11cCasp8fl/fl mice. Thus, these data demonstrate that caspase-8 in DCs maintains tolerance in a manner that is independent of cell death and IRF3, but requires dampening of RIPK1 and MyD88 signaling.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) mice homozygous for loxP flanked caspase-8 allele (Casp8fl/fl) (21) were crossed with mice expressing Cre under control of the CD11c promoter (CreCD11c, Jackson Laboratory, Alexander Chervonsky), generating CreCD11cCasp8fl/fl mice. PCR on FACS-sorted splenic conventional DC populations (B220−CD11c+CD8− and B220−CD11c+CD8+) from CreCD11cCasp8fl/fl mice showed deletion of caspase-8 but not in plasmacytoid DCs (CD11cintermediatePDCA-1+B220+), lymphocytes or macrophages (Supplemental Figure 1). CreCD11cCasp8fl/fl BMDCs showed caspase-8 deletion (Supplemental Figure 1). OT-II/RAG−/− and B6.CD45.1 were purchased (Jackson Laboratory). RIPK3−/− (Genentech), IRF3−/− (a gift from Mike Diamond), IRF7−/− (a gift from Mike Diamond), MyD88fl/fl (Jackson Laboratory) mice were bred to CreCD11cCasp8fl/fl generating RIPK3−/−CreCD11cCasp8fl/fl, IRF3−/−CreCD11cCasp8fl/fl, IRF7−/−CreCD11cCasp8fl/fl and MyD88fl/flCreCD11cCasp8fl/fl mice. Real-time PCR performed by Transnetyx on FACS-sorted splenic conventional DC populations from MyD88fl/flCreCD11cCasp8fl/fl mice showed caspase-8 and MyD88 deletion (Supplemental Figure 1). Female mice were used in all studies. Proteinuria was assessed using uristix (Siemens). Transnetyx performed genotyping. Experiments were approved by Northwestern University IACUC.
Histopathologic Studies
Paraffin-embedded kidney sections (4 µm) were stained with periodic acid Schiff (PAS) and a pathologist blinded to the study scored kidney sections using an Olympus BS40 microscope as previously described (22). Frozen kidney sections (4 µm) were stained with αIgG-FITC (22). All images were photographed at 40X, 200X or 400X magnification on an Olympus BX41 microscope equipped with an Olympus DP20 camera.
Flow Cytometry
Surface staining of cell suspensions and gating strategies were accomplished as previously described (23, 24). At least 100,000 events were acquired on BD LSRII instrument. Data were analyzed with FlowJo software (TreeStar, Inc). Dead cells were excluded using Aqua live/dead staining (Invitrogen). For cell-sorting studies splenocytes pre-incubated with Fc-block were stained with fluorescent antibodies (information available upon request). Splenocyte populations sorted on a BD FACSAria II instrument at University of Chicago’s Flow Core had an average purity of 97%.
Bone Marrow Chimeras
Bone marrow was aseptically harvested from tibias, femurs and humeri from 9-week-old mice, erythrocytes lysed (BD Pharm Lyse buffer), and cells incubated with Fc-block followed by incubation with PE-conjugated antibodies against B220, CD4, CD8, CD11b, Ly6G, NK1.1, Siglec F and Ter119 (BD Biosciences, eBioscience). Cells were incubated with anti-PE microbeads (Miltenyi Biotech) and PE-labeled lineage cells were depleted (AutoMACS separator). Three-month-old B6.CD45.1 received a single 1000 cGy γ-irradiation dose using a Cs-137-based Gammacell-40 irradiator (Nordion). After 12 hours, 1.2×106 lineage-depleted cells were intravenously injected from: Casp8fl/fl, CreCD11cCasp8fl/fl, a mixture of Casp8fl/fl plus B6.CD45.1/2 or CreCD11cCasp8fl/fl plus B6.CD45.1/2 (1:1 ratio). Pre-sorted cells were stained with c-Kit (eBioscience) and Sca-1 (Biolegend) to analyze LSK-fraction. Chimeric mice were maintained on autoclaved water plus antibiotics (Trimetoprim/Sulfamethoxazole, Hi-Tech Pharmacal) for 4 weeks post-transfer and phenotyped 18 weeks post-transfer.
In vivo Assays
For TLR ligand injection studies 3-month-old mice were intraperitoneally injected with LPS, imiquimod or CpG (200 µg/20g body weight, Invivogen) and after 4 hours analyzed by flow cytometry. For oral antibiotic treatment 3-week-old mice were given autoclaved water with ampicillin (1 g/L), vancomycin (0.5 g/L), neomycin sulfate (1 g/L), metronidazole (1 g/L) and sucrose (10 g/L) twice/week for 8 weeks with no observable weight loss. For BrdU assays mice were intravenously injected with 1 mg BrdU (BD Biosciences) for 3 days. On days 0, 1 and 3 post-injection splenocyte and bone marrow suspensions were prepared as described above. After surface staining, cells were processed with BrdU staining kits (BD Biosciences) according to manufacturer’s instructions. FMO controls were used to set gates for BrdU+ populations.
In vitro Assays
For mixed leukocyte reactions splenocytes were incubated with anti-CD19 beads and negative fractions were incubated with anti-CD11c MACS beads (Miltenyi Biotech) to purify antigen-presenting cells (APCs). Purified APCs were pulsed with 10 µg/mL OVA peptide (aa 323–339) for 60 minutes at 37°C. OVA-specific splenic CD4+ T-cells were isolated from B6.CD45.1/OT-II/RAG−/− mice using CD4+ T-cell isolation kits (Miltenyi Biotec) according to manufacturer’s instructions. Purity of APCs and T-cells was 90%. T-cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 500 nM for 12 min at 37°C, Invitrogen). Pulsed APCs at various ratios were incubated with 2×105 CFSE-labeled T-cells ± 5 µg/mL CpG-B (ODN 1668) in triplicate in 96-well flat-bottom plates at 37°C for 3 days. Cell clusters were dissociated with 7.5 mM EDTA for 15 min, and stained with αCD4 (BD Biosciences). 7-Aminoactinomycin D (7-AAD) (0.25 mg/test, BD Biosciences) was used to exclude dead cells. A constant number of CaliBRITE beads (BD Biosciences) were added for acquisition of equal parts/culture. Live T-cells were gated and the number of divided cells, showing less than maximal CFSE fluorescence intensity, was determined. For cell death assays bead-sorted CD4+ T-cells were incubated with αCD3 (0.5 µg/mL, BD Biosciences) and αCD28 (1 µg/mL, BD Biosciences) ± zVAD-FMK (20 µM, Promega) and necrostatin-1 (30 µM, Enzo Life Sciences) and stained with Annexin-V (Invitrogen) and Aqua live/dead after 72 hours according the manufacturer’s instructions.
Bone marrow-derived DCs (BMDCs) were generated as published (25). Briefly, bone marrow was resuspended in complete media with 50 µM 2-ME and cultured for 2 hours. 1×106 nonadherent cells were placed in 24-well plates containing 1 ml complete media plus GM-CSF (10 ng/mL, Peprotech) + Flt3-L (50 ng/mL, Peprotech). Two-thirds of media was replaced on day 3. On days 5 and 7, nonadherent cells were transferred into 6-well plates in media plus cytokines (2.5×106 cells/2 ml/well) for 2 days, and BMDCS were used on day 9 at a concentration of 1.75×106/mL. Supernatants and nuclear lysates from BMDCs stimulated for 6 hours at 37°C in 5% CO2 with LPS (10 ng/mL, Sigma-Aldrich), CpG (5 µg/mL) and imiquimod (5 µg/mL) were evaluated for cytokine levels and transcription factor binding, respectively (see below). Total cell lysates from BMDCs stimulated for 30 and 60 minutes at 37°C in 5% CO2 with imiquimod (5 µg/mL) were evaluated for transcription factor expression levels. BMDCs were also stimulated for 6 hours at 37°C in 5% CO2 with LPS (10 ng/mL), CpG (5 µg/mL), and imiquimod (5 µg/mL) ± necrostatin-1 (30 µM) and/or zIETD-FMK (20 µM, BD Biosciences) and/or 1-Methyl-D-tryptophan (30 µM, Sigma-Aldrich), and supernatants were evaluated for cytokine levels. ATP (5mM, Sigma Aldrich) was added for 30 minutes, then media was replaced for 1 additional hour to evaluate IL-1β levels. For cell death assays 3×106 BMDCs or total splenocytes were stimulated for 10 hours at 37°C in 5% CO2 with superFasL (100 ng/mL, Enzo Life Sciences) or etoposide (10 µM, Alexis Biochemicals) ± necrostatin-1 (30 µM), and then stained with Annexin-V and Aqua live/dead.
Antibody/Cytokine Measurements and Transcription Factor Binding and Expression Quantification
Anti-nuclear antibodies including anti-dsDNA, -ssDNA, -nucleosomes or -histones were measured as previously described (22). Total IgM and IgG isotypes and cytokine/chemokine expression was quantified using Luminex-based assays (Affymetrix). Transcription factor analysis was accomplished using Nuclear Extraction and Procarta Transcription Factor Plex Kits according to manufacturer’s instructions (Affymetrix). Immunoblot analysis was performed as previously described (26) and the concentration of the primary antibodies were as follows: rabbit anti-IRF3 (1:1000, Cell Signaling), rabbit anti-IRF7 (1 mg/mL, Abcam) and mouse anti-GAPDH (1:500, US Biological) antibodies.
Statistical Analysis
GraphPad Prism 5.0 Software was used for statistical analyses. Data are represented as mean ± SD and compared by Mann Whitney test unless otherwise noted.
RESULTS
Mice with conditional deletion of caspase-8 in DCs develop a chronic systemic autoimmune disease
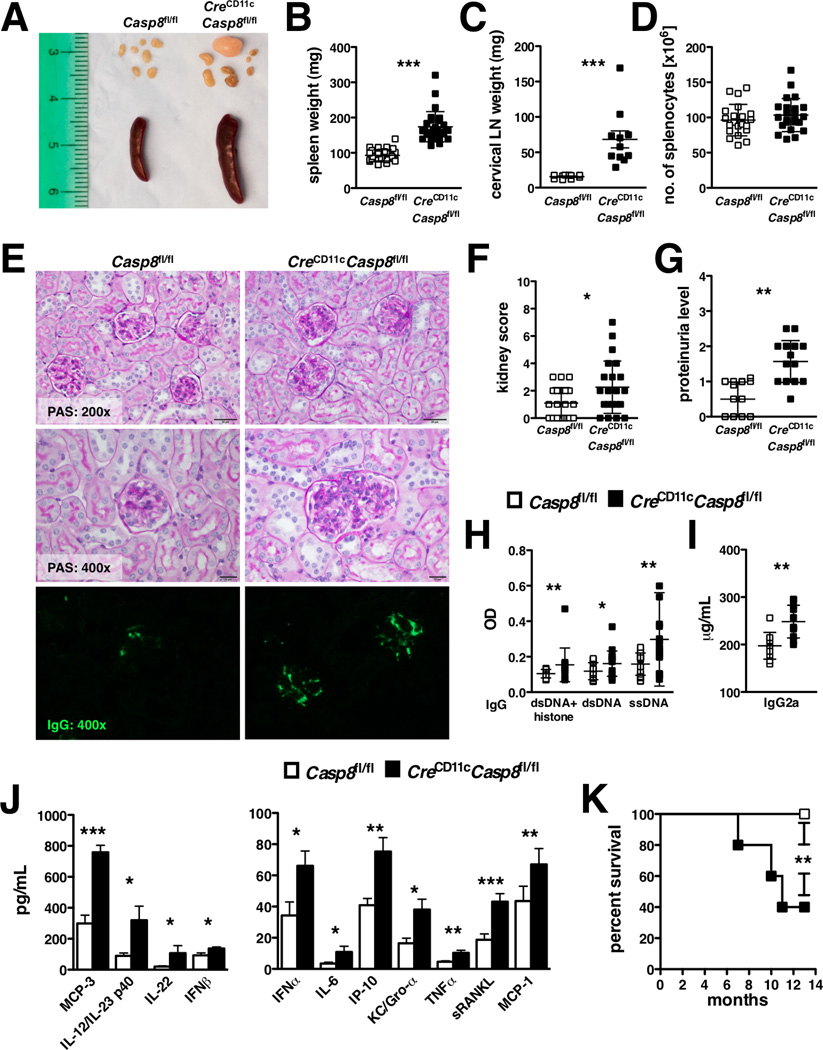
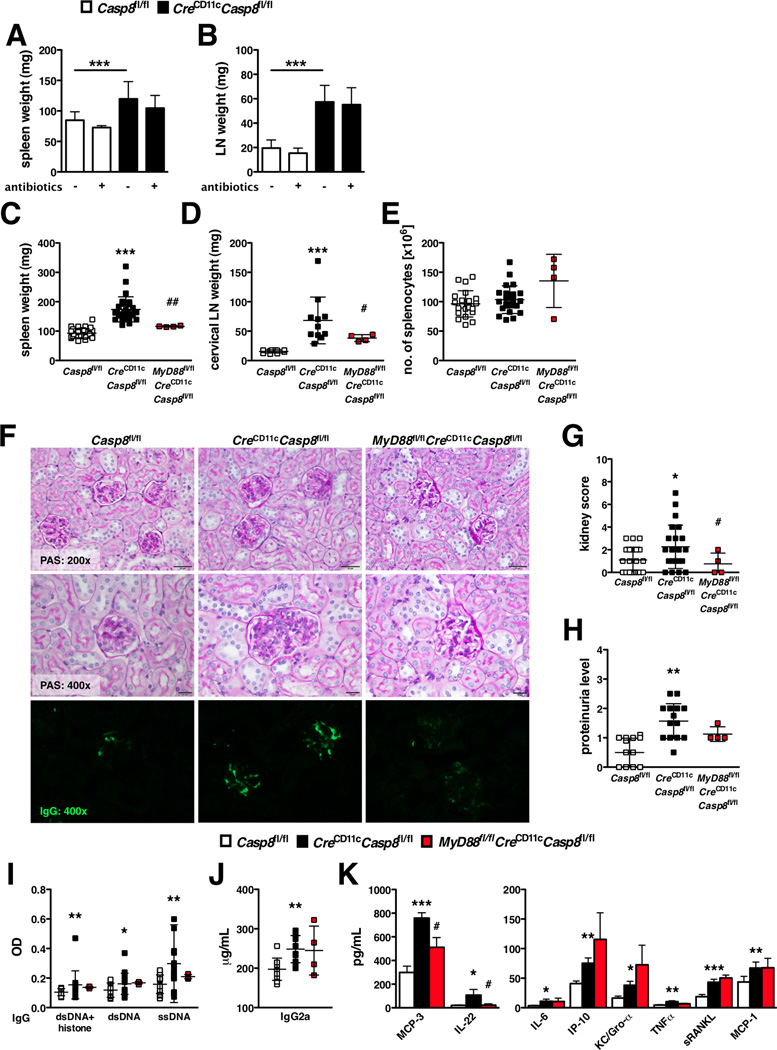
We examined the consequences of DC-specific deletion of caspase-8 (CreCD11cCasp8fl/fl). Loss of caspase-8 in DCs led to splenomegaly and lymphadenopathy in young (Supplemental Figure 2A–B) and aged (Figure 1A–C) mice. However, the observed splenomegaly in aged CreCD11cCasp8fl/fl mice was not attributed to increased numbers of splenocytes (Figure 1D), CD45− cells or Ter119+ cells (Supplemental Figure 2C–D). Additionally, there was a disruption of the splenic architecture in CreCD11cCasp8fl/fl mice compared to control mice, as shown by the expansion of white pulp and reduction of red pulp (Supplemental Figure 2E). There was also a slight increase in splenic collagen deposition in CreCD11cCasp8fl/fl mice compared to control mice and no detectable liver pathology (Supplemental Figure 2E). CreCD11cCasp8fl/fl mice developed glomerulonephritis (Figure 1E–F), IgG deposition in the kidney (Figure 1E) and proteinuria compared to control mice (Figure 1G). CreCD11cCasp8fl/fl mice exhibited markedly elevated levels of serum chromatin-, dsDNA- and ssDNA-reactive IgG antibodies (Figure 1H), pathogenic IgG2a antibodies (Figure 1I) and pro-inflammatory cytokines and chemokines, including MCP-3, IL-12/IL-23p40, IL-22, IFNβ, IFNα, IL-6, IP-10, KC/Gro-α, TNFα, sRANKL and MCP-1 (Figure 1J) compared to control mice. CreCD11cCasp8fl/fl mice also exhibited spontaneous early mortality beginning at 7 months of age and 50% of the mice died by 11 months of age (Figure 1K).
Figure 1. Mice with DC-specific deletion of caspase-8 exhibit systemic autoimmunity.
(A–I) 8-month-old female Casp8fl/fl (control) and CreCD11cCasp8fl/fl mice (n≥10) were evaluated for systemic autoimmune disease phenotypes. (A–B) Splenomegaly. (A and C) Lymphadenopathy. (D) Number of splenocytes. (E) PAS-stained formalin-fixed kidney sections and αIgG-FITC-stained frozen kidney sections. (F) Kidney score. (G) Proteinuria. Serum levels of (H) chromatin, dsDNA- and ssDNA-reactive IgG antibodies, (I) pathogenic IgG2a antibodies and (J) cytokines and chemokines. Data are represented as mean ± SD and compared by Mann Whitney test: *, p<0.05; **, p<0.005; ***, p<0.0005. (K) Survival curve; compared using log-rank Mantel Cox test: **, p<0.005.
Inflammation is independent of DC survival in DC-specific caspase-8 deficient mice
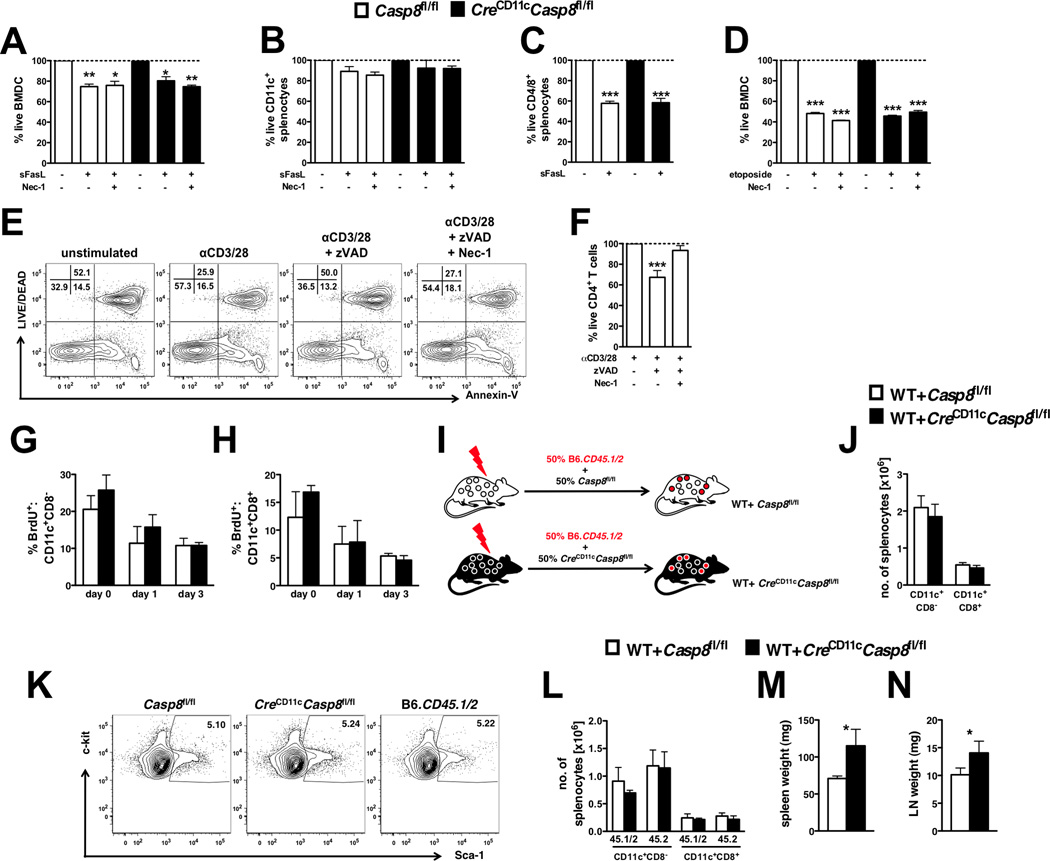
Stimulation with FasL had a minimal effect on the survival of BMDCs (Figure 2A) and splenic DCs (Figure 2B) regardless of the presence of caspase-8, consistent with previous studies (27, 28). Splenic T-cells from control and DC-specific caspase-8-deficient mice displayed similar levels of FasL-induced death (Figure 2C) and etoposide-induced death (Figure 2D). Consistent with recent studies (29), necrostatin-1, an inhibitor of RIPK1 kinase activity and necroptosis, had no effect on the viability of DCs (Figure 2A–B and D) but rescued activated T-cells cultured with the pan-caspase inhibitor zVAD-FMK (Figure 2E and F).
Figure 2. Inflammation related to DC-specific caspase-8 deficiency is independent of DC survival.
Casp8fl/fl (control) and CreCD11cCasp8fl/fl (A) BMDCs (n=4) and (B–C) total splenocytes (n=3) were stimulated with superFasL ± necrostatin-1 (Nec-1) for 10 hours and stained with Annexin-V and Aqua live/dead. Total splenocytes were gated into (B) CD11c+ and (C) CD4/8+ populations for analysis. Additionally, control and CreCD11cCasp8fl/fl BMDCs were stimulated with (D) etoposide for 10 hours and stained with Annexin-V and Aqua live/dead. Data are represented as the percent live compared to unstimulated. (E–F) CD4+ T-cells (n=3) stimulated for 72 hours with αCD3 and αCD28 ± pan-caspase inhibitor zVAD-FMK (zVAD) and Nec-1 were stained with Annexin-V and Aqua live/dead. Data are represented as the percent live compared to αCD3/28 alone. (G–H) Control and CreCD11cCasp8fl/fl mice (n=4) injected with BrdU for 3 days were evaluated for percent splenic BrdU+ (L) CD11c+CD8− and (M) CD11c+CD8+ conventional DCs. (I–M) Mice reconstituted with equal portions of B6.CD45.1/2 (WT) and either control or CreCD11cCasp8fl/fl bone marrow (n=5). (J) Representative Lin−Sca-1+c-kit+ bone marrow cell percentages from 3-month-old female control, CreCD11cCasp8fl/fl and WT mice. Chimeric mice were evaluated 3 months post-transfer for (K) numbers of conventional DCs, (L) distribution of WT (45.1/2) and control or CreCD11cCasp8fl/fl (45.2) derived conventional DCs, (M) splenomegaly and (N) lymphadenopathy. Data are represented as mean ± SD and compared by Mann Whitney test: *, p<0.05; **, p<0.005; ***, p<0.0005.
Previous studies evaluating mice lacking Fas in DCs or overexpressing the p35 caspase inhibitor have suggested a link between an autoimmune disease phenotype and deficiency in DC apoptosis (14, 20). Since caspase-8 is a downstream signaling component of Fas and p35 inhibits all caspase activity, DC survival was examined using in vivo BrdU pulse chase assays and mixed chimeras. BrdU pulse chase assays showed no difference in DC turnover rates between CreCD11cCasp8fl/fl and control conventional DCs (Figure 2G–H). Additionally, loss of caspase-8 in DCs did not result in enhanced survival, as splenic conventional DC numbers remained unchanged in mixed chimera mice (WT+CreCD11cCasp8fl/fl, Figure 2I–J). Of note, the transferred LSK population (Lin−Sca-1+c-kit+) was similar in CreCD11cCasp8fl/fl, Casp8fl/fl and WT mice (Figure 2K). Further, there was no survival advantage attributed to the loss of caspase-8 in DCs, as there were no differences in the number of CreCD11cCasp8fl/fl and WT-derived splenic conventional DCs in mixed chimera mice (Figure 2L), consistent with parallel DC turnover rates in BrdU pulse chase assays. In contrast, hallmarks of systemic autoimmunity including splenomegaly (Figure 2M) and lymphadenopathy (Figure 2N) persisted in mixed chimera mice.
RIPK3 knockout fails to reverse the consequences of DC-specific caspase-8 deletion
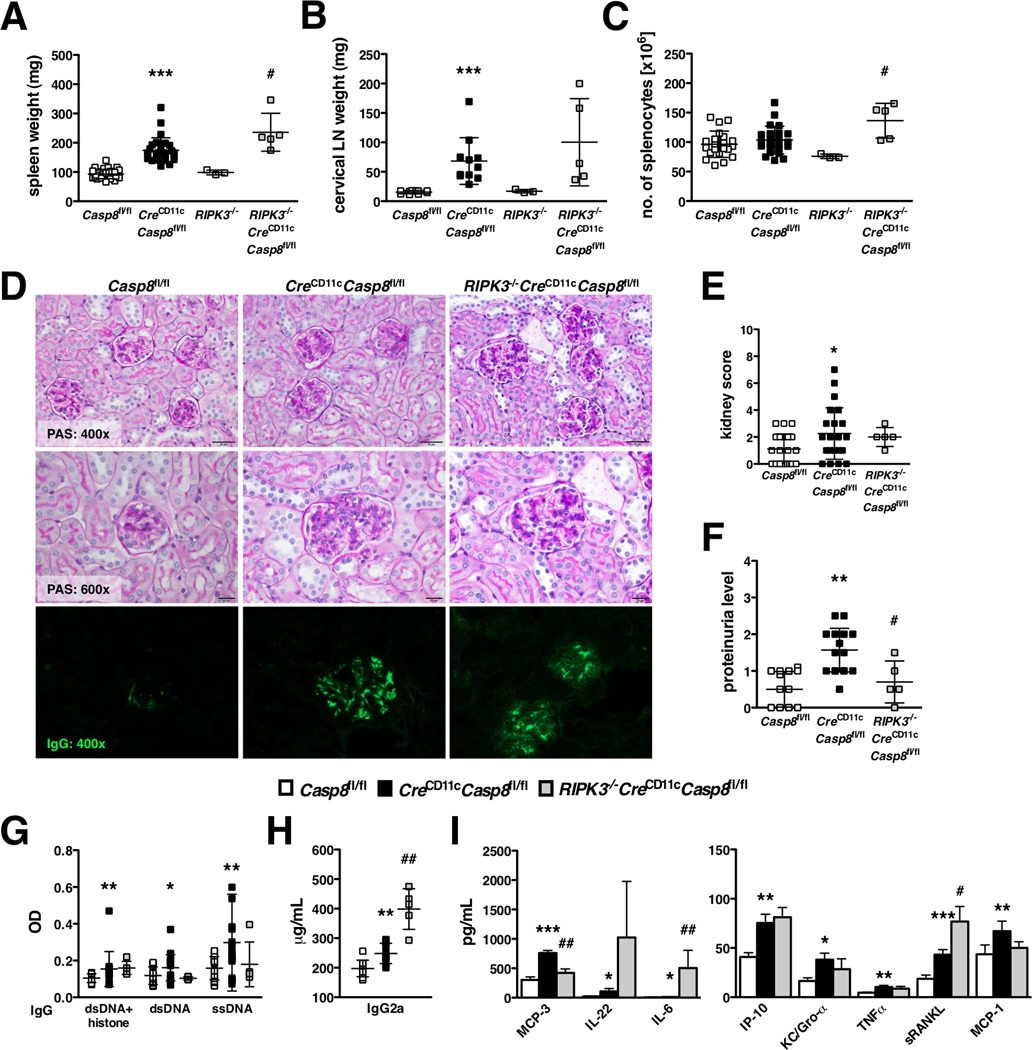
Since RIPK3 knockout reverses the phenotype in global and T-cell-specific caspase-8-deficient mice (18, 19), we crossed CreCD11cCasp8fl/fl mice to RIPK3−/− mice. Young RIPK3−/−CreCD11cCasp8fl/fl mice developed splenomegaly (Supplemental Figure 2A), but lymphadenopathy was abated compared to CreCD11cCasp8fl/fl mice (Supplemental Figure 2B). However with age, RIPK3−/−CreCD11cCasp8fl/fl mice exhibited splenomegaly and lymphadenopathy at unchanged and exacerbated levels, respectively, compared to CreCD11cCasp8fl/fl mice (Figure 3A–C). RIPK3−/−CreCD11cCasp8fl/fl mice also presented with glomerulonephritis (Figure 3D–E) and IgG deposition in the kidney (Figure 3D), although proteinuria was reduced compared to CreCD11cCasp8fl/fl mice (Figure 3F). Further, while serum levels of chromatin-, dsDNA- and ssDNA-reactive IgG antibody levels (Figure 3G) were unaffected by the additional loss of RIPK3, pathogenic IgG2a antibodies (Figure 3H) and pro-inflammatory molecules IL-22, IL-6 and sRANKL (Figure 3I) were heightened compared to CreCD11cCasp8fl/fl mice. Moreover, CreCD11cCasp8fl/fl mice did not present with the characteristic double negative (DN) T-cell population (CD4−CD8−CD3+B220+) associated with deficiencies in Fas (30). In contrast, RIPK3−/−CreCD11cCasp8fl/fl developed a DN T-cell population at a similar proportion to B6.lpr mice (Supplemental Figure 2F–I).
Figure 3. RIPK3 knockout cannot reverse the consequences of DC-specific caspase-8 deletion.
(A–I) 6-month-old female Casp8fl/fl (control), CreCD11cCasp8fl/fl and RIPK3−/−CreCD11cCasp8fl/fl mice (n≥5) were evaluated for systemic autoimmune disease phenotypes. (A) Splenomegaly. (B) Lymphadenopathy. (C) Number of splenocytes. (D) PAS-stained formalin-fixed kidney sections and αIgG-FITC-stained frozen kidney sections. (E) Kidney score. (F) Proteinuria. Serum was evaluated for levels of (G) chromatin, dsDNA- and ssDNA-reactive IgG antibodies, (H) pathogenic IgG2a antibodies and (I) cytokines and chemokines. Data are represented as mean ± SD and compared by Mann Whitney test. * denotes comparison between control and CreCD11cCasp8fl/fl, # denotes comparison between CreCD11cCasp8fl/fl and RIPK3−/−CreCD11cCasp8fl/fl. *, #:p<0.05; **, ##:p<0.005; ***, ###:p<0.0005.
Deletion of IRF3 exacerbates the systemic inflammation in CreCD11cCasp8fl/fl mice
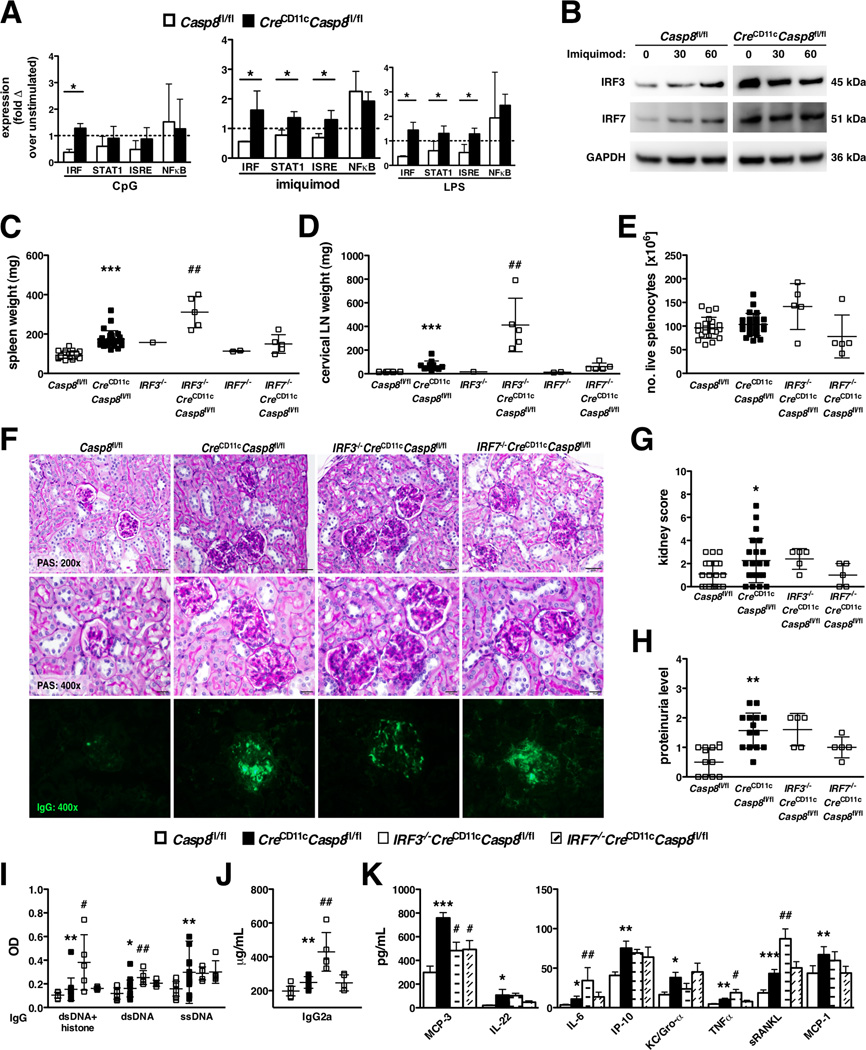
CreCD11cCasp8fl/fl mice present with an interferon signature, indicating that the loss of caspase-8 may increase the activity of interferon regulatory factors (IRFs), a family of transcription factors known to play a role in type 1 interferon production. Caspase-8−/− bone marrow derived DCs (BMDCs) showed sustained DNA binding of IRF in response to treatment with CpG (TLR9 agonist), imiquimod (TLR7 agonist) and LPS (TLR4 agonist). Moreover, STAT1 and IRF signaling response elements (ISRE) exhibited increased DNA binding following TLR7 and TLR4 stimulation in caspase-8-deficient BMDCs (Figure 4A), with no difference in the DNA binding activity of NFκB between control and CreCD11cCasp8fl/fl BMDCs. Further, since previous studies have shown that caspase-8 processes IRF3 for degradation (13), examination of caspase-8-deficient BMDCs revealed constitutively higher expression of IRF3, as well as IRF7, compared to control BMDCs and independent of TLR stimulation (Figure 4B). Therefore, we generated IRF3−/−CreCD11cCasp8fl/fl mice to determine its role in the induction of systemic inflammation. Since IRF7 is a redundant homolog of IRF3, as a control we established IRF7−/−CreCD11cCasp8fl/fl mice. Interestingly, knockout of IRF3 exacerbated the splenomegaly and lymphadenopathy in both young (Supplemental Figure 2A–B) and aged (Figure 4C–E) CreCD11cCasp8fl/fl mice, while IRF7 knockout had no additive effect. Although loss of IRF3 or IRF7 did not dramatically affect the presence of glomerulonephritis (Figure 4F–G), IgG deposition in the kidney (Figure 4F) or proteinuria (Figure 4H) in CreCD11cCasp8fl/fl mice, IRF3−/−CreCD11cCasp8fl/fl, but not IRF7−/−CreCD11cCasp8fl/fl, mice exhibited markedly elevated levels of serum chromatin- and dsDNA-reactive IgG antibodies (Figure 4I), pathogenic IgG2a antibodies (Figure 4J), and pro-inflammatory molecules, including IL-6, TNFα and sRANKL (Figure 4K) compared to CreCD11cCasp8fl/fl mice.
Figure 4. Deletion of IRF3 exacerbates the systemic inflammation in CreCD11cCasp8fl/fl mice.
(A) Casp8fl/fl (control) and CreCD11cCasp8fl/fl BMDCs were stimulated with CpG, imiquimod or LPS for 6 hours and isolated nuclear lysates subjected to a multi-analyte transcription factor bead-based assay, represented as the fold change over unstimulated. (B) Control and CreCD11cCasp8fl/fl BMDCs were stimulated with imiquimod and isolated total cellular lysates subjected to immunoblot analysis for total IRF3. The blot was then stripped for total IRF7 and GAPDH expression and the figures have been cropped and placed together. (C–K) 7-month-old female Casp8fl/fl (control), CreCD11cCasp8fl/flIRF3−/−CreCD11cCasp8fl/fl and IRF7−/−CreCD11cCasp8fl/fl mice (n≥5) were evaluated for systemic autoimmune disease phenotypes. (C) Splenomegaly. (D) Lymphadenopathy. (E) Number of splenocytes. (F) PAS-stained formalin-fixed kidney sections and αIgG-FITC-stained frozen kidney sections. (G) Kidney score. (H) Proteinuria. Serum was evaluated for levels of (I) chromatin, dsDNA- and ssDNA-reactive IgG antibodies, (J) pathogenic IgG2a antibodies and (K) cytokines and chemokines. Data are represented as mean ± SD and compared by Mann Whitney test. * denotes comparison between control and CreCD11cCasp8fl/fl, # denotes comparison between CreCD11cCasp8fl/fl and IRF3−/−CreCD11cCasp8fl/fl or IRF7−/−CreCD11cCasp8fl/fl. *, #:p<0.05; **, ##:p<0.005; ***, ###:p<0.0005.
Caspase-8-deficient DCs are hyper-responsive to TLR activation
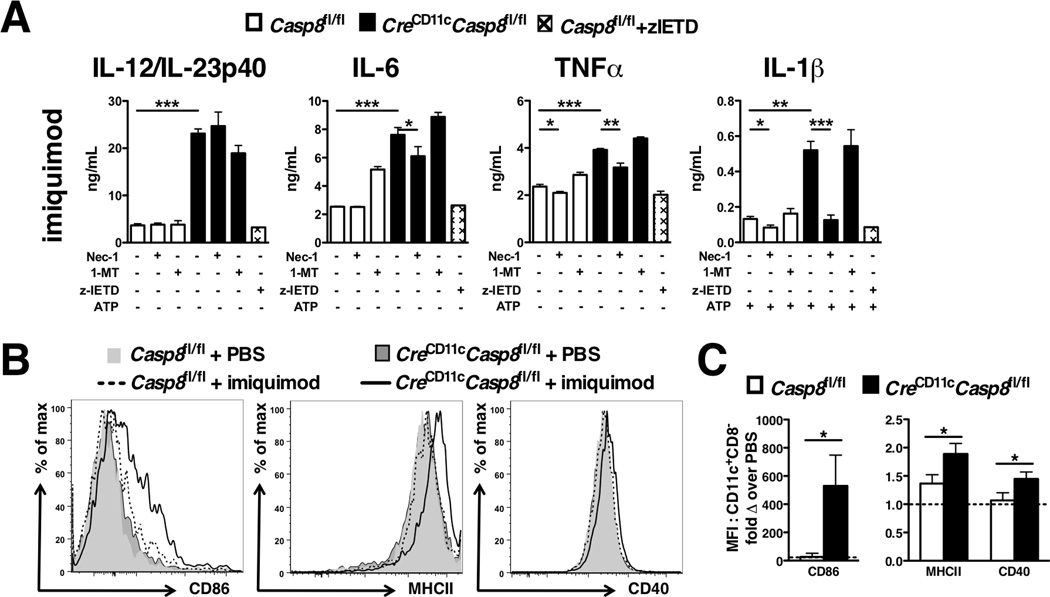
Due to the chronic systemic inflammation observed in CreCD11cCasp8fl/fl mice regardless of the presence of RIPK3, we assessed the response to TLR agonists in the absence of caspase-8. Of note, expression of TLR2/4/7/9 was either unchanged or reduced in CreCD11cCasp8fl/fl splenic DC subsets compared to controls (Supplemental Figure 3A). To determine the functional response of these TLRs in caspase-8−/− DCs, BMDCs were treated with CpG, imiquimod or LPS. CreCD11cCasp8fl/fl BMDCs produced higher levels of IL-12/23p40, IL-6, TNFα and IL-1β compared to control BMDCs in response to TLR7 (Figure 5A), TLR9 and TLR4 activation (Supplemental Figure 3B) without inducing cell death (Supplemental Figure 3C). The loss of caspase-8 was sufficient to induce IL-1β release without the addition of ATP (Supplemental Figure 3D). To expand upon these studies, the aforementioned TLR agonists were intraperitoneally injected into mice. While CpG and LPS had no effect on expression of activation markers in CreCD11cCasp8fl/fl mice (data not shown), imiquimod induced a large increase in CD86 expression and led to elevated MHC class II and CD40 expression on CreCD11cCasp8fl/fl CD11c+CD8− conventional DCs compared to controls (Figure 5B–C).
Figure 5. Caspase-8-deficient DCs are hyper-responsive to TLR activation in a RIPK1-dependent manner.
(A) GM-CSF + Flt3-L-treated BMDCs from Casp8fl/fl (control) and CreCD11cCasp8fl/fl mice were stimulated with imiquimod ± Nec-1 and/or zIETD-FMK (zIETD) and/or 1-Methyl-D-tryptophan (1-MT) for 6 hours ± ATP (5mM) and supernatants evaluated for IL-12/IL-23p40, IL-6, TNFα, and IL-1β. (B–C) 3-month-old control and CreCD11cCasp8fl/fl mice (n=4) injected with imiquimod (200 µg/mouse) or PBS were evaluated 4 hours later for splenic CD11c+CD8− conventional DC CD86, MHCII, and CD40 expression, represented as the fold change over PBS injection. Data are represented as mean ± SD and compared by Mann Whitney test: *, p<0.05; **, p<0.005; ***, p<0.0005.
Although DCs do not undergo necroptosis, we examined whether the necrosome has an effect on proinflammatory cytokine production. Necrostatin-1 inhibited secretion of IL-6, TNFα and IL-1β in all TLR agonist-treated caspase-8-deficient BMDC cultures, while it only reduced IL-12/23p40 secretion mediated by TLR9 activation (Figure 5A and Supplemental Figure 3B). Since necrostatin-1 has been shown to block not only RIPK1 but also indoleamine 2,3-dioxygenase (IDO), the IDO2-specific inhibitor 1-methyl-D-tryptophan (1-MT) (31) was added to BMDC cultures as a control. 1-MT had no effect on cytokine secretion with the exception of CpG-induced IL-12/23p40 production in BMDCs. The caspase-8-specific inhibitor z-IETD-FMK was also added to BMDCs to address the requirement for caspase-8 enzymatic activity in the hyper-secretion of proinflammatory cytokines. Interestingly, specific blockade of caspase-8 activity did not mimic the deletion of caspase-8 in BMDCs (Figure 5A and Supplemental Figure 3B).
Caspase-8 suppresses MyD88 signaling
Over the past several years, gut microflora have been suggested to be a depot for TLR signaling (32). In order to reduce the potential for endogenous TLR ligands from gut microflora to exacerbate SLE-like disease, young CreCD11cCasp8fl/fl mice were treated with oral antibiotics for 2 months. Oral antibiotic treatment had no effect on disease development in CreCD11cCasp8fl/fl mice, as splenomegaly (Figure 6A) and lymphadenopathy (Figure 6B) were unchanged compared to untreated CreCD11cCasp8fl/fl mice.
Figure 6. Caspase-8 suppresses MyD88 signaling.
(A–C) 3-week-old Casp8fl/fl (control) and CreCD11cCasp8fl/fl (n=4) mice treated with oral antibiotics for 8 weeks were evaluated for (A) splenomegaly and (B) lymphadenopathy. (C–K) 8-month-old female control, CreCD11cCasp8fl/fl and MyD88fl/flCreCD11cCasp8fl/fl mice (n≥4) were evaluated for systemic autoimmune disease phenotypes. (C) Splenomegaly. (D) Lymphadenopathy. (E) Number of splenocytes. (F) PAS-stained formalin-fixed kidney sections and αIgG-FITC-stained frozen kidney sections. (G) Kidney score. (H) Proteinuria. Serum was evaluated for levels of (I) chromatin, dsDNA- and ssDNA-reactive IgG antibodies, (J) pathogenic IgG2a antibodies and (K) cytokines and chemokines. Data are represented as mean ± SD and compared by Mann Whitney test. * denotes comparison between control and CreCD11cCasp8fl/fl, # denotes comparison between CreCD11cCasp8fl/fl and MyD88fl/flCreCD11cCasp8fl/fl. *, #:p<0.05; **, ##:p<0.005; ***, ###:p<0.0005.
Due to the heightened production of pro-inflammatory cytokines associated with caspase-8 deficiency, we sought to restrict TLR activation in our model by eliminating MyD88. Concurrent deletion of caspase-8 and MyD88 in DCs was sufficient to reduce splenomegaly and lymphadenopathy in aged CreCD11cCasp8fl/fl mice (Figure 6C–D), but did not affect splenocyte numbers (Figure 6E). Importantly, loss of MyD88 ameliorated kidney disease observed in CreCD11cCasp8fl/fl mice, as shown by reduced glomerulonephritis (Figure 6F–G) and IgG deposition (Figure 6F), though proteinuria levels (Figure 6H) were unchanged. Further, while MyD88fl/flCreCD11cCasp8fl/fl mice exhibited similar levels of anti-chromatin and anti-dsDNA IgG antibodies (Figure 6I) and pathogenic IgG2a antibodies (Figure 6J), there was a trend towards reduced anti-ssDNA IgG antibodies (Figure 6I) and significantly less pro-inflammatory serum cytokines/chemokines, including IL-22 and MCP-3 (Figure 6K) compared to CreCD11cCasp8fl/fl mice.
DC-specific loss of caspase-8 intensifies DC and lymphocyte activation
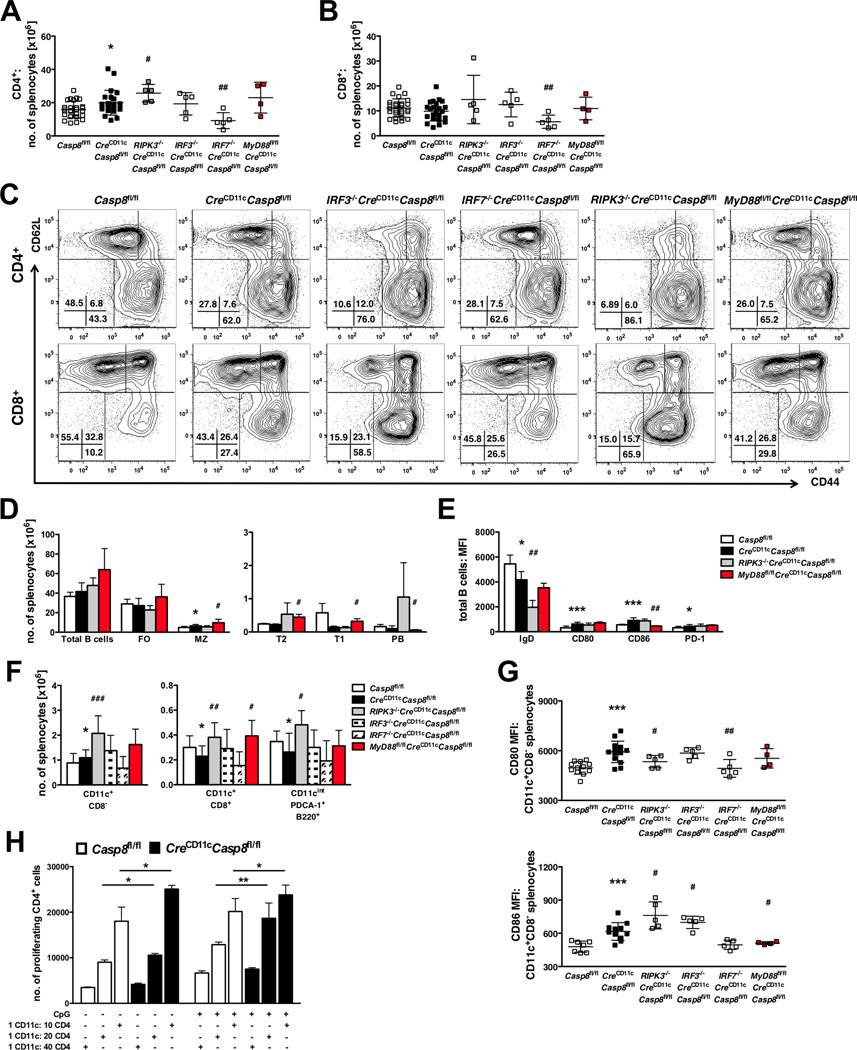
To determine the cellular mechanism responsible for the systemic autoimmunity that develops in CreCD11cCasp8fl/fl mice, multi-parameter flow cytometry was utilized. CreCD11cCasp8fl/fl CD4+ but not CD8+ T-cells were increased in numbers (Figure 7A–B). Further, both T-cell populations showed decreased naïve (CD44−CD62L+) corresponding to increased effector (CD44+CD62L−) subsets compared to controls (Figure 7C, Supplemental Figure 4A–B). Elevated expression of PD-1 on CD4+ T-cells and CD69 and PD-1 on CD8+ T-cells in CreCD11cCasp8fl/fl mice further defined hyperactive T cell subsets (Supplemental Figure 4C–F). Regulatory T-cells (CD4+CD25+Foxp3+) were present at greater numbers in CreCD11cCasp8fl/fl mice and these cells expressed elevated PD-1 (Supplemental Figure 4G–H). Although the loss of caspase-8 in DCs did not affect total B-cell numbers (Figure 7D), CreCD11cCasp8fl/fl mice displayed an increase in marginal zone (MZ) B-cells. CreCD11cCasp8fl/fl total B-cells expressed less IgD, indicating increased maturation and class-switching, and expressed more CD80, CD86 and PD-1 (Figure 7E and Supplemental Figure 4I–L), which correlates with the elevated antibody and autoantibody production observed in CreCD11cCasp8fl/fl mice.
Figure 7. Caspase-8-deficient CD11c+CD8 conventional DCs express increased activation markers and confer a hyperactive phenotype on lymphocytes.
(A–G) Splenocytes from 6-8-month-old female Casp8fl/fl (control), CreCD11cCasp8fl/flRIPK3−/−CreCD11cCasp8fl/flIRF3−/−CreCD11cCasp8fl/flIRF7−/−CreCD11cCasp8fl/fl and MyD88fl/flCreCD11cCasp8fl/fl mice (n≥4) were analyzed by flow cytometry. (A) CD4+ and (B) CD8+ T-cell numbers. (C) Representative naïve (CD44−CD62L+) and activated (CD44+CD62L−) T-cell percentages of total CD4+ and CD8+ populations. (D) Total B-cell (B220+) numbers and subsets: follicular (FO, CD19+CD21/35+CD23+), marginal zone (MZ, CD19+CD21/35+CD23low), transitional 2 (T2, B220+AA4.1+CD23+), transitional 1 (T1, B220+AA4.1+CD23−), plasmablasts (PB, CD19+B220lowCD138+CD21/35−CD23−). (E) B-cell IgD, CD80, CD86 and PD-1 expression. (F) Conventional (CD11c+CD8− and CD11c+CD8−) and plasmacytoid (CD11cintermediatePDCA-1+B220+) DC numbers. (G) CD11c+CD8− conventional DC CD80 and CD86 expression. (H) Bead-separated CD11c+ cells pulsed with OVA were co-cultured with OT-II/RAG−/− CD4+ T-cells. Data are represented as mean ± SD and compared by Mann Whitney test. * denotes comparison between control and CreCD11cCasp8fl/fl, # denotes comparison between CreCD11cCasp8fl/fl and experimental knockouts. *, #:p<0.05; **, ##:p<0.005; ***, ###:p<0.0005.
Since T- and B-cell activation is associated with DC functionality, CreCD11cCasp8fl/fl DC populations were evaluated. CreCD11cCasp8fl/fl mice showed more splenic CD11c+CD8− conventional DCs and less CD11c+CD8+ conventional and plasmacytoid DCs compared to control mice (Figure 7F). Further, both CreCD11cCasp8fl/fl CD11c+CD8− conventional and plasmacytoid DCs were hyperactivated (Figure 7G and Supplemental Figure 4M), as indicated by increased expression of costimulatory molecules CD80 and CD86 and activation marker CD69, respectively. Similar to DC subsets, CreCD11cCasp8fl/fl mice exhibited increased numbers of Ly6Clow and Ly6Chigh splenic monocytes/macrophages and neutrophils compared to control mice (Supplemental Figure 4M). In addition, caspase-8-deficient CD11c+ cells incubated with OVA-peptide induced heightened OT-II-specific CD4+ T-cell proliferation with and without TLR9 activation (Figure 7H) as compared to control CD11c+ cells.
Knockout of RIPK3 or IRF3 not only exacerbated disease phenotypes, but also exaggerated the immune cell dysregulation found in CreCD11cCasp8fl/fl mice. Loss of RIPK3 not only increased the CD4+ (Figure 7A) and regulatory (Supplemental Figure 4G) T-cell populations, but also heightened CD4+ and CD8+ T-cell activation, as seen by increased skewing towards an effector phenotype (Figure 7C and Supplemental Figure 4A–B) and elevated expression of CD69 on CD4+ T-cells (Supplemental Figure 4C) and PD-1 on CD4+, CD8+ and regulatory T-cells (Supplemental Figure 4D, 4F and 4H). RIPK3 deletion also modified B-cells by reducing IgD levels (Figure 7E and Supplemental Figure 4I), indicating increased maturation, compared to caspase-8 deficiency alone. Systemic RIPK3 deletion increased DC numbers (Figure 7F), and modified CD11c+CD8− conventional DC activation by decreasing CD80 expression (Figure 7G) to control levels while increasing CD86 expression (Figure 7G). Although loss of IRF3 did not affect T-cell numbers overall (Figure 7A–B), effector T-cells were increased while naïve T-cells were reduced compared to CreCD11cCasp8fl/fl T-cells (Figure 7C and Supplemental Figure 4A–B). While IRF3 deletion increased neutrophil, eosinophil and Ly6Clow and Ly6Chigh splenic macrophage numbers (Supplemental Figure 4N), DC numbers were unaffected (Figure 7F). However, IRF3 deletion elevated CD11c+CD8− conventional DC CD86 expression (Figure 7G).
Although knockout of IRF7 did not affect disease phenotypes in found in CreCD11cCasp8fl/fl mice, IRF7 deletion not only decreased numbers of CD4+ and CD8+ (Figure 7A–B) T-cells, and reduced expression of PD-1 on CD4+, CD8+ and regulatory T-cells (Supplemental Figure 4D, 4F and 4H), but also reduced CD11c+CD8− conventional DC CD80 expression (Figure 7G) in CreCD11cCasp8fl/fl mice. However, these alterations at the cellular level were insufficient to reduce disease activity.
DC-specific deletion of MyD88 not only partially reversed the SLE-like disease phenotype in CreCD11cCasp8fl/fl mice, but loss of this TLR signaling mediator also altered the observed immune cell dysregulation found in these mice. Although loss of MyD88 did not affect T-cell numbers (Figure 7A–B), CD8+ T-cells expressed less CD69 (Supplemental Figure 4E) and regulatory T-cells expressed less PD-1 (Supplemental Figure 4H) compared to CreCD11cCasp8fl/fl T-cells. MyD88 deletion also altered the B cell pool by augmenting the MZ, transitional 2 (T2) and transitional 1 (T1) subsets, reducing plasmablasts (PB) (Figure 7D) and decreasing CD86 expression on total B cells compared to caspase-8 deficiency alone (Figure 7E and Supplemental Figure 4K). DC-specific loss of MyD88 led to increased numbers of CD11c+CD8+ conventional DCs compared to CreCD11cCasp8fl/fl mice (Figure 7F), and reduced CD11c+CD8− conventional DC CD86 expression (Figure 7G) to that of control DCs. Taken together, these results suggest that caspase-8 dampens MyD88 signaling in DCs, and when caspase-8 is no longer present, unchecked signaling through this TLR mediator occurs leading to onset of systemic autoimmunity.
DISCUSSION
Previous studies have linked increased DC survival to the development of autoimmmune disease. Here, we show that CreCD11cCasp8fl/fl mice develop splenomegaly, lymphadenopathy, autoantibodies, glomerulonephritis, immune complex deposition in the kidney, exacerbated proteinuria levels, heightened amounts of serum pro-inflammatory cytokines and early mortality. In contrast to the other experimental models of apoptotic regulators in DCs, loss of caspase-8 in DCs does not affect their survival. There is no change in DC turnover rates and there are equal numbers of CreCD11cCasp8fl/fl and WT DCs in mixed bone marrow chimera mice. DCs lacking caspase-8 also fail to undergo apoptosis in response to FasL but are equally sensitive to etoposide-induced apoptosis. Thus, these data demonstrate that caspase-8 functions in a vastly different manner in DCs as compared to Fas (14) or pan-caspase inhibitors (20).
Recently, a number of studies have implicated caspase-8 in the regulation of the inflammasome, in particular the Nlrp3 inflammasome, independent of cell death in a number of cell types (29, 33, 34). Our findings are consistent with one such study (29), which showed that deletion of caspase-8 in DCs results in splenomegaly and lymphadenopathy through an apoptosis- and necroptosis-independent mechanism. Both studies show that caspase-8-deficient BMDCs secrete IL-1β without the requirement for secondary ATP stimulation in response to TLR4 activation and that this is abrogated by the addition of necrostatin-1. However, our findings expand on the role of caspase-8 in DCs as we show a novel necrostatin-1-specific inhibitory effect on pro-inflammatory cytokine secretion in response to TLR7/9 activation. In addition, we show that these changes are associated with the development of SLE-like disease, which results in early spontaneous mortality. Further we show that the loss of caspase-8 in DCs leads to their hyperactivation that requires RIPK1 activity, which culminates in development of disease by a MyD88-dependent IRF3-independent mechanism. Differences between the studies may stem from differing cell-specific caspase-8 deletion constructs and/or the chosen transgenic CD11c-Cre line.
TLR7 and 9, which are intracellular receptors known to be activated by nucleic acids, have been linked to both human and murine models of SLE (35). TLR engagement induces RIPK signaling independent of DR activation, thereby leading to formation of a ripoptosome (10). Blocking RIPK1 kinase activity dampens TLR4/7/9-induced secretion of proinflammatory cytokines in caspase-8-deficient DCs without affecting cell survival. Thus, RIPK1 appears to function in a cell-specific manner that is vastly different based on the studies using T-cell caspase-8 insufficient mice (17). However, deletion of RIPK3 in CreCD11cCasp8fl/fl mice is unable to reverse the observed phenotypic outcome, which is contrary to T-cell caspase-8 deficient mice and similar to a recently published study (18, 29). Thus, our data substantiate a new function for caspase-8, namely that it suppresses the inflammatory DC phenotype independent of activating apoptosis or inhibiting necroptosis but requires components of the ripoptosome.
Immunization studies revealed that T cell differentiation by DCs requires TLR activation (36). Teichmann, et al. found that deletion of DCs in a murine model of SLE ameliorates disease by limiting T-cell expansion and subsequent kidney damage (37). While most TLRs signal through MyD88, TLR3 requires the mediator TRIF and TLR4 signals through both MyD88 and TRIF (38). Although DC-specific deletion of MyD88 in MRL.Faslpr mice reduces lymphoproliferation and controls dermatitis, nephritis development persisted (39). However, we are able to suppress lymphoproliferative and end-organ disease by cell-specific deletion of MyD88 in CreCD11cCasp8fl/fl mice. Lyn is a Src-family tyrosine kinase that phosphorylates caspase-8 and blocks its downstream activity in neutrophils (40). While DC-specific deletion of Lyn mimics the systemic autoimmunity induced by deletion of caspase-8, these activities are independent of one another. However, similar to CreCD11cCasp8fl/fl mice, DC-specific MyD88 deletion suppressed the autoimmunity induced in mice by DC-specific Lyn deficiency (41). These data indicate that both Lyn and caspase-8 limit MyD88-dependent TLR signaling in DCs. In contrast to CreCD11cCasp8fl/fl mice, DC-specific deletion of MyD88 is unable to abrogate systemic inflammation caused by DC-specific FADD deficiency (42). Further, administration of broad-spectrum antibiotics suppresses systemic inflammation in DC-specific FADD-deficient mice (42) but has no effect in CreCD11cCasp8fl/fl mice. These results suggest that although caspase-8 and FADD are together intimately involved in cell death, caspase-8 mediates its suppressive action in DCs in part by a MyD88-dependent mechanism, while FADD may function to block MyD88-independent TLR signaling through TRIF. Our in vitro results suggest that the enzymatic activity of caspase-8 is dispensable for its suppressive activity, indicating that caspase-8 may act as a scaffolding protein that in this case may sequester MyD88. Future studies are required to define the exact interaction. Additionally, since we did not observe a complete abrogation of disease with MyD88 deletion, it is possible that caspase-8 may also dampen TRIF-dependent TLR signaling. Therefore, future studies will be to determine whether deletion of TRIF can ameliorate the systemic autoimmunity in CreCD11cCasp8fl/fl mice.
A majority of SLE patients present an IFN signature, namely constitutive production of type I IFNs (IFNα and IFNβ) and increased expression of type I IFN-regulated genes (12). This IFN signature is also detected in DCs from a murine model of SLE (43) and in mice lacking caspase-8 in DCs. Further, caspase-8-deficient BMDCs showed sustained DNA binding of IRF and ISRE following extended TLR4/7/9 stimulation. These studies would suggest that the loss of caspase-8 may increase the transcriptional activity of IRFs. Since previous studies have shown that caspase-8 processes IRF3 for degradation (13), it would follow that IRF3 would be elevated in the absence of caspase-8. Indeed, expression of IRF3 is increased in caspase 8 deficient BMDCs. Thus, we hypothesized that the presence of IRF3 at elevated levels in caspase-8-deficient DCs and the increased potential for deleterious transcriptional products may be the root cause of the autoimmunity observed in CreCD11cCasp8fl/fl mice. However to our surprise, deficiency in IRF3 exacerbates the lymphoproliferative disease in CreCD11cCasp8fl/fl mice. These data indicate that IRF3 may be crucial in providing a compensatory mechanism to dampen inflammation induced by loss of caspase-8. Since recent evidence suggests that IRF3 acts not only as a transcription factor but as an apoptotic mediator through interaction with Bax via its newly discovered BH3 domain (44), a failure to undergo apoptosis may be a potential explanation for the enhanced lymphoproliferative disease in CreCD11cCasp8fl/fl mice. Although deletion of either IRF3 or IRF7 is unable to correct the inflammation associated with DC-specific caspase-8 deficiency, it is possible that other IRFs may be involved. For instance, IRF5 interacts with MyD88 downstream of TLR signaling, and is phosphorylated and activated after TLR engagement (45). Further, polymorphisms in the IRF5 gene in SLE patients result in their constitutive expression, thereby up-regulating type I IFN and pro-inflammatory cytokine production (46).
Both conventional and plasmacytoid DCs from patients with SLE have been shown to possess abnormal phenotypes (47, 48). CD11c+CD8− conventional DCs from SLE patients display a more activated and mature phenotype, including enhanced MHC class II and costimulatory CD80/86 expression (49). Increased numbers of the other major DC subtype, plasmacytoid DCs, are seen in peripheral tissues of SLE patients (49). Furthermore, DCs from SLE patients fail to yield a tolerizing phenotype under experimental conditions (50) and produce high levels of proinflammatory IL-6, which is known to inhibit CD4+CD25+ regulatory T-cells (51). These findings are recapitulated in DCs from mice with SLE-like disease in which the number of DCs is increased as is their secretion of pro-inflammatory cytokines (IL-12 and IL-6), cell-surface activation and maturation markers, induction of T-cell effector responses, and reduction of regulatory T-cell function compared to control DCs (52). While these results support an important role for the expansion and activation of DCs in both human and murine models of SLE, the underlying mechanisms driving these changes are unknown. Our data suggest that in normal DCs following TLR activation presumably via TLR4/7/9, caspase-8 associates with RIPK1 and MyD88 to limit their downstream signaling, thereby preventing the continuous activation of DCs. The removal of this break leads to a self-autoreactive loop in DCs and subsequent onset of autoimmune disease. Thus, our data document a critical role for caspase-8 in DCs in the pathogenesis of murine SLE-like disease, and provide a link between caspase-8 and heightened TLR responses to endogenous ligands leading to disease pathogenesis. Future studies will be required to ascertain whether caspase-8 function or expression is reduced in DCs in SLE patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Perlman Laboratory and Dr. Marcus Peter for their helpful suggestions.
Footnotes
These studies were supported by grants from the National Institutes of Health to Carla Cuda (AR060169), Chandra Mohan (AR055600, AI039824, AR050812), Christian Stehlik (AI092490, GM071723) and Harris Perlman (AR050250, AR054796, AR055503, AI067590), as well as funds provided by the Solovy-Arthritis Research Society Chair in Medicine to Harris Perlman.
REFERENCES
- 1.Hutcheson J, Perlman H. BH3-only proteins in rheumatoid arthritis: potential targets for therapeutic intervention. Oncogene. 2008;27(Suppl 1):S168–S175. doi: 10.1038/onc.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, Kang TBong, Wallach D, Dorfleutner A, Lahti JM, Flynn DC, Frisch SM. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273–4278. doi: 10.1158/0008-5472.CAN-05-4183. [DOI] [PubMed] [Google Scholar]
- 4.Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol. 2005;174:5270–5278. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 5.Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 8.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2011;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sears N, Sen GC, Stark GR, Chattopadhyay S. Caspase-8-mediated cleavage inhibits IRF-3 protein by facilitating its proteasome-mediated degradation. J Biol Chem. 2011;286:33037–33044. doi: 10.1074/jbc.M111.257022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, Liepa J, Wood GA, Casola S, Rajewsky K, Mak TW. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Z, Hampel B, Yagita H, Rajewsky K. T Cell-specific Ablation of Fas Leads to Fas Ligand-mediated Lymphocyte Depletion and Inflammatory Pulmonary Fibrosis. J Exp Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 21.Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Cuda CM, Agrawal H, Misharin AV, Haines GK, 3rd, Hutcheson J, Weber E, Schoenfeldt JA, Mohan C, Pope RM, Perlman H. Requirement of myeloid cell-specific Fas expression for prevention of systemic autoimmunity in mice. Arthritis Rheum. 2012;64:808–820. doi: 10.1002/art.34317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G–based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2011;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 26.Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, Perlman H. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009 doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashany D, Savir A, Bhardwaj N, Elkon KB. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol. 1999;163:5303–5311. [PubMed] [Google Scholar]
- 28.Guo Z, Zhang M, An H, Chen W, Liu S, Guo J, Yu Y, Cao X. Fas ligation induces IL-1beta-dependent maturation and IL-1beta-independent survival of dendritic cells: different roles of ERK and NF-kappaB signaling pathways. Blood. 2003;102:4441–4447. doi: 10.1182/blood-2002-11-3420. [DOI] [PubMed] [Google Scholar]
- 29.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Hauwermeiren FVan, Libert C, Declercq W, Callewaert N, Prendergast GC, Degterev A, Yuan J, Vandenabeele P. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis D, Benoist C. Microbiota and autoimmune disease: the hosted self. Cell Host Microbe. 2011;10:297–301. doi: 10.1016/j.chom.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Gurung P, Anand PK, Malireddi RK, Walle LVande, Opdenbosch NVan, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and Caspase-8 Mediate Priming and Activation of the Canonical and Noncanonical Nlrp3 Inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, Cerundolo V, Sher A. Cutting Edge: Endoplasmic Reticulum Stress Licenses Macrophages To Produce Mature IL-1beta in Response to TLR4 Stimulation through a Caspase-8- and TRIF-Dependent Pathway. J Immunol. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012;53:58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 36.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 37.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annual review of cell and developmental biology. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 39.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia SH, Parodo J, Kapus A, Rotstein OD, Marshall JC. Dynamic regulation of neutrophil survival through tyrosine phosphorylation or dephosphorylation of caspase-8. J Biol Chem. 2008;283:5402–5413. doi: 10.1074/jbc.M706462200. [DOI] [PubMed] [Google Scholar]
- 41.Lamagna C, Scapini P, Ziffle JAvan, DeFranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci U S A. 2013;110:E3311–E3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young JA, He TH, Reizis B, Winoto A. Commensal microbiota are required for systemic inflammation triggered by necrotic dendritic cells. Cell Rep. 2013;3:1932–1944. doi: 10.1016/j.celrep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sriram U, Varghese L, Bennett HL, Jog NR, Shivers DK, Ning Y, Behrens EM, Caricchio R, Gallucci S. Myeloid Dendritic Cells from B6.NZM Sle1/Sle2/Sle3 Lupus-Prone Mice Express an IFN Signature That Precedes Disease Onset. J Immunol. 2012 doi: 10.4049/jimmunol.1101686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay S, Sen GC. IRF-3 and Bax: a deadly affair. Cell cycle. 2010;9:2479–2480. doi: 10.4161/cc.9.13.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paun A, Pitha PM. The IRF family, revisited. Biochimie. 2007;89:744–753. doi: 10.1016/j.biochi.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng D, Stone RC, Eloranta ML, Sangster-Guity N, Nordmark G, Sigurdsson S, Wang C, Alm G, Syvanen AC, Ronnblom L, Barnes BJ. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:562–573. doi: 10.1002/art.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kis-Toth K, Tsokos GC. Dendritic cell function in lupus: Independent contributors or victims of aberrant immune regulation. Autoimmunity. 2010;43:121–130. doi: 10.3109/08916930903214041. [DOI] [PubMed] [Google Scholar]
- 48.Monrad S, Kaplan MJ. Dendritic cells and the immunopathogenesis of systemic lupus erythematosus. Immunol Res. 2007;37:135–145. doi: 10.1007/BF02685895. [DOI] [PubMed] [Google Scholar]
- 49.Chan VS, Nie YJ, Shen N, Yan S, Mok MY, Lau CS. Distinct roles of myeloid and plasmacytoid dendritic cells in systemic lupus erythematosus. Autoimmun Rev. 2012;11:890–897. doi: 10.1016/j.autrev.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Berkun Y, Verbovetski I, Ben-Ami A, Paran D, Caspi D, Krispin A, Trahtemberg U, Gill O, Naparstek Y, Mevorach D. Altered dendritic cells with tolerizing phenotype in patients with systemic lupus erythematosus. Eur J Immunol. 2008;38:2896–2904. doi: 10.1002/eji.200838342. [DOI] [PubMed] [Google Scholar]
- 51.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:1087–1095. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 52.Sang A, Yin Y, Zheng YY, Morel L. Animal models of molecular pathology systemic lupus erythematosus. Progress in molecular biology and translational science. 2012;105:321–370. doi: 10.1016/B978-0-12-394596-9.00010-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.