Abstract
G-protein coupled receptors (GPCRs) represent the largest family of cell surface molecules involved in signal transduction. Surprisingly, open reading frames for multiple GPCRs were hijacked in the process of co-evolution between herpesviridae family viruses and their human and mammalian hosts. Virally encoded GPCRs (vGPCRs) evolved as parts of viral genomes, which allowed harnessing the power of host GPCR signaling circuitries to ensure viral replicative success. Although vGPCRs are phylogenetically related to human chemokine receptors, they feature a number of unique characteristics. Here, we describe the molecular mechanisms underlying vGPCR-mediated viral pathogenesis which include constitutive activity, aberrant coupling to human G-proteins and β-arrestins, binding and activation by human chemokines, and dimerization with human GPCRs expressed in infected cells. The likely structural basis for these molecular events is described for the two closest viral homologs of human GPCRs. This information can be exploited for developing novel targeted therapeutic strategies against viral diseases.
Keywords: Signal Transduction, Structure, Viral-Associated Malignancies, Chemokine Receptors, Constitutive Activity, Human Viruses
The G Protein-Coupled Receptor (GPCR) Signaling System
Approximately 2% of all genes in the human genome encode G protein-coupled receptors (GPCRs), which represent the largest family of cell-surface molecules involved in signal transduction. Members of this receptor family play a central role in many key physiological functions, and the dysregulated activity of GPCRs and downstream signaling circuitries contribute to some of the most prevalent human diseases. Indeed, GPCRs represent the direct or indirect target of 50–60% of all available medicines in the market (1; 2). These receptors share the presence of seven transmembrane spanning regions. Upon ligand binding, GPCRs transduce extracellular signals primarily by stimulating heterotrimeric G proteins that are composed of α subunits and βγ dimers and localized in the inner leaflet of the plasma membrane (1). The 16 human G protein α subunit can be divided into four subfamilies: Gαs (Gαs and Gαolf), Gαi (Gαt, Gαgust, Gαi1–3, Gαo, and Gαz), Gαq (Gαq, Gα11, Gα14, and Gα15/16) and Gα12 (Gα12 and Gα13), while 5 β subunits and 12 γ subunits can form multiple distinct functional βγ dimers (3). Agonist-activated GPCRs act as guanine nucleotide exchange factors (GEFs) for G proteins, catalyzing the dissociation of GDP bound to the α subunit and its replacement for GTP. Both GTP-Gα subunits and Gβγ subunits can then promote the activation of large variety of downstream effectors, thus initiating an intricate network of signaling events that is highly dependent on the G protein-coupling specificity of each receptor.
Virally Encoded GPCRs
Viral genomes have evolved to exploit an extraordinary repertoire of tools designed to ensure successful infectivity and propagation. Thus, it is not surprising that GPCRs have emerged as critical players in viral entry, modulation of the host immune response, and promotion of host cell proliferation or death (4). All ultimately function to ensure viral replicative success and often contribute to viral pathogenesis. Surprisingly, many human viruses harbor open reading frames encoding GPCRs in their viral genomes, hence allowing them to harness the power of GPCR signaling in their host cells and organisms.
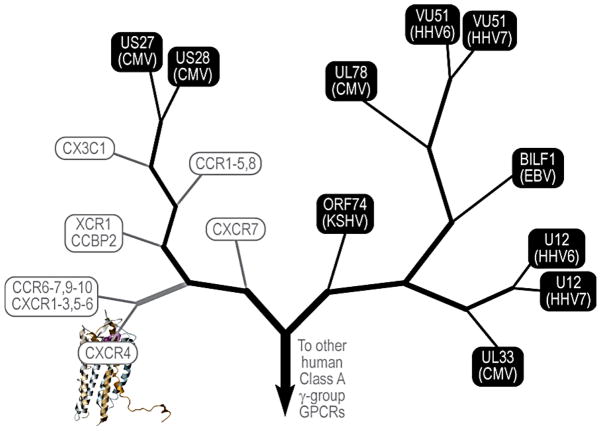
Most viral GPCRs (vGPCRs) have been hijacked and evolved from the comparatively small family of chemokine receptors (Figure 1). The family of human herpesviruses alone encodes multiple vGPCRs. The human herpesvirus-4 (HHV-4), also known as Epstein-Barr virus (EBV), encodes one receptor termed BILF1, while the human cytomegalovirus (HCMV/HHV-5) expresses multiple vGPCRs, including US28, US27, UL33 and UL78. Herpesvirus-6 (HHV-6) and its close relative Herpesvirus-7 (HHV-7) each encode two vGPCRs, U12 and U51, while the Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) or Herpesvirus-8 (HHV-8), encodes a single receptor termed ORF74 or more commonly KSHV vGPCR. Non-human viruses, including Herpesviruses and Poxvirus family members, also express open reading frames for vGPCRs, most of which are highly related to chemokine receptors. Among them, the most widely studied is the murine herpesvirus-68 (MHV-68), which encodes a vGPCR highly related to the KSHV vGPCR, and hence has been often used to investigate the role of ORF74 in viral replication in vivo (5). In this line, the murine cytomegalovirus (MCMV) encodes a vGPCR termed M33 that is highly related to US28, and therefore has served as an excellent model system to study how M33 deletion or replacement with signaling compromised vGPCRs, mimicking US28, affect viral replication in vivo (6). Among the poxviruses, the swinepoxvirus expresses a chemokine receptor-related vGPCRs, termed K2R open reading frame or C3L, the capripoxvirus encodes two vGPCRs, the Q2/3L open reading frame and LSDV01, and the avipoxvirus FPV encodes more than 4 vGPCRs. While we will not review the function of non-human vGPCRs, the widespread retention of open reading frames for GPCRs in the genome of many animal viruses reflects the likely advantage conferred by vGPCRs in viral replication and dissemination.
Figure 1. Phylogenetic tree of herpesviridae-encoded G-protein coupled receptors in context of closest homology human GPCRs.
vGPCRs are distantly related to chemokine receptors and share many of the human chemokine ligands. The two viral receptors with closest homology to human GPCRs are KSHV vGPCR, or ORF74 (26% sequence identity to CXCR2) and CMV US28 (36% sequence identity to CX3CR1). This level of homology enables the construction of reliable 3D models for KSHV vGPCR and CMV US28 based on the X-ray structure of human chemokine receptor CXCR4 (129; 130). For all other vGPCRs, the farther phylogenetic distance to human GPCRs allows tentative conclusions about general principles of activation and ligand binding preferences but not accurate homology modeling.
KSHV GPCR (vGPCR)
vGPCR and its role in Kaposi’s sarcoma
Kaposi’s sarcoma (KS) is a vascular neoplasm invariably associated with infection with the (KS)-human herpesvirus (KSHV/HHV8) and increased prevalence in immunosuppressed patients, including HIV-infected individuals (7; 8). The driving force of the KS lesion is the KSHV-infected spindle-shaped tumor (spindle) cell, which is thought to have a vascular endothelial or endothelial precursor origin. KS tumors are also characterized by infiltrating inflammatory cells, aberrant blood vessels, and extravasated erythrocytes (8; 9). The recruitment of these tumor stromal cells and the promotion of the angiogenic phenotype in KS lesions are thought to be mediated by elevated levels of pro-inflammatory and pro-angiogenic secretions (cytokines, chemokines and growth factors) released by the KS spindle cells. Indeed, KS is often thought to result from reactive endothelial hyperproliferation induced by the chronic release of these inflammatory molecules and has served as a model for tumor- and inflammation-induced angiogenesis (8; 10).
KSHV/HHV8 is a gamma-2-herpesvirus with close homology to Herpesvirus saimiri and Epstein-Barr virus. It was first isolated from human AIDS-KS lesions by Yuan Chang and Patrick Moore (11) and later found to be associated with all four forms of KS (classic, AIDS-associated, endemic (African) and iatrogenic) (10; 12). In addition, KSHV has also been identified as the etiological agent for two lymphoproliferative disorders: primary effusion lymphomas (PELs) and multicentric Castleman’s disease (13).
Similar to other members of the herpesvirus family, KSHV remains in latency in the majority of infected cells, with gene expression limited to a few viral genes that maintain the latent state (12). A small percentage of KSHV-infected cells spontaneously exit latency to enter the lytic cycle, which is accompanied by the expression of viral replicative and structural genes, resulting in the lysis of the host cell and the release of a new progeny of infective virions. The KSHV genome contains more than 80 open reading frames, including those genes required for viral replication and assembly (14). Unlike most tumor viruses, KSHV encodes over a dozen homologues to mammalian proteins likely pirated by KSHV from its cellular host. These genes have be shown to play an important role in regulating host cell immune function, enhancing host cell survival and proliferation, inducing cytokine dysregulation or promoting angiogenesis (8). Among the KSHV proteins that contribute to cytokine upregulation in KS, the virally encoded GPCR (vGPCR) (ORF74) is unique in that it is both transforming and pro-angiogenic (15).
Molecular determinants of KSHV vGPCR constitutive activity
The KSHV vGPCR is highly related to the CXC family of chemokine receptors— in particular, to the CXCR1 and CXCR2 receptors for interleukin-8 (IL-8/CXCL8) (16; 17) — but in addition to being activated upon agonist binding, it shows ligand-independent constitutive activity in a variety of cell-based biochemical assays. This constitutive activity is due to number of unique structural characteristics that make KSHV vGPCR very distinct from other chemokine receptors (Figure 2). KSHV vGPCR features a network of residue substitutions at positions that are highly conserved in other GPCRs and that are believed to be involved in stabilization of the inactive state and in agonist-induced conformational transition from the inactive to the active state. Amazingly, while many individual substitutions are rather dramatic and likely to destabilize the protein and interfere with its proper folding, pairs or larger groups of substitutions observed in KSHV vGPCR appear to have a compensatory effect on one another. Consequently, the general stability and folding of KSHV vGPCR are not compromised; instead, the basal conformational equilibrium of this GPCR is strongly shifted towards the active state, hence resulting in constitutive activity and efficient coupling to multiple human G-proteins.
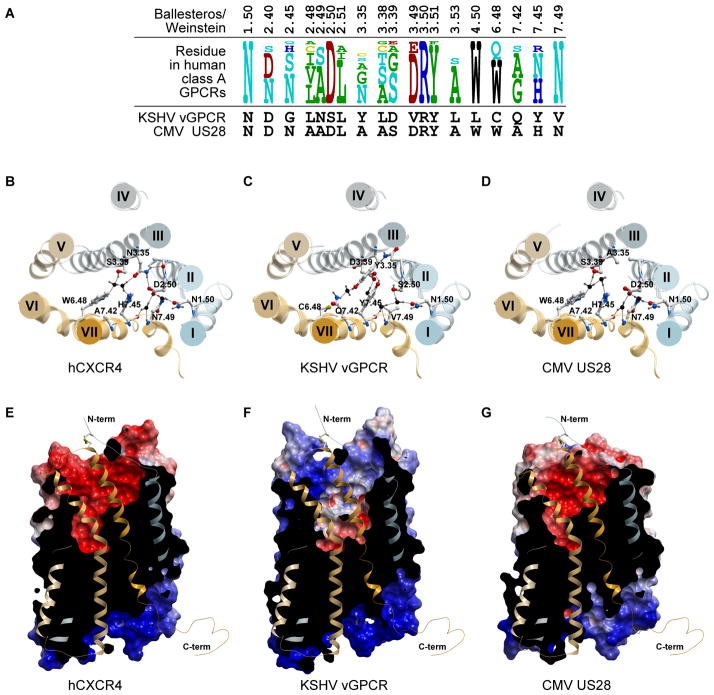
Figure 2. Molecular modeling elucidates the determinants of constitutive activity and ligand binding in the two vGPCRs with the highest level of homology to human chemokine receptors.
(A) Residue comparison in key positions between CMV US28, KSHV vGPCR, and human class A GPCRs. The key residues that are believed to be involved in active-inactive transition are conserved between US28 and human GPCRs, but not in KSHV vGPCR. (B–D) The structure of human chemokine receptor CXCR4 (B) and the 3D models of the two virus-encoded GPCRs, KSHV vGPCR (C) and US28 (D). The proteins are viewed from the extracellular side perpendicular to the membrane plane. (C) The non-conservative residue substitutions lead to gain in interhelical contacts in the extracellular parts of TMs II, III, and VII of KSHV vGPCR with concurrent loss of some important interactions in the intracellular part of these helices (e.g. hydrogen bonding of N7.49V) and in TM VI (e.g.W6.48C). Together, these substitutions result in high level of constitutive activity of KSHV vGPCR. (D) The determinants of activation of US28 highly resemble those of most human GPCRs with the exception of A114 in position 3.35 (Ballesteros-Weinstein notation). (E–G) The overall shape of the binding pockets and the electrostatic properties of CXCR4 (E), KSHV vGPCR (F) and US28 (G). The receptors are shown in lateral view and identical orientation with the front of the skin clipped for clarity. The skins are colored by electrostatic properties (red – negatively charged, blue – positively charged). The models were constructed and analyzed using the ICM molecular modeling package (131; 132)
The substitutions in KSHV vGPCR that stand out as having structural impact include the triple of 2.50, 3.39, and 7.49 (18) linking transmembrane helices (TM)2, TM3, and TM7 via a network of hydrogen bonds and found in most receptors as DSN, DGN, DCN, or DEN, is SDV (S93, D132, and V310) in KSHV vGPCR (Figure 2). This “swap” disrupts the water-mediated hydrogen bonding network and results in the activated conformation of TM7.
Another pair of residues that typically mediates the interhelical hydrogen bonding interactions is located at positions 3.35 (N in 29% of receptors, G, S, C in others) and 7.45 (N in 67% of receptors, H or S in the remaining ones). Both these highly conserved residues are substituted by Y in KSHV vGPCR: Y128 and Y306 (Figure 2). These two bulky aromatic residues greatly modify the nature and the strength of the interhelical interaction which, in KSHV vGPCR, acquires a hydrophobic packing component in addition to the hydrogen bond functionality that is preserved in the phenol groups. The role of a Tyr at position 3.35 is further corroborated by the fact that similar mutations in other GPCRs render them constitutively active (19).
Residue 6.48 which is a W or a Q in most class A GPCRs, and which participates in both stabilization of the inactive state via water-mediated hydrogen bonding network connecting it to D2.50 and in activation transition via change in the steric environment, is substituted with a Cys in KSHV vGPCR. This substitution is partially compensated by the unusual residue at position 7.42: while in the majority of receptors, 7.42 is small (G, A, S, or C), KSHV vGPCR instead has Gln303 which not only partially fills the empty space created by [WQ]6.48C substitution but also makes up for its hydrogen bonding capabilities. However, this interaction does not stabilize the inactive state of TM6; instead, it restricts the rotation of TM7 likely stabilizing it into its activated state.
Finally, basic residues are present in multiple positions at the intracellular end of TM6 (6.30–6.34, 246-RRKVR-250) as well as the intracellular ends of TM3 and TM4 (3.50, 4.37–4.38, R143, 156-KK-157), however, the one and only negatively charged counter-ion in the region is eliminated in KSHV vGPCR by the [ED]3.49V substitution in the DRY motif (V142). Although this feature may have a profound effect on the basal equilibrium, it is not clear whether it preferentially stabilizes active vs inactive state or vice versa; alternatively, it may simply make the G-protein binding more favorable. Introducing a V142D mutation in ORF74 results in ~70% increase in constitutive activity of KSHV vGPCR while a double D83A/V142D mutation makes it 510% more active in signaling assays based on the activation of phospholipase (20). Concurrent with [ED]3.49V substitution, KSHV vGPCR also has a L146 in position 3.53 which is bulkier than commonly occurring A,S,C, and V and is likely to contribute to either the conformational equilibrium or the G-protein binding.
This detailed analysis of the remarkable amino acid substitutions in KSHV vGPCR with respect to highly conserved residues in the GPCRs family suggest that this virally-encoded receptor has evolved to gain multiple molecular changes enhancing its basal activity and G-protein coupling efficiency even in the absence of any bound ligand. While these predictions based on the structural features of KSHV vGPCR warrant further investigation, multiple studies have defined the role of individual receptor residues in chemokine binding and signaling activity, which can guide the search for clinically relevant KSHV vGPCR allosteric modulators or inhibitors halting its constitutive activity and transforming potential (20–24).
Pharmacology of KSHV-vGPCR
The KSHV vGPCR exhibits a potent, ligand-independent constitutive activity. However, certain chemokines can interact with KSHV vGPCR and modulate its function, an event that is dependent on posttranslational modifications by sulfate groups of tyrosine residues within vGPCR N-terminal extracellular domain (25). For example, Groα/CXCL1 and Groγ/CXCL3 can further activate vGPCR and act as full agonists, whereas others, such as CXCL8/IL-8 are neutral ligands or low potency agonists, Groβ/CXCL2 acts as a partial agonist, and inducible protein-10 (IP-10/CXCL10) and SDF-1α/CXCL12, can inhibit KSHV vGPCR signaling and act as reverse agonists (26; 27). KSHV also encodes a chemokine ligand, vMIP-II, which inhibits signaling by KSHV vGPCR, thereby providing the virus with a control mechanism to modulate the activity of this receptor (27; 28). In general, KSHV vGPCR prefers neutral or slightly acidic chemokines, consistent with the charge distribution in its binding pocket (Figure 2F). The high level of chemokine promiscuity distinguishes KSHV vGPCR from CXCR1, CXCR2, CXCR4 and other human chemokine receptors, as the latter typically bind only a restricted number of ligands.
vGPCR as the KSHV tumor-initiating oncogene
Compelling evidence supports an essential role for vGPCR in the initiation and progression of KS in vivo. Expression of vGPCR generates vascular tumors remarkably similar to human KS when expressed in mice using an endothelial-specific (TIE2-tva) gene transfer system (29). Three other vGPCR transgenic models as well as an animal model of virally induced KS also support a role for vGPCR in Kaposi’s sarcomagenesis (30–32; 33. Similar to human KS, analysis of murine vGPCR tumors reveals vGPCR expression only in a few tumor cells and upregulation of a large number of inflammatory and angiogenic molecules (cytokines, chemokines and growth factors), suggestive of a paracrine mechanism for vGPCR oncogenesis {Montaner, 2003 #23). Similarly, conditional transgenic expression of vGPCR has revealed the key role of continued vGPCR expression for the progression of the KS-like phenotype (32). Together, these findings implicate vGPCR as a critical element in KS pathogenesis and suggest that strategies to block its function may represent a novel approach for the treatment of KS.
A complex signaling network underlies the potent sarcomagenic potential of KSHV vGPCR
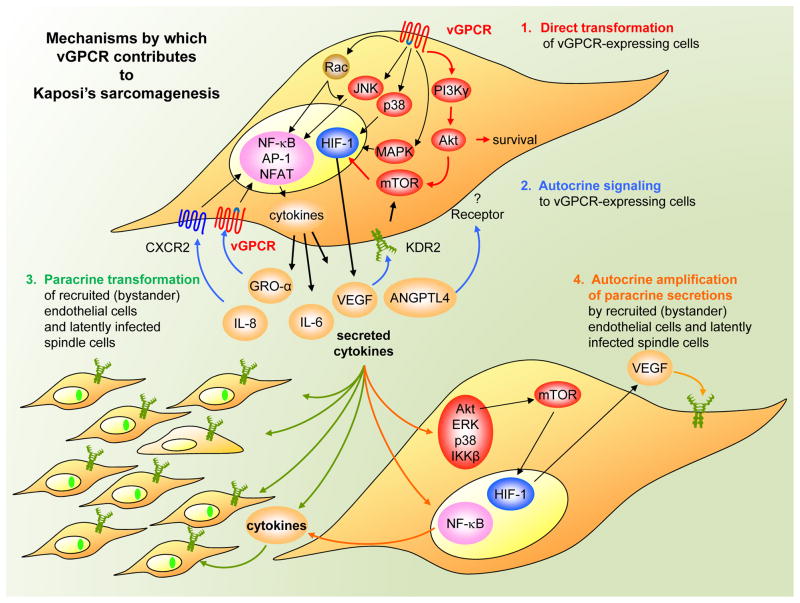
vGPCR enhances the activity of a complex signaling network, the contribution of which to the oncogenic and pro-angiogenic activities of this receptor is beginning to be unraveled (Figure 3). For example, vGPCR potently stimulates the PI3K/AKT pathway in endothelial cells, which thereby protects them from apoptosis (34). Therefore, vGPCR might use this pathway to promote the survival of KSHV-infected endothelial cells. Interestingly, vGPCR can also activate AKT in an autocrine manner by upregulating the expression of the vascular endothelial growth factor (VEGF) receptor KDR2 (35) and by promoting the concomitant release of VEGF (15; 35), which then signals through the VEGF receptor to activate AKT. vGPCR-expressing endothelial cells can then induce activity of that kinase in neighboring endothelial cells in vivo through the release of VEGF and other cytokines and chemokines by a paracrine mechanism (36). Of interest, direct and paracrine AKT activation of the TSC2/mTOR pathway has been shown to be necessary and sufficient for vGPCR oncogenesis (37). Although the different downstream mechanisms by which vGPCR-mediated mTOR drives vGPCR sarcomagenesis are still unclear, one includes the dramatic amplification of VEGF secretion, through the activation by vGPCR cytokines of multiple kinases including AKT, ERK, p38 and IKKβ, all of which can ultimately converge in TSC/mTOR activation and hypoxia-inducible factor (HIF) upregulation in (neighboring) non-vGPCR expressing cells (38). Another novel pro-angiogenic and pro-tumorigenic factor highly upregulated through vGPCR direct and paracrine HIF activation is Angiopoietin-like 4, a novel factor recently found to be involved in tumor progression and tumor metastasis through the regulation of endothelial cell-cell junctions (39; 40). ANGPTL4 has been shown to be highly up-regulated in vGPCR murine tumors and human KS lesions and to have equivalent importance as VEGF in the regulation of angiogenesis and vascular permeability in KS (39; 40). Collectively, these data demonstrate that drugs targeting proteins of the AKT/mTOR/HIF axis or HIF downstream targets may represent an effective mechanism-based therapy for the treatment of patients with KS (37; 38; 41–44). In addition, PI3Kγ, a PI3K isoform with restricted tissue distribution, has also been strictly required for signaling from the KSHV-encoded GPCR oncogene to AKT/mTOR, suggesting that PI3Kγ may represent an alternative molecular target for therapeutic intervention in KS (45).
Figure 3.
ORF74 of KSHV encodes a vGPCR which utilizes multiple diverse but interdependent strategies to promote dysregulated angiogenesis and endothelial cell transformation of both expressing and recruited (bystander) endothelial cells, thereby promoting Kaposi’s sarcomagenesis. See text for details.
The transforming, pro-survival and angiogenic effects of vGPCR are also highly dependent on its ability to stimulate MAPKs and, consequently, the activity of transcription factors that are regulated by these kinases. vGPCR induction of VEGF expression is dependent on ERK and p38, which phosphorylate HIF1α, thereby increasing transcription from a HIF-responsive element within the VEGF promoter (46). vGPCR can also activate the AP-1, NF-κB, CREB and NFAT transcription factors (15; 34; 47–50). AP-1 and NF-κB can stimulate the expression of pro-inflammatory cytokines such as IL-1β, IL-2, IL-4, IL-6, TNFα, CCL3/MIP-1 and IL-8/CXCL8, as well as bFGF — all of which are important mediators in KS (8). Interestingly, vGPCR stimulates an NFκB gene expression signature in endothelial cells expressing vGPCR or non-expressing cells exposed to vGPCR secretions (51). These events may help propagate a vGPCR-initiated paracrine signaling network to the surrounding and even distant endothelial cells, thereby promoting their unrestricted growth. Furthermore, as small guanosine triphosphate (GTP)-binding proteins represent critical links between GPCRs and nuclear transcription factors, the involvement of members of the Rho family of small guanosine triphosphatases (GTPases) in vGPCR-induced transcriptional regulation has been investigated. These experiments have revealed that vGPCR potently activates the small G protein, Rac1 (47). Preventing the activation of Rac1 by vGPCR blocks the stimulation of the transcriptional activity of NF-kB, AP-1, and NFAT, resulting in the inhibition of cytokine secretion in vitro and vGPCR sarcomagenesis in vivo (47). Moreover, expression of activated Rac1 is sufficient to generate tumors in mice with a phenotype similar to KS (47; 52). More recently, an animal model with endothelial cell-specific deletion of Rac1 has demonstrated an essential role for this GTPase in endothelial cell function and vascular development, corroborating a role for Rac1-mediated pathways in aberrant neovascularization (53).
vGPCR and other KSHV-related malignancies
vGPCR might have a distinct role in two other KSHV-associated malignancies — primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD), two rare B-cell lymphomas with increased incidence in AIDS patients (8; 13). The gene-expression profile of vGPCR-expressing B cells is quite different from that observed in endothelial cells, suggesting that signaling by this receptor may make different contributions to the pathogenesis of KSHV-related endothelial and lymphoproliferative lesions (54). Still, in PEL-derived cell lines, vGPCR also activates ERK and p38, and the transcription factors AP-1, NF-κB, CREB, and NFAT (55). The activation of AP-1 and CREB is mediated cooperatively by the Gαq–ERK and the Gαi–PI3K signalling pathways. However, unlike in other cell types, NF-κB activation by vGPCR seems not to be mediated by Gαi or by PI3K–AKT/PKB (56), but might require Rac1 (47). Conversely, it has also been shown that NFAT activation in lymphocytes requires signaling through the PI3K–AKT/PKB pathway to glycogen synthase kinase-3 (GSK3) and results in increased expression of NFAT-dependent cell-surface molecules (such as CD25, CD29 and the FAS ligand), pro-inflammatory cytokines (such as IL-2 and IL-4), and proangiogenic factors (such as granulocyte/macrophage colony-stimulating factor (GM-CSF) and TNFα (57). vGPCR might therefore upregulate the expression of potent lymphocyte chemoattractants and mitogens that can promote B-cell recruitment and proliferation in KSHV-induced lymphoproliferative disorders. In addition to promoting the secretion of VEGF, vGPCR also augments the expression of another KSHV gene product, vIL-6, in KSHV-infected PEL cells (55). This IL-6 homologue shows sequence similarity (25% amino-acid identity) to human IL-6, a cytokine that enhances B-cell survival and proliferation (8). Similar to human IL-6, vIL-6 upregulates intracellular signaling pathways by stimulating a transmembrane protein co-receptor, gp130, which is also shared by other cytokines. However, whereas human IL-6 requires both its specific receptor, gp80, and the gp130 co-receptor, vIL-6 bypasses the requirement for gp80 by binding directly to the gp130 subunit (7; 10; 58). In both cases, activation of gp130 stimulates Janus kinase (JAK), which phosphorylates transcription factors of the signal transducer and activator of transcription (STAT) family (59). PEL-derived cell lines are dependent on this viral cytokine for proliferation and survival, which implies that vGPCR might promote the development of PEL and MCD indirectly through the upregulation of vIL-6. vIL-6 does not seem to have a significant role in Kaposi’s sarcoma, so the complex interplay among viral and host-cell genes probably contributes to the diversity and complexity of KSHV-associated diseases.
HCMV vGPCRs
Human cytomegalovirus
Human cytomegalovirus (HCMV) is a widespread herpesvirus, as reflected by the presence of antibodies against HCMV proteins in 50–95% of the population (60). This virus causes asymptomatic or subclinical infections in most immunocompetent subjects. However, HCMV infection can cause severe manifestations in immunocompromised individuals, such as pneumonitis or obliterative bronchiolitis. HCMV also remains as the leading cause of congenital viral infection, as it can be transmitted during pregnancy from the mother with primary (and also recurrent) infection to the fetus (61). HCMV infection has been linked to glioma pathogenesis (62), and is often detected in arterial tissues in severe atherosclerotic patients where it is believed to promote arterial smooth muscle cell focal proliferation (63). Among the HCMV-encoded proteins, four GPCRs — US27, US28, UL33 and UL78 — stand out as likely candidates for involvement in HCMV-induced pathogenesis (64).
US28
Pharmacology of US28
US28 is a homolog of CCR1, CCR2 and CX3CR1 that was identified as a promiscuous calcium-mobilizing beta-chemokine receptor for chemokines such as CCL5/RANTES, macrophage inflammatory protein-1 α (MIP-α), macrophage inflammatory protein-1 β (MIP1-β), monocyte chemoattractant protein-1 (MCP-1) and CX3CL1/Fractalkine (65; 66). Its binding pocket indeed closely resembles those of several human chemokine receptors by both shape and residue property distribution (Figure 2G). Furthermore, recent efforts have shown that even though US28 is a constitutively active GPCR, this vGPCRs is druggable, as it can be inhibited by small nonpeptidergic molecules, such as VUF2274, which behaves as a full inverse agonist for US28 (67). This molecule also inhibits 60% of the US28-mediated HIV entry. These initial studies sparked the search for new pharmacological compounds targeting vGPCRs, leading to the synthesis and pharmacological characterization of many additional novel US28 inhibitors, which hold promise for future potential clinical applications (68; 69).
Molecular determinants of US28 vGPCR constitutive activity
Unlike KSHV vGPCR, US28 represents a fairly “classical” G-protein coupled receptor in which the determinants of activation are fully conserved (Figure 2A). This, however, does not preclude it from being constitutively active similarly to many human GPCRs for which the phenomena of basal ligand-independent activity and inverse agonism have been described (70). A possible reason for the constitutive activity of US28 is the small hydrophobic residue (A114) in position 3.35 of the transmembrane helix TM3 that does not support the sufficient TM2/TM3/TM7 interhelical interactions (Figure 2C). While the preferred residue at this position is N3.35, Ala or Val are observed in several human GPCRs with documented constitutive activity, including muscarinic M2 and M3 receptors and histamine receptor H1. A N119A mutation at the identical position of CXCR4 renders this normally non-constitutively active receptor noticeable basal agonist-independent activity (71); similar result is observed for AT1A angiotensin II receptor (72) and α1B adrenergic receptor (19). Another residue that may correlate with constitutive activity is A77 in position 2.48 (normally a larger hydrophobic residue). Although facing the lipid bilayer, this residue is known to affect the basal signaling in some human receptors (73) as well as in KSHV vGPCR (ORF74) (23). Additional mutagenesis studies will be necessary to confirm these predictions,
Signaling capacity of US28
US28 is a typical example of a constitutively active receptor (74; 75). The surface expression and β-arrestin-dependent endocytosis of US28 is regulated by constitutive receptor phosphorylation in C-terminal serine and threonine residues (76–78). The deletion of the US28 C terminus results in reduced constitutive endocytosis and consequently enhanced signaling capacity. The receptor activity can be further enhanced by certain CC chemokines, including RANTES and MCP-1. Because of its high affinity, US28 can modify the chemokine environment of infected cells through intense sequestering of those chemokines, downregulating their extracellular levels, thereby facilitating immune evasion at sites of infection and contributing to the latent presence of the virus (79–81).
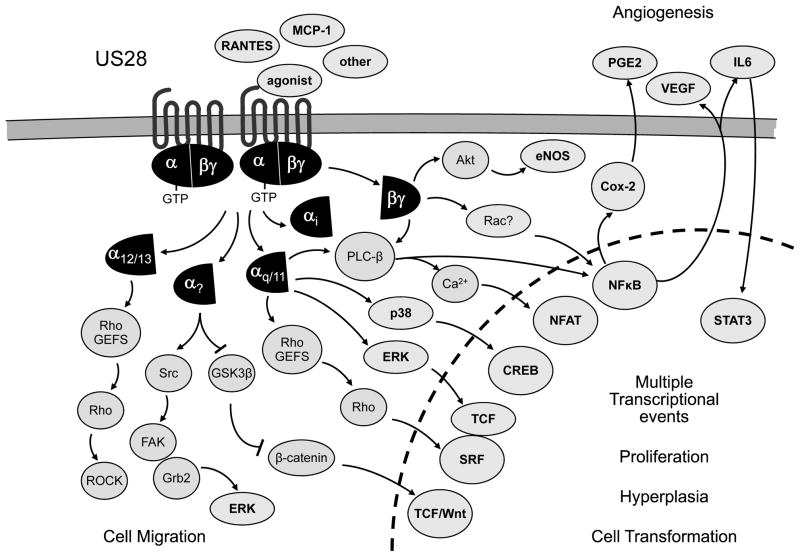
Similar to KSHV vGPCR, US28 has been shown to activate numerous signaling cascades (Figure 4). US28 activates NF-κB through βγ dimers that are released from Gαq/11 and activates CREB through p38 (75) — which indicates that US28 might regulate various transcription factors through G-protein-initiated signaling routes that control MAPKs. Other transcription factors activated by US28 are NFAT (82) and SRF (83). Activation of SRF is mediated by Gαq proteins likely acting though RhoA. However, the stimulatory effect of US28 and Gαq appears to be independent on phospholipase C-βbeta (PLCβ) activation and is sensitive to inhibition by wild-type Gα16, which may compete for Gαq binding uncoupling it from US28 (83). US28 appears to display a more promiscuous coupling selectivity, as cellular migration is increased by the expression of Gα12/13 proteins, which may enhance the ability of US28 to activate RhoA/p160ROCK, an event that is dependent on US28 stimulation with CCL5/RANTES (84).
Figure 4. The US28 CMV vGPCR utilized a complex intracellular network to promote angiogenesis, migration, and aberrant cell growth.
The US28 CMV vGPCR utilized a complex intracellular network to promote angiogenesis, migration, and aberrant cell growth. US28 acts as a constitutively active GPCR activating multiple signaling pathways, either in the absence of agonist binding or when bound to its ligands. See text for details.
Signaling capacity of US28 in cell migration
US28 promotes the migration of infected cells towards CC-chemokine-secreting tissues, thereby assisting virus dissemination. Interestingly, US28-mediated cell migration provides a molecular basis for the correlative evidence that links HCMV to the acceleration of vascular disease (85). Enhanced cellular migration is observed in HCMV-infected primary arterial SMCs but not in SMCs that have been infected by mutants of HCMV that lack US28-although this latter effect can be rescued by expression of US28, but not a cellular homologue. US28 has been shown to signal through Src and focal adhesion kinase (FAK) and that this activity is necessary for US28-mediated SMC migration (86). In the presence of CCL5/RANTES, US28 stimulates the production of a FAK-Src kinase complex. Interestingly, Src co-immunoprecipitates with US28 in a ligand-dependent manner. This association occurs earlier than the formation of the FAK-Src kinase complex, suggesting a hierarchy in which US28 activates Src before FAK. US28 binding to CCL5/RANTES also promotes the formation of a Grb2-FAK complex, which is sensitive to treatment with the Src inhibitor PP2, further highlighting the critical role of Src in US28 activation of FAK. Collectively, these findings demonstrate that activation of FAK and Src plays a critical role in US28-mediated signaling and SMC migration. This pathway may contribute to the resulting infiltration of SMCs into the innermost membrane of the vessel, which can then cause the narrowing of the large blood vessels and hence contributed to vascular pathologies.
A potential role for US28 signaling in tumorigenesis
Several studies implicate the role of US28 in tumorigenesis. For example, US28 expression induces a proangiogenic and transformed phenotype by up-regulating the expression of VEGF and enhancing cell growth and cell cycle progression (87). Constitutive activity is important for tumor development as cells expressing an uncoupled constitutively inactive mutant of US28 exhibit a delayed and attenuated tumor formation in nude mice compared to US28-expressing cells. Recent data obtained by microarray analysis suggests an important role for US28-induced upregulation of COX-2 through NFκB in the production of VEGF (87). Indeed, pharmacological inhibition of COX-2 with Celecoxib can inhibit the angiogenic activity and tumorigenesis of US28-transfected NIH-3T3 cells fibroblasts, suggesting that the development of HCMV-related proliferative diseases may involve the activation of COX-2 by US28 and the consequent production of lipid inflammatory mediators (87).
US28 has also been found to induce an invasive and angiogenic phenotype in glioblastoma multiforme (GBM), a tumor often associated with HCMV infections (88; 89). In either uninfected GBM cells or neural progenitor cells, thought to be the GBM precursor cells, HCMV infection or US28 overexpression was sufficient to promote secretion of biologically active VEGF and to activate multiple cellular kinases that promote glioma growth and invasion, including phosphorylated STAT3 (p-STAT3) and endothelial nitric oxide synthase (e-NOS). Consistent with these findings, US28 overexpression increased primary GBM cell invasion in Matrigel assays. Notably, this invasive phenotype was further enhanced by exposure to CCL5/RANTES, and associated with poor patient outcome in GBM. Conversely, RNA interference-mediated knockdown of US28 in human glioma cells persistently infected with HCMV leads to an inhibition in VEGF expression and glioma cell invasion in response to CCL5/RANTES stimulation. US28 colocalizes in situ in GBM with several markers of angiogenesis and inflammation, including VEGF, p-STAT3, COX2, and e-NOS. The emerging picture from these studies and other recent findings (90) suggest that US28 expression from the HCMV genome could contribute to GBM pathogenesis by inducing an invasive, angiogenic phenotype. This possibility requires further clinical evaluation, as targeting US28 may provide therapeutic benefits in some GBM patients. Other studies also suggests that US28 mediates proliferative signaling through the activation of the IL-6-STAT3 axis (91), primarily by promoting the release of IL-6 through the activation of NFκB in model cellular systems as well as in HCMV-infected cells. In particular for glioblastoma, patient tumor specimens demonstrated colocalization of US28 and phosphorylated STAT3 in the vascular niche of these tumors, suggesting that US28 may help establish a positive feedback loop through activation of the IL-6-STAT3 signaling axis that increase tumor growth.
Recent evidence underscores the potential role of US28 in tumor development (92), as transgenic mice in which US28 expression was targeted to intestinal epithelial cells (IECs) develop intestinal neoplasia, in vivo. US28 expression in IECs inhibited GSK-3β, promoting β-catenin accumulation, and increased expression of Wnt target genes involved in the control of the cell proliferation. These mice showed a hyperplastic intestinal epithelium and, strikingly, developed adenomas and adenocarcinomas. When exposed to an inflammation-driven tumor model (azoxymethane/dextran sodium sulfate), these mice develop a significantly higher tumor burden than control littermates. As transgenic co-expression of the US28 ligand CCL2 (an inflammatory chemokine) increased IEC proliferation and tumor burden, we can speculate that the oncogenic activity of US28 can be further modulated by locally produced inflammatory factors.
UL33
UL33 is a glycoprotein whose gene is conserved among all beta-herpesviruses. UL33 might not bind chemokines, but it can activate several signaling cascades in a ligand-independent manner, including phospholipase C (PLC) through Gαq proteins and partially through Gαi/o (67). In addition, UL33 constitutively upregulates CREB-mediated transcription by coupling to Gαi/o and Gαs, thereby controlling the intracellular levels of cAMP. UL33 was found to enhance CRE activation also through the Rho/p38 pathway, via Gβγ. Infection with an UL33-deficient variant of HCMV confirmed that HCMV-induced CRE activation is, at least in part, dependent on UL33 expression (67). Activation of CRE, in turn, might promote the expression of molecules that stimulate cell growth, such as cyclin D. It is tempting to speculate that HCMV US28 and UL33 might contribute to the observed transformation of SMCs in atherosclerosis by activating ERK- and p38-dependent proliferative signaling pathways. However, a definitive role for these pathways in HCMV-induced SMC proliferation remains unclear.
US27
HMCV US27 is a heterogeneously N-glycosylated receptor expressed during the late phase of replication that does not show constitutive activity (75; 93; 94). No cellular ligands have been identified either for this viral receptor, a fact that may be due to the presence in the C-terminal domain of the HCMV US27 protein of a di-leucine endocytic sorting motif that is both necessary and sufficient for intracellular localization {Stapleton, 2012 #328). Interestingly, recent data shows that US27, and also UL33 and UL78, can co-localize and heteromerize with US28 in vitro (95). Furthermore, it has been shown that HCMV mutants lacking pUS27 rely primarily on direct cell-to-cell spread, indicating that US27 may act at a late stage of the HCMV replication cycle to support efficient spread of virus by the extracellular route (96).
UL78
HMCV UL78 does not show any constitutive signaling activity either, but can also co-localize and heteromerize with US28 (75; 93; 95). Indeed, while the constitutive activation of the US28-mediated Gαq/phospholipase C pathway is not affected by receptor heteromerization, UL33 and UL78 are able to silence US28-mediated activation of the transcription factor NF-κB, suggesting that the interaction between receptors may have an important regulatory capacity on the function of US28 and as a consequence, may ultimately impact on HCMV viral life cycle (95). Conversely, another study shows that this receptor has been shown not to be essential for virus replication in fibroblasts or ex vivo-cultured sections of human renal arteries (97).
EBV BILF1
In 1958, Denis Burkitt first described a childhood B cells malignancy in Uganda, now known as Burkitt’s lymphoma, which he suspected to have a viral etiology closely associated with malaria (98). In 1965, Tony Epstein and Yvonne Barr identified herpesvirus particles by electron microscopy in Burkitt’s lymphoma cells. This virus was distinct from other previously described herpesviruses and was named Epstein-Barr virus (EBV) or human herpesvirus 4 (HHV-4) (99). Currently, we know that EBV is associated with infectious mononucleosis and multiple human malignancies, including nasopharyngeal carcinoma, gastric carcinoma, almost half of the cases of Hodgkin’s lymphoma, and B-cell lymphoma in immunocompromised patients (100). Like other herpesviruses, EBV has pirated and modified host genes encoding important regulatory cellular proteins to take over cellular control after infection. Although EBV was believed to be the only γ-herpesvirus that does not encode a chemokine receptor, an open reading frame within EBV genome encoding a constitutively active GPCR, BILF1, has been recently described (101; 102). This receptor has turned to be a glycosylated protein with expression predominantly during lytic infection and ability to block PKA or stimulate NFκB activity in a pertussis toxin-sensitive manner, implying Gi-dependent signaling (101).
Signaling capacity of BILF1
In spite of its recent identification, several functions have been already attributed to this viral receptor (Figure 5). BILF1 contributes to immune evasion during the establishment of EBV infection by physical associating with MHC class I molecules, promoting their endocytosis and lysosomal degradation (103). In addition, BILF1 has the ability of targeting newly synthesized MHC-I/peptide complexes en route to the cell surface (104). Both mechanisms lead to the inhibition CD8(+) T cell recognition of endogenous target antigens. A BILF1 mutant unable to activate G protein signaling pathways retains the ability to downregulate MHC class I, indicating that the immune-modulating and GPCR-signaling properties are two distinct functions of BILF1 (105). Of interest, BILF1 is the third EBV lytic gene (along with BGLF5 and BNLF2A) that interfere with MHC class I antigen processing, which indicates the importance for EBV to evade CD8+ T cell responses during the lytic replication cycle (103). While the BILF1 protein of the closely related CeHV15 gamma(1)-herpesvirus of the Rhesus Old World primate (80% amino acid sequence identity) downregulated surface MHC class I similarly to EBV BILF1, the KSHV vGPCR (the most closely herpesvirus-encoded GPCR related to EBV BILF1; 15% amino acid sequence identity) did not affect levels of surface MHC class I (105). In addition, BILF1 has been shown to block the phosphorylation of RNA-dependent protein kinase (PKR) (101). This event causes the inhibition of PKR function, which may help preventing mounting a cellular antiviral response, supporting then an efficient EBV infection.
Figure 5.

EBV BILF1 is a unique viral GPCR which functions to: A) regulate immune evasion by physical associating with MHC class I molecules and promoting their endocytosis and lysosomal degradation (top) or by directly targeting newly synthesized MHC-I/peptide complexes en route to the cell surface (bottom); B) inhibit CXCR4 signaling by forming hetero-oligomeric complexes with CXCR4, thereby inhibiting binding to CXCL12, or by scavenging Gαi proteins; and C) promote angiogenesis and cell transformation. See text for details.
BILF1 is still an orphan GPCR, as no direct ligands have yet been identified. Instead, recent evidence also suggests that BILF1 can heterodimerize with host chemokine receptors, thereby modifying their intracellular signaling (106). In particular, BILF1 can form hetero-oligomeric complexes with human CXCR4, inhibiting CXCL12 binding to CXCR4 and, consequently, CXCL12-induced signaling (107). BILF1-mediated CXCR4 inhibition is a consequence of its constitutive activity, suggesting a functional cross-regulation of GPCRs by BILF1 at the level of the G proteins (probably by scavenging Gαi proteins). Ultimately, impairment of CXCR4 function by interacting with BILF1 may control the responsiveness of B lymphocytes to chemokines, thereby altering homing and homeostasis of infected B lymphocytes, which might be essential for EBV dissemination and EBV-induced pathogenesis. In addition, a recent report shows that BILF1 exerts transforming potential in vitro and in vivo, an effect that depends on its constitutive activity (108). Interestingly, similarly to KSHV vGPCR, BILF1 expression induces VEGF secretion in a constitutively active manner. These data suggest that BILF1 could indeed play a direct role in the pathogenesis of EBV-associated malignancies, supporting the idea that inverse agonists for BILF1 could be relevant for the treatment of such cancers.
EBV infection of B cells also leads to the upregulated expression of endogenous cellular GPCR chemokine receptors including CCR6, CCR7 and CCR10, as well as an orphan GPCR termed EBI2 (109–112). In addition to activating JAK–STAT signaling (99R), induction of CCR7 activity by its endogenous ligands can stimulate AKT/PKB and ERK (113–115). Upregulated expression of CCR7 might therefore help to promote survival and proliferation of EBV-infected cells. CCR7 has also been implicated in lymphocyte migration through the activation of several Rho GTPases, including Rho, Rac and Cdc42 (116; 117), an event that may facilitate viral spread. Besides EBV, HHV6 and HHV7 can also upregulate CCR7 expression, indicating that CCR7 might have a similar role in the pathogenesis of several herpesviruses. Nonetheless, the potential contribution of upregulation of cellular GPCRs in herpesviral diseases requires further investigation.
HHV6 and HHV7 U12 and U51
Clinically, HHV6 (mostly variant B) has been recognized as the cause of exanthem subitum in infants and other febrile illnesses in young children (118; 119). Over 90% of the population is infected within the first 18 months of life (119; 120). While the infection by HHV-6 is indolent in most cases, it can give rise to an infectious-mononucleosis-like illness in some adults. Furthermore, in immunosuppressed patients HHV-6 reactivation is often associated with a worse outcome (HHV-6 reactivation occurs in 33–48% of immunocompromised patients undergoing hematopoietic stem cell transplantation (121). Clinical conditions associated with HHV-6 reactivation in this population include hepatitis, idiopathic pneumonitis, bone marrow suppression, and encephalitis (118). HHV7 has also been associated with the development of exanthem subitum and mononucleosis-like illness, although its link to human disease is less established.
Signaling and pharmacology of HHV6 and HHV7 vGPCRs
HHV6 and HHV7 each encode two GPCRs, which are known as open reading frames U12 and U51. HHV6 U12 shows the highest homology with CCR3 and is a promiscuous high-affinity CC-chemokine receptor, which increases intracellular Ca2+ concentrations through a pertussis-toxin-insensitive pathway (122). This receptor can bind the CC chemokines CCL2, CCL3, CCL4, and CCL5 (122). The HHV7 U12 gene also encodes a Ca2+-mobilizing receptor which responds to MIP-3β/CCL19, although this receptor may also bind the CC chemokines CCL17, CCL21, and CCL22 (123; 124). The role of these receptors and its downstream signaling events in viral disease is still obscure.
The HHV6 U51 chemokine receptor is quite different to other virally encoded GPCRs, as its primary sequence is closer to that of the opioid receptors than to chemokine receptors (27; 125). Yet, HHV-6 U51 is able to bind chemokines from the CC (CCL2, 5, 7, 11, 13, 19, and 22), CX3C (CX3CL1), and XC (XCL1) families, as well as the KSHV encoded chemokine vCXCL2/vMIPII (125; 126). In contrast, HHV-7 U51 has been only shown to bind chemokines from the CC family (CCL17, 19, 21, and 22) (124). HHV-6 U51 has been shown to have constitutive signaling activity via the activation of Gq and Gi proteins (127). However, the activity and coupling specificity of this receptor can be modulated upon chemokine binding. CCL5 increases the activation of both Gq and Gi initiated pathways, while CCL2 and CCL11 likely favor an active conformation of the HHV-6 encoded vGPCR that specifically couples to Gi proteins, hence diminishing its Gq coupling (127). These observations reflect nicely the emerging view that ligand binding can bias the signaling selectivity of GPCRs, likely depending on the access of the different G protein subunits to the conformation adopted by the GPCR bound to each of its ligands.
In epithelial cells, HHV-6 U51 expression may contribute to immune evasion by causing transcriptional down-regulation of the RANTES/CCL5 gene and the scavenging of RANTES/CCL5 protein (125). Regulation of RANTES levels may alter selective recruitment of circulating inflammatory cells that the virus can infect and thus could mediate the systemic spread of the virus from initial sites of infection; or alternatively, chemokine regulation could modulate a protective inflammatory response to aid the spread of virus (125). In addition, HHV-6 U51-induced downregulation of FOG-2, a hematopoietic transcriptional repressor involved in the development of Th1 and Th2 immune responses, which has been found by microarray analysis to be restricted to targets of infection and immunity in human leukocytes (126). Finally, stable expression of an shRNA for HHV-6 U51 in human T cells prior to infection with HHV-6 has been shown to reduce viral DNA replication (128).
Concluding Remarks
In the process of co-evolution between viruses and their human and mammalian hosts, the Herpesviridae family of viruses hijacked multiple GPCRs. Viruses have evolved to exploit these vGPCRs to their advantage, harnessing the extraordinary signaling capacity of GPCRs to redirect normal cellular programs to evade immunodetection and to carry out the replicative needs of the virus. The latter includes the increased motility, growth, and survival of the infected cells, which in turn may contribute to viral pathogenesis. The unique molecular features of vGPCRs allow them to integrate into and modify host signaling pathways by physically interacting with host chemokines and G-proteins as well as possibly oligomerizing with host GPCRs. The study of GPCRs and GPCR-initiated signaling circuitries in viral diseases has already afforded the opportunity to identify effective therapeutic options interfering with GPCR downstream targets for treating human viral-associated pathologies, such as Kaposi’s sarcoma. The recent breakthroughs in GPCR crystallography provided unprecedented knowledge of the structural determinants of GPCR ligand binding and G protein activation. We can expect that this knowledge will soon afford the opportunity to identify vGPCR allosteric modulators halting the constitutive activity of vGPCRs without affecting the normal function of host chemokine receptors. Ultimately, vGPCRs may represent excellent candidates for pharmacological intervention in a wide variety of human viral-associated diseases.
Acknowledgments
I.K. and R.A. were supported by the NIH grants U01 GM094612 and R01 GM071872; S.M. was supported by NIH grant R01 CA119911; JSG was supported by a National Institutes of Health Intramural AIDS Targeted Antiviral Program and the National Institute of Dental and Craniofacial Research. We thank Akrit Sodhi for his help and expert advice in every aspect of this review, and Daniel Martin and Tracy M. Handel for their insightful suggestions.
Bibliography
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–50. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Flower DR. Modelling G-protein-coupled receptors for drug design. Biochimica et biophysica acta. 1999;1422:207–34. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, et al. Insights into G protein structure, function, and regulation. Endocrine reviews. 2003;24:765–81. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 4.Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nature reviews. Molecular cell biology. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- 5.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annual review of immunology. 2011;29:351–97. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 6.Sherrill JD, Stropes MP, Schneider OD, Koch DE, Bittencourt FM, et al. Activation of intracellular signaling pathways by the murine cytomegalovirus G protein-coupled receptor M33 occurs via PLC-{beta}/PKC-dependent and -independent mechanisms. Journal of virology. 2009;83:8141–52. doi: 10.1128/JVI.02116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenner RG, Boshoff C. The molecular pathology of Kaposi’s sarcoma-associated herpesvirus. Biochim Biophys Acta. 2002;1602:1–22. doi: 10.1016/s0304-419x(01)00040-3. [DOI] [PubMed] [Google Scholar]
- 8.Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nature reviews. Cancer. 2010;10:707–19. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganem D. Human herpesvirus 8 and its role in the genesis of Kaposi’s sarcoma. Current clinical topics in infectious diseases. 1998;18:237–51. [PubMed] [Google Scholar]
- 10.Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67:175–212. doi: 10.1128/MMBR.67.2.175-212.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 12.Moore PS, Chang Y. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesarman E. The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 2002;159:27–37. doi: 10.1007/978-3-642-56352-2_4. [DOI] [PubMed] [Google Scholar]
- 14.Ganem D. KSHV-induced oncogenesis. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: 2007. Number of. [PubMed] [Google Scholar]
- 15.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, et al. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–9. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 16.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–50. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 17.Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, et al. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–23. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballesteros JA, Weinstein H. Methods in Neurosciences. Vol. 25. Academic Press; 1995. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors; pp. 366–428. Number of 366–428. [Google Scholar]
- 19.Porter JE, Hwa J, Perez DM. Activation of the alpha1b-adrenergic receptor is initiated by disruption of an interhelical salt bridge constraint. The Journal of biological chemistry. 1996;271:28318–23. doi: 10.1074/jbc.271.45.28318. [DOI] [PubMed] [Google Scholar]
- 20.Ho HH, Ganeshalingam N, Rosenhouse-Dantsker A, Osman R, Gershengorn MC. Charged residues at the intracellular boundary of transmembrane helices 2 and 3 independently affect constitutive activity of Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. The Journal of biological chemistry. 2001;276:1376–82. doi: 10.1074/jbc.M007885200. [DOI] [PubMed] [Google Scholar]
- 21.Verzijl D, Pardo L, van Dijk M, Gruijthuijsen YK, Jongejan A, et al. Helix 8 of the Viral Chemokine Receptor ORF74 Directs Chemokine Binding. Journal of Biological Chemistry. 2006;281:35327–35. doi: 10.1074/jbc.M606877200. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Sandford G, Fei G, Nicholas J. Gα Protein Selectivity Determinant Specified by a Viral Chemokine Receptor-Conserved Region in the C Tail of the Human Herpesvirus 8 G Protein-Coupled Receptor. Journal of Virology. 2004;78:2460–71. doi: 10.1128/JVI.78.5.2460-2471.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenkilde MM, Kledal TN, Holst PJ, Schwartz TW. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. The Journal of biological chemistry. 2000;275:26309–15. doi: 10.1074/jbc.M003800200. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkilde MM, Schwartz TW. Potency of ligands correlates with affinity measured against agonist and inverse agonists but not against neutral ligand in constitutively active chemokine receptor. Molecular pharmacology. 2000;57:602–9. doi: 10.1124/mol.57.3.602. [DOI] [PubMed] [Google Scholar]
- 25.Feng H, Sun Z, Farzan MR, Feng P. Sulfotyrosines of the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promote tumorigenesis through autocrine activation. Journal of virology. 2010;84:3351–61. doi: 10.1128/JVI.01939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Invest. 1998;102:1469–72. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenkilde MM, Waldhoer M, Luttichau HR, Schwartz TW. Virally encoded 7TM receptors. Oncogene. 2001;20:1582–93. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- 28.Sodhi A, Montaner S, Gutkind JS. Does dysregulation of a deregulated GPCR trigger Kaposi’s sarcomagenesis. FASEB J. 2004 doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- 29.Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 30.Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi’s sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol. 2003;77:2631–9. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191:445–54. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen KK, Manfra DJ, Grisotto MG, Martin AP, Vassileva G, et al. The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi’s sarcoma. Journal of immunology. 2005;174:3686–94. doi: 10.4049/jimmunol.174.6.3686. [DOI] [PubMed] [Google Scholar]
- 33.Mutlu AD, Cavallin LE, Vincent L, Chiozzini C, Eroles P, et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi’s sarcoma. Cancer Cell. 2007;11:245–58. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–8. [PubMed] [Google Scholar]
- 35.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, et al. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell. 2003;3:131–43. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 36.Sodhi A, Montaner S, Patel V, Gomez-Roman J, Li Y, et al. Akt plays a central role in vGPCR-induced sarcomagenesis: a novel therapeutic target for Kaposi’s sarcoma. PNAS. 2004 doi: 10.1073/pnas.0400835101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, et al. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10:133–43. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Jham BC, Ma T, Hu J, Chaisuparat R, Friedman ER, et al. Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi’s sarcoma. PloS one. 2011;6:e19103. doi: 10.1371/journal.pone.0019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma T, Jham BC, Hu J, Friedman ER, Basile JR, et al. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14363–8. doi: 10.1073/pnas.1001065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J, Jham BC, Ma T, Friedman ER, Ferreira L, et al. Angiopoietin-like 4: a novel molecular hallmark in oral Kaposi’s sarcoma. Oral oncology. 2011;47:371–5. doi: 10.1016/j.oraloncology.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: a successful treatment for posttransplantation Kaposi’s sarcoma. Transplantation. 2004;77:760–2. doi: 10.1097/01.tp.0000115344.18025.0b. [DOI] [PubMed] [Google Scholar]
- 42.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. The New England journal of medicine. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 43.Chaisuparat R, Hu J, Jham BC, Knight ZA, Shokat KM, Montaner S. Dual inhibition of PI3Kalpha and mTOR as an alternative treatment for Kaposi’s sarcoma. Cancer research. 2008;68:8361–8. doi: 10.1158/0008-5472.CAN-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaner S. Akt/TSC/mTOR activation by the KSHV G protein-coupled receptor: emerging insights into the molecular oncogenesis and treatment of Kaposi’s sarcoma. Cell cycle. 2007;6:438–43. doi: 10.4161/cc.6.4.3843. [DOI] [PubMed] [Google Scholar]
- 45.Martin D, Galisteo R, Molinolo AA, Wetzker R, Hirsch E, Gutkind JS. PI3Kgamma mediates kaposi’s sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell. 2011;19:805–13. doi: 10.1016/j.ccr.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, et al. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–80. [PubMed] [Google Scholar]
- 47.Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, et al. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–11. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- 48.Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. The Journal of biological chemistry. 2001;276:33805–11. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- 49.Pati S, Cavrois M, Guo HG, Foulke JS, Jr, Kim J, et al. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol. 2001;75:8660–73. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz M, Murphy PM. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505–13. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- 51.Martin D, Galisteo R, Ji Y, Montaner S, Gutkind JS. An NF-kappaB gene expression signature contributes to Kaposi’s sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene. 2008;27:1844–52. doi: 10.1038/sj.onc.1210817. [DOI] [PubMed] [Google Scholar]
- 52.Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, et al. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi’s sarcoma. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8683–8. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:1829–38. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 54.Polson AG, Wang D, DeRisi J, Ganem D. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2002;62:4525–30. [PubMed] [Google Scholar]
- 55.Cannon M, Philpott NJ, Cesarman E. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J Virol. 2003;77:57–67. doi: 10.1128/JVI.77.1.57-67.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cannon ML, Cesarman E. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene. 2004;23:514–23. doi: 10.1038/sj.onc.1207021. [DOI] [PubMed] [Google Scholar]
- 57.Pati S, Foulke JS, Jr, Barabitskaya O, Kim J, Nair BC, et al. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J Virol. 2003;77:5759–73. doi: 10.1128/JVI.77.10.5759-5773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz TF. KSHV/HHV8-associated lymphoproliferations in the AIDS setting. European journal of cancer. 2001;37:1217–26. doi: 10.1016/s0959-8049(01)00115-0. [DOI] [PubMed] [Google Scholar]
- 59.Hideshima T, Chauhan D, Teoh G, Raje N, Treon SP, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:1180–9. [PubMed] [Google Scholar]
- 60.Goodrum F, Caviness K, Zagallo P. Human cytomegalovirus persistence. Cellular microbiology. 2012;14:644–55. doi: 10.1111/j.1462-5822.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–9. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 62.Cobbs CS. Evolving evidence implicates cytomegalovirus as a promoter of malignant glioma pathogenesis. Herpesviridae. 2011;2:10. doi: 10.1186/2042-4280-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Streblow DN, Orloff SL, Nelson JA. Do pathogens accelerate atherosclerosis? The Journal of nutrition. 2001;131:2798S–804S. doi: 10.1093/jn/131.10.2798S. [DOI] [PubMed] [Google Scholar]
- 64.Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774–7. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- 65.Gao JL, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. The Journal of biological chemistry. 1994;269:28539–42. [PubMed] [Google Scholar]
- 66.Kuhn DE, Beall CJ, Kolattukudy PE. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211:325–30. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- 67.Casarosa P, Gruijthuijsen YK, Michel D, Beisser PS, Holl J, et al. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. The Journal of biological chemistry. 2003;278:50010–23. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- 68.Hulshof JW, Vischer HF, Verheij MH, Fratantoni SA, Smit MJ, et al. Synthesis and pharmacological characterization of novel inverse agonists acting on the viral-encoded chemokine receptor US28. Bioorg Med Chem. 2006;14:7213–30. doi: 10.1016/j.bmc.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 69.Vischer HF, Hulshof JW, Hulscher S, Fratantoni SA, Verheij MH, et al. Identification of novel allosteric nonpeptidergic inhibitors of the human cytomegalovirus-encoded chemokine receptor US28. Bioorg Med Chem. 2010;18:675–88. doi: 10.1016/j.bmc.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 70.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends in pharmacological sciences. 2006;27:92–6. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. The Journal of biological chemistry. 2002;277:24515–21. doi: 10.1074/jbc.M200889200. [DOI] [PubMed] [Google Scholar]
- 72.Groblewski T, Maigret B, Larguier R, Lombard C, Bonnafous JC, Marie J. Mutation of Asn111 in the third transmembrane domain of the AT1A angiotensin II receptor induces its constitutive activation. The Journal of biological chemistry. 1997;272:1822–6. doi: 10.1074/jbc.272.3.1822. [DOI] [PubMed] [Google Scholar]
- 73.Savarese TM, Wang CD, Fraser CM. Site-directed mutagenesis of the rat m1 muscarinic acetylcholine receptor. Role of conserved cysteines in receptor function. The Journal of biological chemistry. 1992;267:11439–48. [PubMed] [Google Scholar]
- 74.Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, et al. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. The Journal of biological chemistry. 2001;276:1133–7. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- 75.Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76:8161–8. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mokros T, Rehm A, Droese J, Oppermann M, Lipp M, Hopken UE. Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. The Journal of biological chemistry. 2002;277:45122–8. doi: 10.1074/jbc.M208214200. [DOI] [PubMed] [Google Scholar]
- 77.Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, et al. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. The Journal of biological chemistry. 2003;278:19473–82. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- 78.Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. The Journal of biological chemistry. 2003;278:21663–71. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- 79.Bodaghi B, Jones TR, Zipeto D, Vita C, Sun L, et al. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–66. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Billstrom MA, Johnson GL, Avdi NJ, Worthen GS. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72:5535–44. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Randolph-Habecker JR, Rahill B, Torok-Storb B, Vieira J, Kolattukudy PE, et al. The expression of the cytomegalovirus chemokine receptor homolog US28 sequesters biologically active CC chemokines and alters IL-8 production. Cytokine. 2002;19:37–46. doi: 10.1006/cyto.2002.0874. [DOI] [PubMed] [Google Scholar]
- 82.McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325:241–51. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 83.Moepps B, Tulone C, Kern C, Minisini R, Michels G, et al. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16) Cell Signal. 2008;20:1528–37. doi: 10.1016/j.cellsig.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol. 2004;78:8382–91. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, et al. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–20. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- 86.Streblow DN, Vomaske J, Smith P, Melnychuk R, Hall L, et al. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. The Journal of biological chemistry. 2003;278:50456–65. doi: 10.1074/jbc.M307936200. [DOI] [PubMed] [Google Scholar]
- 87.Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, et al. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009;69:2861–9. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- 88.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, et al. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13068–73. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, et al. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71:6643–53. doi: 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dziurzynski K, Wei J, Qiao W, Hatiboglu MA, Kong LY, et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4642–9. doi: 10.1158/1078-0432.CCR-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Science signaling. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 92.Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, et al. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest. 2010;120:3969–78. doi: 10.1172/JCI42563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vischer HF, Leurs R, Smit MJ. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends in pharmacological sciences. 2006;27:56–63. doi: 10.1016/j.tips.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Margulies BJ, Gibson W. The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles. Virus Res. 2007;123:57–71. doi: 10.1016/j.virusres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tschische P, Tadagaki K, Kamal M, Jockers R, Waldhoer M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol. 2011;82:610–9. doi: 10.1016/j.bcp.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Connor CM, Shenk T. Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread. J Virol. 2011;85:3700–7. doi: 10.1128/JVI.02442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michel D, Milotic I, Wagner M, Vaida B, Holl J, et al. The human cytomegalovirus UL78 gene is highly conserved among clinical isolates, but is dispensable for replication in fibroblasts and a renal artery organ-culture system. J Gen Virol. 2005;86:297–306. doi: 10.1099/vir.0.80436-0. [DOI] [PubMed] [Google Scholar]
- 98.Burkitt D. A tumour syndrome affecting children in tropical Africa. Postgraduate medical journal. 1962;38:71–9. doi: 10.1136/pgmj.38.436.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Epstein MA, Henle G, Achong BG, Barr YM. Morphological and Biological Studies on a Virus in Cultured Lymphoblasts from Burkitt’s Lymphoma. The Journal of experimental medicine. 1965;121:761–70. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niedobitek G, Young LS. Epstein-Barr virus persistence and virus-associated tumours. Lancet. 1994;343:333–5. doi: 10.1016/s0140-6736(94)91167-3. [DOI] [PubMed] [Google Scholar]
- 101.Beisser PS, Verzijl D, Gruijthuijsen YK, Beuken E, Smit MJ, et al. The Epstein-Barr virus BILF1 gene encodes a G protein-coupled receptor that inhibits phosphorylation of RNA-dependent protein kinase. Journal of virology. 2005;79:441–9. doi: 10.1128/JVI.79.1.441-449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. Journal of virology. 2005;79:536–46. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ressing ME, Horst D, Griffin BD, Tellam J, Zuo J, et al. Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Seminars in cancer biology. 2008;18:397–408. doi: 10.1016/j.semcancer.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Zuo J, Quinn LL, Tamblyn J, Thomas WA, Feederle R, et al. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. Journal of virology. 2011;85:1604–14. doi: 10.1128/JVI.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS pathogens. 2009;5:e1000255. doi: 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vischer HF, Nijmeijer S, Smit MJ, Leurs R. Viral hijacking of human receptors through heterodimerization. Biochemical and biophysical research communications. 2008;377:93–7. doi: 10.1016/j.bbrc.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 107.Nijmeijer S, Leurs R, Smit MJ, Vischer HF. The Epstein-Barr virus-encoded G protein-coupled receptor BILF1 hetero-oligomerizes with human CXCR4, scavenges Galphai proteins, and constitutively impairs CXCR4 functioning. The Journal of biological chemistry. 2010;285:29632–41. doi: 10.1074/jbc.M110.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lyngaa R, Norregaard K, Kristensen M, Kubale V, Rosenkilde MM, Kledal TN. Cell transformation mediated by the Epstein-Barr virus G protein-coupled receptor BILF1 is dependent on constitutive signaling. Oncogene. 2010;29:4388–98. doi: 10.1038/onc.2010.173. [DOI] [PubMed] [Google Scholar]
- 109.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. Journal of virology. 1993;67:2209–20. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–43. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama T, Fujisawa R, Izawa D, Hieshima K, Takada K, Yoshie O. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J Virol. 2002;76:3072–7. doi: 10.1128/JVI.76.6.3072-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation--a global toggle switch model. Annual review of pharmacology and toxicology. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 113.Stein JV, Soriano SF, M’Rini C, Nombela-Arrieta C, de Buitrago GG, et al. CCR7-mediated physiological lymphocyte homing involves activation of a tyrosine kinase pathway. Blood. 2003;101:38–44. doi: 10.1182/blood-2002-03-0841. [DOI] [PubMed] [Google Scholar]
- 114.Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, et al. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313–24. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adachi S, Kuwata T, Miyaike M, Iwata M. Induction of CCR7 expression in thymocytes requires both ERK signal and Ca(2+) signal. Biochem Biophys Res Commun. 2001;288:1188–93. doi: 10.1006/bbrc.2001.5912. [DOI] [PubMed] [Google Scholar]
- 116.Bardi G, Niggli V, Loetscher P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett. 2003;542:79–83. doi: 10.1016/s0014-5793(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 117.Yanagawa Y, Onoe K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood. 2003;101:4923–9. doi: 10.1182/blood-2002-11-3474. [DOI] [PubMed] [Google Scholar]
- 118.Levy JA. Three new human herpesviruses (HHV6, 7, and 8) Lancet. 1997;349:558–63. doi: 10.1016/S0140-6736(97)80119-5. [DOI] [PubMed] [Google Scholar]
- 119.Dockrell DH. Human herpesvirus 6: molecular biology and clinical features. J Med Microbiol. 2003;52:5–18. doi: 10.1099/jmm.0.05074-0. [DOI] [PubMed] [Google Scholar]
- 120.de Pagter PJ, Schuurman R, Visscher H, de Vos M, Bierings M, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:831–9. doi: 10.1016/j.bbmt.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 121.Betts BC, Young JA, Ustun C, Cao Q, Weisdorf DJ. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2011;17:1562–8. doi: 10.1016/j.bbmt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 1998;72:6104–12. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakano K, Tadagaki K, Isegawa Y, Aye MM, Zou P, Yamanishi K. Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 2003;77:8108–15. doi: 10.1128/JVI.77.14.8108-8115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tadagaki K, Nakano K, Yamanishi K. Human herpesvirus 7 open reading frames U12 and U51 encode functional beta-chemokine receptors. Journal of virology. 2005;79:7068–76. doi: 10.1128/JVI.79.11.7068-7076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J Immunol. 2000;164:2396–404. doi: 10.4049/jimmunol.164.5.2396. [DOI] [PubMed] [Google Scholar]
- 126.Catusse J, Spinks J, Mattick C, Dyer A, Laing K, et al. Immunomodulation by herpesvirus U51A chemokine receptor via CCL5 and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human leukocytes. European journal of immunology. 2008;38:763–77. doi: 10.1002/eji.200737618. [DOI] [PubMed] [Google Scholar]
- 127.Fitzsimons CP, Gompels UA, Verzijl D, Vischer HF, Mattick C, et al. Chemokine-directed trafficking of receptor stimulus to different g proteins: selective inducible and constitutive signaling by human herpesvirus 6-encoded chemokine receptor U51. Molecular pharmacology. 2006;69:888–98. doi: 10.1124/mol.105.015222. [DOI] [PubMed] [Google Scholar]
- 128.Zhen Z, Bradel-Tretheway B, Sumagin S, Bidlack JM, Dewhurst S. The human herpesvirus 6 G protein-coupled receptor homolog U51 positively regulates virus replication and enhances cell-cell fusion in vitro. Journal of virology. 2005;79:11914–24. doi: 10.1128/JVI.79.18.11914-11924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kufareva I, Rueda M, Katritch V, Stevens RC, Abagyan R. Status of GPCR modeling and docking as reflected by community-wide GPCR Dock 2010 assessment. Structure. 2011;19:1108–26. doi: 10.1016/j.str.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuznetsov DA, Abagyan RA. A technique for identifying atoms from a screen image. Journal of molecular graphics. 1993;11:245–7. doi: 10.1016/0263-7855(93)80004-b. [DOI] [PubMed] [Google Scholar]
- 132.Abagyan R, Batalov S, Cardozo T, Totrov M, Webber J, Zhou Y. Homology modeling with internal coordinate mechanics: deformation zone mapping and improvements of models via conformational search. Proteins Suppl. 1997;1:29–37. doi: 10.1002/(sici)1097-0134(1997)1+<29::aid-prot5>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]