Abstract
p63 expression has been identified in several cohorts as a predictor of poorer prognosis in Merkel cell carcinoma. We used multivariate analysis in a large independent cohort to determine the clinical utility of this parameter. Immunohistochemistry was used to determine p63 expression on MCC tumors from 128 patients. Of these, 33% had detectable p63 expression. p63 positivity was associated with an increased risk of death from MCC (hazard ratio 2.05, p = 0.02) in a multivariate Cox regression model considering stage at presentation, age at diagnosis, and gender. Although p63 expression correlated with diminished survival in this largest cohort reported thus far, the effect was weaker than that observed in prior studies. Indeed, within a given stage, p63 status did not predict survival in a clinically or statistically significant manner. It thus remains unclear whether this test should be integrated into routine MCC patient management.
Keywords: Merkel cell carcinoma, survival, prognosis, p63
Introduction
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine skin cancer, likely derived from epidermis1, and with disease-associated mortality of 46%2. An international consensus staging system was adopted in 20102, however, there remains a clinical need to identify biomarkers to refine prognosis. p63 is a transcription factor with functionally distinct alternative splice products that are expressed in the dividing basal layer of the epidermis3 as well as in many cancers. p63 expression in cancer cells has been reported to be associated with worse prognosis in certain types of cancer including squamous cell carcinoma of the head and neck and a subset of B-cell lymphomas, but with better prognosis in other cancers including estrogen receptor-positive breast cancer4-9. The expression of p63 can be tested immunohistochemically and is a standard laboratory assay used in the diagnosis of prostate and breast cancers10,11.
Asioli et al reported p63 as a potential prognostic factor for Merkel cell carcinoma12,13. p63 expression was detected in 61% of MCCs (43 of 70 cases) and demonstrated a strong association with poor survival in a univariate analysis for localized MCC (20% 5-year survival for patients with p63 positive tumors; n=21 vs. 100% for those with p63 negative tumors; n=19; p<.0001). Hall et al recently reported (n=42) that p63 expression represents a strong risk factor (p<0.0001) for shortened survival14. Another study of 17 cases suggested a correlation between p63 expression and poor prognosis in MCC15. In contrast, Lim et al reported no significant association of p63 expression with disease outcome, however, in this study only 9% cases were positive for p63 expression16. None of these studies carried out multivariate analysis for p63 and survival in which stage was included. The goals of the present study were to test whether p63 independently predicts survival for a larger MCC cohort in a multivariate analysis and to explore its potential clinical utility.
Materials and methods
Patient characteristics
All studies were performed in accordance with Helsinki principles and were approved by the Institutional Review Board (IRB study number: 6585) at the Fred Hutchinson Cancer Research Center. A total of 128 patients were included in this study. These included patients who had enrolled in Merkel cell carcinoma research while seeking care at a tertiary care center affiliated with this study or via an informational website (www.merkelcell.org). A total of 156,301 days (5139 months) of detailed follow-up were obtained. Median follow-up was 41 months for patients who did not die of MCC (35 months among all patients). There were 11 cases in which patients died, and it was not possible to obtain the cause of death. Based on statistical analysis of their stage and age, as compared to US population data, it is likely that the majority of these deaths were attributable to non-MCC related causes. Therefore, these 11 cases were attributed to the non-MCC death category.
MCC diagnosis
Diagnosis of MCC required agreement of two independent pathologists and, in the vast majority of cases, was supported by CK20 dot-like perinuclear staining as well as negative immunohistochemistry for TTF-1 and/or CK7. In cases in which these characteristic immunohistochemistry findings were not present, the pathologists determined the skin lesion was consistent with MCC based on morphology, neuron specific enolase, chromogranin, or synaptophysin positivity. In addition, if CK20 was not positive, to ensure that there was no evidence that this was a metastasis from a neuroendocrine tumor from lung or another primary site, careful radiologic evaluations were carried out.
Staging
Tumor staging was performed using the 2010 American Joint Commission on Cancer guidelines for MCC2.
Tissue selection
Formalin-fixed, paraffin-embedded tissues were obtained from >50 pathology labs across the United States. Effort was made in all cases to obtain the primary tumor sample when feasible. In the event that more than one tissue was received for a particular patient, the following hierarchy was employed for tissue selection: primary tumor > regional nodal metastasis > recurrence > distant metastasis. A total of 101 primary tumors, 15 nodal metastases, 4 recurrences, and 8 distant metastases were studied. For 11 of 15 patients who had only nodal metastases studied, the patient had no clinically identifiable primary tumor.
p63 staining
All tissues were processed in a uniform manner in 4 batches at a single institution (University of Washington Department of Pathology). 5 micrometer sections were baked onto charged glass slides. Samples were then deparaffinized, and heat-induced epitope retrieval was performed (incubation at 98° C for 18 minutes in a temperature-controlled microwave oven in citrate buffer, pH 6.0). Slides were stained for 40 minutes with mouse monoclonal anti-p63 (Clone 4A4, Dakopatts) at a dilution of 1:500 in PBS/1% BSA. This primary antibody detects all known isoforms of p63, and is the same clone used in the largest prior Merkel cell carcinoma studies12,13. Secondary detection was with avidin/biotin. All staining was performed within 9 days of slide cutting to minimize signal loss as previously described by Burford et al17. A stain with normal mouse serum in place of primary antibody was employed as negative control, and normal basal epidermis, present in most cases, served as an on-slide positive staining control.
p63 scoring
Expression of p63 was scored semi-quantitatively using the modified histochemical score (H-score) of McCarthy et al18. In brief, scores range from 0-300 and are calculated using the following formula: 0*(% of MCC nuclei with no staining) + 1*(% of MCC nuclei with weak staining) + 2*(% of MCC nuclei with moderate staining) + 3*(% of MCC nuclei with moderate staining). Three separate high power fields were scored for each sample independently by two pathologists. The median score was then calculated from these six reported high power fields in order to obtain a single score for each sample. Agreement between the two pathologists was generally very strong (R2 value of 0.84 for linear regression comparing the numeric median scores).
An H-score of 10 or greater was considered to be positive. This cut-point was selected prior to scoring or statistical analyses and was chosen because it was employed as the cut-point in Asioli et al12,13 in order to be comparable to their prior studies. An H-score of 10 roughly translates to 1 in 10 MCC nuclei staining weakly or 1 in 33 MCC nuclei staining strongly for p63. Six percent (8 of 128) of the p63 stained slides were discrepant between the two pathologists regarding the critical H score threshold of 10. These discrepant cases were re-reviewed and a consensus H score was determined.
Statistical analysis
All statistical analyses were performed using STATA software (StataCorp LP). Merkel cell carcinoma specific mortality was analyzed using univariate and multivariate Cox regression. Deaths known to be due to non-MCC causes (n=3) were treated as a competing risk. Deaths where the cause of death was unknown (n=8) were treated as presumptive MCC deaths. Cumulative incidence of MCC-specific mortality was estimated using standard methods. All p-values are two-sided and derived from likelihood ratio tests.
Results
P63 expression was detectable in 33% of MCC tumors
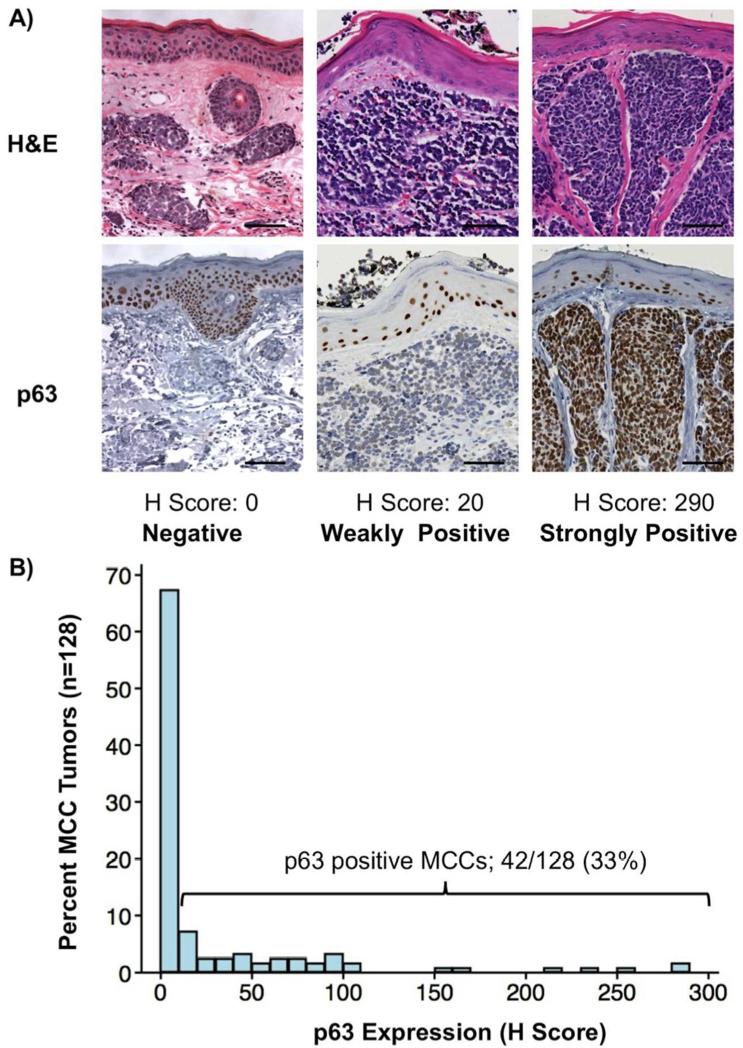
MCC tumors from a total of 128 patients were studied for p63 expression (Figure 1). Of these, 33% (n=42) expressed p63 at least weakly (Table 1; Figure 1B). 79% of p63 positive MCC tumors expressed p63 at levels significantly lower than that of on-slide epidermis controls (H-score of 10-99, n=33), while 21% of positive tumors had p63 expression that was comparable to basal epidermis (H-score ≥100, n=9).
Figure 1. Expression of p63 in Merkel cell carcinoma tumors.
P63 expression in three Merkel cell carcinoma tumors is shown. In the tumor at left, no p63 expression was detected. In the tumor at center, p63 expression was weak and near the threshold of determining positive and negative (which was defined as a H-score of 10, corresponding to 10% of cells staining weakly). In contrast, the tumor at right demonstrates strong p63 expression. As expected, epidermal cells in the basal epidermis overlying all three tumors demonstrated consistent strong nuclear stain and served as an internal positive control. Scale bar = 75 micrometers, photographs taken with 20× objective.
Table 1. Patient and tumor characteristics.
A total of 128 patients were studied. The gender and age distributions were similar between p63 positive and negative patients (p>0.05 for both comparisons). Staging was performed as per 2010 American Joint Commission on Cancer guidelines 2, staging information was not available for one patient. Stage at presentation differed significantly between p63 positive and negative patients, p = 0.03 by Fisher’s exact test.
| P63 positive (n=42) | P63 negative (n=86) | |
|---|---|---|
| Male gender | 76% | 69% |
|
Age at diagnosis
(median, range) |
67 years (40-88 years) | 66 years (31-99 years) |
| Stage at diagnosis* | ||
| Stage I n=42 | 34% | 33% |
| Stage II n=21 | 5% | 22% |
| Stage III n=56 | 49% | 42% |
| Stage IV n=8 | 12% | 3% |
No significant differences in p63 expression were observed comparing MCC tumors by age or gender (Table 1). However, p63 was more likely to be positive in patients who presented with advanced MCC (Table 1, p = 0.03). Specifically, 61% of patients with p63 positive tumors had advanced disease (stage III-IV) at presentation as compared to 45% of patients whose tumors were p63 negative (p = 0.03).
Expression of p63 is associated with poorer MCC-specific survival
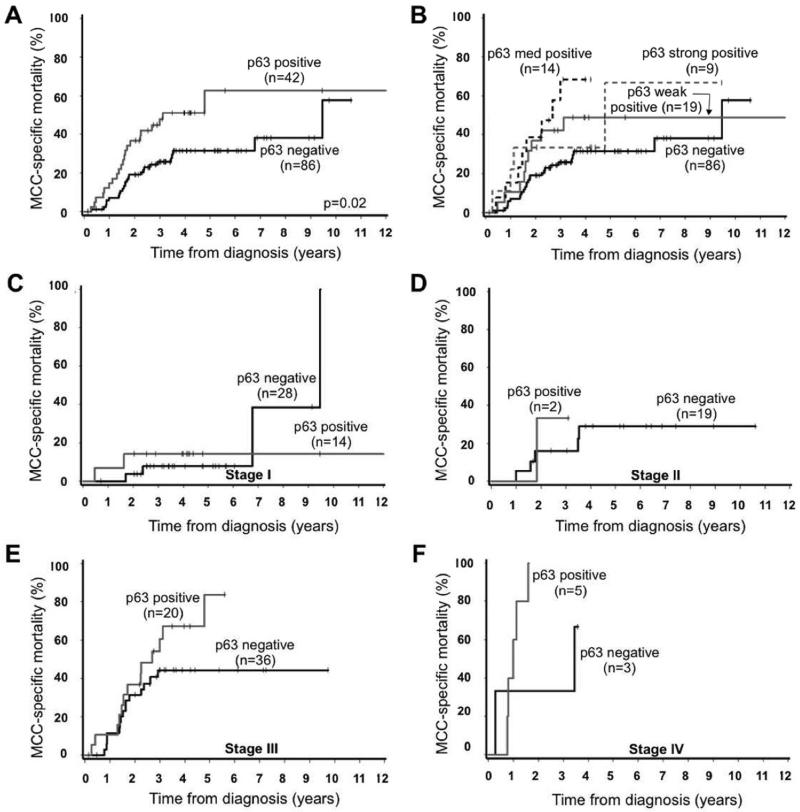
In univariate analysis, presence of p63 expression was associated with significantly higher MCC-specific mortality (hazard ratio 2.05, p = 0.02, Table 2). Cumulative mortality at 5 years was 63% among those with p63-positive tumors (n=42) as compared to 31% for those with p63-negative tumors (n=86; Figure 2A).
Table 2. p63 is an independent predictor of MCC-specific survival.
Univariate and multivariate Cox regression analyses of MCC-specific mortality. HR: hazard ratio. 95% CI: 95% confidence interval.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Stage II (vs. I) | 1.91 (0.6-5.9) | 0.27 | 2.33 (0.7-7.4) | 0.16 |
| Stage III (vs. I) | 4.94 (2.0-12) | <0.001 | 4.92 (2.0-12) | <0.001 |
| Stage IV (vs. I) | 15.8 (5.2-48) | <0.001 | 13.7 (4.3-44) | <0.001 |
| Female (vs. male) | 0.53 (0.3-1.1) | 0.07 | 0.67 (0.3-1.4) | 0.27 |
|
Age at diagnosis (per 5 years older) |
1.11 (1.0-1.3) | 0.10 | 1.04 (0.9-1.2) | 0.61 |
|
p63 expressed in
tumor |
2.05 (1.1-3.6) | 0.02 | 2.05 (1.1-3.8) | 0.02 |
Figure 2. Expression of p63 is associated with worsened Merkel cell carcinoma specific survival.
Panel A. Presence of p63 expression was a statistically significant prognostic factor in both univariate and multivariate analyses, p<= 0.01 (see Table 2). Panel B. Survival as broken down by strength of p63 expression. Panel C-F. Data from panel A, broken down by stage at diagnosis. C. Stage I, local disease, 2cm; D. Stage II, local disease, >2cm; E. Stage III, regional nodal disease; F. Stage IV, distant metastatic. Staging performed as per 2010 American Joint Committee on Cancer guidelines2.
We further tested whether the degree of p63 expression, as manifested by staining intensity, was associated with MCC-specific mortality. The strength of p63 expression was associated with MCC-specific outcome (Figure 2B) with a trend for higher mortality associated with higher levels of expression (ptrend = 0.04).
The present cohort included patients who presented with nodal disease with no identified skin primary. Analysis was repeated restricting patients to only those for whom a primary skin tumor was available for study; similar results were observed (n=101, hazard ratio for p63 expression 2.57, p = 0.02).
Multivariate analyses were performed in order to determine whether p63 expression is an independent predictor of survival that adds information to current tumor-node-metastasis based staging2. In a model also considering stage at diagnosis, age at diagnosis, and gender, p63 expression remained a statistically significant predictor of MCC-specific mortality (hazard ratio 2.05, p = 0.02, Table 2).
In order to determine the clinical utility of p63 expression for individual stages we performed subgroup analyses (Figure 2C-2F). This analysis demonstrated that p63 expression did not statistically differentiate outcomes among patients for all stages. 5-year MCC-specific mortality is described in Figure 2 (Panel C-E; Stage I: 14% mortality for p63 positive tumors versus 8% for p63 negative tumors; Stage II: neither p63-positive patient survived to five years versus 19% mortality for p63 negative patients; Stage III: 84% 5-mortality for p63 positive stage III tumors (n=20) as compared to 44% among those with p63 negative stage III tumors (n=36).
Discussion
The present study of 128 MCC tumors examines the role of p63 as a prognostic biomarker in Merkel cell carcinoma in a multivariate model. p63 expression in MCC tumor cells was found to be significantly associated with poorer disease-specific survival (p=0.02) with a roughly two-fold increase in MCC-specific mortality in this model. However, analysis of this large cohort raises questions as to whether and how p63 should routinely be used in the management of this cancer.
Although the results of this study were consistent with prior studies12,13,14 in that we found p63 to be a prognostic factor for MCC, this effect was much smaller in our cohort (hazard ratio of 2.0 versus 22.212 and 7.2613 in Asioli et al,, and ∞ in Hall et al14. This correlates to a 5 year mortality difference of 32% in this cohort (comparing p63 pos vs negative) to 91% in the initial Asioli et al study12. These studies shared many features, including use of the same antibody for p63 staining (Clone 4A4, Dakopatts) and the same scoring algorithm12,15. Our study however included more patients than individual prior studies (128 in the present study versus 7013, 3614 and 1715), which may explain the difference in observed effect size. This phenomenon of difference between initial study result and subsequent validation studies has been previously described in the literature19. Although several prior studies validate the initial Asioli et al results, Lim et al16 reported no association between p63 expression and clinical outcome in a 105 patient cohort. Potential explanations include their use of a different antibody clone (Clone 7-Jul, Novocastra vs Dakopatts) and variable time between tissue cutting and immunohistochemiccal staining (a relevant factor for p63 as discussed below).
p63 tends to be positive in more advanced disease as shown in Table 1. Specifically, patients with ‘local’ (Stage I & II; n=63) MCC at presentation were p63-positive in 39% of cases whereas patients with advanced disease (nodal or distant disease at presentation; n=64) were p63-positive in 61% of cases (p=0.03). Although statistically significant, the variability in stage at presentation among p63-positive and -negative tumors does not fully account for the survival effect because this effect persisted in multivariate analyses that include stage at presentation (Table 2).
Among the 63 patients presenting with local-only disease (stage I or II), p63 expression was not found to be associated with survival outcome (Fig 2C-D). This finding differs from the results of Asioli et al12,13, where p63 expression was associated with a significantly worse prognosis for localized MCC. Thus p63 appears to have limited clinical utility for local only disease. Furthermore, while there was improved survival among p63 negative patients presenting with advanced disease (stages III or IV, Fig 2E-F) this effect was only noted 1-3 years after diagnosis and it is unclear how p63 status could affect clinical management decisions.
There are several limitations of the present study. Although the number of tumors studied is relatively high given the rarity of Merkel cell carcinoma, the results might be affected by sampling limitations associated with studies on rare diseases. The patients participating in our study were younger on average than the overall MCC patient population (median age of 66 years vs. 76)20, possibly reflecting a recruitment bias for patients seeking care at tertiary institutions or information via the internet. Additional limitations to this study relate to challenges that are intrinsic to this p63 antibody. Although p63 immunohistochemistry is used routinely in clinical laboratories, p63 staining is challenging and its expression can vary in tissues as this antibody is known to lose sensitivity if tissue sections were not cut recently17. To minimize these effects, we stained all sample tissues in four large batches, with at most 9 days between sectioning and staining.
The association of p63 with poorer survival in MCC is logical given that this transcription factor is associated with proliferation in the epidermis. Furthermore, Merkel cells were recently shown to be of the epidermal lineage1. These data support a role (direct or indirect) in MCC biology for p63. However, study of a possible role for p63 in this cancer will be challenging because this transcription factor has multiple functionally distinct isoforms, the regulation of which is complex. The antibody used in this and prior studies detects all p63 isoforms. It is possible that an isoform-specific study might reveal more nuanced results and provide further insight into the role of p63 in MCC biology.
In conclusion, similar to prior studies, we found that p63 expression in Merkel cell carcinoma predicts poorer survival, however, its role as a independent prognostic marker is limited among patients of a given stage. Since stage is almost always known at the time of diagnosis, based on presently available data, it is unclear how routine p63 assessment would meaningfully alter management of this cancer.
Acknowledgments
We thank Farinaz Shokri, Elizabeth Donato, Ashley Warcola, Sherry Lee, and Andrew Tegeder for their contributions towards this study.
Funding Sources: Supported by American Cancer Society (ACS) RSG-08-115-01-CCE; National Institutes of Health K24-CA139052; F30ES017385 (to KP), the David & Rosalind Bloom Endowment for MCC Research, Michael Piepkorn Endowment fund, and the University of Washington MCC Patient Gift Fund.
Footnotes
Authors’ disclosures of potential conflicts of interest: The author(s) indicated no potential conflicts of interest.
References
- 1.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemos BD, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010 doi: 10.1016/j.jaad.2010.02.056. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol. 2010;5:349–371. doi: 10.1146/annurev-pathol-121808-102117. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Di Como CJ, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 5.Lo Muzio L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Human pathology. 2005;36:187–194. doi: 10.1016/j.humpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, et al. Elevated expression of p63 protein in human esophageal squamous cell carcinomas. International journal of cancer. Journal international du cancer. 2002;102:580–583. doi: 10.1002/ijc.10739. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima N, et al. Clinico-pathological characteristics of p63 expression in B-cell lymphoma. Cancer Science. 2006;97:1050–1055. doi: 10.1111/j.1349-7006.2006.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moergel M, Abt E, Stockinger M, Kunkel M. Overexpression of p63 is associated with radiation resistance and prognosis in oral squamous cell carcinoma. Oral Oncol. 2010;46:667–671. doi: 10.1016/j.oraloncology.2010.06.012. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Hanker L, et al. Clinical relevance of the putative stem cell marker p63 in breast cancer. Breast Cancer Res Treat. 122:765–775. doi: 10.1007/s10549-009-0608-6. [DOI] [PubMed] [Google Scholar]
- 10.Leite KR, et al. The use of immunohistochemistry for diagnosis of prostate cancer. Int Braz J Urol. 36:583–590. doi: 10.1590/s1677-55382010000500008. [DOI] [PubMed] [Google Scholar]
- 11.de Biase D, et al. p63 short isoforms are found in invasive carcinomas only and not in benign breast conditions. Virchows Arch. 456:395–401. doi: 10.1007/s00428-010-0900-1. [DOI] [PubMed] [Google Scholar]
- 12.Asioli S, Righi A, Volante M, Eusebi V, Bussolati G. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer. 2007;110:640–647. doi: 10.1002/cncr.22828. [DOI] [PubMed] [Google Scholar]
- 13.Asioli S, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I-II) Merkel cell carcinomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1451–1461. doi: 10.1038/modpathol.2011.100. [DOI] [PubMed] [Google Scholar]
- 14.Hall BJ, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39:911–917. doi: 10.1111/j.1600-0560.2012.01964.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, McNiff JM. Nuclear expression of survivin portends a poor prognosis in Merkel cell carcinoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21:764–769. doi: 10.1038/modpathol.2008.61. [DOI] [PubMed] [Google Scholar]
- 16.Lim CS, et al. Increasing Tumor Thickness is Associated with Recurrence and Poorer Survival in Patients with Merkel Cell Carcinoma. Annals of surgical oncology. 2012 doi: 10.1245/s10434-012-2509-x. [DOI] [PubMed] [Google Scholar]
- 17.Burford HN, Adams AL, Hameed O. Effect of storage on p63 immunohistochemistry: a time-course study. Appl Immunohistochem Mol Morphol. 2009;17:68–71. doi: 10.1097/PAI.0b013e31818110de. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy K, Miller L, Cox E, Konrath J, McCarthy KS. Estrogen receptor analysis: correlation of biochemical and immunohistochemical methods using monoclonal anti-receptor antibodies. Arch Pathol Lab Med. 1985:716–721. [PubMed] [Google Scholar]
- 19.Ioannidis JP. Why most published research findings are false. PLoS medicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albores-Saavedra J, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009 doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]